Abstract
Zebrafish have long been used as a model vertebrate organism in cardiovascular research. The technical difficulties of isolating individual cells from the zebrafish cardiovascular tissues have been limiting in studying their electrophysiological properties. Previous methods have been described for dissection of zebrafish hearts and isolation of ventricular cardiac myocytes. However, the isolation of zebrafish atrial and vascular myocytes for electrophysiological characterization was not detailed. This work describes new and modified enzymatic protocols that routinely provide isolated juvenile and adult zebrafish ventricular and atrial cardiomyocytes, as well as vascular smooth muscle (VSM) cells from the bulbous arteriosus, suitable for patch-clamp experiments. There has been no literary evidence of electrophysiological studies on zebrafish cardiovascular tissues isolated at embryonic and larval stages of development. Partial dissociation techniques that allow patch-clamp experiments on individual cells from larval and embryonic hearts are demonstrated.
Introduction
Zebrafish are small teleost fish that have long been used as a model vertebrate organism1 and have recently come to prominence as a viable vertebrate system for high throughput screening of genes and drugs2, 3. However, physiological analysis of zebrafish tissues is not well developed. In the cardiovascular system, methods have been described for dissection of zebrafish hearts4 and isolation of ventricular cardiac myocytes5, 6, 7. There are few detailed descriptions of the effective isolation of atrial myocytes and no reports of vascular smooth muscle (VSM) preparations for patch-clamp studies.
The current work describes methodology for the isolation of zebrafish cardiac and vascular myocytes, viable for electrophysiological and functional studies. This approach includes modifications of previously reported protocols for zebrafish ventricular myocyte isolation5, 6 and adapts methods from mammalian VSM cell isolations8, allowing for the isolation of zebrafish vascular smooth muscle cells from the bulbous arteriosus (BA). The protocols result in efficient yields of isolated atrial, ventricular, and VSM cells from zebrafish that can be reliably used in patch-clamp studies for up to 8 h9.
Despite their nearly transparent larvae that develop entirely outside the parental organism, exploring their promised ontogenetic potential in studying cardiovascular development has been limited by challenges in extracting and analyzing tissues at a young age. The current article addresses this limitation by demonstrating patch-clamp experiments on zebrafish hearts isolated as early as 3 days post-fertilization (dpf), using an adapted, published extraction method10.
Protocol
All zebrafish (wild type strain AB, both male and female) were raised, maintained, and handled for the experiments following the guidelines of the Washington University Institutional Animal Care and Use Committee (IACUC).
1. Isolation of atrium, ventricle, and bulbous arteriosus from adult, juvenile, and larval zebrafish
Euthanize fish using cold-shock, i.e., by immersing in 4 °C water, for ~10 s.
- Using curved forceps, transfer fish into a large Petri dish partially filled with Perfusion Buffer (PB) (Table 1) and place under a dissecting microscope.
- Decapitate the fish just posterior to the eyes, using a pair of scissors.
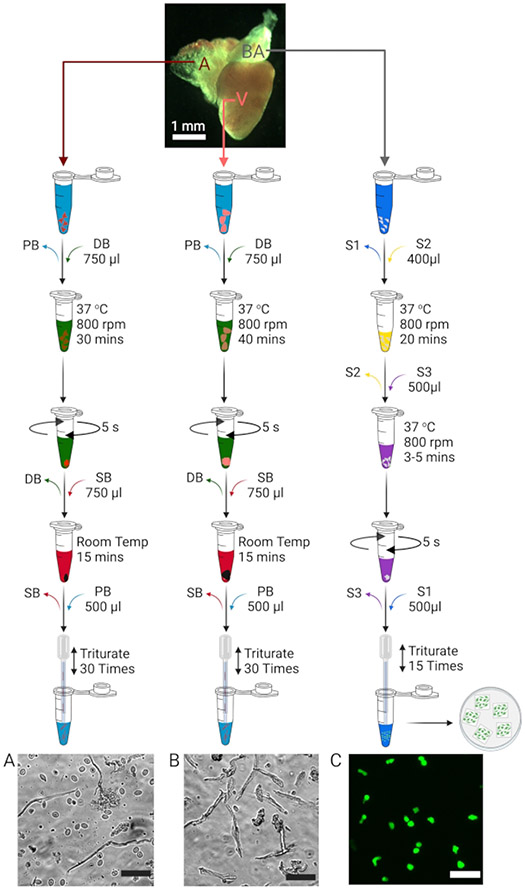
Using curved forceps (fine forceps for larval fish), hold the fish in the non-dominant hand with the ventral side facing the dissection scope. With the second pair of fine forceps (or superfine for larval fish) in the dominant hand, gently tear open the pectoral muscles and fins to reveal the cardiovascular (CV) tissues (atrium, ventricle, and bulbous arteriosus, BA) (Figure 1).
-
Placing the fine forceps in the dominant hand, gently pull the BA at the intersecting tip of the BA and the ventral aorta.
NOTE: The BA and heart will come out of the body cavity.- Carefully separate the BA from the aorta by pinching the forceps and locating the atrium's tip into which multiple venous branches converge (sinus venosus). Using the fine forceps, 'pluck' the tip of the atrium off the sinus venosus. This isolates the CV tissues from the rest of the body (Figure 1).
Table 1:
Solutions for isolation of zebrafish cardiomyocytes.
| PERFUSION BUFFER (PB) | 10 mM HEPES, 30 mM Taurine, 5.5 mM Glucose, 10 mM BDM. Make the solution in phosphate-buffered saline (PBS), adjust pH to 7.4 |
| DIGESTION BUFFER (DB) | PB + 12.5 μM CaCl2 + 5 mg/mL Col II + 5 mg/mL Col IV + 5 ng/mL Insulin |
| STOPPING BUFFER (SB) | PB + 10% FBS + 12.5 μM CaCl2 + 10 mg/ml BSA + 5 ng/mL Insulin |
Figure 1: Isolation of atrial, ventricular, and bulbous arteriosus cells.
Schematic to illustrate the isolation of atrial (A), ventricular (B), and bulbous arteriosus (C) cells. The images depicting each isolated cell type (bottom) have scale bars = 50 μm in each case. BA cells isolated from smooth muscle cell transgenic reporter zebrafish lines (Tg(tagln:egfp)11, 12) are positive for green fluorescence.
2. Dissociation of cardiomyocytes from adult, juvenile, and larval zebrafish for electrophysiological studies
-
Using the fine forceps, 'pluck' the atrium and ventricle out of the CV tissue isolated in the earlier step.
NOTE: The process allows for clean dissection of each tissue with minimal cross-contamination and feels akin to plucking fruit from a tree.- Make a gentle tear into the ventricle using fine forceps to drain excess blood, which can be ensured when the cardiac tissue turns pale salmon color from bright red.
-
Pool the isolated atrium and ventricle into separate 1.5 mL centrifuge tubes containing Perfusion Buffer (PB; 1.5 mL).
NOTE: To guarantee successful dissociation of adult zebrafish atrial cardiomyocytes (ACMs) and ventricular cardiomyocytes (VCMs), typically four atria and three ventricles are needed. In the case of juvenile zebrafish (28-90 days), this number is doubled.- In the case of larval zebrafish (14-28 days), collect as much tissue as possible from ~30 larvae.
Replace the PB in the tubes with 750 μL of Digestion Buffer (DB) (Table 1) each and place the tubes on a thermoshaker at 37 °C and 800 rpm. Allow digestion of the tissues until they become translucent (~30 min for the atria and ~40 min for the ventricles).
-
End the digestion by gently spinning down the tissues in a benchtop mini centrifuge (2,000 x g at ambient temperature) for 3-5 s and replace the supernatant DB with 750 μL of Stopping Buffer (SB) each (Table 1).
NOTE: This centrifugation step is optional for VCM isolation.
Incubate the pelleted tissues in the SB at ambient temperature for 15 min.
-
Gently replace the SB with 500-750 μL of PB each (depending on the desired density of the final cells) and triturate the tissues (~30 times) using a flame-polished Pasteur pipette to disperse the cells.
NOTE: The cardiomyocytes (CMs) are now ready for electrophysiological studies and stored at room temperature for up to 8 h.
For uniform sampling, gently pipette the cells up and down a couple of times to resuspend before adding a drop of them for corresponding studies.
3. Dissociation of vascular smooth muscle (VSM) cells from adult and juvenile zebrafish for electrophysiological studies
-
Pluck bulbous arteriosus (BA) from the CV tissues using the same approach as step 2.1 and pool five adult BAs into a 1.5 mL centrifuge tube containing S1 buffer (1.5 mL) (Table 2). In the case of juvenile zebrafish, pool ten BAs and, for larvae older than 2 weeks, pool 30-40 BAs.
NOTE: It is not practically feasible to isolate VSM cells from zebrafish larvae younger than 2 weeks.
Replace S1 with 400 μL of S2 buffer (Table 2) and allow papain (see Table of Materials) digestion of the BAs on a thermoshaker at 37 °C and 800 rpm for ~20 min.
Allow the partially digested BAs to settle down for 1 min and replace the supernatant S2 with 500 μL of S3 buffer (Table 2) containing collagenase. Digest the tissues on a thermoshaker at 37 °C and 800 rpm for 3-5 min.
End the digestion by gently spinning down the tissues in a benchtop mini centrifuge (2,000 x g at ambient temperature) for 3-5 s and replacing supernatant S3 with 500 μL of S1.
-
Gently triturate the pelleted tissues (~15 times) to disperse VSM cells and plate onto glass coverslips of appropriate size.
NOTE: The coverslip size is determined by the size of the patch-clamp recording chamber, in this case, 5 x 5 mm was used).- Keep the coverslips at room temperature for 30 min for the cells to attach and use them within the next 6 h.
Table 2:
Solutions for isolation of zebrafish VSM cells.
| S1 BUFFER | 0.1% BSA (w/v), 145 mM NaCl, 4 mM KCl, 10 mM HEPES, 10 mM Glucose, 0.05 mM CaCl2, 1 mM MgCl2, adjusted to pH 7.4 using NaOH |
| S2 BUFFER | 2 mL S1, 4 mg Papain, 2 mg DTT |
| S3 BUFFER | 2 mL S1, 3 mg Collagenase Type H, 2 mg Trypsin Inhibitor, 1 mg Elastase |
4. Isolation of hearts from embryonic zebrafish for electrophysiological studies
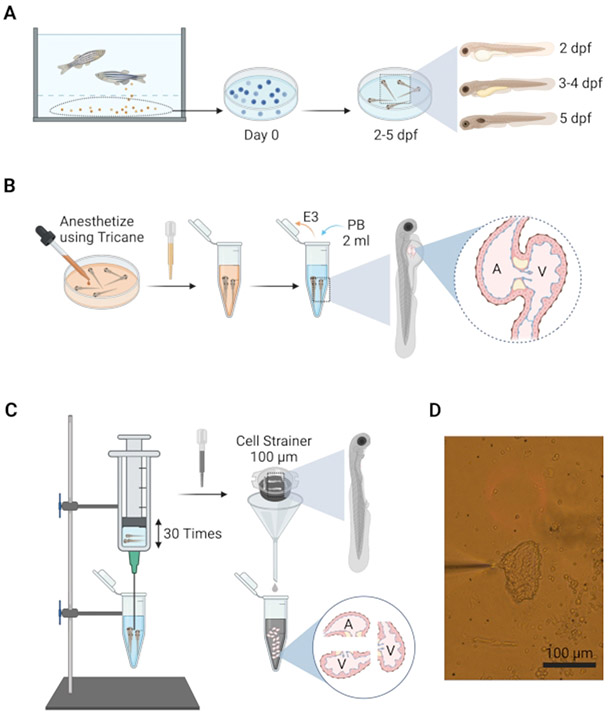
- Add tricaine (150 mg/L; 1 ml per 100 mm x 20 mm Petri dish) to ~300 embryos (2-5 dpf), collected from their incubation conditions (Figure 2A).
- Concentrate the anesthetized embryos by transferring them to a 5 mL centrifuge tube using a transfer pipette, removing excess media (E3) from the centrifuge tube (Figure 2B).
Wash the embryos with 3 mL of cold Perfusion Buffer (PB) twice and resuspend in 2 mL of PB (Figure 2B).
Using the embryonic heart isolation apparatus (Figure 2C), draw ~1 mL of the embryos into the syringe needle and immediately expel them back into the tube. Repeat this process 30 times, at a rate of 1 s per syringe motion (Figure 2C).
Pass the fragmented embryos through a 100 μm cell-strainer sieve placed in a funnel and collect the filtered hearts in another 5 mL centrifuge tube. Rinse the syringe with 1 mL of PB and collect this rinse as well into the tube through the sieve (Figure 2C).
Mix well and separate into 1.5 mL centrifuge tubes, with each tube containing 1 mL of the contents. Centrifuge all the tubes on a benchtop mini centrifuge for 5 s (2,000 x g at ambient temperature) and discard the supernatants.
Carry out serial resuspension of the pellets in the tubes using 1 mL of PB, i.e., resuspend the pellet in one tube using 1 mL of PB and use that resuspended PB to resuspend the pellet in the next tube.
Gently pipette the hearts up and down two times before adding a drop of them for corresponding studies.
Figure 2: Schematic to illustrate the isolation of embryonic hearts.
(A) Fertilized embryos are removed from the breeding tank and placed in a Petri dish containing E3 water and maintained at 28 °C for up to 5 days. (B) At desired age, embryos are anesthetized in situ using tricaine and transferred into PB in 5 mL centrifuge tubes for dissociation. (C) Embryonic heart isolation apparatus consists of a 10 mL syringe attached to a 19 G needle mounted above the dissociation tube on a bench stand, allowing the hands to perform the trituration. (D) Image of the isolated heart (day 4) attached to a patch-clamp pipette.
5. Patch-clamp studies on isolated cardiovascular cells
NOTE: Inside-out and whole-cell patch-clamp recordings from the isolated cells can be obtained as previously reported for KATP channel currents in zebrafish cardiovasculature9.
For adult and juvenile cardiomyocytes, add the dissociated cells (from step 2) directly to the recording chamber from suspension. For VSM cells, place the coverslip containing the plated VSM cells (from step 3) into the recording chamber.
Add isolated intact embryonic hearts (from step 4) to the chamber from suspension, and make patch-clamp recordings from the epicardial myocytes (Figure 2D).
Representative Results
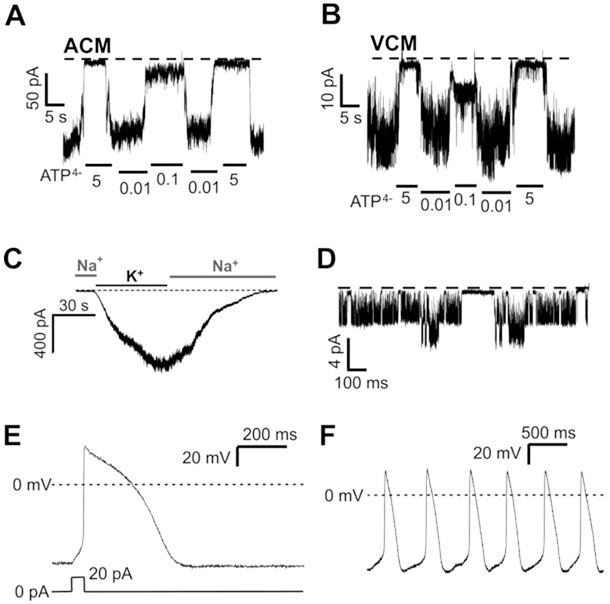
The above protocols reliably and routinely provide sufficient cardiac and vascular myocytes of consistent quality amenable for patch-clamp studies as recently reported in extensive studies of ATP-sensitive potassium (KATP) Channels in wild-type and mutant zebrafish cardiovasculature9. Representative traces of recordings of such KATP Channel activity from isolated cardiomyocytes are shown in Figure 3A-C. In the case of cells isolated from bulbous arteriosus, the vascular smooth muscle cells were confirmed by Tg(tagln:egfp) expression11, 12 (Figure 1). Successful single-channel recordings were obtained (n = 8 recordings from N = 5 preparations) in patches excised from the embryonic hearts (Figure 3D). Action potentials were recorded from isolated adult zebrafish ventricular and atrial myocytes in whole-cell, current-clamp mode, using a patch-clamp amplifier along with a compatible digitizer (see Table of Materials). The extracellular recording solution for the action potential (AP) measurements contained NaCl (140 mM), KCl (4 mM), CaCl2 (1.8 mM), MgCl2 (1 mM), Glucose (10 mM), HEPES (10 mM), and Blebbistatin (0.01 mM, pH 7.4); the intracellular recording solution contained KCl (120 mM), EGTA (5 mM), K2ATP (5 mM), MgCl2 (5 mM), and HEPES (10 mM, pH 7.2). Patch pipettes were pulled from soda-lime glass and had resistances of 3 - 5 MΩ when filled with intracellular solution. Ventricular myocytes exhibit stable hyperpolarized membrane potentials, and action potentials were stimulated via current injection through the patch pipette (Figure 3E), whereas atrial myocytes exhibited spontaneous action potential firing (Figure 3F). Action potential properties are summarized in Table 3.
Figure 3: Representative patch-clamp recordings from isolated zebrafish cardiovascular tissues.
(A, B) ATP-sensitive KATP channels from inside-out excised patch-clamp recordings of atrial (A) and ventricular (B) cardiomyocytes isolated from adult zebrafish. (C) KATP channel conductance was recorded from whole-cell patch-clamping of VSM cells isolated from adult zebrafish. (D) Single K+ channel activity in membrane patches excised from zebrafish embryonic hearts (4 dpf). All excised patch recordings were made with 140 mM KCl on both sides of the membrane, at −50 mV membrane potential. Whole-cell currents were recorded with the membrane potential clamped at −70 mV. (E) Example current-clamp recording from isolated ventricular myocyte. Action potentials were stimulated by current injection as shown below. (F) Example current-clamp recording from isolated atrial myocyte showing spontaneous action potential firing.
Table 3: Action potential properties from isolated zebrafish cardiomyocytes.
Recorded in current clamp mode. Data shown as mean ± S.D. Vm = membrane potential; APA = action potential amplitude; APD50 = duration of action potential to 50% repolarization; MDP = maximal diastolic potential.
| Ventricular Myocytes (n = 3 Recordings) | |||
|---|---|---|---|
| Vm (mV) | APA (mV) | APD50 (ms) | |
| −75.7 ± 3.9 | −119.6 ± 3.8 | 329 ± 163 | |
| Atrial Myocytes (n = 3 Recordings) | |||
| AP Rate (min−1) | MDP (mV) | APA (mV) | APD50 (ms) |
| 107.3 ± 32.6 | −71.2 ± 5.1 | 98.4 ± 4.7 | 141 ± 12 |
Discussion
Previous methods for isolating zebrafish ventricular myocytes5, 6, aimed at generating myocytes for culture or electrophysiological studies, provided cells of lower yield and involved lengthy steps of multiple centrifugations that adversely affected the cell quality and viability. The protocols described here are reliable, cover each of the significant cardiovascular tissues (ventricle, atria, and VSM), and importantly are quite practical for acute isolation of live cells. Isolation approaches involving cannulation of the ventricle via the BA13 can be a sophisticated alternative for ventricular cardiomyocytes but require a higher degree of dexterity and may negatively affect atrial isolation. The approach detailed in the current protocol provides a simple and efficient alternative.
Additional considerations for larval cardiac tissue isolation are: (1) In step 1.4, depending on the age of the fish and magnification available, the tissues might be visible even before removing the pectoral muscles, in which case the tissues can be directly "plucked" out of the fish without prior surgery, and then the non-CV tissues can be removed using superfine forceps; (2) In step 2.2, for larvae younger than 14 days, the hearts can be used directly for electrophysiological studies without need for enzymatic dissociation.
As noted above, and typically true of enzymatic dissociation methods in general, it has proven important to use fresh buffers and enzymes each time. However, Perfusion Buffer (PB) and S1 Buffer can be prepared in advance and stored at 4-8 °C for 1 month.
Successful tissue isolation time should not exceed ~90 s per fish, and tissues should not be exposed to air. When dissociation takes longer, the cell quality (i.e. survival) is reduced. When triturating tissues, care should be taken to avoid generating air bubbles, which also reduces cell quality. VSM cell isolation is sensitive to the digestion by collagenase in S3 buffer. After the first 3 min of digestion, the tube should be taken out of the thermoshaker every minute and examined for floating dilated and translucent tissues. Once tissue fragmentation is apparent, the digestion step should be ceased.
It is important to note that these protocols are not extensively optimized for isolating calcium-tolerant cardiomyocytes, as would be required for contractility studies. However, as shown, reliable recording of cardiomyocyte action potentials can be achieved in the presence of physiological extracellular calcium concentrations7, 14. Blebbistatin, included to decouple excitation and contraction, and improve giga-Ohm seal formation and recording efficiency in these experiments, has previously been shown to not significantly affect the AP parameters in intact zebrafish hearts14. For electrophysiology, the absolute yield of cells is also not routinely rate-limiting. This protocol has not been optimized for yield, as might be necessary for biochemical studies of isolated cells. Still, the yield achieved with these methods is well suited for electrophysiological studies and likely multiple other approaches.
Supplementary Material
Acknowledgments
This work was supported by NIH grants HL140024 to CGN and HL150277 to CMC. Figure 1 and Figure 2 were created with BioRender.com.
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/63225.
Disclosures
The authors declare no competing financial interests.
References
- 1.Vascotto SG, Beckham Y, Kelly GM The zebrafish's swim to fame as an experimental model in biology. Biochemistry and Cell Biology. 75 (5), 479–485 (1997). [PubMed] [Google Scholar]
- 2.Love DR, Pichler FB, Dodd A, Copp BR, Greenwood DR Technology for high-throughput screens: The present and future using zebrafish. Current Opinion in Biotechnology. 15 (6), 564–571 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Keßler M, Rottbauer W, Just S Recent progress in the use of zebrafish for novel cardiac drug discovery. Expert Opinion on Drug Discovery. 10 (11), 1231–1241 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Singleman C, Holtzman NG Heart dissection in larval, juvenile and adult zebrafish, Danio rerio. Journal of Visualized Experiments. 55, e3165 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brette F et al. Characterization of isolated ventricular myocytes from adult zebrafish (Danio rerio). Biochemical and Biophysical Research Communications. 374 (1) 143–146 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sander V, Sune G, Jopling C, Morera C, Izpisua Belmonte JC Isolation and in vitro culture of primary cardiomyocytes from adult zebrafish hearts. Nature protocol. 8, 800–809 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Nemtsas P, Wettwer E, Christ T, Weidinger G, Ravens U Adult zebrafish heart as a model for human heart? An electrophysiological study. Journal of Molecular and Cellular Cardiology. 48 (1), 161–171 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Huang Y et al. Cardiovascular consequences of KATP overactivity in Cantu syndrome. JCI insight. 3 (15), e121153 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singareddy SS et al. ATP-sensitive potassium channels in zebrafish cardiac and vascular smooth muscle. The Journal of Physiology. 10.1113/jp282157 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns CG, MacRae CA Purification of hearts from zebrafish embryos. BioTechniques. 40 (3), 274–282 (2006). [PubMed] [Google Scholar]
- 11.Seiler C, Abrams J, Pack M Characterization of zebrafish intestinal smooth muscle development using a novel sm22α-b promoter. Developmental Dynamics. 239, 2806–2812 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang XY et al. Whole amount in situ hybridization and transgene via microinjection in zebrafish. Shi Yan Sheng Wu Xue Bao. 36 (3), 243–247 (2003). [PubMed] [Google Scholar]
- 13.Kompella SN, Brette F, Hancox JC, Shiels HA Phenanthrene impacts zebrafish cardiomyocyte excitability by inhibiting IKr and shortening action potential duration. The Journal of General Physiology. 153 (2), e202012733 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jou CJ, Spitzer KW, Tristani-Firouzi M Blebbistatin effectively uncouples the excitation-contraction process in zebrafish embryonic heart. Cellular Physiology and Biochemistry. 25 (4-5), 419–424 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.