Abstract
Background
Respiratory syncytial virus (RSV) is a major cause of hospitalisation in infants. The burden of RSV infection in healthy term infants has not yet been established. Accurate health-care burden data in healthy infants are necessary to determine RSV immunisation policy when RSV immunisation becomes available.
Methods
We performed a multicentre, prospective, observational birth cohort study in healthy term-born infants (≥37 weeks of gestation) in five sites located in different European countries to determine the health-care burden of RSV. The incidence of RSV-associated hospitalisations in the first year of life was determined by parental questionnaires and hospital chart reviews. We performed active RSV surveillance in a nested cohort to determine the incidence of medically attended RSV infections. The study is registered with ClinicalTrials.gov, NCT03627572.
Findings
In total, 9154 infants born between July 1, 2017, and April 1, 2020, were followed up during the first year of life and 993 participated in the nested active surveillance cohort. The incidence of RSV-associated hospitalisations in the total cohort was 1·8% (95% CI 1·6–2·1). There were eight paediatric intensive care unit admissions, corresponding to 5·5% of 145 RSV-associated hospitalisations and 0·09% of the total cohort. Incidence of RSV infection in the active surveillance cohort confirmed by any diagnostic assay was 26·2% (24·0–28·6) and that of medically attended RSV infection was 14·1% (12·3–16·0).
Interpretation
RSV-associated acute respiratory infection causes substantial morbidity, leading to the hospitalisation of one in every 56 healthy term-born infants in high-income settings. Immunisation of pregnant women or healthy term-born infants during their first winter season could have a major effect on the health-care burden caused by RSV infections.
Funding
Innovative Medicines Initiative 2 Joint Undertaking, with support from the EU's Horizon 2020 research and innovation programme and European Federation of Pharmaceutical Industries and Associations.
Introduction
Respiratory syncytial virus (RSV) causes a substantial burden of disease in infants worldwide with an estimated annual mortality of 101 400 in children younger than 5 years.1 Although more than 97% of RSV-attributable deaths occur in low-income and middle-income countries, the health-care burden of RSV infection in high-income countries is considerable, with an estimated annual hospitalisation rate of three per 1000 children younger than 5 years in the USA.2 Passive immunisation against RSV with palivizumab is available for high-risk groups, including premature infants and children with congenital heart disease or bronchopulmonary dysplasia. Because the majority of children hospitalised with RSV have no pre-existing conditions, a high morbidity is seen in infants younger than 6 months despite the availability of palivizumab.2 Various maternal vaccine and passive immunisation trials, which aim to protect all infants in the first months of life, are currently in phase 3 or submitted for regulatory approval.3, 4, 5 Expectations are that within 1–3 years one or several of these products will be approved by regulatory authorities and governments will have to decide whether these newly available prevention strategies should be implemented into their national immunisation schedule.6 Accurate information about RSV health-care burden in healthy infants is essential for decision makers to evaluate the health and economic benefit of these new prevention strategies.
Most large studies that aimed to determine RSV-associated hospitalisation rates in young children included children with comorbidities, were country-specific, and partly based on estimates instead of actual numbers.2, 7, 8 Birth cohort studies estimate disease incidence more accurately, but previous prospective birth cohorts in healthy infants were relatively small (158–1143 participants) and done in one centre or country, restricting generalisability.9, 10, 11, 12, 13, 14, 15, 16, 17, 18 To our knowledge, the largest prospective birth cohort determining RSV burden was a South African, single-centre study that reported 54 RSV-associated hospitalisations in 1143 children (17% with comorbidity) in the first 2 years of life.13 To prepare for the introduction of RSV immunisation, the Respiratory Syncytial virus Consortium in Europe (RESCEU) international consortium was funded by the EU Commission to obtain accurate data on the incidence and long-term consequences of RSV infection in healthy term infants.
Research in context.
Evidence before this study
We searched PubMed, using the terms “RSV” or “respiratory syncytial virus”, “hospitalisations”, and “infant” or “first year of life”, on May 31, 2022, for studies published between Jan 1, 1993, and May 31, 2022, with no language restrictions. The results, 4957 articles, included mostly retrospective analyses of RSV-coded hospitalisations from health registries or prospective studies conducted in a single country. These studies emphasised the large morbidity and mortality burden in young children associated with RSV. In a systematic review and meta-analysis from The Lancet, RSV was estimated to be associated with 3·6 million hospitalisations for acute lower respiratory infections and 101 400 in-hospital or out-of-hospital deaths in children younger than 5 years, annually, worldwide. A gap exists in the knowledge of the RSV burden in healthy term infants, the largest population of RSV-infected infants. We identified ten birth cohort studies that reported RSV-associated hospitalisation in infants with estimates varying between 0·6% and 5%. These birth cohorts had relatively small sample sizes with 156 to 1143 participants, and only five included only healthy term-born children. The reliability and the precision of these estimates can be improved by large prospective birth cohorts conducted in multiple countries. Several maternal vaccines and passive immunisation against RSV are currently at advanced stages of clinical development or under review for licensure. To decide how these new prevention strategies should be included in national vaccination programmes, precise estimates of the health-care burden of RSV infections in the first months of life are required.
Added value of this study
The RESCEU birth cohort study is the largest multicentre prospective birth cohort that evaluated the incidence of RSV-associated hospitalisations and medically attended acute respiratory infections. It was designed to provide a precise and up-to-date estimate of the total RSV incidence and health-care burden in Europe. Almost 10 000 participants were enrolled in five European countries and 97% were successfully followed up during the first year of life. To estimate the incidence of medically attended RSV infections, we actively followed up a nested cohort of approximately 1000 participants. The incidence of RSV-confirmed hospitalisations in the first year of life was 1·8% (95% CI 1·6–2·1). About half of hospitalisations for respiratory tract infection in the first year of life were associated with RSV. The majority (57·9%) of RSV-associated hospitalisations occurred in children younger than 3 months. The incidence of medically attended RSV infections was 14·1% (12·3–16·0).
Implications of all the available evidence
This study provides the precise estimates of the health-care burden of RSV required to decide on future RSV immunisation programmes. The health-care burden of RSV among healthy infants is considerable in Europe, with one in 56 healthy term-born infants hospitalised for RSV infection annually. As the incidence of severe RSV infection is highest in the first months of life, maternal vaccination as well as passive infant immunisation could have a major effect on the health of healthy term infants.
The primary objective of this study was to determine the incidence of medically attended and hospitalised RSV-associated respiratory infections in healthy term infants in Europe. Secondary objectives included estimating the incidence of symptomatic RSV infections, the incidence of all-cause respiratory infections, and the proportion of respiratory infections attributable to RSV.
Methods
Study design
The study design and protocol have been described previously.19 In short, healthy term-born infants were enrolled at birth between July 1, 2017, and July 31, 2020, in five sites each located in a different European country representing western, northern, and southern Europe (Spain, Finland, England, Scotland, and the Netherlands). Children born at 37 weeks or more of gestation with no evidence of significant cardiovascular, respiratory, renal, gastrointestinal, haematological, neurological, endocrine, immunological, musculoskeletal, oncological, or congenital disorders were considered healthy term-born.18 All participating children were followed up for at least 1 year. Children diagnosed with comorbidities later were not systematically excluded. We used parental questionnaires to screen for hospitalisation for acute respiratory infection (ARI) during the first year of life at the age of 1 year. Hospital records, including RSV testing results, were retrospectively assessed in case of hospitalisation for ARI. All participating hospitals tested for RSV during the RSV season as part of standard care and were situated in a distinct geographical area to ensure that children were preferentially referred to that hospital if inpatient care was needed. For infants whose parents did not complete the 1-year questionnaire, hospital records were screened for ARI hospitalisations within the first year of life in participating hospitals.
At enrolment at all five sites, participants of the birth cohort were also invited to participate in a nested cohort (referred to as active surveillance cohort). Participants of the birth cohort and the active surveillance cohort were recruited on a voluntary basis and therefore were a convenience sample of term-born children living in the catchment area of the sites. To obtain a cohort with evenly distributed months and years of birth over the recruitment period, sites were instructed to recruit 15–20 participants per week, including two participants in the active surveillance cohort. Enrolment in the active surveillance cohort continued until the planned sample size was reached in each site (200 per site). Infants were actively followed up until their first birthday during the RSV seasons of 2017–18, 2018–19, and 2019–20. Between Oct 1 and May 1 (or longer if RSV was still circulating), parents were contacted weekly to report ARI symptoms of their child. In case of an ARI, a study visit was planned within 72 h of notification to obtain a nasal swab for RSV testing. Parents completed a diary with respiratory symptoms and health-care usage for 14 days after symptoms onset.18 Written or electronic informed consent was obtained from the parents of all study participants.
RSV detection in active surveillance cohort
At all sites, a nasal sample was collected during each ARI episode by using minitip flocked swabs (FLOQSwab, Copan Diagnostics, California, USA), and directly stored in viral transport medium (MicroTest M4RT [Remel, 3 mL]). All samples were stored at –80°C. After the end of the study, all samples were tested with in-house RSV quantitative reverse transcription PCR (RT-qPCR; appendix p 2).20, 21 In addition, a point of care test (POCT, Alere i RSV assay [Alere, Waltham, MA, USA]) was performed at the time of sample collection at the three sites in Spain, England, and the Netherlands. If the infant had an RSV-positive ARI episode, POCT was not performed during further ARIs. An RSV-positive ARI episode was defined as a positive test result from either in-house RT-qPCR or POCT, or both.
Outcomes and statistical analysis
An ARI episode was defined as the onset or worsening of any of the following symptoms for at least 1 day: runny or blocked nose, coughing, wheezing, or dyspnoea.19 Episodes were associated with RSV if a POCT or in-house PCR test was positive for RSV. Samples taken more than 10 days after onset were excluded from analysis. Medically attended ARI were defined as ARI episodes with at least one visit to a health-care provider (outpatient clinics, emergency department visits, general practitioner visits) or hospitalisation. RSV-associated hospitalisations, RSV-associated ARI, and medically attended RSV-associated ARI were reported as incidence (ie, the proportion of infants experiencing the event at least once during their first year of life) and as incidence rate per 1000 infant-months (number of events per 1000 infant-months of follow-up). The use of incidence rates in addition to incidence was pre-defined in the statistical analysis plan to account for possible variation in follow-up time due to early dropouts of participants and for participants experiencing outcomes more than once (appendix pp 17–44). Wheezing during the first year of life was defined as at least one wheezing episode reported by parents in the 1-year questionnaire.
Statistical analyses were performed according to the predefined statistical analysis plan (appendix pp 17–44). For sample size calculation of the total cohort, a yearly incidence of hospitalisations of 0·7% was assumed on the basis of previous literature.2, 22 A sample size of 8700 would produce a two-sided 95% Clopper-Pearson CI with a half-width of 0·2% for this incidence. If accounting for 10% loss to follow-up 10 000 infants were to be included.19 Similarly, a sample size of 1000 infants was estimated for the active surveillance cohort, which would produce a two-sided 95% Clopper-Pearson CI with a half-width of 2%, for an assumed incidence of medically attended ARI of 10%.2, 9, 22 Baseline characteristics and clinical parameters were summarised by frequency and percentage for categorical variables and mean (SD) or median (IQR) for continuous variables. Baseline characteristics were compared between groups using χ2 tests for categorical variables, Student's t tests for normally distributed continuous variables and Mann-Whitney U tests for not normally distributed continuous variables. RSV status was assumed negative when hospitalisation occurred outside of the RSV season. RSV status of hospitalisations during the RSV season and ARI in the active surveillance cohort with invalid or missing RSV test results were imputed using multiple imputation based on site, sex, age, and meteorological season at time of hospitalisation or ARI. Any missing observations for medical attendance of ARIs was subsequently imputed using the same set of predictors to which RSV status was added. Imputation yielded ten complete datasets for each of the two cohorts. After imputation, pooled 95% Wilson-score CIs were calculated for the proportion of infants with at least one RSV-associated hospitalisation or ARI in the first year. Incidence rates were calculated together with 95% CIs based on a Poisson distribution and compared between subgroups of infants using Poisson generalised linear models. Statistical analyses were performed using SPSS (version 26) and R statistical software (version 3.5.1).
The study was approved by the Institutional Review Board of the University Medical Center Utrecht (ref 17/069), National Health Service National Research Ethics Service Oxfordshire Committee A (ref 17/SC/0335) and South East Scotland Research Ethics Committee (ref 17/SS/0086), the Ethics Committee of the Hospital District of Southwest Finland (ref 17201), and Hospital Clínico Universitario de Santiago de Compostela (ref 2017/175).
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies (appendix pp 11–16).
The study is registered with ClinicalTrials.gov, NCT03627572.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report or the decision to submit for publication.
Results
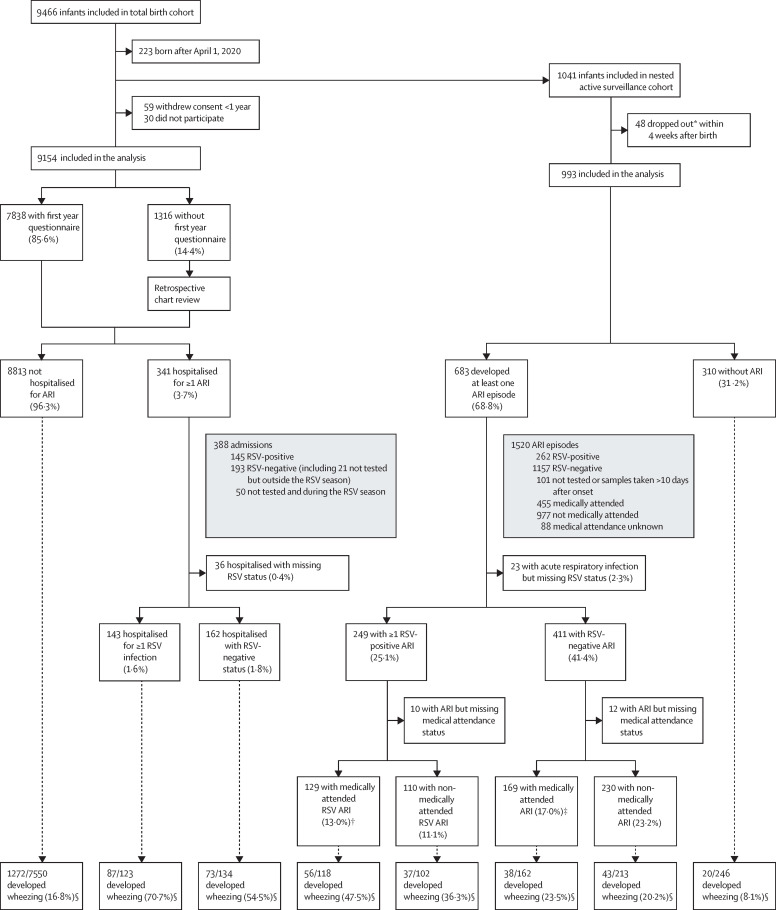
Between July 1, 2017, and July 31, 2020, 9466 healthy term infants were recruited at birth, of whom 9154 (96·7%) were included in the primary analysis (figure 1 ). Because of the COVID-19 pandemic, 223 infants born after April 1, 2020, were excluded as RSV was not circulating during their first year of life. Between Sept 1, 2017, and Nov 30, 2019, 1041 infants were enrolled in the active surveillance cohort and 993 (95·4%) who participated for at least 4 weeks were included in the analysis (figure 1). Five deaths occurred in study participants, none was related to RSV. There was substantial and expected variation in baseline characteristics between countries (table 1 ). Non-exhaustively, the most common ethnic origin was according to country geographical location, smokers in the family were more common in Spain, and maternal vaccination was almost never reported in the Netherlands where it was not recommended at the time. Compared with the rest of the cohort, participants of the active surveillance cohort more frequently reported maternal vaccination against influenza or pertussis, multiple births, a family history of atopy, and parental university level of education, whereas parental smoking and parental origin from northwest Europe were reported less frequently; they also had fewer siblings and were born later in the year than other participants.
Figure 1.
Flow chart of participants in RESCEU birth cohort study for total cohort and active surveillance cohort
ARI=acute respiratory infection. RSV=respiratory syncytial virus. *Did not continue with active surveillance. †Including 16 RSV admissions (also counted in RSV admissions). ‡Including seven ARI admissions (also counted in RSV-negative admission). §Number of children with wheezing of total number of children with known wheezing status.
Table 1.
Baseline characteristics of participants by recruitment sites based on participants with available information
|
Total cohort |
Active surveillance cohort |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scotland | England | Spain | Finland | Netherlands | All | Scotland | England | Spain | Finland | Netherlands | All | |||
| Total number of participants | 2130 | 1972 | 1080 | 2093 | 1879 | 9154 | 203 | 198 | 205 | 200 | 187 | 993 | ||
| Follow-up time (infant-months) | 25 498 | 23 458 | 12 949 | 25 119 | 22 484 | 109 507 | 2408 | 2288 | 2404 | 2384 | 2245 | 11 729 | ||
| Pregnancy | ||||||||||||||
| Vaccination* | 1815/2127 (85%) | 1766/1947 (91%) | 632/1037 (61%) | 933/2072 (45%) | 626/1827 (34%) | 5772/9010 (64%) | 188/203 (93%) | 183/196 (93%) | 118/200 (59%) | 129/198 (65%) | 57/186 (31%) | 675/983 (69%) | ||
| Influenza | 1444/2127 (68%) | 1417/1947 (73%) | 294/1037 (28%) | 931/2072 (45%) | 25/1827 (1%) | 4111/9010 (46%) | 154/203 (76%) | 142/196 (72%) | 37/200 (19%) | 128/198 (65%) | 5/186 (3%) | 466/983 (47%) | ||
| Pertussis | 1742/2127 (82%) | 1672/1947 (86%) | 597/1037 (58%) | 3/2072 (<1%) | 617/1827 (34%) | 4631/9010 (51%) | 181/203 (89%) | 179/196 (91%) | 113/200 (57%) | 1/198 (1%) | 55/186 (30%) | 529/983 (54%) | ||
| Smoking during pregnancy | 153/2129 (7%) | 102/1954 (5%) | 109/1049 (10%) | 112/2086 (5%) | 65/1827 (4%) | 541/9045 (6%) | 9/203 (4%) | 10/198 (5%) | 18/204 (9%) | 13/198 (7%) | 4/186 (2%) | 54/989 (5%) | ||
| Birth | ||||||||||||||
| Month of birth* | ||||||||||||||
| Oct–Dec | 509/2130 (24%) | 437/1972 (22%) | 285/1080 (26%) | 435/2093 (21%) | 518/1879 (28%) | 2184/9154 (24%) | 30/203 (15%) | 26/198 (13%) | 69/205 (34%) | 38/200 (19%) | 62/187 (33%) | 225/993 (23%) | ||
| Jan–March | 658/2130 (31%) | 565/1972 (29%) | 254/1080 (24%) | 324/2093 (15%) | 612/1879 (33%) | 2413/9154 (26%) | 32/203 (16%) | 28/198 (14%) | 33/205 (16%) | 58/200 (29%) | 63/187 (34%) | 214/993 (22%) | ||
| April–June | 468/2130 (22%) | 543/1972 (28%) | 157/1080 (15%) | 615/2093 (29%) | 310/1879 (16%) | 2093/9154 (23%) | 70/203 (34%) | 60/198 (30%) | 29/205 (14%) | 68/200 (34%) | 29/187 (16%) | 256/993 (26%) | ||
| July–Sept | 495/2130 (23%) | 427/1972 (22%) | 384/1080 (36%) | 719/2093 (34%) | 439/1879 (23%) | 2464/9154 (27%) | 71/203 (35%) | 84/198 (42%) | 74/205 (36%) | 36/200 (18%) | 33/187 (18%) | 298/993 (30%) | ||
| Male sex | 1108/2130 (52%) | 1036/1944 (53%) | 555/1080 (51%) | 1093/2093 (52%) | 933/1869 (50%) | 4725/9116 (52%) | 106/203 (52%) | 108/197 (55%) | 107/205 (52%) | 106/200 (53%) | 85/187 (45%) | 512/992 (52%) | ||
| Female sex | 1022/2130 (48%) | 908/1944 (47%) | 525/1080 (49%) | 1000/2093 (48%) | 936/1869 (50%) | 4391/9116 (48%) | 97/203 (48%) | 89/197 (45%) | 98/205 (48%) | 94/200 (47%) | 102/187 (55%) | 480/992 (48%) | ||
| Multiple birth* | 52/2120 (2%) | 65/1940 (3%) | 36/1080 (3%) | 17/2093 (1%) | 27/1872 (1%) | 197/9105 (2%) | 18/203 (9%) | 5/195 (3%) | 7/205 (3%) | 2/200 (1%) | 6/187 (3%) | 38/990 (4%) | ||
| Caesarean delivery* | 927/2126 (44%) | 742/1941 (38%) | 238/1080 (22%) | 293/2091 (14%) | 409/1872 (22%) | 2607/9110 (29%) | 83/203 (41%) | 76/197 (39%) | 65/205 (32%) | 28/200 (14%) | 44/187 (24%) | 296/992 (30%) | ||
| Birth weight <2500 g | 40/2092 (2%) | 54/1938 (3%) | 27/1080 (3%) | 22/2091 (1%) | 26/1831 (1%) | 169/9032 (2%) | 4/201 (2%) | 6/197 (3%) | 9/205 (4%) | 3/200 (2%) | 3/183 (2%) | 25/986 (3%) | ||
| Antibiotics <72 h post-partum | 8/2130 (<1%) | 146/1972 (7%) | 6/1080 (1%) | 6/1080 (5%) | 41/1879 (2%) | 305/9154 (3%) | 0/203 | 14/198 (7%) | 1/205 (<1%) | 8/200 (4%) | 14/198 (1%) | 24/993 (2%) | ||
| Intention to breastfeed* | 1681/2129 (79%) | 1659/1947 (85%) | 739/1051 (70%) | 2025/2082 (97%) | 1368/1826 (75%) | 7472/9035 (83%) | 182/203 (90%) | 182/198 (92%) | 146/205 (71%) | 196/198 (99%) | 154/186 (83%) | 860/990 (87%) | ||
| Family | ||||||||||||||
| Any siblings | 924/2130 (43%) | 979/1959 (50%) | 549/1055 (52%) | 1103/2091 (53%) | 892/1849 (48%) | 4447/9084 (49%) | 104/203 (51%) | 89/198 (45%) | 99/205 (48%) | 95/200 (48%) | 118/186 (63%) | 505/992 (51%) | ||
| Number of siblings* | 1 (1–2) | 1 (1–2) | 1 (1–1) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–2) | 1 (1–1) | ||
| Siblings in daycare or primary school | 799/2130 (38%) | 817/1959 (42%) | 474/1055 (45%) | 849/2091 (41%) | 823/1849 (45%) | 3762/9084 (41%) | 92/203 (45%) | 70/198 (35%) | 87/205 (42%) | 70/200 (35%) | 106/186 (57%) | 425/992 (43%) | ||
| Smokers in the family* | 320/2129 (15%) | 274/1947 (14%) | 299/1050 (28%) | 261/2085 (13%) | 301/1826 (16%) | 1455/9037 (16%) | 15/203 (7%) | 20/198 (10%) | 58/204 (28%) | 23/198 (12%) | 21/186 (11%) | 137/989 (14%) | ||
| Mother | 83/2129 (4%) | 47/1947 (2%) | 61/1050 (6%) | 50/2085 (2%) | 59/1826 (3%) | 300/9037 (3%) | 5/203 (2%) | 2/198 (1%) | 8/204 (4%) | 4/198 (2%) | 1/186 (1%) | 20/989 (2%) | ||
| Father | 265/2129 (12%) | 218/1947 (11%) | 254/1050 (24%) | 235/2085 (11%) | 257/1826 (14%) | 1229/9037 (14%) | 12/203 (6%) | 5/198 (8%) | 50/204 (25%) | 21/198 (11%) | 19/186 (10%) | 117/989 (12%) | ||
| Other family member | 21/2129 (1%) | 45/1947 (2%) | 34/1050 (3%) | 0/2085 | 26/1826 (1%) | 126/9037 (1%) | 1/203 (<1%) | 4/198 (2%) | 6/204 (3%) | 0/198 | 1/186 (1%) | 12/989 (1%) | ||
| Smoking in the house | 29/2129 (1%) | 15/1947 (1%) | 41/1050 (4%) | 9/2085 (<1%) | 4/1826 (<1%) | 98/9037 (1%) | 1/203 (<1%) | 4/198 (2%) | 6/204 (3%) | 0/198 | 1/186 (1%) | 12/989 (1%) | ||
| Family history of atopy* | 1568/2129 (74%) | 1409/1951 (72%) | 578/1037 (56%) | 1319/2079 (63%) | 1292/1831 (71%) | 6166/9027 (68%) | 163/203 (80%) | 150/198 (76%) | 121/203 (60%) | 132/198 (67%) | 142/186 (76%) | 708/988 (72%) | ||
| Siblings use or used respiratory medicine | 172/2130 (8%) | 212/1959 (11%) | 112/1055 (11%) | 167/2091 (8%) | 198/1849 (11%) | 861/9084 (9%) | 11/203 (5%) | 15/198 (8%) | 17/205 (8%) | 10/200 (5%) | 28/186 (15%) | 81/992 (8%) | ||
| Ethnic origin of the mother* | ||||||||||||||
| Northwest Europe | 1643/2124 (77%) | 1473/1952 (75%) | 31/1048 (3%) | 2029/2086 (97%) | 1416/1827 (78%) | 6592/9037 (73%) | 146/203 (72%) | 143/198 (72%) | 9/205 (4%) | 195/198 (98%) | 163/186 (88%) | 656/990 (66%) | ||
| Southern Europe | 94/2124 (4%) | 46/1952 (2%) | 943/1048 (90%) | 10/2086 (<1%) | 29/1827 (2%) | 1122/9037 (12%) | 11/203 (5%) | 6/198 (3%) | 179/205 (87%) | 0/198 | 4/186 (2%) | 200/990 (20%) | ||
| Other | 393/2124 (19%) | 453/1952 (23%) | 106/1048 (10%) | 54/2086 (3%) | 434/1827 (24%) | 1440/9037 (16%) | 46/203 (23%) | 49/198 (25%) | 17/205 (8%) | 5/198 (3%) | 20/186 (11%) | 137/990 (14%) | ||
| Ethnic origin of the father* | ||||||||||||||
| Northwest Europe | 1664/2124 (78%) | 1475/1952 (76%) | 35/1048 (3%) | 1979/2086 (95%) | 1414/1827 (77%) | 6567/9037 (73%) | 155/203 (76%) | 156/198 (79%) | 9/205 (4%) | 192/198 (97%) | 165/186 (89%) | 677/990 (68%) | ||
| Southern Europe | 79/2124 (4%) | 53/1952 (3%) | 946/1048 (90%) | 13/2086 (1%) | 23/1827 (1%) | 1114/9037 (12%) | 7/203 (3%) | 3/198 (2%) | 181/205 (88%) | 0/198 | 2/186 (1%) | 193/990 (19%) | ||
| Other | 387/2124 (18%) | 444/1952 (23%) | 99/1048 (9%) | 101/2086 (5%) | 442/1827 (24%) | 1473/9037 (16%) | 41/203 (20%) | 39/198 (20%) | 15/205 (7%) | 8/198 (4%) | 20/186 (11%) | 123/990 (12%) | ||
| Highest level of education of the mother* | ||||||||||||||
| Secondary or vocational school | 780/2125 (37%) | 743/1954 (38%) | 540/1049 (51%) | 721/2085 (35%) | 580/1826 (32%) | 3364/9039 (37%) | 36/203 (18%) | 40/198 (20%) | 103/205 (50%) | 62/198 (31%) | 46/186 (25%) | 287/990 (29%) | ||
| University of (applied) sciences | 1336/2125 (63%) | 1202/1954 (62%) | 471/1049 (45%) | 1315/2085 (63%) | 1230/1826 (67%) | 5554/9039 (61%) | 167/203 (82%) | 158/198 (80%) | 94/205 (46%) | 133/198 (67%) | 140/186 (75%) | 692/990 (70%) | ||
| Highest level of education of the father* | ||||||||||||||
| Secondary or vocational school | 1000/2102 (48%) | 917/1928 (48%) | 685/1037 (66%) | 982/2059 (48%) | 732/1818 (40%) | 4316/8944 (48%) | 58/202 (29%) | 67/197 (34%) | 138/203 (68%) | 90/197 (46%) | 68/185 (37%) | 421/984 (43%) | ||
| University of (applied) sciences | 1096/2102 (52%) | 1001/1928 (52%) | 253/1037 (24%) | 986/2059 (48%) | 1063/1818 (58%) | 4399/8944 (49%) | 143/202 (71%) | 129/197 (65%) | 54/203 (27%) | 101/197 (51%) | 116/185 (63%) | 543/984 (55%) | ||
| Employment of the mother before birth | ||||||||||||||
| Full-time | 1384/2129 (65%) | 1250/1954 (64%) | 619/1045 (59%) | 1432/2084 (69%) | 763/1827 (42%) | 5448/9039 (60%) | 140/203 (69%) | 142/198 (72%) | 109/205 (53%) | 137/198 (69%) | 83/186 (45%) | 611/990 (62%) | ||
| Part-time | 519/2129 (24%) | 511/1954 (26%) | 168/1045 (16%) | 264/2084 (13%) | 902/1827 (49%) | 2364/9039 (26%) | 51/203 (25%) | 48/198 (24%) | 38/205 (19%) | 29/198 (15%) | 93/186 (50%) | 259/990 (26%) | ||
| Employment of the father before birth | ||||||||||||||
| Full-time | 1933/2129 (91%) | 1843/1954 (94%) | 955/1045 (91%) | 1827/2084 (88%) | 1520/1827 (83%) | 8078/9039 (89%) | 193/203 (95%) | 187/198 (94%) | 187/205 (91%) | 164/198 (83%) | 150/186 (81%) | 881/990 (89%) | ||
| Part-time | 82/2129 (4%) | 48/1954 (2%) | 37/1045 (4%) | 74/2084 (4%) | 244/1827 (13%) | 485/9039 (5%) | 3/203 (1%) | 8/198 (4%) | 7/205 (3%) | 8/198 (4%) | 31/186 (17%) | 57/990 (6%) | ||
Data are n/N (%), and median (IQR).
p<0·05 total active surveillance versus total passive (without active) cohort.
We observed 388 ARI hospitalisations (figure 1 and 2, appendix pp 3–4). Of these, 145 (37·4%) were positive for RSV, 193 (49·7%) were negative or occurred outside the RSV season, and 50 (12·9%) occurred during the RSV season but were not tested for RSV (and status was imputed). Among 145 RSV-associated hospitalisations, RSV was detected during admission by hospital laboratory PCR tests in 71 (49·0%) and by POCT in 67 (46·2%). The test used was not documented for seven RSV-associated hospitalisations. Overall, 143 (1·6%) children were hospitalised with confirmed RSV, including two who were admitted twice with RSV. After imputing missing RSV test results, the incidence of RSV-associated hospitalisation was 1·8% (95% CI 1·6–2·1), corresponding to an RSV-associated hospitalisation incidence rate of 1·6 per 1000 infant-months (1·3–1·8; table 2 ). RSV-associated hospitalisation incidence in countries varied between 1·1% (0·7–1·5) in Finland and 2·5% (1·8–3·4) in Spain (table 3 ). RSV-associated hospitalisation incidence rate was higher in children born in autumn (2·6 per 1000 infant-months, 2·0–3·3) than in children born in winter (1·1 per 1000 infant-months, 0·8–1·6, Bonferroni adjusted p=0·002) and spring (0·8 per 1000 infant-months, 0·5–1·3, Bonferroni adjusted p=0·001; table 3, appendix p 10). RSV-associated hospitalisation incidence rate was highest in 2017–18 (2·7 per 1000 infant-months, 1·9–4·0) when the proportion of participating children younger than 6 months was high, and lowest in 2019–20 (1·5 per 1000 infant-months, 1·1–1·8; table 3).
Table 2.
Incidence and incidence rates of RSV-associated ARI, medically attended ARI, and hospitalised ARI in the first year of life
| RSV incidence after imputation*† | RSV incidence before imputation‡ | Cohort size or person-time | Number of hospitalisations or number of ARI episodes | Number of RSV-positive (observed) | |
|---|---|---|---|---|---|
| RSV-associated hospitalisation in total cohort | |||||
| Incidence§ | 1·8% (1·6–2·1) | 1·6% (1·3–1·8) | 9154 infants | 341 infants hospitalised | 143 infants with RSV-associated hospitalisation |
| Incidence rate¶ per 1000 infant-months | 1·6 (1·3–1·8) | 1·3 (1·1–1·6) | 109 507 infant-months | 388 hospitalisations | 145 RSV-associated hospitalisations |
| Medically attended RSV-positive ARI in active surveillance cohort | |||||
| Incidence§ | 14·1% (12·3–16·0) | 13·0% (11·0–15·2) | 993 infants | 683 infants with ARI | 129 infants with medically attended RSV-associated ARI |
| Incidence rate¶ per 1000 infant-months | 12·1 (10·2–14·3) | 11·2 (9·3–13·3) | 11 728 infant-months | 1520 ARI | 131 medically attended RSV-associated ARI |
| RSV-positive ARI in active surveillance cohort | |||||
| Incidence§ | 26·2% (24·0–28·6) | 25·1% (22·4–27·9) | 993 infants | 683 infants with ARI | 249 infants with RSV-associated ARI |
| Incidence rate¶ per 1000 infant-months | 23·7 (21·0–26·7) | 22·3 (19·7–25·2) | 11 728 infant-months | 1520 ARI | 262 RSV-associated ARI |
ARI=acute respiratory infection. RSV=respiratory syncytial virus.
Missing RSV status imputed using multiple imputation based on site, sex, age, and meteorological season at time of hospitalisation or ARI, and missing medical attendance imputed using site, sex, age, meteorological season at time of hospitalisation or ARI and RSV status (observed or imputed).
Outcomes that required imputations included: 50 hospitalisations with missing RSV status, 166 ARI episodes with missing RSV status or missing medical attendance status, and 101 ARI episodes with missing RSV status.
Assuming all missing outcomes were negative.
Incidence as proportion of infants experiencing the event at least once during their first year of life.
Incidence rate as number of events per 1000 infant-months of follow-up.
Table 3.
Incidence and incidence rates after imputation for missing RSV test results and missing medical attendance status of RSV-associated hospitalised ARIs, medically attended ARIs, and ARIs by age group, according to season, recruitment site, cohort, and season of birth
|
RSV-associated hospitalised ARI |
Medically attended RSV-associated ARI |
RSV-associated ARI |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <3 months | 3 to <6 months | 6 to <12 months | <12 months | <3 months | 3 to <6 months | 6 to <12 months | <12 months | <3 months | 3 to <6 months | 6 to <12 months | <12 months | ||
| RSV incidence proportion | |||||||||||||
| Overall | 0·97% (0·82–1·16) | 0·49% (0·38–0·63) | 0·39% (0·29–0·52) | 1·80% (1·58–2·05) | 3·39% (2·56–4·49) | 4·55% (3·55–5·80) | 6·32% (5·13–7·77) | 14·07% (12·31–16·03) | 5·05% (4·01–6·33) | 9·29% (7·84–10·97) | 12·61% (10·93–14·51) | 26·22% (23·95–28·63) | |
| Site | |||||||||||||
| Scotland | 1·15% (0·83–1·60) | 0·47% (0·28–0·79) | 0·73% (0·48–1·10) | 2·31% (1·83–2·92) | 1·48% (0·59–3·64) | 5·72% (3·55–9·11) | 6·75% (4·30–10·45) | 13·74% (10·17–18·31) | 3·50% (1·91–6·33) | 12·69% (9·17–17·3) | 13·60% (9·88–18·43) | 29·21% (24·05–34·97) | |
| England | 1·03% (0·71–1·51) | 0·71% (0·44–1·14) | 0·43% (0·23–0·81) | 1·97% (1·50–2·57) | 2·58% (1·26–5·20) | 5·05% (2·97–8·46) | 3·03 % (1·48–6·09) | 10·4% (7·18–14·84) | 3·99% (2·21–7·11) | 9·95% (6·89–14·15) | 7·61% (4·93–11·55) | 20·51% (15·96–25·94) | |
| Spain | 1·20% (0·77–1·88) | 1·00% (0·60–1·65) | 0·28% (0·11–0·69) | 2·48% (1·81–3·40) | 6·00% (3·77–9·43) | 6·65% (4·27–10·21) | 5·35% (3·22–8·76) | 17·71% (13·65–22·65) | 7·71% (5·10–11·49) | 11·15% (7·98–15·37) | 11·80% (8·50–16·16) | 29·56% (24·49–35·19) | |
| Finland | 0·62% (0·4–0·97) | 0·24% (0·12–0·49) | 0·19% (0·08–0·44) | 1·05% (0·74–1·49) | 1·00% (0·33–2·98) | 1·01% (0·33–2·99) | 4·95% (2·95–8·19) | 6·90% (4·48–10·49) | 1·00% (0·33–2·98) | 2·51% (1·23–5·07) | 7·07% (4·62–10·68) | 10·50% (7·45–14·61) | |
| Netherlands | 0·97% (0·65–1·43) | 0·26% (0·12–0·57) | 0·25% (0·11–0·56) | 1·47% (1·07–2·03) | 6·04% (3·73–9·63) | 4·28% (2·43–7·43) | 11·66% (8·32–16·10) | 21·98% (17·38–27·39) | 9·25% (6·30–13·38) | 10·16% (7·08–14·38) | 23·32% (18·6–28·81) | 42·19% (36·35–48·26) | |
| RSV incidence rate per 1000 infant-months | |||||||||||||
| Overall | 3·26 (2·63–4·04) | 1·67 (1·23–2·27) | 0·65 (0·45–0·92) | 1·56 (1·33–1·82) | 11·69 (8·34–16·38) | 15·21 (11·28–20·52) | 10·77 (8·36–13·88) | 12·11 (10·24–14·34) | 17·55 (13·34–23·1) | 31·69 (25·76–38·98) | 22·81 (19·16–27·17) | 23·70 (21·02–26·73) | |
| Site | |||||||||||||
| Scotland | 3·88 (2·60–5·80) | 1·55 (0·82–2·92) | 1·21 (0·73–2·00) | 1·96 (1·48–2·61) | 4·95 (1·60–15·35) | 19·10 (10·63–34·32) | 11·47 (6·62–19·87) | 11·75 (8·06–17·12) | 11·70 (5·58–24·56) | 44·82 (30·18–66·56) | 24·77 (16·78–36·56) | 26·52 (20·54–34·25) | |
| England | 3·46 (2·20–5·45) | 2·56 (1·47–4·47) | 0·72 (0·34–1·51) | 1·87 (1·38–2·55) | 8·61 (3·58–20·71) | 17·00 (8·89–32·54) | 5·04 (2·09–12·10) | 8·98 (5·69–14·18) | 13·31 (6·44–27·51) | 34·07 (21·68–53·55) | 12·99 (7·63–22·1) | 18·39 (13·4–25·23) | |
| Spain | 4·01 (2·33–6·90) | 3·34 (1·81–6·14) | 0·46 (0·15–1·44) | 2·07 (1·41–3·03) | 20·11 (11·37–35·55) | 22·22 (12·92–38·24) | 8·93 (4·80–16·61) | 15·09 (10·82–21·06) | 27·46 (16·81–44·88) | 37·28 (24·56–56·59) | 20·58 (13·77–30·75) | 26·49 (20·63–34·03) | |
| Finland | 2·07 (1·20–3·56) | 0·80 (0·33–1·92) | 0·31 (0·1–0·9) | 0·87 (0·57–1·33) | 3·34 (0·84–13·35) | 3·35 (0·84–13·41) | 8·24 (4·37–15·52) | 5·79 (3·40–9·85) | 3·34 (0·84–13·35) | 8·38 (3·49–20·14) | 11·78 (6·98–19·89) | 8·81 (5·74–13·51) | |
| Netherlands | 3·23 (2·02–5·18) | 0·86 (0·33–2·27) | 0·40 (0·14–1·15) | 1·23 (0·83–1·81) | 21·93 (12·46–38·57) | 14·27 (7·14–28·54) | 20·28 (13·43–30·64) | 19·20 (14·21–25·93) | 32·63 (20·57–51·77) | 33·90 (21·62–53·15) | 44·48 (33·62–58·85) | 38·89 (31·49–48·02) | |
| Season | |||||||||||||
| 2017–18 | 3·90 (2·51–6·08) | 2·49 (1·21–5·09) | 0* | 2·71 (1·85–3·98) | 15·01 (7·81–28·86) | 11·98 (4·49–31·94) | 0* | 12·05 (7·00–20·75) | 20·75 (11·75–36·67) | 18·08 (8·03–40·72) | 0* | 17·15 (10·79–27·26) | |
| 2018–19 | 3·17 (2·30–4·38) | 1·41 (0·83–2·41) | 0·90 (0·50–1·62) | 1·76 (1·38–2·25) | 8·36 (4·75–14·71) | 9·79 (5·50–17·46) | 10·37 (6·64–16·19) | 9·60 (7·12–12·95) | 12·10 (7·56–19·38) | 20·32 (13·60–30·37) | 21·30 (15·62–29·05) | 18·19 (14·67–22·55) | |
| 2019–20 | 3·03 (2·10–4·36) | 1·79 (1·17–2·76) | 0·74 (0·47–1·15) | 1·45 (1·14–1·83) | 14·90 (8·66–25·64) | 21·24 (14·44–31·24) | 12·65 (9·26–17·29) | 15·06 (12·04–18·83) | 24·32 (15·89–37·22) | 46·16 (35·79–59·54) | 27·20 (21·99–33·66) | 31·25 (26·81–36·42) | |
| Cohort | |||||||||||||
| Cohort A | 2·92 (1·48–5·77) | 2·45 (1·13–5·29) | 0·72 (0·27–1·91) | 1·71 (1·08–2·69) | .. | .. | .. | .. | .. | .. | .. | .. | |
| Cohort P without cohort A | 3·30 (2·63–4·14) | 1·57 (1·13–2·19) | 0·64 (0·44–0·93) | 1·54 (1·30–1·82) | .. | .. | .. | .. | .. | .. | .. | .. | |
| Sex | |||||||||||||
| Female | 3·16 (2·31–4·32) | 1·44 (0·90–2·30) | 0·55 (0·32–0·93) | 1·42 (1·13–1·80) | 10·68 (6·45–17·71) | 11·37 (6·94–18·63) | 11·49 (8·07–16·37) | 11·26 (8·77–14·46) | 17·39 (11·66–25·92) | 28·39 (20·73–38·89) | 23·99 (18·8–30·61) | 23·43 (19·71–27·84) | |
| Male | 3·38 (2·53–4·51) | 1·89 (1·24–2·88) | 0·74 (0·47–1·17) | 1·69 (1·37–2·08) | 12·65 (8·05–19·86) | 18·82 (12·87–27·52) | 10·09 (7·04–14·48) | 12·92 (10·31–16·19) | 17·73 (12·08–26·03) | 34·16 (25·81–45·21) | 21·72 (16·98–27·78) | 23·82 (20·16–28·14) | |
| Season of birth† | |||||||||||||
| Spring | 0·47 (0·15–1·45) | 0·77 (0·31–1·95) | 1·02 (0·56–1·83) | 0·82 (0·51–1·31) | 0* | 6·15 (2·45–15·40) | 18·52 (12·77–26·86) | 10·72 (7·60–15·12) | 0* | 16·71 (9·70–28·77) | 42·87 (33·49–54·87) | 25·43 (20·31–31·83) | |
| Summer | 1·55 (0·86–2·80) | 4·24 (2·92–6·15) | 0·29 (0·10–0·82) | 1·60 (1·18–2·16) | 8·17 (3·90–17·14) | 36·82 (25·64–52·88) | 2·03 (0·65–6·30) | 12·32 (9·01–16·83) | 14·99 (8·66–25·95) | 78·13 (61·17–99·79) | 4·92 (2·39–10·15) | 25·81 (20·85–31·95) | |
| Autumn | 8·53 (6·60–11·04) | 1·35 (0·70–2·61) | 0·17 (0·04–0·65) | 2·57 (2·03–3·25) | 31·56 (20·95–47·55) | 11·37 (5·69–22·73) | 1·48 (0·37–5·91) | 11·55 (8·19–16·27) | 46·95 (33·56–65·67) | 17·83 (9·98–31·88) | 4·22 (1·90–9·40) | 18·41 (13·99–24·23) | |
| Winter | 2·03 (1·18–3·48) | 0·15 (0·02–1·05) | 1·17 (0·7–1·95) | 1·13 (0·78–1·62) | 7·23 (2·71–19·29) | 0* | 25·22 (17·40–36·55) | 14·41 (10·17–20·41) | 7·23 (2·71–19·29) | 0* | 46·33 (35·24–60·90) | 24·97 (19·17–32·51) | |
| Birthweight | |||||||||||||
| <2500 g | 5·78 (1·86–17·91) | 0* | 0* | 1·49 (0·48–4·63) | 0* | 38·45 (11·15–132·56) | 6·94 (0·98–49·29) | 13·42 (4·87–36·98) | 0* | 72·07 (30·01–173·09) | 7·44 (1·05–52·97) | 22·04 (9·96–48·75) | |
| ≥2500 g | 3·18 (2·55–3·96) | 1·69 (1·25–2·30) | 0·66 (0·47–0·95) | 1·55 (1·32–1·82) | 12·04 (8·59–16·88) | 14·72 (10·77–20·12) | 10·94 (8·47–14·13) | 12·16 (10·25–14·43) | 18·10 (13·75–23·82) | 30·54 (24·63–37·87) | 23·17 (19·43–27·62) | 23·73 (21·01–26·81) | |
Data are % (95% CI) or incidence rate (95% CI). ARI=acute respiratory infection. A=active surveillance. P=passive surveillance. RSV=respiratory syncytial virus.
Incidence rate estimated as 0, 95% CI not determined because of 0 cases.
Season of birth was defined as: spring from March 21 to June 20, summer from June 21 to Sept 20, autumn from Sept 21 to Dec 20, and winter from Dec 21 to March 20.
Out of 145 RSV-associated hospitalisations, 84 (57·9%) were in children younger than 3 months (appendix p 5, 10). In that age group, incidence of RSV-associated hospitalisations peaked at 1 month to less than 2 months of age (appendix p 10). Median duration of hospitalisation was 3 days (range 1–19 days, IQR 2–5 days). Hospitalisations lasted longer in Spain (median 6 days, IQR 5–6 days) than in the Netherlands (median 3 days, IQR 2–6 days; p<0·003), Finland (median 2 days, IQR 1–4 days), England (median 3 days, IQR 2–4 days), and Scotland (median 2 days, IQR 1–3 days; p<0·001). Duration of hospitalisation and other measures of severity were not found to be associated with the incidence rate of RSV-associated hospitalisations. Length of hospitalisation was longer in infants younger than 3 months when compared with infants aged 6 months to younger than 12 months (p=0·004), but not when compared with infants aged 3 months to younger than 6 months (p=0·27). Eight of 145 RSV-associated hospitalisations (5·5%) led to admission to the paediatric intensive care unit (0·09% of total cohort [n=9154 infants]), and three (2%) required mechanical ventilation (0·03% of total cohort). Six of eight infants admitted to the intensive care unit were aged younger than 3 months (median age 1 month). Any respiratory support was more frequently used in RSV-positive than RSV-negative hospitalisations (77 [53·1%] of 145 vs 45 [23·3%] of 193, p<0·001). Coinfections with other respiratory viruses were tested as part of routine care in 85 (58·6%) and found in 34 (23·4%) of 145 RSV-associated hospitalisations. Rhinovirus was most frequently co-detected. In RSV-negative hospitalisations, rhinovirus, influenza, and parainfluenza were the three most prevalent viruses (appendix p 5).
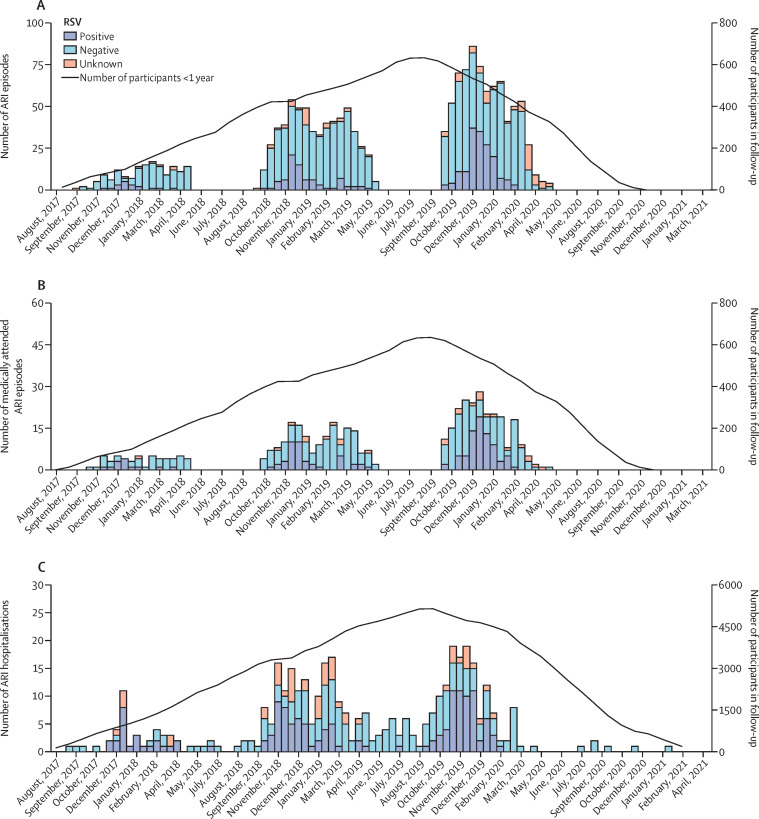
We registered 1520 ARI episodes in 993 infants in the active surveillance cohort (Figure 1, Figure 2 ). A nasal swab was collected during 1442 (94·9%) episodes. Missed episodes was the main reason for not collecting a swab. 23 samples collected later than 10 days after start of symptoms were excluded. Most samples (87·7%) were collected within 7 days after the start of symptoms. In total, 262 (18·5%) of 1419 episodes were positive for RSV in 249 infants (figure 1). Among the 840 episodes tested by PCR and POCT, RSV was detected only by POCT in five (0·6%).
Figure 2.
Number of all-cause and RSV-associated ARI by month for ARI (A), medically attended ARI (B), and hospitalised ARI (C)
ARI and medically attended ARI are derived from the active surveillance cohort; hospitalised ARI is derived from the passive surveillance cohort. ARI=acute respiratory infection. RSV=respiratory syncytial virus.
RSV-A was detected in 142 (54·2%) of RSV-associated ARI and RSV-B in 111 (42·4%). One sample was positive for both RSV-A and RSV-B. RSV subtype was unknown for ten ARI episodes: five were only tested by POCT, four were only tested in hospital as part of routine care, and for one the RSV subtype could not be determined. Information about medical attendance was available for 1432 (94·2%) episodes. For 1353 (89·0%) ARI episodes both RSV and medical attendance status were available. Medical attendance was reported in 131 (52·2%) of 251 RSV-positive ARI, which was more frequent than in RSV-negative ARI (298 [27·0%] of 1102, p<0·001).
After imputing missing RSV test results, the incidence of medically attended RSV-associated ARI was 14·1% (95% CI 12·3–16·0) with an incidence rate of 12·1 per 1000 infant-months (10·2–14·3; table 2). The incidence of RSV-associated ARI overall was 26·2% (24·0–28·6) with an incidence rate of 23·7 per 1000 infant-months (21·0–26·7). Incidence rate of RSV-associated ARI and medically attended RSV-associated ARI were similar for infants younger than 6 and those aged 6 months and older (table 3). The incidence rates for RSV-associated ARI and medically attended RSV-associated ARI episodes were highest in the Netherlands (38·9 per 1000 infant-months [31·5–48·0] and 19·2 per 1000 infant-months [14·2–25·9], respectively) and lowest in Finland (8·8 per 1000 infant-months [5·7–13·5] and 5·8 per 1000 infant-months [3·4–9·9] respectively, Bonferroni adjusted p<0·05; table 3).
Information on wheezing was available for 7838 children whose parents completed the 1-year questionnaire (85·6% of the 9154 participants), which included 7807 participants of the total cohort with complete information on hospitalisations for ARI and 841 participants of the active surveillance cohort with complete information on ARI episodes (figure 1). Wheezing was reported in 87 (70·7%) of 123 infants admitted with RSV. Wheezing was less frequent in infants hospitalised for RSV-negative ARI only (73 [54·5%] of 134, p=0·008) and in infants never admitted for an ARI (1272 [16·8%] of 7550, p<0·001, figure 1). In the active surveillance cohort, wheezing was reported for 56 (47·5%) of 118 infants with medically attended RSV-associated ARI and 37 (36·3%) of 102 infants with non-medically attended RSV-associated ARI (p=0·09). This occurrence was more frequent than in children who had no ARI (20 [8·1%] of 246, p<0·001 and p<0·001), had medically attended RSV-negative ARI (38 [23·5%] of 162, p<0·001 and p=0·03) or had non-medically attended RSV-negative ARI (43 [20·2%] of 213, p<0·001 and p=0·002). When adjusted for family history of atopy and smoking household members at birth, the difference in wheezing between RSV-positive and RSV-negative or no ARI remained significant (p=0·003 and p<0·001 for hospitalisations, p<0·001 and p<0·001 for medically attended ARI, and p=0·002 and p<0·001 for non-medically attended ARI).
Discussion
To our knowledge, this is the first international birth cohort study powered to accurately estimate the health-care burden of RSV in healthy term-born infants. Our results showed an incidence of RSV-associated hospitalisation of 1·8% in the first year of life. Almost half of all ARI hospitalisations in the first year of life were RSV-associated. The burden of RSV-associated hospitalisation was highest in infants younger than 3 months with an incidence rate of 3·3 per 1000 infant-months. Children born in autumn had a significantly higher risk of hospitalisation than children born in other seasons. One quarter of infants experienced an RSV-associated ARI, of which half were medically attended. Wheezing during the first year of life was associated with RSV hospitalisation, medically attended RSV-associated ARI, and overall RSV-associated ARI.
Our findings are consistent with previous literature. Although not a birth cohort study, a study conducted in the USA reported an incidence of RSV-associated hospitalisations of 1·7% in infants younger than 6 months (1·5% in our study), and 0·5% in infants aged 6 to younger than 12 months (0·4% in our study).2 The higher admission rate in infants younger than 6 months reported by Hall and colleagues2 might be related to the 35% of higher-risk infants included. In our study, incidence of RSV-associated hospitalisations per country varied between 1·1% and 2·5%, which was in line with previous findings from these countries.9, 11, 18, 22 In other birth cohort studies, RSV-associated hospitalisation incidence in the first year of life varied between 0·6% and 5%. Some studies also included high-risks infants (appendix p 6).10, 12, 13, 14, 15, 16, 17 The two largest birth cohort studies in healthy term-born infants showed an incidence of RSV-associated hospitalisations of 1·9% in an Indian birth cohort of 310 infants and 1% in 298 infants of a Dutch birth cohort.9, 14
Wheezing in the first year of life was associated with RSV infection irrespective of severity. The association between severe RSV infections and wheezing has been described earlier.23 Whether this is also associated with development of childhood asthma remains unclear, as well as whether RSV immunisation will prevent wheezing during later childhood.24 Intervention studies are required to define the causal association between RSV infection during infancy and wheezing in healthy term-born infants.
The major strength of our study is the prospective design with the power to accurately estimate RSV incidence in European countries over several seasons. We used active surveillance to capture mild RSV disease to provide a precise estimate of total RSV incidence and disease burden. Follow-up rates were high with collection of swabs in 95% of reported ARI episodes and more than 85% completion of the 1-year questionnaire in the total cohort. In addition to parental report, we screened the study participants’ hospital charts to ensure no ARI hospitalisation was missed. This study also has limitations. First, in 50 of 388 ARI hospitalisations during the RSV season, no RSV test was performed. When using a cohort study design with RSV testing results as primary outcome, missing test results will systematically lead to an underestimation of true incidence if assumed negative. To avoid this systematic bias, primary outcomes were reported after using multiple imputation for missing RSV test results and medical attendance status. As the proportion of missing information was small, using multiple imputation resulted in a small increase in incidence compared with estimating incidence assuming all cases with missing RSV status were RSV-negative. Two of the five sites did not use POCT, which could have led to underestimating incidence in those countries; however, that effect was probably small. Of 840 episodes tested by PCR and POCT, five (0·6%) were detected by POCT only. Assuming a similar rate, two additional RSV cases would have been detected by POCT among the 415 episodes tested by PCR only at the sites not using POCT. Second, data on coinfection with other respiratory viruses were scarce. Third, the participants in the study might not be representative of the country population and not all countries in Europe were represented. The education level of participants, especially in the active surveillance cohort, was high with 70% of mothers reporting university education and is therefore not necessarily representative of the whole population. Lower socioeconomic status and younger age of the mother have been reported as risk factors for RSV-associated hospitalisation in infancy.25 Other risk factors like parental smoking were less frequently reported by active surveillance cohort participants than the rest of the study population. This could have resulted in an underestimation of RSV incidence in the study population compared with the country population and in the active cohort compared with the entire cohort. Although children with evidence of significant comorbidities at birth were excluded, we cannot rule out that a minority of participants had comorbidities diagnosed later in life. Fourth, it is possible that we missed ARI episodes despite weekly contacts with parents during the period of active surveillance (October to May, or longer if RSV was still circulating). We cannot rule out that some participants could have stopped reporting ARI of their children, which could result in underestimating incidence rate and would be more pronounced in the older infants. However, participation to the 1-year questionnaire was 89% in the active surveillance cohort, suggesting a high retention rate. ARI episodes occurring outside of the active surveillance period would not have been captured, which probably contributed to the finding of 31% of active cohort participants with no ARI in the first year of life. However, it is unlikely that those uncaptured ARI episodes were associated with RSV infection. Fifth, the COVID-19 pandemic impacted RSV incidence in 2020. The 2019–20 RSV season was virtually finished in the participating countries when the COVID-19 pandemic started, except for Finland, where the usual continuation of the RSV outbreak into late spring was abruptly terminated because of the COVID-19 pandemic.26, 27 The COVID-19 pandemic might have contributed to the lower incidence of RSV-associated hospitalisations, medically attended ARIs, and RSV-associated ARIs in the study in Finland. Participants born after April 1, 2020, were excluded as RSV did not circulate during their first year of life. Follow-up time after Nov 1, 2020, represented less than 3% of total follow-up time of the cohort and concerned only participants aged 6 months or older. Sixth, health-care burden does not reflect the total burden of RSV. Health-care burden is key information to estimate economic and societal burden, and the incidence of medically attended and hospitalised RSV infections is expected to be a major part of the health-care burden in Europe where RSV-related deaths are rare. Overall, study limitations have possibly resulted in a modest underestimation of actual RSV burden.
In conclusion, the health-care burden of RSV in healthy term-born infants in Europe is considerable with an incidence of RSV-associated hospitalisation of 1·8% in the first year of life, which means that one in 56 healthy term-born infants is hospitalised with RSV annually. Because the highest burden is seen in infants in their first months of life, maternal vaccination and passive immunisation could have a profound impact on the RSV burden.
Data sharing
The anonymised data of the RESCEU birth cohort study will be made available for research purposes after the end of the long-term follow-up. The data will be stored on the Elixir data platform at https://elixir-europe.org/platforms/data. Requests to access the data should be sent via Elixir to the RESCEU consortium.
Declaration of interests
LJB has regular interaction with pharmaceutical and other industrial partners. He has not received personal fees or other personal benefits. UMCU has received major funding (>€100 000 per industrial partner) for investigator initiated studies from AbbVie, MedImmune, AstraZeneca, Sanofi, Janssen, Pfizer, MSD, and MeMed Diagnostics. UMCU has received major funding for the RSV GOLD study from the Bill & Melinda Gates Foundation. UMCU has received major funding as part of the public private partnership IMI-funded RESCEU and PROMISE projects with partners GSK, Novavax, Janssen, AstraZeneca, Pfizer, and Sanofi. UMCU has received major funding by Julius Clinical for participating in clinical studies sponsored by MedImmune and Pfizer. UMCU received minor funding (€1000–25 000 per industrial partner) for consultation and invited lectures by AbbVie, MedImmune, Ablynx, Bavaria Nordic, MabXience, GSK, Novavax, Pfizer, Moderna, Astrazeneca, MSD, Sanofi, Genzyme, and Janssen. LJB is the founding chairman of the ReSViNET Foundation. SC has provided consultancy or investigator roles in relation to product development for Ablynx, Janssen, MedImmune, AstraZeneca, Pfizer, GSK, Vertex, AbbVie, Valneva, Fibrogen, and Boehringer Ingelheim, with fees paid to the University of Edinburgh. FM-T reports honoraria from GSK group, Pfizer, Sanofi Pasteur, MSD, Seqirus, Biofabri, and Janssen for taking part in advisory boards and expert meetings and for acting as a speaker in congresses, outside the submitted work; and principal investigator-role in randomised controlled trials for GSK, Pfizer, Sanofi Pasteur, MSD, Seqirus, Biofabri, Janssen, Ablynx, Gilead, Regeneron, Roche, Abbott, Novavax, and MedImmune, with honoraria paid to his institution. MDS acts as an investigator on behalf of the University of Oxford on research studies funded by vaccine manufacturers including GSK, Janssen, MCM vaccines, Novavax, AtraZeneca, and Pfizer. MDS was an National Institute for Heath and Care Research (NIHR) Senior Investigator and received salary support from the NIHR Oxford Biomedical Research Centre during the course of this work. MDS is currently an employee of Moderna. SBD had received honoraria from MSD and Sanofi Pasteur for taking part in advisory boards and has provided consultancy or investigator roles in relation to product development for Janssen, AstraZeneca, Pfizer, Valneva, MSD, and Sanofi Pasteur with fees paid to St George's University of London. TH has received honoraria for lectures or participation in advisory boards or data monitoring committees from Janssen, Sanofi Pasteur, Enanta, and MSD. BR is a full-time employee of the GSK group and holds shares and restricted shares in the GSK group as part of their employee remuneration. AJP is currently Chair of the Department of Health Social Cares Joint Committee on Vaccination and Immunisation and was previously a member of WHO Scientific Advisory Group for Emergencies and chair of the European Medicine's Agency Scientific Advisory Group on Vaccines. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank all infants and parents who participated in this study. We thank the local study teams who were responsible for local patient recruitment and follow-up. This manuscript reflects only the views of the authors. The EU and the Innovative Medicines Initiative are not responsible for any use that might be made of the information it contains. RESCEU has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 116019. This Joint Undertaking receives support from the EU's Horizon 2020 research and innovation programme and European Federation of Pharmaceutical Industries and Associations.
The RESCEU Investigators
Belgium Philippe Beutels (University of Antwerp, Antwerp), Jeroen Aerssens (Janssen, Beerse), Bishoy Rizkalla (GlaxoSmithKline, Wavre); Denmark Thea Kølsen Fischer (Statens Serum Institut, Copenhagen); Finland Terho Heikkinen (University of Turku and Turku University Hospital, Turku); France Charlotte Vernhes, Scott Gallichan (Sanofi Pasteur, Lyon); Italy Carlo Giaquinto (Fondazione PENTA for the Treatment and Care of Children with HIV-ONLUS, Padua); Netherlands Joanne Wildenbeest, Marie-Noelle Billard, Roy Zuurbier, Koos Korsten, Marlies van Houten, Annefleur Langedijk, Peter van de Ven, Louis Bont (University Medical Center Utrecht, Utrecht), Maarten van den Berge (Academisch Ziekenhuis Groningen, Groningen), Adam Meijer (National Institute for Public Health and the Environment, Utrecht); Spain Ana Dacosta-Urbieta, Irene Rivero-Calle, Alberto Gómez-Carballa, Sara Pischedda, Carmen Rodriguez-Tenreiro, Federico Martinón-Torres (Servicio Galego de Saude, Santiago de Compostela), Eva Molero (Team-It Research, Barcelona); UK Simon Drysdale, Joseph McGinley, Gu-Lung Lin, Matthew Snape, Andrew Pollard, Andrew Ives, Helen Wolfenden, Sanjay Salgia, Rohoth Shetty (University of Oxford, Oxford); Steve Cunningham, Harish Nair, Harry Campbell (University of Edinburgh, Edinburgh); Thom O’Neill, Margaret Miller, Julie Baggott, Catherine Beveridge, Rachael McKernan (Children's Clinical Research Facility, NHS Lothian, Edinburgh), Peter Openshaw (Imperial College London, London); USA Michael Abram (AstraZeneca, Gaithersburg, MD), Kena Swanson (Pfizer, New York, NY); Veena Kumar (Novavax, Washington, DC).
Contributors
JGW, AP, TH, SC, FMT, MS, and LJB designed the study. JGW, RZ, MvH, TH, SC, MS, SC, FMT, KK, SD, HR, ADU, and TON collected data. JGW, MB, PvdV, and LJB analysed and interpreted data. JGW wrote the first draft. AP, TH, SC, FMT, MS, RZ, MvH, KK, SD, HR, ADU, BR, and TON reviewed and commented on the manuscript. JGW and MB accessed and verified the data. JGW and LJB were responsible for the decision to submit the manuscript.
Contributor Information
RESCEU Investigators:
Joanne Wildenbeest, Marie-Noëlle Billard, Roy Zuurbier, Koos Korsten, Marlies van Houten, Annefleur Langedijk, Peter van de Ven, Louis Bont, Simon Drysdale, Joseph McGinley, Gu-Lung Lin, Matthew Snape, Andrew Pollard, Andrew Ives, Helen Wolfenden, Sanjay Salgia, Rohoth Shetty, Ana Dacosta-Urbieta, Irene Rivero-Calle, Alberto Gómez-Carballa, Sara Pischedda, Carmen Rodriguez-Tenreiro, Federico Martinón-Torres, Terho Heikkinen, Steve Cunningham, Harish Nair, Harry Campbell, Thomas O'Neill, Margaret Miller, Julie Baggott, Catherine Beveridge, Rachael McKernan, Bishoy Rizkalla, Philippe Beutels, Peter Openshaw, Adam Meijer, Thea Kølsen Fischer, Maarten van den Berge, Carlo Giaquinto, Michael Abram, Kena Swanson, Jeroen Aerssens, Charlotte Vernhes, Scott Gallichan, Veena Kumar, and Eva Molero
Supplementary Material
References
- 1.Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399:2047–2064. doi: 10.1016/S0140-6736(22)00478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madhi SA, Polack FP, Piedra PA, et al. Respiratory Syncytial Virus Vaccination during Pregnancy and Effects in Infants. N Engl J Med. 2020;383:426–439. doi: 10.1056/NEJMoa1908380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N Engl J Med. 2022;386:837–846. doi: 10.1056/NEJMoa2110275. [DOI] [PubMed] [Google Scholar]
- 5.RSV Vaccine and mAb Snapshot. https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/
- 6.EMA . European Medicines Agency; 2022. New medicine to protect babies and infants from respiratory syncytial virus (RSV) infection.https://www.ema.europa.eu/en/news/new-medicine-protect-babies-infants-respiratory-syncytial-virus-rsv-infection published online Sept 16. [Google Scholar]
- 7.Haerskjold A, Kristensen K, Kamper-Jørgensen M, Nybo Andersen A-M, Ravn H, Graff Stensballe L. Risk Factors for Hospitalization for Respiratory Syncytial Virus Infection: A Population-based Cohort Study of Danish Children. Pediatr Infect Dis J. 2016;35:61–65. doi: 10.1097/INF.0000000000000924. [DOI] [PubMed] [Google Scholar]
- 8.Hardelid P, Verfuerden M, McMenamin J, Smyth RL, Gilbert R. The contribution of child, family and health service factors to respiratory syncytial virus (RSV) hospital admissions in the first 3 years of life: birth cohort study in Scotland, 2009 to 2015. Euro Surveill. 2019;24 doi: 10.2807/1560-7917.ES.2019.24.1.1800046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houben ML, Bont L, Wilbrink B, et al. Clinical prediction rule for RSV bronchiolitis in healthy newborns: prognostic birth cohort study. Pediatrics. 2011;127:35–41. doi: 10.1542/peds.2010-0581. [DOI] [PubMed] [Google Scholar]
- 10.Nokes DJ, Okiro EA, Ngama M, et al. Respiratory syncytial virus infection and disease in infants and young children observed from birth in Kilifi District, Kenya. Clin Infect Dis. 2008;46:50–57. doi: 10.1086/524019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toivonen L, Karppinen S, Schuez-Havupalo L, et al. Respiratory syncytial virus infections in children 0–24 months of age in the community. J Infect. 2020;80:69–75. doi: 10.1016/j.jinf.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Kubale J, Kuan G, Gresh L, et al. Assessing the Incidence of Symptomatic Respiratory Syncytial Virus Illness Within a Prospective Birth Cohort in Managua, Nicaragua. Clin Infect Dis. 2020;70:2029–2035. doi: 10.1093/cid/ciz585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zar HJ, Nduru P, Stadler JAM, et al. Early-life respiratory syncytial virus lower respiratory tract infection in a South African birth cohort: epidemiology and effect on lung health. Lancet Glob Health. 2020;8:e1316–e1325. doi: 10.1016/S2214-109X(20)30251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar P, Medigeshi GR, Mishra VS, et al. Etiology of Acute Respiratory Infections in Infants: A Prospective Birth Cohort Study. Pediatr Infect Dis J. 2017;36:25–30. doi: 10.1097/INF.0000000000001359. [DOI] [PubMed] [Google Scholar]
- 15.Regamey N, Kaiser L, Roiha HL, et al. Viral etiology of acute respiratory infections with cough in infancy: a community-based birth cohort study. Pediatr Infect Dis J. 2008;27:100–105. doi: 10.1097/INF.0b013e31815922c8. [DOI] [PubMed] [Google Scholar]
- 16.Kusel MMH, de Klerk NH, Holt PG, Kebadze T, Johnston SL, Sly PD. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25:680–686. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 17.Takashima MD, Grimwood K, Sly PD, et al. Epidemiology of respiratory syncytial virus in a community birth cohort of infants in the first 2 years of life. Eur J Pediatr. 2021;180:2125–2135. doi: 10.1007/s00431-021-03998-0. [DOI] [PubMed] [Google Scholar]
- 18.Thomas E, Mattila J-M, Lehtinen P, Vuorinen T, Waris M, Heikkinen T. Burden of Respiratory Syncytial Virus Infection During the First Year of Life. J Infect Dis. 2021;223:811–817. doi: 10.1093/infdis/jiaa754. [DOI] [PubMed] [Google Scholar]
- 19.Wildenbeest JG, Zuurbier RP, Korsten K, et al. Respiratory Syncytial Virus Consortium in Europe (RESCEU) Birth Cohort Study: Defining the Burden of Infant Respiratory Syncytial Virus Disease in Europe. J Infect Dis. 2020;222:S606–S612. doi: 10.1093/infdis/jiaa310. [DOI] [PubMed] [Google Scholar]
- 20.Korsten K, Adriaenssens N, Coenen S, et al. Burden of respiratory syncytial virus infection in community-dwelling older adults in Europe (RESCEU): an international prospective cohort study. Eur Respir J. 2021;57 doi: 10.1183/13993003.02688-2020. [DOI] [PubMed] [Google Scholar]
- 21.Zuurbier RP, Korsten K, Verheij TJM, et al. Performance Assessment of a Rapid Molecular Respiratory Syncytial Virus Point-of-Care Test: A Prospective Community Study in Older Adults. J Infect Dis. 2022;226:S63–S70. doi: 10.1093/infdis/jiab600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zomer-Kooijker K, Uiterwaal CSPM, van der Gugten AC, Wilbrink B, Bont LJ, van der Ent CK. Decreased lung function precedes severe respiratory syncytial virus infection and post-respiratory syncytial virus wheeze in term infants. Eur Respir J. 2014;44:666–674. doi: 10.1183/09031936.00009314. [DOI] [PubMed] [Google Scholar]
- 23.Blanken MO, Rovers MM, Molenaar JM, et al. Respiratory Syncytial Virus and Recurrent Wheeze in Healthy Preterm Infants. N Engl J Med. 2013;368:1791–1799. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 24.Brunwasser SM, Snyder BM, Driscoll AJ, et al. Assessing the strength of evidence for a causal effect of respiratory syncytial virus lower respiratory tract infections on subsequent wheezing illness: a systematic review and meta-analysis. Lancet Respir Med. 2020;8:795–806. doi: 10.1016/S2213-2600(20)30109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitzpatrick T, McNally JD, Stukel TA, et al. Family and Child Risk Factors for Early-Life RSV Illness. Pediatrics. 2021;147 doi: 10.1542/peds.2020-029090. [DOI] [PubMed] [Google Scholar]
- 26.Haapanen M, Renko M, Artama M, Kuitunen I. The impact of the lockdown and the re-opening of schools and day cares on the epidemiology of SARS-CoV-2 and other respiratory infections in children - a nationwide register study in Finland. EClinicalMedicine. 2021;34 doi: 10.1016/j.eclinm.2021.100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Summeren J, Meijer A, Aspelund G, et al. Low levels of respiratory syncytial virus activity in Europe during the 2020/21 season: what can we expect in the summer and autumn/winter? Eurosurveillance. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.29.2100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The anonymised data of the RESCEU birth cohort study will be made available for research purposes after the end of the long-term follow-up. The data will be stored on the Elixir data platform at https://elixir-europe.org/platforms/data. Requests to access the data should be sent via Elixir to the RESCEU consortium.