ABSTRACT
To report on the therapy used for penicillin- and cephalosporin-resistant pneumococcal meningitis, we conducted an observational cohort study of patients admitted to our hospital with pneumococcal meningitis between 1977 and 2018. According to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommendations, we defined pneumococci as susceptible and resistant to penicillin with MIC values of ≤0.06 mg/L and > 0.06 mg/L, respectively; the corresponding values for cefotaxime (CTX) were ≤0.5 mg/L and >0.5 mg/L. We treated 363 episodes of pneumococcal meningitis during the study period. Of these, 24 had no viable strain, leaving 339 episodes with a known MIC for inclusion. Penicillin-susceptible strains accounted for 246 episodes (73%), penicillin-resistant strains for 93 (27%), CTX susceptible for 58, and CTX resistant for 35. Nine patients failed or relapsed and 69 died (20%), of whom 22% were among susceptible cases and 17% were among resistant cases. During the dexamethasone period, mortality was equal (12%) in both susceptible and resistant cases. High-dose CTX (300 mg/Kg/day) helped to treat failed or relapsed cases and protected against failure when used as empirical therapy (P = 0.02), even in CTX-resistant cases. High-dose CTX is a good empirical therapy option for pneumococcal meningitis in the presence of a high prevalence of penicillin and cephalosporin resistance, effectively treating pneumococcal strains with MICs up to 2 mg/L for either penicillin or CTX.
KEYWORDS: antibiotic resistance, cefotaxime, ceftriaxone, bacterial meningitis
INTRODUCTION
Streptococcus pneumoniae remains one of the most feared causes of bacterial meningitis, historically showing the highest morbidity and mortality among community-acquired cases and still presenting as the most frequent cause in Europe (1, 2). Treating bacterial meningitis has become even more difficult since penicillin-resistant strains arose as causes of meningitis in the late 1970s (3, 4), with many case reports or case series in the 1980s and 1990s reporting treatment failures. Crucially, these strains were also resistant to antibiotics such as cotrimoxazole, tetracycline, and chloramphenicol (5–9) and may be fully susceptible, intermediate, or resistant to third-generation cephalosporins and other beta-lactams (8). The numbers differed by country and geography, and although the causes are not clear-cut, the overuse of beta-lactam antibiotics and the spread of efficient strains have been widely invoked as major contributors (10–16).
Penicillin-resistant pneumococci represent a major threat in terms of both antimicrobial resistance and global public health (17, 18). In Spain, the number of resistant cases had grown to almost40% of pneumococcal meningitis cases by 1997 (11), with similar growth seen in many other countries (10, 13–15). After confirming penicillin failure (5), we have several therapeutic options based on research, recommendations, and clinical assumptions (19–25). Failure has also been reported with standard meningeal doses of third-generation cephalosporins (9, 21). However, clinical trials are difficult to perform due to the low number of cases and dramatic disease course of pneumococcal meningitis, resulting in limited evidence-based knowledge about how to treat penicillin- and cephalosporin-resistant disease. Animal models have shed some light (26–34), with guidelines for bacterial meningitis often relying on results from these studies or expert opinion (35, 36). Current European guidelines recommend standard meningeal doses of a third-generation cephalosporin to treat cephalosporin-resistant strains.
Given our near 40-year experience treating penicillin-resistant pneumococcal meningitis, we sought to report on the outcomes of therapy for penicillin- and/or cephalosporin-resistant pneumococcal meningitis in a real-world setting.
RESULTS
Clinical and laboratory characteristics.
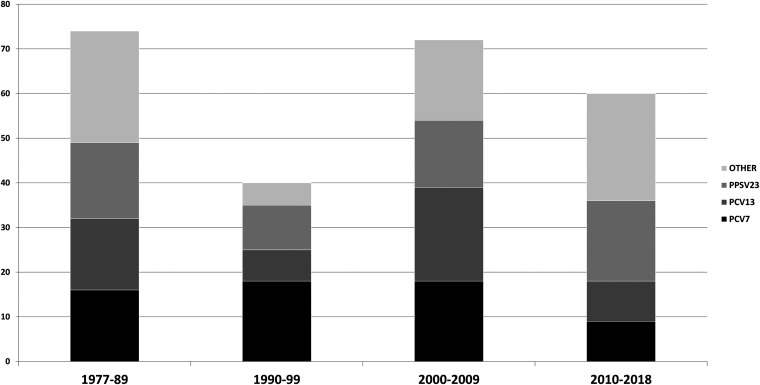
Between 1977 and 2018, we attended 363 episodes of pneumococcal meningitis, of which 24 were diagnosed by means without viable strain (pneumococcal antigen, Gram stain, or the strain was not available for MIC determination), leaving 339 episodes with known susceptibility data. Among these episodes, 246 (73%) were due to susceptible strains and 93 (27%) were due to resistant strains. Data on vaccination status prior to the episode are available from 2004 to 2018, corresponding to 128 episodes. Forty-one (32%) had a pneumococcal vaccine previous to the meningitis episode, and it was the pneumococcal polysaccharide vaccine (Pneumovax 23) in all cases.
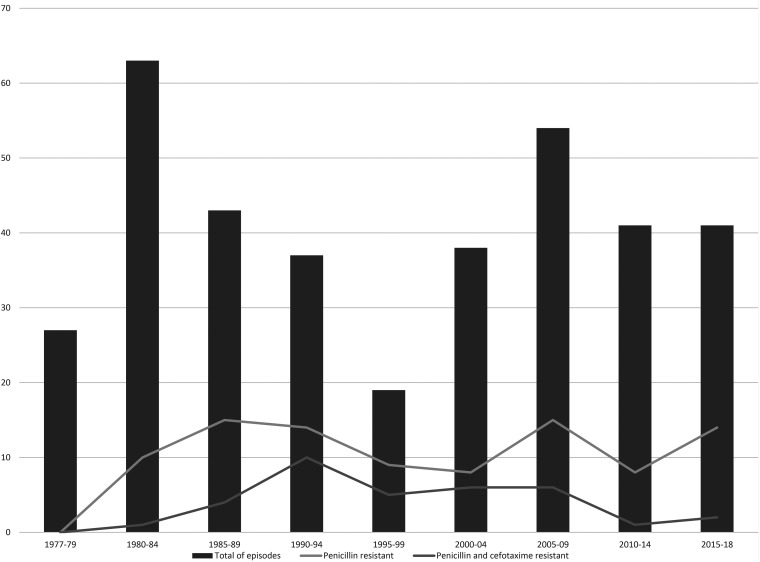
Fig. 1 and 2 summarizes the evolution over time. Tables 1 to 3 summarize the demographic characteristics, infection source, clinical and laboratory data, therapy and evolution, and comparison of penicillin-susceptible and penicillin-resistant cases. The groups had no major differences. However, cases due to resistant strains were associated with higher prehospital antibiotic use and lower cerebrospinal fluid (CSF) culture positivity, were more common in cases with solid or hematological neoplasm or with immunodeficiency, and were less often associated with posttherapy seizures and the need for antiseizure therapy. Clinical characteristics were similar on admission, with 9.8% presenting with shock, 34% having a Glasgow coma scale score ≤8, and 13% presenting with seizures before therapy. Patients with cefotaxime (CTX)-resistant strains had fewer seizures after therapy despite groups receiving antiseizure prophylaxis in the same proportions. Blood cultures were positive in 79% of episodes, again without differences between groups.
FIG 1.
Evolution of penicillin and cefotaxime resistance and total number of episodes of S. pneumoniae meningitis.
FIG 2.
Number of meningitis episodes caused by PCV7 serotypes, additional PCV13 serotypes, additional PPSV23 serotypes, and nonvaccine types (other).
TABLE 1.
Clinical and laboratory characteristics and outcomes of patients with meningitis due to penicillin-susceptible and -resistant S. pneumoniae
| Susceptibleb | Resistantc | P valuea | Total | |
|---|---|---|---|---|
| Characteristics | N = 246 (73%) | N = 93 (27%) | N = 339 | |
| Age median (IQR) | 58 (42 68) | 56 (40 66) | 58 (42 67) | |
| Gender (female) | 104 (43%) | 47 (51%) | 0.184 | 151 (45%) |
| Hospital acquired | 11 (4%) | 8 (9%) | 0.138 | 19 (6%) |
| Recurrent episode | 31 (13%) | 13 (14%) | 0.710 | 44 (13%) |
| Any underlying disease | 99 (40%) | 40 (43%) | 0.624 | 139 (41%) |
| Solid or hematologic neoplasm | 10 (4%) | 9 (10%) | 0.044 | 19 (6%) |
| Immunodeficiency | 1 (0.4%) | 4 (4%) | 0.021 | 5 (1.5%) |
| Previous antibiotic | 49 (20%) | 31 (33%) | 0.010 | 80 (24%) |
| Source of infection | ||||
| Unknown | 41 (17%) | 14 (15%) | 0.719 | 55 (16%) |
| Pericranial fistula | 73 (30%) | 30 (32%) | 0.644 | 103 (30%) |
| Sinusitis | 8 (3%) | 5 (5%) | 0.103 | 13 (4%) |
| Otitis | 96 (39%) | 32 (34%) | 0.446 | 128 (38%) |
| Pneumonia | 24 (10%) | 8 (9%) | 0.746 | 32 (9%) |
| Endocarditis | 2 (1%) | 2 (2%) | 0.308 | 4 (1%) |
| Other | 2 (1%) | 2 (2%) | 0.308 | 4 (1%) |
| Other clinical characteristics | ||||
| Fever | 209 (87%) | 81 (88%) | 0.814 | 290 (87%) |
| Shock | 24 (10%) | 9 (10%) | 0.988 | 33 (10%) |
| Headache | 168 (72%) | 66 (74%) | 0.632 | 234 (72%) |
| Nuchal stiffness | 209 (85) | 77 (84% | 0.530 | 286 (85%) |
| Nausea/vomiting | 135 (63%) | 53 (64%) | 0.939 | 188 (64%) |
| Coma admission (Glasgow ≤ 8) | 88 (36%) | 25 (27%) | 0.130 | 113 (34%) |
| Cranial nerve palsy | 25 (10%) | 6 (6%) | 31 (9%) | |
| Hemiparesis | 40 (16.5%) | 9 (9.8%) | 0.123 | 49 (14.6%) |
| Seizures | 75 (30.7%) | 23 (25.3%) | 0.328 | 98 (29.3%) |
| Before therapy | 30 (12%) | 15 15 (17%) | 0.340 | 45 45 (13%) |
| Seizures after therapy | 44 (18.4%) | 8 (8.8%) | 0.031 | 52 (15.8%) |
| Laboratory test | ||||
| CSF cloudy/purulent | 205 (88%) | 39 (91%) | 284 (89%) | |
| Median CSF WBC (IQR) | 2435 (504 5798) | 2168 (509 6900) | 2400 (530 5800) | |
| CSF hypoglycorrhachia | 189 (83%) | 82 (91%) | 0.076 | 271 (86%) |
| CSF proteinorrhachia >5 g | 86 (39%) | 39 (44%) | 0.452 | 125 (41%) |
| Positive CSF Gram’s stain | 187 (82%) | 73 (81%) | 0.837 | 260 (82%) |
| Positive CSF culture | 216 (92%) | 73 (81%) | 0.008 | 289 (89%) |
| Positive blood culture | 177 (79%) | 66 (79%) | 0.918 | 243 (79%) |
| Thrombocytopenia | 23 (10%) | 5 (6%) | 0.208 | 28 (9%) |
| CT scan on admission | 149 (61%) | 61 (66%) | 210 (62%) | |
| Abnormal CT scan on admission | 66 (44%) | 26 (43%) | 92 (44%) | |
| Median total hospital days (IQR) | 17 (11 25) | 18 (12 29) | 17 (11 26) | |
| Relapse or failure | 4 (1.6%) | 5 (6.6%) | 0.041 | 9 (2.6%) |
| Sequelae | 46 (24%) | 11 (14%) | 0.231 | 57 (21%) |
| Epilepsy | 5 (3%) | 2 (3%) | 7 (3%) | |
| Cranial nerve palsy | 7 (4%) | 3 (4%) | 10 (4%) | |
| Hearing impairment | 13 (7%) | 1 (1%) | 0.082 | 14 (5%) |
| Hemiparesis | 3 (2%) | 2 (3%) | 5 (2%) | |
| Hydrocephalus shunt | 4 (2%) | 0 | 4 (1%) | |
| Combination/other | 14 (7%) | 3 (4%) | 13 (5%) | |
| Total mortality | 53 (22%) | 16 (17%) | 0.344 | 69 (20%) |
P < 0.05 was considered significant.
Susceptible MIC ≥0.06 mg/L, range <0.03 to 0.06 mg/L.
Resistant MIC >0.06 mg/L, range 0.12 to 4 mg/L.
TABLE 2.
Therapy for S. pneumoniae meningitis both susceptible and resistant to penicillin
| Initial antibiotic therapy | Susceptibled N = 246 | Resistante N = 93 | P valuec | Total N = 339 |
|---|---|---|---|---|
| Penicillin | 79 | 7 | 86 | |
| Ampicillin | 2 | 2 | 4 | |
| Total penicillins | 81 | 9 | 90 | |
| Failure or relapse | 2 | 1 | 3 | |
| Cured | 52 | 5a | 55 | |
| Dead | 27 | 3 | 30 | |
| Cefotaxime/HDCTX | 105/99 | 50/47 | 155/146 | |
| Failure or relapse | 0 | 0 | 0 | |
| Cured | 89/86 | 41/39 | 130/125 | |
| Dead | 16/13 | 9/8 | 25/21 | |
| Ceftriaxone | 26 | 9 | 35 | |
| Failure or relapse | 0 | 2b | 2 | |
| Cured | 22 | 5 | 27 | |
| Dead | 4 | 2 | 6 | |
| Total 3rdG cephalosporins | 132 | 59 | 191 | |
| Ceftriaxone + ampicillin | 12 | 2 | 14 | |
| Total beta-lactams | 225 (91%) | 70 (75%) | 0.00 | 295 (87%) |
| Chloramphenicol | 1 | 1 | 2 | |
| Vancomycin | 7 | 8 | 15 | |
| Failure or relapse | 3 | 1b | 4 | |
| Cured | 3 | 7 | 10 | |
| Dead | 1 | 0 | 1 | |
| Vancomycin + rifampicin | 5 | 5 | 10 | |
| Failure or relapse | 0 | 1b | 1 | |
| Cured | 4 | 4 | 8 | |
| Dead | 1 | 0 | 1 | |
| CRO/CTX + vancomycin | 5 | 7 | 12 | |
| Failure or relapse | 0 | 0 | 0 | |
| Cured | 5 | 7 | 12 | |
| Dead | 0 | 0 | 0 | |
| Other | 4 | 2 | 6 | |
| Final antibiotic therapy | ||||
| Penicillin | 86 | 3 | 89 | |
| Ampicillin | 3 | 2 | 5 | |
| Total penicillins | 89 | 5 | 94 | |
| Cefotaxime | 18 | 39 | 57 | |
| Ceftriaxone | 130 | 32 | 162 | |
| Total 3rdG cephalosporins | 148 | 71 | 219 | |
| Total Beta-lactams | 238 (97%) | 76 (82%) | 0.00 | 313 (92%) |
| Chloramphenicol | 0 | 2 | 2 | |
| Vancomycin | 4 | 8 | 12 | |
| Vancomycin + rifampicin | 5 | 4 | 9 | |
| CRO/CTX + vancomycin | 0 | 3 | 3 | |
| Median antibiotic days (IQR) | 10 (10 14) | 10 (10 14) | 10 (10 14) | |
| Other therapies | ||||
| Dexamethasone ± mannitol | 147 (125) (60%) | 74 (50) (80%) | 0.00 | 221 (175) (65%) |
| Antiseizures therapy | 73 (30.8%) | 18 (20%) | 0.051 | 91 (27.9 %) |
| Antiseizures prophylaxis | 118 (49.2%) | 59 (66.3%) | 0.006 | 177 (53.8%) |
| Mechanical ventilation | 84 (35.3%) | 30 (33.7%) | NS | 114 (34.8%) |
Three increasing doses of penicillin and two switching to third-generation (3rdG) cephalosporin when MIC was known.
All cured switching to high dose of cefotaxime (300 mg/Kg/d).
P < 0.05 was considered significant.
Susceptible MIC ≥ 0.06mg/L, range <0.03 to 0.06 mg/L.
Resistant MIC >0.06 mg/L, range 0.12 to 4 mg/L.
TABLE 3.
Outcome by causative S. pneumoniae susceptibility to penicillin and third-generation cephalosporin, total episodes, and dexamethasone period
| Total n = 339 |
Dexamethasone period n = 220 |
|||||
|---|---|---|---|---|---|---|
| Outcome | Penicillin susceptibleb n = 246 |
Penicillin resistantc cefotaxime susceptibled n = 58 | Cefotaxime resistante n = 35 |
Penicillin susceptible n = 147 | Penicillin resistant cefotaxime susceptible n = 42 | Cefotaxime resistant n = 31 |
| Mortality | 53 (22%) | 9 (16%) | 7 (20%) | 18 (12%) | 4 (10%) | 5 (16%) |
| Early mortality | 36 (14.6%) | 3 (5.3%) | 4 (11.4%) | 11 (7.5%) | 1 (2.4%) | 2 (6.5%) |
| Therapeutical failure | 4 (2%) | 1 (2.2%) | 4 (13.8%)*a | 2 (1.5%) | 0 | 4 (14.8%)** |
| Sequelae | 46 (24%) | 5 (10.9%) | 6 (22.2%) | 28 (22%) | 3 (8.1%)*** | 6 (24%) |
| Hearing loss | 13 (7%) | 1 (2.2%) | 0 | 8 (6.3%) | 1 (2.7%) | 0 (0%) |
| Seizures before therapy | 30 (12%) | 10 (17%) | 4 (12%) | 21 (14%) | 6 (14%) | 4 (13%) |
| Seizures after therapy | 44 (18%) | 6 (11%) | 2 (6%) | 18 (12%) | 6 (14%) | 2 (7%) |
| Seizures (total) | 74 (31%) | 16 (28%) | 6 (18%) | 39 (26%) | 12 (28%) | 6 (20%) |
*, P = 0.001; **, P = 0.005; ***, P = 0.044.
Penicillin susceptible MIC ≥0.06 mg/L, range <0.03 to 0.06 mg/L.
Penicillin resistant MIC >0.06 mg/L, range 0.12 to 4 mg/L.
Cefotaxime susceptible MIC ≤0.5 mg/L, range <0.03 to 0.5 mg/L.
Cefotaxime resistant >0.5 mg/L range 1 to 2 mg/L.
Since 1987, 220 cases (65%) received corticosteroids and 54% received antiseizure prophylaxis. Sequelae developed in 21% of cases, with hearing loss the most frequent. Overall, 69/339 patients (20%) died, with neurological causes being more frequent than systemic. Resistance to penicillin or a third-generation cephalosporin was unrelated to mortality (P values of 0.344 and 0.742, respectively). Strains with a penicillin MIC > 1 mg/L or resistance to a third-generation cephalosporin were associated with therapeutic failure (P values of 0.001 and 0.009, respectively).
Microbiological characteristics.
The penicillin MICs were 0.03 mg/L for 173 strains, 0.06 mg/L for 73 strains, 0.12 mg/L for 12 strains, 0.25 mg/L for 12 strains, 0.5 mg/L for 17 strains, 1 mg/L for 13 strains, 2 mg/L for 29 strains, and 4 mg/L for 10 strains. In total, 35 strains were resistant to a third-generation cephalosporin (MICs of 1 mg/L for 29 strains and 2 mg/L for six strains), but none were both resistant to a third-generation cephalosporin and susceptible to penicillin.
The capsular type could be determined in 241 cases. Nevertheless, for some isolates collected before 1990, only serogroup data were available. Overall, serotype 3 was most frequent with 25 isolates, followed by serotype 8 (n = 16), 9V (n = 14), 19A (n = 11), 10A (n = 9), 23F (n = 9), 14, 18C, and 24F and 6B (n = 8, each). Among penicillin resistant strains (MIC > 0.06 mg/L) serotypes 9V (n = 14), 14, 19A, 23F,and 6B (n = 7, each) were the most frequent. Furthermore, these five serotypes were also associated with cefotaxime resistance (MIC > 0.5 mg/L) serotypes 9V (n = 7), 14 (n = 5), 19A (n = 5), 23F (n = 4),0 and 6B (n = 4). Including oldest strains of serogroups 19, 23, and 9, these five serotypes accounted for 67% and 100% of penicillin- and cefotaxime-resistant strains. Molecular typing (either PFGE or MLST) could be determined for 175 strains, the most frequent clonal complexes (CC) were: CC156 (n = 20, serotypes 9V, 14, 11A), ST97 (n = 9, serotype 10A), CC81 (n = 8, serotypes 23F and 19A), CC260 (serotype 3, n = 8), and CC180 (serotype 3, n = 7). All strains belonging to CC156 and CCT81 were resistant to penicillin. Mortality was present among patients with strains belonging to at least 18 serogroups. The most frequent among patients who died was serotype 8 with seven patients followed by serotypes 12, 14, and 19A with three each.
Therapy outcomes.
Table 2 shows the role of different antibiotics as initial or final therapy in penicillin and/or cephalosporin-resistant pneumococcal meningitis. Beta-lactams were used as empirical therapy alone in 75% of episodes and as final therapy in 82%.
Since introducing dexamethasone (1987 to 2018), we used the following therapeutic regimens as empirical therapy in 220 episodes: penicillin (n = 3), third-generation cephalosporin (n = 163; of these, 135 with high-dose CTX and 28 with meningeal doses of ceftriaxone (CRO) to a 4 g maximum dose), vancomycin (n = 12), a third-generation cephalosporin plus vancomycin (n = 12), vancomycin plus rifampin (n = 10), CRO plus ampicillin (n = 14), and the rest with other antibiotics. Among the cases treated during the dexamethasone period, 200 patients (90%) received beta-lactam monotherapy as their final treatment: 36 received high-dose CTX and the rest received meningeal doses of CRO (usually 4 g). Another eight patients received vancomycin and another nine received vancomycin plus rifampin as part of different studies (19), while three received vancomycin plus CRO.
Table 3 shows the evolution of the different episodes by penicillin and cephalosporin susceptibility. Global mortality was similar between penicillin-susceptible (53/246; 22%) and penicillin-resistant (16/93; 17%) cases and between cephalosporin-susceptible (9/58; 16%) and cephalosporin-resistant (7/35; 20%) cases. Among the cephalosporin-resistant cases, mortality was 24% (7/29) in cases with an MIC of 1 mg/L (six received high-dose CTX and one received CRO). Six cases with an MIC of 2 mg/L survived after receiving high-dose CTX (n = 4), high-dose CTX plus vancomycin (n = 1), or vancomycin plus rifampicin (n = 1). Concerning early mortality, penicillin-resistant cases (7/93; 8%) tended to have lower mortality than penicillin-susceptible cases (36/246; 15%) (P = 0.081), including mortality due to early sepsis (1 [<1%] versus 9 [4%] cases, respectively).
Overall mortality was also lower in the dexamethasone period (27/220; 12%), and we found no differences among penicillin-susceptible (18/147; 12%), penicillin-resistant cephalosporin-susceptible (4/42; 10%), and cephalosporin-resistant (5/31, 16%) cases. No major differences in mortality existed among the different empirical therapy groups.
Therapeutic failures and case analysis.
Among the nine therapeutic failures (2.6%), four had penicillin-susceptible disease (4/246; 2%), two relapsed after penicillin therapy due to unresolved otic pathology (cases 1 and 2; Table 4), and two received vancomycin (cases 5 and 6; Table 4). In the penicillin-resistant cephalosporin-susceptible group, one patient (1/58 2.2%) with a penicillin MIC of 4 mg/L experienced penicillin treatment failure (case 3, Table 4). In the cephalosporin-resistant group, four cases failed (4/35, 14% P = 0.001): two receiving vancomycin (alone or with rifampicin) with low vancomycin levels (cases 4 and 9) and two receiving CRO with MICs of 1 and 2 mg/L. Ultimately, all four cases had good outcomes with high doses of CTX.
TABLE 4.
Analysis of treatment failuresa
| No. (yr) | Age/sex | Source | MIC PENb (mg/L) |
MIC CTX (mg/L) |
Serotype | Initial therapy | Observations | Second therapy | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 (1977) | 50/F | AOM | 0.03 | 0.03 | NA | PEN | Relapse due to unsolved otological problem | PEN | Cured, after ENT surgery |
| 2 (1984) | 52/F | AOM | 0.03 | 0.03 | 3 | PEN | Relapse due to unsolved otological problem | PEN | Cured, after ENT surgery |
| 3 (1984) | 11/F | Unknown | 4 | 0.5 | 23 | PEN | No improvement with PEN, fever persisted, and relapsed | High dose CTX/VAN | Cured |
| 4 (1988) | 66/F | Unknown | 4 | 2 | 9V | VAN | Clinical failure with VAN | High dose CTX | Cured |
| 5 (1989) | 27/M | Fistula | 0.03 | 0.03 | 3 | VAN | Clinical failure with VAN | PEN | Cured |
| 6 (1989) | 17/M | Fistula | 0.03 | 0.03 | 18C | VAN | Clinical failure with VAN | PEN | Cured |
| 7 (1991) | 37/M | Fistula | 2 | 1 | 6 | CRO | Clear initial improvement, 48 h of therapy, reappearance of fever, reduced consciousness, and greater meningeal signs. CSF Gram stain at 72 h indicated diplococci. CRO dose represented 65 mg/Kg. | High dose CTX | Cured |
| 8 (1992) | 62/F | Fistula | 4 | 2 | 23F | CRO | After clear initial improvement, the fever, coma, and meningeal signs reappeared and worsened. CRO dose represented 55 mg/Kg. | High dose CTX | Cured |
| 9 (1994) | 56/F | AOM | 2 | 1 | 6B | VAN+RIF | Failure due to low VAN serum levels | High dose CTX | Cured |
No cases of failures after the implementation of high dose CTX as empirical therapy.
PEN, penicillin; CTX, cefotaxime; VAN, vancomycin; CRO, ceftriaxone; RIF, rifampin.
Due to the relapses in these cases, we use high-dose CTX (300 mg/Kg/d) routinely as both empirical therapy and for highly resistant cases. No patients treated with high doses of CTX failed or relapsed and its use appeared to protect against treatment failure (P = 0.02). Vancomycin alone as initial therapy was statistically significantly related to treatment failure (P = 0.002). Among patients in the dexamethasone era, six out of 220 (2.7%) experienced therapeutic failure, with a higher frequency among cephalosporin-resistant cases (4/31; 15%) (P = 0.005). In fact, cephalosporin resistance was significantly associated with therapeutic failure (P = 0.002).
Analysis of sequelae.
The number of sequelae varied between groups, occurring in 46 of 193 (24%) survivors with susceptible strains and six of 28 (22.2%) survivors with CTX resistance, and both being higher than the five of 49 (11%) with penicillin-resistant strains susceptible to CTX. The numbers were similar in the dexamethasone period, with 28 of 129 (22%) susceptible cases, six of 26 (24%) cephalosporin-resistant cases, and three of 38 (8%) penicillin-resistant but cephalosporin-susceptible cases experiencing sequelae (P = 0.04). Hearing loss was 13/246 (5%) in susceptible cases and 1/93 (2.2%) in resistant cases (P = 0.082). Globally, we observed a pattern of less mortality, less early mortality, fewer sequelae, and less hearing loss among patients with penicillin-resistant CTX-susceptible strains.
DISCUSSION
This study included a large series of patients with beta-lactam-resistant pneumococcal meningitis and covered the evolution of resistance and therapy in a real-world setting over 3 decades. This revealed that patients with penicillin resistance and susceptibility are similar except for the former having more comorbidities and/or contact with the health system. However, patients with strains resistant to penicillin but susceptible to cephalosporins seemed to have better outcomes, with a clear tendency to have less mortality, less early mortality, and fewer sequelae, including hearing loss.
Our experience also revealed that penicillin and ampicillin should be avoided due to the high prevalence of therapeutic failure from the beginning of the resistance era (5). In addition, vancomycin monotherapy appeared unsafe and produced multiple therapeutic failures, usually due to erratic CSF vancomycin levels (19). Using dexamethasone may contribute to erratic CSF levels (27, 28, 37), but given that this treatment is mandatory in pneumococcal meningitis, vancomycin monotherapy should be avoided. An alternative is to use vancomycin plus rifampin, which has superior efficacy to vancomycin monotherapy despite concomitant dexamethasone use. Only one therapeutic failure occurred due to low serum vancomycin levels when using this approach, but this was readily cured with high doses of CTX. The short experience with the other nine patients, all cured without major problems, may suggest that vancomycin plus rifampin might be a good alternative in cases with true allergy or toxicity associated with beta-lactam use. Experience in an animal model corroborates our experience (28).
Standard meningeal doses of CRO (50 mg/Kg/d) or CTX (200 mg/Kg/d) were useful for treating pneumococcal meningitis due to penicillin-resistant strains fully susceptible to third-generation cephalosporins (MIC ≤ 0.5 mg/L); however, our experience revealed that they could fail in cases with higher MICs. This experience prompted us to use high-dose CTX (300 mg/Kg/d) as empirical therapy in all cases until the MIC was known and to maintain this therapy in cases due to strains with MICs of 1 or 2 mg/L. Our experience with this practice has been very good, with no cases of therapeutic failure when used for empirical therapy and evidence that it was a good final therapy option. Consistent with this, doses of 300 mg/Kg/d are recommended in French guidelines (38) for empirical therapy. The equivalent dose of CRO (100 mg/Kg/d) has not been used by us because the manufacturer advised against using doses exceeding 4g/24 h, which makes the 100 mg/Kg/d option available only to people weighing ≤40kg. Recent experience in France, where high doses of CRO are recommended for empirical therapy (39), has revealed excellent evolution and a very low number of adverse events. The high doses of CTX we use have also proven to be safe with the concomitant use of dexamethasone, and in cases resistant to CTX/CRO, without adding vancomycin. Current European guidelines (36) recommend standard meningeal doses in cases with MICs <2mg/L to third-generation cephalosporins, which may be not enough according to our experience, and also recommend adding vancomycin in cases with an MIC of 2 mg/L, which may not be necessary.
Based on our experience, we have proposed some updates to treatment recommendations, as shown in Table 5. Using high-dose CTX, and possibly CRO given the experience in both this study and in French settings, and especially if confirmed by wider experience, might require considering S. pneumoniae with MICs of 1 and 2 mg/L to CTX/CRO as “susceptible at increased doses” rather than “resistant.” Vancomycin plus a third-generation cephalosporin might be another good option for empirical and definitive therapy, consistent with recommendations in several guidelines (36). We use vancomycin with a third-generation cephalosporin (high-dose CTX) because standard meningeal doses of third-generations cephalosporin represent a more dangerous option in this context. Using high-dose CTX then makes concomitant vancomycin unnecessary, at least up to an MIC of 2 mg/L for CTX. In cases with higher MICs, it would be safe to add the second antibiotic later. Despite these informative results, our study is limited by the cohort design and a long study period that has seen many important changes in medical practice; however, this is also a major strength of the study, showing how penicillin and cephalosporin resistance can be handled effectively.
TABLE 5.
Recommendations for definitive therapy by the MIC of cefotaxime
| Cefotaxime MIC | Therapya |
|---|---|
| <1 mg/L | Ceftriaxone 100 mg/Kg with 4 g as maximum dose |
| 1 to 2 mg/L | Cefotaxime 300 mg/Kg/d in 4 divided doses |
| >2 mg/L | Cefotaxime 300 mg/Kg/d in 4 divided doses + vancomycin 30 mg/Kg/12 h |
| Allergy | Vancomycin 30 mg/Kg/12 h + rifampicin15 mg/Kg/24 h 900 mg/24 h maximum dose |
Always check serum vancomycin levels when using vancomycin. Avoid penicillin, ampicillin, and vancomycin as monotherapy.
In conclusion, high-dose CTX (300 mg/Kg/d) is a good empirical therapy option for pneumococcal meningitis in settings with high levels of penicillin and cephalosporin resistance. It is also a good option for treating cases where the MIC is ≤2mg/L for penicillin or CTX/CRO. Care should be taken to avoid penicillin, ampicillin, or vancomycin monotherapy.
MATERIALS AND METHODS
This is an observational cohort study of patients admitted with pneumococcal meningitis to a large university hospital in Barcelona between 1977 and 2018, where all cases of bacterial meningitis have been routinely recorded using a 120-variable protocol. Our hospital admitted patients as young as 7 years old between 1977 and 1994, but since then, it has only admitted patients aged ≥14 years. For analysis, we included all episodes of meningitis due to S. pneumoniae, defined as follows: (i) clinical findings of meningitis with S. pneumoniae isolated in the blood or CSF; (ii) in the absence of positive cultures, detecting Gram-positive diplococci in the CSF Gram stain; or (iii) the detection of pneumococcal antigen in the CSF or PCR determination in the CSF.
Meningitis was diagnosed by inflammatory parameters in the CSF, as the presence of at least 5 white blood cells (WBC)/mm3 or positive CSF culture. Pneumococcal isolates were identified and serotyped at the Spanish Pneumococcal Reference Laboratory. Antibiotic susceptibility was determined systematically. MICs for penicillin, CTX, and other antibiotics were determined by microdilution, using commercial panels from the Sensititre system (TREK Diagnostic Systems Ltd.) according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommendations and criteria (40). We therefore considered S. pneumoniae strains penicillin-susceptible with MICs ≤0.06 mg/L, penicillin-resistant with MICs >0.06 mg/L, CTX-susceptible with MICs ≤0.5 mg/L, and CTX-resistant with MICs >0.5 mg/L.
Since 1987, all patients with either suspected pneumococcal meningitis or a CSF pressure ≥30cmH2O received dexamethasone (4 mg every 6 h for 48 h, started before or with the first antibiotic dose) and seizures prophylaxis with phenytoin.
Patients admitted between March 1988 and January 1989 have been included in a clinical trial of intravenous (IV) vancomycin (19), while patients admitted between March 1993 and March 1996 were included in a trial of vancomycin plus rifampin versus high-dose CTX. Otherwise, empirical and final therapy consisted of beta-lactams in all remaining periods: IV penicillin (150 to 180 mg/Kg/day), high-dose IV penicillin (300 mg/Kg/day), or a third-generation cephalosporin, which usually involved 4 g CRO or a standard meningeal dose (200 mg/Kg/day) or a high dose of CTX (300 mg/Kg/day). Between 1977 and 1986, patients received 14 days of therapy, but between 1987 and 2018, they received 10 days of therapy.
All patients were evaluated daily and underwent complete hematological and biochemical tests. All patients surviving pneumococcal meningitis were monitored as outpatients and followed to resolution of all symptoms or consideration of sequelae 3 months after the initial infection. Neurological sequelae included epilepsy, hearing loss, cranial nerve palsy, hydrocephalus needing a shunt, and/or hemiparesis.
Mortality during hospitalization was recorded and the mechanism of death was classified as early sepsis or neurological (first 48 h), late neurological, or not neurological. Therapeutic failure was considered if the illness recurred, which we defined as a rise in fever and meningeal signs and symptoms after a clear initial improvement or after symptom resolution and therapy completion.
Statistical analysis.
Continuous variables are expressed as means ± standard deviations or as median with interquartile ranges (Q1 to Q3). For descriptive analysis, categorical data were compared using the chi-square test or Fisher exact test, and continuous data with the t test or Mann–Whitney U test.
Ethics.
The Ethics Committee of our center approved this study (EOM016/21) and waived the necessity of informed consent due to the observational nature of the study with the guarantee of anonymous data collection.
ACKNOWLEDGMENTS
We thank the Centro de Investigación Biomédica en Red de Enfermedades Infecciosas (CIBERINFEC; CB/13/00009), Instituto de Salud Carlos III. We thank all the past and present staff of the Infectious Diseases and Microbiology Departments of Hospital Universitari de Bellvitge who contributed to this project on a daily basis.
L.G. had personal funding from grant PI18/00814 Instituto de Salud Carlos III. This study was supported by grants from Instituto de Salud Carlos III (PI18/00814; PI18/00339) and from Centro de Investigación Biomédica en Red (CIBER) de Enfermedades Infecciosas (CIBERINFEC; CB/13/00009) and de Enfermedades Respiratorias (CIBERES; CB06/06/0037) an initiative of the Instituto de Salud Carlos III. Cofunded by the European Regional Development Fund/European Social Fund (ERDF/ESF, “Investing in your future”). We thank CERCA Program/Generalitat de Catalunya for institutional support.
C.A. has participated as scientific advisor of Pfizer and MSD, and has received research funding from MSD, unrelated to the present study. All other authors declare no conflicts of interest.
Contributor Information
Carmen Cabellos, Email: ccabellos@bellvitgehospital.cat.
Lluïsa Guillem, Email: lui.gui.ti@gmail.com.
REFERENCES
- 1.Domingo P, Pomar V, Benito N, Coll P. 2013. The changing pattern of bacterial meningitis in adult patients at a large tertiary university hospital in Barcelona, Spain (1982–2010). J Infect 66:147–154. doi: 10.1016/j.jinf.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 2.Brouwer MC, Tunkel AR, van de Beek D. 2010. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev 23:467–492. doi: 10.1128/CMR.00070-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansman D, Bullen MM. 1967. A resistant pneumococcus. Lancet 290:264–265. doi: 10.1016/S0140-6736(67)92346-X. [DOI] [PubMed] [Google Scholar]
- 4.Appelbaum PC, Bhamjee A, Scragg JN, Hallett AF, Bowen AJ, Cooper RC. 1977. Streptococcus pneumoniae resistant to penicillin and chloramphenicol. Lancet 2:995–997. doi: 10.1016/s0140-6736(77)92892-6. [DOI] [PubMed] [Google Scholar]
- 5.Viladrich PF, Gudiol F, Liñares J, Rufí G, Ariza J, Pallarés R. 1988. Characteristic and antibotic therpay of adult meningitis due to penicillin-resisitant pneumococci. Am J Med 84:839–846. doi: 10.1016/0002-9343(88)90061-7. [DOI] [PubMed] [Google Scholar]
- 6.Sloas MM, Barrett FF, Chesney PJ, English BK, Hill BC, Tenover FC, Leggiadro RJ. 1992. Cephalosporin treatment failure in penicillin- and cephalosporin-resistant Streptococcus pneumoniae meningitis. Pediatr Infect Dis J 11:662–666. [PubMed] [Google Scholar]
- 7.Liñares J, Garau J, Domínguez C, Pérez JL. 1983. Antibiotic resistance and serotypes of Streptococcus pneumoniae from patients with community-acquired pneumococcal disease. Antimicrob Agents Chemother 23:545–547. doi: 10.1128/AAC.23.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liñares J, Pallares R, Alonso T, Perez JL, Ayats J, Gudiol F, Viladrich PF, Martin R. 1992. Trends in antimicrobial resistance of clinical isolates of Streptococcus pneumoniae in Bellvitge Hospital, Barcelona, Spain (1979–1990). Clin Infect Dis 15:99–105. doi: 10.1093/clinids/15.1.99. [DOI] [PubMed] [Google Scholar]
- 9.Lonks JR, Durkin MR, Meyerhoff AN, Medeiros AA. 1995. Meningitis due to CRO-resistant Streptococcus pneumoniae. N Engl J Med 332:893–894. doi: 10.1056/NEJM199503303321317. [DOI] [PubMed] [Google Scholar]
- 10.McCormick AW, Whitney CG, Farley MM, Lynfield R, Harrison LH, Bennett NM, Schaffner W, Reingold A, Hadler J, Cieslak P, Samore MH, Lipsitch M. 2003. Geographic diversity and temporal trends of antimicrobial resistance in Streptococcus pneumoniae in the United States. Nat Med 9:424–430. doi: 10.1038/nm839. [DOI] [PubMed] [Google Scholar]
- 11.Fenoll A, Granizo JJ, Aguilar L, Giménez MJ, Aragoneses-Fenoll L, Hanquet G, Casal J, Tarragó D. 2009. Temporal trends of invasive Streptococcus pneumoniae serotypes and antimicrobial resistance patterns in Spain from 1979 to 2007. J Clin Microbiol 47:1012–1020. doi: 10.1128/JCM.01454-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liñares J, Ardanuy C, Pallares R, Fenoll A. 2010. Changes in antimicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-year period. Clin Microbiol Infect 16:402–410. doi: 10.1111/j.1469-0691.2010.03182.x. [DOI] [PubMed] [Google Scholar]
- 13.Pinto TCA, Neves FPG, Souza ARV, Oliveira LMA, Costa NS, Castro LFS, Mendonça-Souza CRdV, Peralta JM, Teixeira LM. 2019. Evolution of penicillin not-susceptibility among streptococcus pneumoniae isolates recovered from asymptomatic carriage and invasive disease over 25 years in Brazil, 1990–2014. Front Microbiol 10:486. doi: 10.3389/fmicb.2019.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sallam M, Abbadi J, Natsheh A, Ababneh NA, Mahafzah A, Sahin GO. 2019. Trends in antimicrobial drug resistance of Streptococcus pneumoniae isolates at Jordan University Hospital (2000–2018). Antibiotics 8:41. doi: 10.3390/antibiotics8020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jayaraman R, Varghese R, Kumar JL, Neeravi A, Shanmugasundaram D, Ralph R, Thomas K, Veeraraghavan B. 2019. Invasive pneumococcal disease in Indian adults: 11 years' experience. J Microbiol Immunol Infect 52:736–742. doi: 10.1016/j.jmii.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Càmara J, Grau I, González-Díaz A, Tubau F, Calatayud L, Cubero M, Domínguez MÁ, Liñares J, Yuste J, Pallarés R, Ardanuy C. 2021. A historical perspective of MDR invasive pneumococcal disease in Spanish adults. J Antimicrob Chemother 76:507–515. doi: 10.1093/jac/dkaa465. [DOI] [PubMed] [Google Scholar]
- 17.CDC. 2019. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA, USA. [Google Scholar]
- 18.WHO. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics.
- 19.Viladrich PF, Gudiol F, Liñares J, Pallarés R, Sabaté I, Rufí G, Ariza J. 1991. Evaluation of vancomycin for therapy of adult pneumococcal meningitis. Antimicrob Agents Chemother 35:2467–2472. doi: 10.1128/AAC.35.12.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klugman KP, Friedland IR, Bradley JS. 1995. Bactericidal activity against cephalosporin-resistant Streptococcus pneumoniae in cerebrospinal fluid of children with acute bacterial meningitis. Antimicrob Agents Chemother 39:1988–1992. doi: 10.1128/AAC.39.9.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viladrich PF, Cabellos C, Pallares R, Tubau F, Martínez-Lacasa J, Liñares J, Gudiol F. 1996. High doses of cefotaxime in the treatment of adult menigitis due to Streptococcus pneumoniae with decreased susceptibility to third feneration cephalosporins. Antimicrob Agents Chemother 40:218–220. doi: 10.1128/AAC.40.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed A. 1997. A critical evaluation of vancomycin for treatment of bacterial meningitis. Pediatr Infect Dis J 16:895–903. doi: 10.1097/00006454-199709000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Appelbaum PC. 2002. Resistance among Streptococcus pneumoniae: implications for drug selection. Clin Infect Dis 34:1613–1620. doi: 10.1086/340400. [DOI] [PubMed] [Google Scholar]
- 24.Ricard J-D, Wolff M, Lacherade J-C, Mourvillier B, Hidri N, Barnaud G, Chevrel G, Bouadma L, Dreyfuss D. 2007. Levels of vancomycin in cerebrospinal fluid of adult patients receiving adjunctive corticosteroids to treat pneumococcal meningitis: a prospective multicenter observational study. Clin Infect Dis 44:250–255. doi: 10.1086/510390. [DOI] [PubMed] [Google Scholar]
- 25.Erdem H, Elaldi N, Öztoprak N, Sengoz G, Ak O, Kaya S, Inan A, Nayman-Alpat S, Ulu-Kilic A, Pekok AU, Gunduz A, Gozel MG, Pehlivanoglu F, Yasar K, Yılmaz H, Hatipoglu M, Cicek-Senturk G, Akcam FZ, Inkaya AC, Kazak E, Sagmak-Tartar A, Tekin R, Ozturk-Engin D, Ersoy Y, Sipahi OR, Guven T, Tuncer-Ertem G, Alabay S, Akbulut A, Balkan II, Oncul O, Cetin B, Dayan S, Ersoz G, Karakas A, Ozgunes N, Sener A, Yesilkaya A, Erturk A, Gundes S, Karabay O, Sirmatel F, Tosun S, Turhan V, Yalci A, Akkoyunlu Y, Aydın E, Diktas H, Kose S, Ulcay A, et al. 2014. Mortality indicators in pneumococcal meningitis: therapeutic implications. Int J Infect Dis 19:13–19. doi: 10.1016/j.ijid.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Friedland IR, Paris M, Ehrett S, Hickey S, Olsen K, McCracken GH. 1993. Evaluation of antimicrobial regimens for treatment of penicillin and cephalosporin resistant pneumococcal meningitis. Antimicrob Agents Chemother 37:1630–1636. doi: 10.1128/AAC.37.8.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.París M, Hickey SM, Uscher MI, Shelton S, Olsen KD, McCracken GH. Jr.. 1994. Effect of dexamethasone on therapy of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother 38:1320–1324. doi: 10.1128/AAC.38.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martínez-Lacasa J, Cabellos C, Martos A, Fernández A, Tubau F, Viladrich PF, Liñares J, Gudiol F. 2002. Experimental study of the efficacy of vancomycin, rifampin and dexamethasone in the therapy of pneumococcal meningitis. J Antimicrob Chemother 49:507–513. doi: 10.1093/jac/49.3.507. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt H, Dalhoff A, Stuertz K, Trostdorf F, Chen V, Schneider O, Kohlsdorfer C, Brück W, Nau R. 1998. Moxifloxacin in the therapy of experimental pneumococcal meningitis. Antimicrob Agents Chemother 42:1397–1407. doi: 10.1128/AAC.42.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedland IR, Paris M, Ehrett S. 1999. Evaluation of antimicrobial regimens for treatment of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother 43:2372–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerber J, Smirnov A, Wellmer A, Ragheb J, Prange J, Schütz E, Wettich K, Kalich S, Nau R. 2001. Activity of LY333328 in experimental meningitis caused by a Streptococcus pneumoniae strain susceptible to penicillin. Antimicrob Agents Chemother 45:2169–2172. doi: 10.1128/AAC.45.7.2169-2172.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kühn F, Cottagnoud M, Acosta F, Flatz L, Entenza J, Cottagnoud P. 2003. CTX acts synergistically with levofloxacin in experimental meningitis due to penicillin-resistant pneumococci and prevents selection of levofloxacin-resistant mutants in vitro. Antimicrob Agents Chemother 47:2487–2491. doi: 10.1128/AAC.47.8.2487-2491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cottagnoud P, Pfister M, Acosta F, Cottagnoud M, Flatz L, Kühn F, Müller H-P, Stucki A. 2004. Daptomycin is highly efficacious against penicillin-resistant and penicillin- and quinolone-resistant pneumococci in experimental meningitis. Antimicrob Agents Chemother 48:3928–3933. doi: 10.1128/AAC.48.10.3928-3933.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ribes S, Taberner F, Domenech A, Cabellos C, Tubau F, Liñares J, Viladrich PF, Gudiol F. 2006. Evaluation of fosfomycin alone and in combination with CRO or vancomycin in an experimental model of meningitis caused by two strains of cephalosporin-resistant Streptococcus pneumoniae. 57:931–936. doi: 10.1093/jac/dkl047. [DOI] [PubMed] [Google Scholar]
- 35.Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, Whitley RJ. 2004. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 39:1267–1284. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 36.van de Beek D, Cabellos C, Dzupova O, Esposito S, Klein M, Kloek AT, Leib SL, Mourvillier B, Ostergaard C, Pagliano P, Pfister HW, Read RC, Sipahi OR, Brouwer MC, ESCMID Study Group for Infections of the Brain (ESGIB) . 2016. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect 22 Suppl 3:S37–62. doi: 10.1016/j.cmi.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Cabellos C, Martínez-Lacasa J, Tubau F, Fernández A, Viladrich PF, Liñares J, Gudiol F. 2000. Evaluation of combined CRO and dexamethasone therapy in experimental cephalosporin-resistant pneumococcal meningitis. J Antimicrob Chemother 45:315–320. doi: 10.1093/jac/45.3.315. [DOI] [PubMed] [Google Scholar]
- 38.Société de pathologie infectieuse de langue française (SPILF), organisateur de la 17e Conférence de consensus en thérapeutique anti-infectieuse. 2008. Prise en charge des méningites bactériennes aiguës communautaires (à l’exclusion du nouveau-né). http://www.infectiologie.com/site/medias/_documents/consensus/2008-Meningites-court.pdf. [DOI] [PubMed]
- 39.Le Turnier P, Navas D, Garot D, Guimard T, Bernard L, Tattevin P, Vandamme YM, Hoff J, Chiffoleau A, Dary M, Leclair-Visonneau L, Grégoire M, Pere M, Boutoille D, Sébille V, Dailly E, Asseray N, High-Dose Ceftriaxone CNS Infections Study Group . 2019. Tolerability of high-dose CRO in CNS infections: a prospective multicentre cohort study. J Antimicrob Chemother 74:1078–1085. doi: 10.1093/jac/dky553. [DOI] [PubMed] [Google Scholar]
- 40.The European Committee on Antimicrobial Susceptibility Testing. 2021. Breakpoint tables for interpretation of MICs and zone diameters. Version 11.0. http://www.eucast.org.