Abstract
Background
Little is known about the risk of SARS-CoV-2 infection and transmission in educational settings. Public Health England initiated a study, COVID-19 Surveillance in School KIDs (sKIDs), in primary schools when they partially reopened from June 1, 2020, after the first national lockdown in England to estimate the incidence of symptomatic and asymptomatic SARS-CoV-2 infection, seroprevalence, and seroconversion in staff and students.
Methods
sKIDs, an active, prospective, surveillance study, included two groups: the weekly swabbing group and the blood sampling group. The swabbing group underwent weekly nasal swabs for at least 4 weeks after partial school reopening during the summer half-term (June to mid-July, 2020). The blood sampling group additionally underwent blood sampling for serum SARS-CoV-2 antibodies to measure previous infection at the beginning (June 1–19, 2020) and end (July 3–23, 2020) of the summer half-term, and, after full reopening in September, 2020, and at the end of the autumn term (Nov 23–Dec 18, 2020). We tested for predictors of SARS-CoV-2 antibody positivity using logistic regression. We calculated antibody seroconversion rates for participants who were seronegative in the first round and were tested in at least two rounds.
Findings
During the summer half-term, 11 966 participants (6727 students, 4628 staff, and 611 with unknown staff or student status) in 131 schools had 40 501 swabs taken. Weekly SARS-CoV-2 infection rates were 4·1 (one of 24 463; 95% CI 0·1–21·8) per 100 000 students and 12·5 (two of 16 038; 1·5–45·0) per 100 000 staff. At recruitment, in 45 schools, 91 (11·2%; 95% CI 7·9–15·1) of 816 students and 209 (15·1%; 11·9–18·9) of 1381 staff members were positive for SARS-CoV-2 antibodies, similar to local community seroprevalence. Seropositivity was not associated with school attendance during lockdown (p=0·13 for students and p=0·20 for staff) or staff contact with students (p=0·37). At the end of the summer half-term, 603 (73·9%) of 816 students and 1015 (73·5%) of 1381 staff members were still participating in the surveillance, and five (four students, one staff member) seroconverted. By December, 2020, 55 (5·1%; 95% CI 3·8–6·5) of 1085 participants who were seronegative at recruitment (in June, 2020) had seroconverted, including 19 (5·6%; 3·4–8·6) of 340 students and 36 (4·8%; 3·4–6·6) of 745 staff members (p=0·60).
Interpretation
In England, SARS-CoV-2 infection rates were low in primary schools following their partial and full reopening in June and September, 2020.
Funding
UK Department of Health and Social Care.
Introduction
The declaration of COVID-19 as a pandemic led most countries to close their schools as part of their national lockdown measures,1, 2, 3 with more than 1 billion children affected worldwide.4 Although children are recognised to contribute to only a small proportion of confirmed COVID-19 cases and rarely develop severe or fatal disease,5, 6 their role in asymptomatic infection and transmission is uncertain. The proximity of young children in educational settings could lead to rapid transmission between the children and staff, their household contacts, and potentially the wider community. This is well described for other viral infections, including influenza, in which children are the main drivers of infection.7, 8 In previous coronavirus outbreaks, school closure did not contribute to the control of these epidemics.3 School closure affects educational attainment as well as the physical, social, and mental wellbeing of children,3 especially among those from vulnerable and disadvantaged backgrounds.9
In England, a rapid increase in SARS-CoV-2 infections from early March, 2020, led to school closures on March 20, 2020, and wider lockdown on March 23, 2020.10 However, children of key workers, including health-care workers, could attend school throughout lockdown.11 Nationally, COVID-19 cases plateaued in mid-April, 2020, and then declined in May, 2020, allowing gradual easing of lockdown measures.12 Preschool and some primary school years (nursery [age 3–4 years], reception [4–5 years], year 1 [5–6 years], and year 6 [10–11 years]) reopened from June 1, 2020, and some secondary school years (years 10 [14–15 years] and 12 [16–17 years]) reopened from June 15, 2020, until the end of the summer half-term in mid-July, 2020.13 Strict physical distancing and infection control measures were implemented, including smaller class sizes and clustering of staff and students into so-called bubbles.13 These measures, along with low community infection rates (0·7 per 100 000 population), were associated with very few SARS-CoV-2 infections14 or outbreaks during the summer half-term,15 leading to full reopening of all school years in September, 2020,13 when daily SARS-CoV-2 infection rates were 0·6 per 100 000 population, increasing to 4·7 per 100 000 by December, 2020.14
Research in context.
Evidence before this study
We searched PubMed for articles published in English between Jan 1 and Dec 31, 2020, with the terms “COVID-19” or “SARS-CoV-2” with “school”, “education”, or “student” to identify publications relating to SARS-CoV-2 infections and COVID-19 cases in educational settings. The majority of publications were reviews and opinion pieces on the effects of school closures on disease transmission and child health. Some countries, such as Sweden and Iceland, kept their schools open during their lockdowns and found no increase in confirmed SARS-CoV-2 infections, COVID-19 cases, or severe cases in school-aged children (younger than 18 years). Other countries, such as Germany and England, reported limited infections and transmission in educational settings following the reopening of schools after the first lockdown. Published studies have been limited to surveillance and reporting of active infections in children and provide no information on asymptomatic infection and transmission in educational settings.
Added value of this study
In addition to swabbing for SARS-CoV-2 RNA to test for acute infection, we measured serum SARS-CoV-2 antibodies in students and staff in primary schools across England as a marker of previous infection. We found very low rates of SARS-CoV-2 infection estimated through nasal swab RT-PCR during the summer half-term (June–July) when some primary school years returned to school and during the autumn term (September–December) when all primary school years returned to school. In seronegative staff and students at recruitment, we found almost no seroconversions during the summer half-term and only 5% seroconversions during the autumn term.
Implications of all the available evidence
We found very low rates of symptomatic or asymptomatic SARS-CoV-2 infection in students and staff following partial and full reopening of primary schools in England. Community SARS-CoV-2 infection rates were low during the summer half-term and high during the autumn term. Our results indicate that primary schools were not sites of significant transmission, before the emergence of new variants of SARS-CoV-2 in the UK. Further work is needed to understand the effect of new variants within educational settings.
The decision to reopen schools has been divisive.2, 9 Despite the clear benefits of children returning to school, parents and school staff remain concerned about putting the students, staff, and their household members at risk of infection.16, 17 To investigate this risk, Public Health England (PHE) initiated a study, COVID-19 Surveillance in School KIDs (sKIDs), to monitor the incidence of acute asymptomatic and symptomatic SARS-CoV-2 infection and transmission in primary schools when they partially reopened in June, 2020.
Methods
Study design and population
sKIDs, an active, prospective surveillance study, involved convenience sampling of primary schools into two groups. One group received weekly swabbing to estimate the incidence of acute asymptomatic and symptomatic SARS-CoV-2 infection and transmission in students and staff. Because of concerns about asymptomatic infections and transmission, another group of schools received both nasal swabbing and blood sampling for SARS-CoV-2 antibodies to assess previous infection at recruitment and at the end of each school term. Serum antibodies provide a robust measure of past exposure to SARS-CoV-2, capturing symptomatic, asymptomatic, and mild, transient infections.
In both groups, head teachers sent the study information pack to staff and parents and asked them to return a signed consent form before the first sampling day.18 The protocol is available online and was approved by PHE Research Ethics Governance Group (reference NR0209; May 16, 2020).
Procedures
In the weekly swabbing group, 200 primary schools across English regions with 30 or more students attending during the summer half-term (from June 15 to mid-July, 2020) for at least 4 weeks were approached by the UK Department of Education to take part. A nasal swab was taken on the same day every week and couriered to PHE for testing. The sKIDs investigators worked with the Department of Education, local health-care trusts, health protection teams, and the local authority to identify a local experienced person, such as a local nurse or first aider, to take nasal swabs from students and supervise staff members taking their own swabs.
In the blood sampling group, schools were approached in five regions where a paediatric investigation team could be assembled: North London, East London, Oxford, Derby, and Manchester. Participating staff and students had nose and throat swabs and a blood sample taken by the investigation team in June (round 1: June 1–19), mid-July (round 2: July 3–23), and end of the autumn term (round 3: Nov 23–Dec 18). Blood samples and swabs were taken by sKIDs investigators (doctors, nurses, and phlebotomists) in school premises. Local anaesthetic cream was offered to all students.
In both groups, before the first sampling day, staff and parents completed a questionnaire about demographics, risk factors, and school attendance during lockdown, as well as COVID-19 symptoms or confirmed infections in the household. Questionnaires about COVID-19 symptoms and positive tests for participants and their household members were completed at each testing round.
Throughout the summer half-term, investigators worked closely with schools to test unwell staff and students for SARS-CoV-2 infection through local testing centres or by posting swab kits to their homes. Head teachers and participants were asked to notify PHE if any participant tested positive for SARS-CoV-2 or was a contact of a positive case. SARS-CoV-2-positive staff and students were invited to enrol in a household transmission study, in which all household members were swabbed by the sKIDs investigation team and had blood samples taken for antibody testing 4–6 weeks later. The weekly swabbing group was stopped after the summer half-term because very few positive results were yielded.
The swabs were tested by a quantitative RT-PCR (RT-qPCR) assay on an Applied Biosystems 7500 FAST system (ThermoFisher Scientific, CA, USA) targeting a conserved region of the open reading frame 1ab (ORF1ab) gene of SARS-CoV-2.19 Positive RT-qPCR results were reported to the participant, local investigator, head teacher, and local PHE health protection team, typically within 48 h; any positive results with a cycle threshold value greater than 35 were retested by extraction and concentration to verify the initial result. The participant and household members self-isolated as per national guidance. Public health risk assessment was undertaken with the school to decide additional measures, including isolation of the participant's class bubble. Serological tests were performed on the Abbott Architect using a chemiluminescent microparticle immunoassay to detect IgG antibodies to SARS-CoV-2 nucleoprotein (Abbott Commerce, Chicago, IL, USA). This assay has a seropositivity threshold of 0.8.20
Statistical analysis
Participants were linked to the national surveillance database held at PHE, which includes daily reports of all SARS-CoV-2 RT-qPCR tests. Participants were linked by name, date of birth, sex, and region to ascertain whether they had tested positive for SARS-CoV-2 between testing rounds. Analysis of swab positivity rates during the summer half-term included both study groups. Where participant status was unknown, we assumed that the student-to-staff ratio in participants with missing information was the same as the proportion with available information; these participants with missing data were included as denominators to estimate weekly infection rates.
Data that did not follow a normal distribution are described as median with IQR. Categorical data are described as proportions and compared with the χ2 test or Fisher's exact test. We tested for predictors of SARS-CoV-2 antibody positivity using logistic regression. We built a multivariable regression model using likelihood ratio tests and included factors that were significant, or of a priori interest, in the univariable analysis. These factors included demographics, school attendance during lockdown, and additional children in the household (students only). Being unwell with symptoms of COVID-19, having confirmed COVID-19, and having household members with previous confirmed COVID-19 were assessed in the univariable analysis only because of their strong correlation with seropositivity. The univariable analysis included all participants, followed by multivariable analysis including participants with complete questionnaire data to allow consistent results. We tested differences between schools by adjusting for clustering in the final multivariable models. Wald tests were used to generate global p values. The 95% CIs for antibody positivity include clustered SEs by school. We analysed characteristics of dropouts in students and staff in subsequent rounds using univariate and multivariable logistic regression, adjusted for sex, age, ethnicity, region, and round 1 antibody test results. Participants were classified as included in rounds two and three if they provided a blood or swab sample during those visits. We calculated antibody seroconversion rates for all participants who were tested in at least two rounds and were negative in the first round. We assessed factors associated with seroconversion using Poisson regression, adjusted for sex, ethnicity, region, participant type, proportion of students at the school who receive free school meals, and follow-up time, and clustered by school. An overall seroconversion rate was estimated using participants who were tested at the beginning and end of the surveillance (rounds 1 and 3); all other analyses included seroconversions in participants who were tested at least twice during the surveillance period.
Data were managed in Microsoft Access and analysed using Stata (version 15.0).
Role of funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
In June, 2020, 11 966 participants in 131 schools were recruited across England (appendix p 2), with a median of 93 (IQR 61–156) participants in 86 schools taking part in weekly swabbing and 43 (28–68) participants in 45 schools involved in serology testing. Of the 11 355 (94·9%) participants with completed questionnaires, 6727 (59·2%) were students and 4628 (40·8%) were staff (table 1 ). Of the 40 501 swabs taken in June and July, 2020, 23 339 (57·6%) with available information were from students and 15 288 (37·7%) were from staff. The number of swabs taken in the weekly swabbing group of the surveillance increased weekly as more participants agreed to take part until the first week of July, 2020, and then declined as schools closed for the summer holidays. One student and five staff members had detectable SARS-CoV-2 from their nose or throat swab. Three staff members (two previously symptomatic and one asymptomatic) had very high RT-qPCR cycle threshold values (>39), consistent with very low viral load, and when the samples were concentrated and re-analysed they tested negative; all three were also antibody negative 4–6 weeks later. The asymptomatic student was a child of a health-care worker who had been symptomatic and RT-qPCR positive 4 weeks previously. Therefore, after adjusting for missing status (ie, staff or student), one student (of 24 463 swabs taken) and two staff members (of 16 038 swabs taken) had confirmed SARS-CoV-2 infection. We estimated a SARS-CoV-2 infection rate of 4·1 (95% CI 0·1–22·8) per 100 000 students and 12·5 (1·5–45·0) per 100 000 staff per week of testing during the summer half-term. All household contacts were offered a nasal swab and tested negative; none became symptomatic during follow-up (appendix p 3).
Table 1.
Characteristics of staff and students in the participating schools (June to mid-July, 2020)
| Students (n=6727) | Staff (n=4628) | Unknown (n=611) | Total (n=11 966) | ||
|---|---|---|---|---|---|
| Sex | |||||
| Female | 3374/6653 (50·7%) | 3902/4600 (84·8%) | 2 | 7278/11 255 (64·7%) | |
| Male | 3279/6653 (49·3%) | 698/4600 (15·2%) | 0 | 3977/11 255 (35·3%) | |
| Missing sex | 74 | 28 | 609 | 711 | |
| Ethnicity | |||||
| White | 4016/5389 (74·5%) | 3403/4019 (84·7%) | 1 | 7420/9409 (78·9%) | |
| Mixed or multiple ethnic groups | 415/5389 (7·7%) | 94/4019 (2·3%) | 0 | 509/9409 (5·4%) | |
| Black or Black British | 217/5389 (4·0%) | 128/4019 (3·2%) | 0 | 345/9409 (3·7%) | |
| Asian or Asian British | 576/5389 (10·7%) | 344/4019 (8·6%) | 0 | 920/9409 (9·8%) | |
| Other ethnic group | 165/5389 (3·1%) | 50/4019 (1·2%) | 0 | 215/9409 (2·3%) | |
| Missing ethnicity | 1338 | 609 | 610 | 2557 | |
| Study group | |||||
| Weekly swabbing only (86 schools) | 5911 (87·9%) | 3247 (70·2%) | 611 | 9769 (81·6%) | |
| Participant numbers in swabs schools | 61 (33–102) | 36 (23–48) | 4 (2–8) | 93 (61–156) | |
| Swabs per person in swab schools | 4 (3–5) | 4 (4–5) | 3 (2–4) | 4 (3–5) | |
| Blood sampling (45 schools) | 816 (12·1%) | 1381 (29·8%) | 0 | 2197 (18·4%) | |
| Participant numbers in blood schools | 13 (8–36) | 28 (17–36) | .. | 43 (28–68) | |
| Swabs per person in blood schools | 2 (1–2) | 2 (1–2) | 1 (1–1) | 2 (1–2) | |
| Total numbers of swabs taken | 23 339 | 15 288 | 1874 | 40 501 | |
| Swabs per school | 127 (30–306) | 93 (55–163) | 8 (4–21) | 227 (103–467) | |
| Age, years | |||||
| 4–6 | 2515/5949 (42·3%) | NA | 0 | .. | |
| 7–9 | 1377/5949 (23·1%) | NA | 0 | .. | |
| 10–12 | 2057/5949 (34·6%) | NA | 0 | .. | |
| 20–29 | NA | 68/489 (13·9%) | 0 | .. | |
| 30–39 | NA | 139/489 (28·4%) | 0 | .. | |
| 40–49 | NA | 121/489 (24·7%) | 0 | .. | |
| 50–59 | NA | 125/489 (25·6%) | 0 | .. | |
| ≥60 | NA | 36/489 (7·4%) | 0 | .. | |
| Missing | 778 | 4139 | 0 | 4917 | |
| Region in England | |||||
| East Midlands | 1194 (17·7%) | 611 (13·2%) | 52 | 1857 (15·5%) | |
| East of England | 259 (3·9%) | 141 (3·0%) | 29 | 429 (3·6%) | |
| London | 950 (14·1%) | 1280 (27·7%) | 73 | 2303 (19·2%) | |
| North East | 583 (8·7%) | 282 (6·1%) | 79 | 944 (7·9%) | |
| North West | 405 (6·0%) | 375 (8·1%) | 1 | 781 (6·5%) | |
| South East | 513 (7·6%) | 298 (6·4%) | 40 | 851 (7·1%) | |
| South West | 523 (7·8%) | 332 (7·2%) | 39 | 894 (7·5%) | |
| West Midlands | 1985 (29·5%) | 1152 (24·9%) | 19 | 3156 (26·4%) | |
| Yorkshire and the Humber | 315 (4·7%) | 157 (3·4%) | 279 | 751 (6·3%) | |
Data are n (%), n, or median (IQR). Percentages might not sum to 100% due to rounding. NA=not applicable.
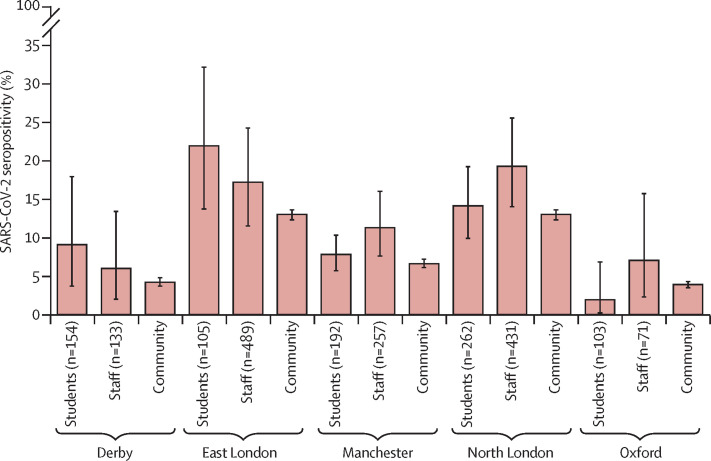
In blood sampling schools, at recruitment (the first two weeks of June, 2020), 300 (13·7%; 95% CI 10·8–16·9) of 2197 participants tested positive for SARS-CoV-2 antibodies: 91 (11·2%; 7·9–15·1) of 816 students and 209 (15·1%; 11·9–18·9) of 1381 staff members. Antibody positivity varied across the different English regions (p<0·001) and between students and staff (p=0·009), but was similar to the regional seroprevalence during the same week (figure 1 ).
Figure 1.
Seropositivity in staff and students in primary schools in five English regions compared with regional seroprevalence during the first two weeks of June, 2020
Community seroprevalence data were obtained from Ward et al (2020).21 Error bars indicate 95% CIs.
For both students (table 2 ) and staff (table 3 ), after adjusting for the other variables in the final model, a school clustering effect was significant for staff members (p=0·0002) but not students (p=1·00). For both students and staff, seropositivity was associated with non-White ethnicity and health-care workers in the household (Table 2, Table 3). For students, region was a significant predictor of antibody positivity (p=0·020; table 2) and for staff seropositivity was significantly lower in Derby than in North London (adjusted OR 0·27, 95% CI 0·10–0·73; table 3). Seropositivity was not associated with school attendance during lockdown (p=0·13 for students and p=0·20 for staff) or staff contact with students (p=0·37). 20 (22·0%) of 91 seropositive students reported COVID-19-like illness compared with 122 (58·4%) of 209 staff members (p<0·001).
Table 2.
Risk factors for SARS-CoV-2 antibody positivity in students participating in school surveillance during the summer half-term (June to mid-July, 2020)
|
Antibody positive* |
Univariable analysis (n=816) |
Complete case univariable analysis adjusted for clustering by school (n=714) |
Multivariable analysis adjusted for clustering by school (p=1·00; n=714) |
|||||
|---|---|---|---|---|---|---|---|---|
| n/N (%)* | OR (95% CI) | p value† | OR (95% CI) | p value† | OR (95% CI) | p value† | ||
| Sex | .. | .. | 0·24 | .. | 0·21 | .. | 0·16 | |
| Female | 41/415 (9·9%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| Male | 50/401 (12·5%) | 1·30 (0·84–2·01) | .. | 1·35 (0·84–2·17) | .. | 1·42 (0·87–2·30) | .. | |
| Missing | 0 | NA | NA | NA | NA | NA | NA | |
| Age, years | .. | .. | 0·039 | .. | 0·049 | .. | 0·14 | |
| 4–6 | 27/322 (8·4%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| 7–9 | 34/220 (15·5%) | 2·00 (1·17–3·42) | .. | 2·03 (1·12–3·68) | .. | 1·79 (0·98–3·25) | .. | |
| ≥10 | 30/274 (10·9%) | 1·34 (0·78–2·32) | .. | 1·18 (0·63–2·19) | .. | 1·16 (0·63–2·14) | .. | |
| Missing | 0 | NA | NA | NA | NA | NA | NA | |
| Ethnicity | .. | .. | <0·0001 | .. | 0·0004 | .. | 0·0010 | |
| White | 37/497 (7·4%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| Mixed or multiple ethnic groups | 9/81 (11·1%) | 1·55 (0·72–3·36) | .. | 1·63 (0·73–3·65) | .. | 1·08 (0·47–2·49) | .. | |
| Black or Black British | 10/51 (19·6%) | 3·03 (1·41–6·54) | .. | 3·30 (1·41–7·75) | .. | 2·72 (1·13–6·59) | .. | |
| Asian or Asian British | 15/101 (14·9%) | 2·17 (1·14–4·13) | .. | 2·26 (1·12–4·56) | .. | 1·56 (0·76–3·23) | .. | |
| Other ethnic group | 20/75 (26·7%) | 4·52 (2·45–8·33) | .. | 4·45 (2·22–8·92) | .. | 4·02 (2·03–7·94) | .. | |
| Missing | 0/11 | NA | NA | NA | NA | NA | NA | |
| Region in England | .. | .. | 0·0002 | .. | 0·0056 | .. | 0·020 | |
| Derby | 14/154 (9·0%) | 0·61 (0·32–1·17) | .. | 0·64 (0·31–1·34) | .. | 0·48 (0·23–0·99) | .. | |
| East London | 23/105 (21·9%) | 1·71 (0·96–3·04) | .. | 1·69 (0·89–3·23) | .. | 1·48 (0·74–2·97) | .. | |
| Manchester | 15/192 (7·8%) | 0·52 (0·27–0·97) | .. | 0·65 (0·31–1·35) | .. | 0·72 (0·35–1·50) | .. | |
| North London | 37/262 (14·1%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| Oxford | 2/103 (1·9%) | 0·12 (0·03–0·51) | .. | 0·14 (0·03–0·67) | .. | 0·22 (0·05–0·97) | .. | |
| Attended school during lockdown | .. | .. | 0·40 | .. | 0·53 | .. | 0·13 | |
| No | 18/126 (14·3%) | 1 (ref) | 1 (ref) | .. | 1 (ref) | |||
| Yes | 71/613 (11·6%) | 0·79 (0·45–1·37) | 0·82 (0·45–1·51) | .. | 0·61 (0·32–1·15) | |||
| Missing | 2/77 (2·6%) | NA | NA | NA | NA | NA | NA | |
| Frequency of school attendance during lockdown | .. | .. | 0·52 | .. | .. | .. | .. | |
| Did not attend | 18/126 (14·3%) | 1 (ref) | .. | .. | .. | .. | .. | |
| ≥1 per week | 18/192 (9·4%) | 0·62 (0·31–1·24) | .. | .. | .. | .. | .. | |
| Less than half the week | 9/96 (9·4%) | 0·62 (0·27–1·45) | .. | .. | .. | .. | .. | |
| More than half the week | 18/136 (13·2%) | 0·92 (0·45–1·85) | .. | .. | .. | .. | .. | |
| Everyday | 26/189 (13·8%) | 0·96 (0·50–1·83) | .. | .. | .. | .. | .. | |
| Missing | 2/77 (2·6%) | NA | NA | .. | .. | .. | .. | |
| Previous confirmed COVID-19 in household | .. | .. | 0·007 | .. | .. | .. | .. | |
| No | 84/792 (10·6%) | 1 (ref) | .. | .. | .. | .. | .. | |
| Yes | 7/24 (29·2%) | 3·47 (1·40–8·61) | .. | .. | .. | .. | .. | |
| Number of children at home‡ | .. | .. | .. | .. | ||||
| Per one child increase in household | .. | 1·19 (0·96–1·48) | 0·12 | 1·14 (0·91–1·44) | 0·25 | 1·12 (0·89–1·42) | 0·33 | |
| Mean (SD) in negative households | 2·30 (0·93) | .. | .. | .. | .. | .. | .. | |
| Mean (SD) in positive households | 2·48 (1·12) | .. | .. | .. | .. | .. | .. | |
| Parental occupation | .. | .. | 0·0056 | .. | 0·0063 | .. | 0·0046 | |
| Not a health-care or key worker | 52/555 (9·4%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| Health-care worker | 23/115 (20·0%) | 2·42 (1·41–4·14) | .. | 2·58 (1·42–4·68) | .. | 2·66 (1·44–4·91) | .. | |
| Key worker (excluding health-care workers) | 16/146 (11·0%) | 1·19 (0·66–2·15) | .. | 1·10 (0·57–2·12) | .. | 1·01 (0·52–1·98) | .. | |
NA=not applicable. OR=odds ratio.
Unless otherwise specified.
Global p values calculated using the Wald test.
60 children had missing data.
Table 3.
Risk factors for antibody positivity in staff participating in school surveillance during the summer half-term (June to mid-July, 2020)
|
Antibody positive |
Univariable analysis (n=1381) |
Complete case univariable analysis adjusted for clustering by school (n=1307) |
Multivariable analysis adjusted for clustering by school (p=0·0002; n=1307) |
|||||
|---|---|---|---|---|---|---|---|---|
| n/N (%) | OR (95% CI) | p value* | OR (95% CI) | p value* | OR (95% CI) | p value* | ||
| Sex | .. | .. | 0·066 | .. | 0·11 | .. | 0·20 | |
| Female | 158/1107 (14·3%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| Male | 51/272 (18·8%) | 1·39 (0·98–1·96) | .. | 1·36 (0·94–1·96) | .. | 1·28 (0·88–1·87) | .. | |
| Missing | 0/2 | NA | NA | NA | NA | NA | NA | |
| Ethnicity | .. | .. | 0·0004 | .. | 0·0028 | .. | 0·018 | |
| White | 140/1048 (13·4%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| Mixed or multiple ethnic groups | 13/49 (26·5%) | 2·34 (1·21–4·53) | .. | 2·32 (1·15–4·67) | .. | 1·99 (0·98–4·04) | .. | |
| Black or Black British | 22/71 (31·0%) | 2·91 (1·71–4·96) | .. | 2·78 (1·56–4·94) | .. | 2·49 (1·38–4·46) | .. | |
| Asian or Asian British | 26/163 (16·0%) | 1·23 (0·78–1·94) | .. | 1·22 (0·73–2·02) | .. | 1·16 (0·69–1·95) | .. | |
| Other ethnic group | 5/31 (16·1%) | 1·25 (0·47–3·30) | .. | 1·43 (0·51–4·02) | .. | 1·39 (0·49–3·93) | .. | |
| Missing | 3/19 (15·8%) | NA | NA | NA | NA | NA | NA | |
| Region | .. | .. | 0·0003 | .. | 0·051 | .. | 0·085 | |
| Derby | 8/133 (6·0%) | 0·27 (0·13–0·57) | .. | 0·26 (0·10–0·69) | .. | 0·27 (0·10–0·73) | .. | |
| East London | 84/489 (17·2%) | 0·87 (0·62–1·22) | .. | 0·81 (0·47–1·42) | .. | 0·70 (0·39–1·24) | .. | |
| Manchester | 29/257 (11·3%) | 0·53 (0·34–0·84) | .. | 0·55 (0·28–1·09) | .. | 0·55 (0·28–1·09) | .. | |
| North London | 83/431 (19·3%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| Oxford | 5/71 (7·0%) | 0·32 (0·12–0·81) | .. | 0·40 (0·09–1·81) | .. | 0·48 (0·11–2·14) | .. | |
| Attended school during lockdown | .. | .. | 0·048 | .. | 0·21 | .. | 0·20 | |
| No | 41/203 (20·2%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| Yes | 164/1116 (14·7%) | 0·68 (0·47–1·00) | .. | 0·77 (0·50–1·16) | .. | 0·73 (0·48–1·12) | .. | |
| Missing | 4/62 (6·5%) | NA | NA | NA | NA | NA | NA | |
| Frequency of school attendance during lockdown | .. | .. | 0·14 | .. | .. | .. | .. | |
| Only home | 41/203 (20·2%) | 1 (ref) | .. | .. | .. | .. | .. | |
| Mainly home | 66/502 (13·1%) | 0·60 (0·39–0·92) | .. | .. | .. | .. | .. | |
| Equal school and home | 53/318 (16·7%) | 0·79 (0·50–1·24) | .. | .. | .. | .. | .. | |
| Mainly school | 31/183 (16·9%) | 0·81 (0·48–1·35) | .. | .. | .. | .. | .. | |
| Full time | 14/113 (12·4%) | 0·56 (0·29–1·08) | .. | .. | .. | .. | .. | |
| Missing | 4/62 (6·5%) | NA | NA | .. | .. | .. | .. | |
| Student contact during lockdown | .. | .. | 0·37 | .. | .. | .. | .. | |
| None | 69/385 (17·9%) | 1 (ref) | .. | .. | .. | .. | .. | |
| Occasional | 105/698 (15·0%) | 0·81 (0·58–1·13) | .. | .. | .. | .. | .. | |
| Regular | 28/198 (14·1%) | 0·75 (0·47–1·22) | .. | .. | .. | .. | .. | |
| Missing | 7/100 (7·0%) | NA | NA | .. | .. | .. | .. | |
| Other household occupation | .. | .. | 0·0023 | .. | 0·0020 | .. | 0·0069 | |
| Not a health-care or key worker | 158/1135 (13·9%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| Health-care worker | 14/43 (32·6%) | 2·99 (1·54–5·77) | .. | 3·45 (1·69–7·03) | .. | 3·08 (1·48–6·41) | .. | |
| Key worker (excluding health-care workers) | 37/203 (18·2%) | 1·38 (0·93–2·04) | .. | 1·35 (0·88–2·06) | .. | 1·35 (0·88–2·07) | .. | |
NA=not applicable. OR=odds ratio.
Global p values calculated using Wald test.
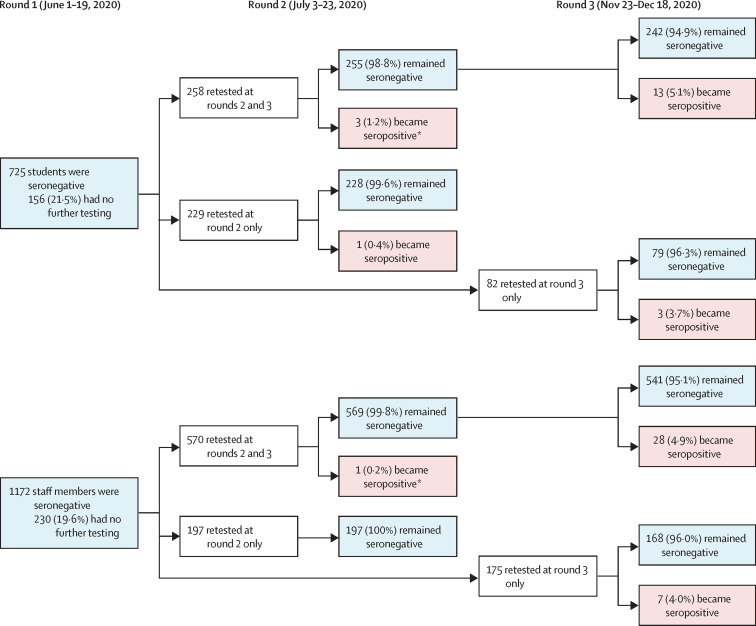
1618 (73·6%) of 2197 individuals were still participating in the surveillance in round 2 (end of summer half-term), 603 (73·9%) of 816 students and 1015 (73·5%) of 1381 staff members (appendix p 4). Multivariable logistic regression found the school region was a significant predictor of dropout for staff and students in round 2, and ethnicity for students only (appendix pp 5–6). 173 (12·1%; 95% CI 9·3–15·3) of 1430 returning participants were seropositive, including 56 (10·4%; 6·8–15·0) of 540 students and 117 (13·1%; 9·9–17·0) of 890 staff members. Of the 1897 seronegative participants in round 1, 1254 were retested in round 2 and five (0·4%; 95% CI 0·1–0·9) seroconverted (figure 2 ). Seroconversion rates were not significantly different in students (four [0·8%] of 487; 95% CI 0·2–2·1) and staff (one [0·1%] of 767; 0·0–0·7; p=0·08).
Figure 2.
Flow chart of SARS-CoV-2 antibody results in students and staff who were seronegative at recruitment
*These participants were tested again in round 3.
In round 3 (end of autumn term), 1361 (61·9%) of 2197 participants were retested, including 442 (54·2%) of 816 students and 919 (66·5%) of 1381 staff members (appendix p 4). School region was a significant predictor of participation for staff and students (appendix pp 5–6). In students, age was also a significant predictor of participation in round 3 because children moving to secondary school in the 2020–21 academic year were not included. One (0·1%; 95% CI 0·0–0·6) of 900 participants, a staff member, was SARS-CoV-2 RT-qPCR positive in round 3. Linkage with surveillance data for students identified five additional cases during the autumn term, one of whom participated in round 3. There were no reinfections in staff or students. 136 (10·7%; 95% CI 8·7–13·0) of 1274 participants were seropositive, including 33 (8·6%; 5·8–12·2) of 384 students and 103 (11·2%; 9·2–14·3) of 890 staff members. Among students who were seronegative at round 1, 13 (5·1%; 95% CI 2·7–8·6) of 255 among those attending rounds 2 and 3, and three (3·7%; 0·8–10·3) of 82 among those attending rounds 1 and 3 seroconverted (figure 2). Among staff members who were seronegative at round 1, 28 (4·9%; 95% CI 3·3–7·0) of 569 members attending rounds 2 and 3 and seven (4·0%; 1·6–8·1) of 175 members attending round 3 seroconverted (figure 2).
Of participants who were seronegative in round 1 and tested in round 3, 55 (5·1%; 95% CI 3·8–6·5) of 1085 participants seroconverted: 19 (5·6%; 3·4–8·6) of 340 students and 36 (4·8%; 3·4–6·6) of 745 staff members (p=0·60; figure 2). In participants who were tested at least twice over the study period (56 of 1511), seroconversion incidence was 1·5 (95% CI 1·1–1·9) per 1000 weeks of follow-up: 1·4 (0·9–2·2) per 1000 weeks for students and 1·5 (1·1–2·1) per 1000 weeks for staff. Among the 56 participants who seroconverted (including one student who seroconverted in round 2 but was not retested in round 3), 44 (78·6%) completed the final questionnaire; a higher proportion of staff reported COVID-19 symptoms than students (16 [53·3%] of 30 vs two [14·3%] of 14; p=0·021) in the period between testing. In a multivariable Poisson regression, seroconversion was associated with region (p=0·012) and ethnicity (p=0·0023; appendix p 7). No difference was reported by sex (p=0·33), or between staff and students (p=0·36). Participants in Manchester were significantly more likely to seroconvert than those from North London (incidence rate ratio 2·38; 95% CI 1·24–4·55).
Discussion
Active, prospective surveillance identified very low rates of SARS-CoV-2 infection in primary schools during the summer half-term in England, when schools reopened only for certain year groups. Only three of 40 501 swabs from 11 966 participants in 131 schools had confirmed SARS-CoV-2 infection. At recruitment, SARS-CoV-2 seropositivity was 11·2% in students and 15·1% in staff, similar to local community seroprevalence. We found no significant association between antibody positivity and school attendance during lockdown. The degree of staff contact with students (regular, occasional, or none) was also not associated with seropositivity. There were only five seroconversions among staff and students during the summer half-term. SARS-CoV-2 infection and seroconversion rates in staff and students remained at or below 5% even after primary schools reopened fully during the autumn term.
In England, some primary schools reopened in June, 2020, when community SARS-CoV-2 infection rates were generally low nationally. Because of concerns about asymptomatic infections and silent transmission in schools, we rapidly initiated SARS-CoV-2 surveillance and successfully recruited large numbers of students and staff in primary schools across all English regions. Large-scale weekly mass testing identified only three people with SARS-CoV-2 infections, whereas three others with an initial positive swab (with very high cycle threshold values), which were then repeat tested as negative following concentration, showed that false positivity does exist, but procedures are in place within the laboratory to investigate these aberrant results. Further evidence of no antibody development 4–6 weeks later shows that the results of retesting were correct.22
Despite the challenges of blood sampling large numbers of staff and students, we used serum SARS-CoV-2 antibodies to assess previous infection because this method would also capture asymptomatic and mild, transient infections. At recruitment, seropositivity rates in students and staff were similar and reflected local community seroprevalence. This finding is important because children represent less than 5% of confirmed COVID-19 cases,23, 24 and the similar seropositivity rates in students and staff indicate that children are as likely to be infected with SARS-CoV-2 as adults, but more likely to develop asymptomatic or mild, transient illness, which would also reduce their likelihood of getting tested for SARS-CoV-2 infection. The higher seropositivity in minority ethnic groups is consistent with published literature,25 although to our knowledge this is the first report in children. A higher risk of SARS-CoV-2 infection in students and staff living with a health-care worker is unsurprising since they were more likely to be infected with SARS-CoV-2,26 and develop COVID-19,27 especially early in the UK epidemic when personal protective equipment in health-care settings was limited.
Seropositivity rates in staff were similar to community seroprevalence at recruitment, indicating that they were not at higher risk of infection than people in other professions.26 In our cohort, both staff and students who did not attend school during lockdown had higher albeit non-significant seropositivity rates than those who attended school, possibly because of increased exposure to household members at high risk of COVID-19, more opportunities for infection in the community than in school (because of small numbers of students and staff attending during lockdown, with strict infection control practices in place), or both. A systematic review, for example, found infection risk from household contacts to be ten times higher than contacts from any other setting.28 Additionally, at recruitment, there was significant clustering of seropositive cases by school in staff but not in students. This could be due to increased transmission risk between staff in school but could also reflect the local community seroprevalence rates in adults at the time.
There are few other similar serosurveillance studies for comparison, but in Sweden, which kept preschools and primary schools open during lockdown, repeated serosurveys among primary care patients without COVID-19 showed similar seropositivity rates in individuals younger than 20 years and working-age adults during April–May, 2020.1, 29 In England, a national cross-sectional survey of 105 schools found less than 1% SARS-CoV-2 infection rates in primary school students and staff attending school during November, 2020.30 Low infection, seroconversion, and outbreak rates in primary schools15 contrast with other institutional settings, such as care homes, where widespread asymptomatic infection and silent transmission were common even before an outbreak was recognised. In London care homes reporting a COVID-19 outbreak, for example, half of the residents and 20% of staff were SARS-CoV-2 RT-qPCR positive when tested and half of them never developed symptoms.31 Additionally, two-thirds of asymptomatic and RT-qPCR-negative residents and staff had SARS-CoV-2 antibodies at the end of the outbreak.32 Reassuringly, 96–100% of RT-qPCR-positive residents and staff developed SARS-CoV-2 antibodies irrespective of whether they were symptomatic or asymptomatic, highlighting the added value of antibody testing to measure the spread of infection in institutional settings.32
The autumn term saw all children returning to school, making physical distancing and other infection control measures difficult to implement. In primary schools, staff and students were not required to wear face masks or face coverings.13 In England, SARS-CoV-2 infection rates are reported weekly for all age-groups,33 but the national reports only include testing of symptomatic cases and thus do not provide any insight into asymptomatic infections or silent transmissions. We used the Abbott assay, which detects nucleoprotein antibodies within 7–14 days after infection; it is quicker than other assays, such as those measuring spike protein antibodies, and would therefore detect seroconversion quickly between antibody testing rounds.20 The low seroconversion rates during the autumn term, in addition to the negligible seroconversions during the summer half-term, provides further reassurance against high rates of asymptomatic infections among students or silent transmission to staff in primary schools. The emergence and rapid spread of a novel SARS-CoV-2 variant of concern (VUI-202012/01; VOC B.1.1.7) since December, 2020, which is more transmissible than previously circulating strains, will require careful monitoring now that schools have reopened (on March 8, 2021) after the latest national lockdown.34
The strength of this surveillance study is the large numbers of schools and participants recruited rapidly when schools reopened from June, 2020, highlighting the willingness of parents to allow their children to take part in school surveillance. The low infection and seroconversion rates during the summer half-term helped to support the full reopening primary schools during the autumn term. A limitation of the study was that the participating schools were not selected to be nationally representative. In particular, the schools in the blood sampling group were recruited in regions where we had paediatric teams to collect blood samples. We also did not collect samples at the start of the lockdown and therefore cannot comment on whether seropositive participants might have been exposed to SARS-CoV-2 in school or the community before lockdown. Moreover, the study was open to all staff and students, but the characteristics of those who took part might be different to those who declined. Additionally, not all participants returned for rounds 2 and 3 and, because of differences in returns among seropositive and seronegative participants, the seroprevalence rates during subsequent visits should be interpreted with caution. The retention of seronegative participants, however, provided a useful baseline for estimating seroconversion during the summer and autumn terms. Finally, our findings cannot be extrapolated to senior schools,2 because older children have a higher risk of infection and disease than younger children,35, 36 with higher propensity for SARS-CoV-2 transmission and outbreaks in secondary compared with primary schools.37, 38
We found a very low risk of SARS-CoV-2 infection in students or staff attending primary schools during both partial reopening in the summer half-term and full opening in the autumn term. The similar seropositivity rates indicate that students are as likely to get infected as staff but more likely to have asymptomatic or mild illness. Similar studies are needed in secondary schools and higher-education settings, in which the risk of infection, transmission, and disease are likely to be different.
Data sharing
Applications for relevant anonymised data should be submitted to the Public Health England Office for Data Release.
Acknowledgments
Acknowledgments
This study was funded by the UK Department of Health and Social Care. We thank all those who contributed to the study; the PHE team, the schools, head teachers, staff, families, and their very brave children who took part in sKIDs. We also thank Sir Jeremy Farrar, Sir Patrick Vallance, members of the UK Department for Education, UK Department of Health and Social Care, London School of Hygiene and Tropical Medicine (London, UK), UK Office for National Statistics, and the UK Scientific Advisory Group for Emergencies for their input and support for sKIDs.
Contributors
SNL, FB, JB, IOO, SA, JG, AJB, BB, GA, VS, JLB, KEB, and MER were responsible for conceptualisation and study design and methodology. SNL, FB, JB, IOO, SA, JG, AJB, BB, GI, FA, ZA-C, LL, JF, SEIJ, RB, EL, MZ, and JP contributed to project administration (including laboratory colleagues). SNL, JW, and GI contributed to the original draft of the report, and JW, NA, and GI did the formal analysis. GI, JW, JP, and SNL were responsible for data validation and verification. All authors contributed to reviewing and editing of the manuscripts. All authors had access to the data; SNL and GI had final responsibility to submit for publication.
Declaration of interests
MER reports that the Immunisation and Countermeasures Division (PHE) has provided vaccine manufacturers with post-marketing surveillance reports on pneumococcal and meningococcal infection, which the companies are required to submit to the UK licensing authority in compliance with their risk management strategy. A cost-recovery charge is made for these reports. AJB reports that he is chair of governors of one of the schools included in the study. RB and EL report that the PHE Vaccine Evaluation Unit does contract research on behalf of GlaxoSmithKline, Sanofi, and Pfizer, which is outside the submitted work. JW reports grants from the UK National Institute for Health Research Health Protection Research Unit in Immunisation during the conduct of this study. All other authors declare no competing interests.
Supplementary Material
References
- 1.European Centre for Disease Control and Prevention COVID-19 in children and the role of school settings in COVID-19 transmission. August 6, 2020. https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-schools-transmission-August%202020.pdf
- 2.Levinson M, Cevik M, Lipsitch M. Reopening primary schools during the pandemic. N Engl J Med. 2020;383:981–985. doi: 10.1056/NEJMms2024920. [DOI] [PubMed] [Google Scholar]
- 3.Viner RM, Russell SJ, Croker H, et al. School closure and management practices during coronavirus outbreaks including COVID-19: a rapid systematic review. Lancet Child Adolesc Health. 2020;4:397–404. doi: 10.1016/S2352-4642(20)30095-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United Nations Educational. Scientific and Cultural Organization COVID-19 education: from disruption to recovery. https://en.unesco.org/covid19/educationresponse
- 5.Munro APS, Faust SN. Children are not COVID-19 super spreaders: time to go back to school. Arch Dis Child. 2020;105:618–619. doi: 10.1136/archdischild-2020-319474. [DOI] [PubMed] [Google Scholar]
- 6.Viner RM, Mytton OT, Bonell C, et al. Susceptibility to and transmission of COVID-19 amongst children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2020;175:143–156. doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson C, Mangtani P, Hawker J, Olowokure B, Vynnycky E. The effects of school closures on influenza outbreaks and pandemics: systematic review of simulation studies. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cauchemez S, Ferguson NM, Wachtel C, et al. Closure of schools during an influenza pandemic. Lancet Infect Dis. 2009;9:473–481. doi: 10.1016/S1473-3099(09)70176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viner RM, Bonell C, Drake L, et al. Reopening schools during the COVID-19 pandemic: governments must balance the uncertainty and risks of reopening schools against the clear harms associated with prolonged closure. Arch Dis Child. 2021;106:111–113. doi: 10.1136/archdischild-2020-319963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prime Minister's Office Prime Minister's statement on coronavirus (COVID-19): 23 March 2020. March 23, 2020. https://www.gov.uk/government/speeches/pm-address-to-the-nation-on-coronavirus-23-march-2020
- 11.UK Department for Education Guidance: Critical workers who can access schools or educational settings. June 16, 2020. https://www.gov.uk/government/publications/coronavirus-covid-19-maintaining-educational-provision/guidance-for-schools-colleges-and-local-authorities-on-maintaining-educational-provision
- 12.Public Health England National COVID-19 surveillance reports 2020. April 23, 2020. https://www.gov.uk/government/publications/national-covid-19-surveillance-reports
- 13.UK Department for Education Guidance for full opening: schools. July 27, 2020. https://www.gov.uk/government/publications/actions-for-schools-during-the-coronavirus-outbreak/guidance-for-full-opening-schools
- 14.Coronavirus (COVID-19) Infection Survey pilot: England, 21 May 2020. May 21, 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/england21may2020
- 15.Ismail S, Saliba V, Lopez-Bernal J, Ramsay M, Ladhani S. SARS-CoV-2 infection and transmission in educational settings: cross-sectional analysis of clusters and outbreaks in England. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30882-3. published online Dec 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Educational Institute of Scotland School-based member survey results. November, 2020. https://www.eis.org.uk/Content/images/corona/Nov20Report.pdf
- 17.Redfield & Wilton Strategies Up to two-thirds of parents could send their children back to school this summer. June 22, 2020. https://redfieldandwiltonstrategies.com/up-to-two-thirds-of-parents-could-to-send-their-children-back-to-school-this-summer/
- 18.Ladhani SN, Amin-Chowdhury Z, Amirthalingam G, Demirjian A, Ramsay ME. Prioritising paediatric surveillance during the COVID-19 pandemic. Arch Dis Child. 2020;105:613–615. doi: 10.1136/archdischild-2020-319363. [DOI] [PubMed] [Google Scholar]
- 19.Niu P, Lu R, Zhao L, et al. Three novel real-time RT-PCR assays for detection of COVID-19 virus. China CDC Wkly. 2020;2:453–457. doi: 10.46234/ccdcw2020.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Public Health England Evaluation of the Abbott SARS-CoV-2 IgG for the detection of anti-SARS-CoV-2 antibodies. June 8, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/890566/Evaluation_of_Abbott_SARS_CoV_2_IgG_PHE.pdf
- 21.Ward H, Atchison C, Whitaker M, et al. Antibody prevalence for SARS-CoV-2 following the peak of the pandemic in England: REACT2 study in 100,000 adults. Nat Commun. 2021;12:905. doi: 10.1038/s41467-021-21237-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen AN, Kessel B. False positives in reverse transcription PCR testing for SARS-CoV-2. medRxiv. 2020 doi: 10.1101/2020.04.26.20080911. published online May 1. (preprint). [DOI] [Google Scholar]
- 23.Ladhani SN, Amin-Chowdhury Z, Davies HG, et al. COVID-19 in children: analysis of the first pandemic peak in England. Arch Dis Child. 2020;105:1180–1185. doi: 10.1136/archdischild-2020-320042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 25.Pan D, Sze S, Minhas JS, et al. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClinicalMedicine. 2020;23 doi: 10.1016/j.eclinm.2020.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Office for National Statistics Which occupations have the highest potential exposure to the coronavirus (COVID-19)? May 11, 2020. https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentandemployeetypes/articles/whichoccupationshavethehigestpotentialexposuretothecoronaviruscovid19/2020-05-11
- 27.Office for National Statistics Coronavirus (COVID-19) related deaths by occupation, England and Wales: deaths registered up to and including 20 April 2020. May 11, 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/causesofdeath/bulletins/coronaviruscovid19relateddeathsbyoccupationenglandandwales/deathsregistereduptoandincluding20april2020
- 28.Lei H, Xu X, Xiao S, Wu X, Shu Y. Household transmission of COVID-19-a systematic review and meta-analysis. J Infect. 2020;81:979–997. doi: 10.1016/j.jinf.2020.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folkhälsomyndigheten Påvisning av antikroppar efter genomgången covid-19 i blodprov från öppenvården (Delrapport 1) June 18, 2020. https://www.folkhalsomyndigheten.se/contentassets/9c5893f84bd049e691562b9eeb0ca280/pavisningantikroppar-genomgangen-covid-19-blodprov-oppenvarden-delrapport-1.pdf
- 30.Office for National Statistics COVID-19 Schools Infection Survey Round 1, England: November 2020. Dec 17, 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/covid19schoolsinfectionsurveyround1england/november2020
- 31.Ladhani SN, Chow JY, Janarthanan R, et al. Investigation of SARS-CoV-2 outbreaks in six care homes in London, April 2020. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ladhani SN, Jeffery-Smith A, Patel M, et al. High prevalence of SARS-CoV-2 antibodies in care homes affected by COVID-19: Prospective cohort study, England. EClinicalMedicine. 2020;28 doi: 10.1016/j.eclinm.2020.100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Public Health England Official Statistics: national flu and COVID-19 surveillance reports. National influenza and COVID-19 report, monitoring COVID-19 activity, seasonal flu and other seasonal respiratory illnesses. Oct 8, 2020. https://www.gov.uk/government/statistics/national-flu-and-covid-19-surveillance-reports
- 34.Public Health England Investigation of novel SARS-COV-2 variant: variant of concern 202012/01. December, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/959438/Technical_Briefing_VOC_SH_NJL2_SH2.pdf
- 35.Park YJ, Choe YJ, Park O, et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26:2465–2468. doi: 10.3201/eid2610.201315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS-CoV-2 in the Icelandic Population. N Engl J Med. 2020;382:2302–2315. doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fontanet A, Tondeur L, Madec Y, et al. Cluster of COVID-19 in northern France: a retrospective closed cohort study. medRxiv. 2020 doi: 10.1101/2020.04.18.20071134. published online April 23. (preprint). [DOI] [Google Scholar]
- 38.Stein-Zamir C, Abramson N, Shoob H, et al. A large COVID-19 outbreak in a high school 10 days after schools' reopening, Israel, May 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.29.2001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Applications for relevant anonymised data should be submitted to the Public Health England Office for Data Release.