ABSTRACT
This phase I study evaluated the safety of the optimal ceftazidime-avibactam (CZA) with aztreonam (ATM) regimens identified in hollow fiber infection models of MBL-producing Enterobacterales. Eligible healthy subjects aged 18 to 45 years were assigned to one of six cohorts: 2.5 g CZA over 2 h every 8 h (approved dose), CZA continuous infusion (CI) (7.5 g daily), 2 g ATM over 2 h every 6 h, ATM CI (8 g daily), CZA (approved dose) with 1.5 g ATM over 2 h every 6 h, and CZA (approved dose) with 2 g ATM over 2 h every 6 h. Study drug(s) were administered for 7 days. The most frequently observed adverse events (AEs) were hepatic aminotransferase (ALT/AST) elevations (n = 19 subjects). Seventeen of the 19 subjects with ALT/AST elevations received ATM alone or CZA-ATM. The incidence of ALT/AST elevations was comparable between the ATM-alone and CZA-ATM cohorts. Two subjects in the ATM CI cohort experienced severe ALT/AST elevation AEs. All subjects with ALT/AST elevations were asymptomatic with no other findings suggestive of liver injury. Most other AEs were of mild to moderate severity and were similar across cohorts, except for prolonged prothrombin time (more frequent in CZA-ATM cohorts). These results suggest that CZA-ATM administered as 2-h intermittent infusions is safe and that some caution should be exercised with the use of ATM CI at an ATM dose of 8 g daily. If CZA-ATM is prescribed, clinicians are advised to monitor liver function, hematologic, and coagulation parameters. Future controlled studies are required to better define the safety and efficacy of the CZA-ATM regimens evaluated in this phase I study.
KEYWORDS: aztreonam, ceftazidime-avibactam, clinical trials, safety
INTRODUCTION
Treatment of patients with serious infections due to highly resistant Enterobacterales remains problematic and is a major public health concern (1–3). The rise in resistant Enterobacterales is driven in large part by the increasing prevalence and complexity of a variety of β-lactamases (4, 5). While drugs have been developed to combat extended-spectrum β-lactamase (ESBL)-producing and Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacterales infections, only cefiderocol has some activity against metallo-β-lactamase (MBL)-producing Gram-negative bacteria (3, 6, 7). Antibiotics with activity against MBL-producing Enterobacterales and other Gram-negative bacteria are in development, but they are years away from introduction into clinical practice (6, 8). In the meantime, existing agents will have to be deployed in innovative ways to meet the needs of patients today (9).
Ceftazidime-avibactam (CZA) combined with aztreonam (ATM) currently serves as a “bridge” treatment for MBL-producing Enterobacterales and other Gram-negative bacteria (8, 10–13). Aztreonam is not hydrolyzed by MBLs, but many MBL-bearing Gram-negative bacteria co-produce β-lactamases which hydrolyze ATM (4, 8, 10, 14–16). When ATM is administered with CZA, ATM is active against MBL-producing Gram-negative bacteria because the avibactam (AVI) component of CZA inhibits ESBL and KPC β-lactamases that are often present in these organisms (8, 10, 12–14, 16–18). Although CZA combined with ATM has been shown to be effective against MBL-producing Gram-negative bacteria in pre-clinical and observational studies (8, 10, 12, 13, 18, 19), the optimal dosage remains unknown. We recently conducted hollow fiber infection model (HFIM) studies of MBL-producing strains of Escherichia coli and K. pneumoniae to identify CZA-ATM combination regimens which resulted in maximum bacterial killing and resistance suppression. The two combination regimens which showed maximal bacterial killing and resistance suppression over 7 days were (i) 2.5 g CZA intravenously (i.v.) as a 2-h infusion every 8 h combined with 1.5 to 2 g ATM i.v. as a 2-h infusion every 6 h, and (ii) CZA combined with ATM, each administered as a continuous infusion (CI) (7.5 g/day CZA CI combined with 8 g/day ATM CI).
Before the optimal CZA-ATM combination regimens from the HFIM studies (19) are uniformly adopted into clinical practice, it was important to establish the safety and pharmacokinetics (PK) of these regimens in humans. Although CZA and ATM are generally safe and well-tolerated, self-limiting, mild-to-moderate asymptomatic serum hepatic aminotransferase elevations are common with ATM (20–22). It is unclear whether CZA combined with ATM further exacerbates liver enzyme elevations or leads to other adverse events (AEs) due to potential cumulative toxicity from dual-β-lactam treatment (9). This phase I study in healthy adult volunteers was conducted to evaluate the safety and pharmacokinetics of the two optimal CZA-ATM combination regimens (COMBINE) identified in the HFIM studies (19). The manuscript focuses on the safety aspects of COMBINE. The PK findings are described separately (23). Eligible subjects were assigned to one of six dosing cohorts (Table 1), and the tested products were administered for 7 days. Four of the six cohorts were single-agent dosing cohorts: 2.5 g CZA intravenously (i.v.) over 2 h every 8 h (cohort 1), 2.5 g CZA i.v. over 2 h × 1, then 7.5 g daily as a continuous infusion (cohort 2), 2 g ATM i.v. over 2 h every 6 h (cohort 3), and 2 g ATM i.v. × 1, then 8 g daily as a CI (cohort 4). Cohorts 5 and 6 were CZA-ATM combination regimens and included 2.5 g CZA i.v. over 2 h every 8 h with 1.5 g ATM i.v. over 2 h every 6 h (cohort 5) and 2.5 g CZA i.v. over 2 h every 8 h with 2 g ATM i.v. over 2 h every 6 h (cohort 6).
TABLE 1.
Investigational product cohorts (8 subjects/cohort) in COMBINEa
| Cohort | Drug regimen |
|---|---|
| 1 | 2.5 g CZA i.v. as 2-h infusions every 8 h for 7 days |
| 2 | 2.5 g CZA i.v. as 2-h infusion × 1, then 0.32 g/h i.v. daily as CI (7.5 g/day) for 7 days |
| 3 | 2 g ATM i.v. as 2-h infusions every 6 h for 7 days |
| 4 | 2 g ATM i.v. as a 2-h infusion × 1, then 0.33 g/h i.v. daily as a CI (8 g/day) for 7 days |
| 5b | 2.5 g CZA i.v. as 2-h infusions every 8 h for 7 days and 1.5 g ATM i.v. as 2-h infusions every 6 h for 7 days |
| 6b | 2.5 g CZA i.v. as 2-h infusions every 8 h for 7 days and 2 g ATM i.v. as 2-h infusions every 6 h for 7 days |
CZA, ceftazidime-avibactam; i.v., intravenously; CI, continuous infusion; ATM, aztreonam.
Cohorts 5 and 6 reflect modified treatment regimens following recommendations of the Safety Monitoring Committee (SMC). Initial regimen for cohort 5: 2.5 g CZA i.v. as 2-h infusions every 8 h and 2 g ATM i.v. as 2-h infusions every 6 h for 7 days. Initial regimen for cohort 6: 2.5 g CZA i.v. as a 2-h infusion × 1, 0.32 g/h i.v. daily as CI (7.5 g/day) and 2 g ATM i.v. as 2-h infusion × 1, and then 0.33 g/h i.v. daily as CI (8 g/day) for 7 days. A halting rule was observed in cohort 4 (2 subjects experienced grade 3 elevations in ALT/AST values deemed to be related to the study product), and a SMC meeting was convened. In response to the observed study halt, the SMC recommended that dosing in cohorts 5 and 6 be changed, and additional safeguards were incorporated into the study protocol.
RESULTS
Study population.
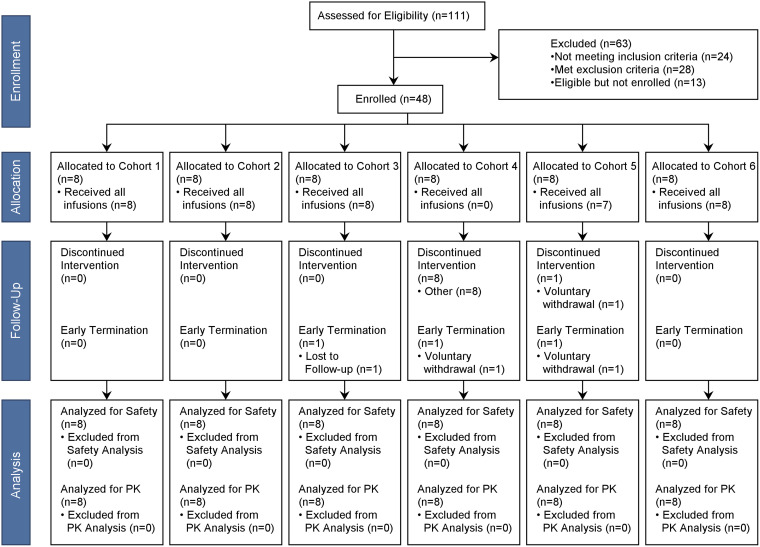
A total of 111 subjects were screened and 48 who met all eligibility criteria were enrolled in the study. Eight subjects were enrolled in each cohort (Fig. 1). Of the 48 enrolled subjects (safety population), 3 subjects terminated the study early. The reasons for early termination included voluntary withdrawal by subject (n = 2) and lost to follow-up (n = 1). Twelve subjects did not receive all scheduled doses. Eight subjects discontinued dosing on day 6 in cohort 4 due to triggering of halting criteria. Four subjects in cohort 5 did not receive the planned 7 days of CZA and/or ATM. One subject in cohort 5 voluntarily withdrew on study day 6 prior to completion of CZA-ATM dosing. Although the electronic case report form indicated that the ATM dose was administered, all plasma ATM concentrations after dosing on day 7 were below the limit of quantification for 3 subjects in cohort 5, indicating that ATM was not administered on day 7. Seventeen subjects received a partial dose of study product, nearly all of whom (n = 15) were in the CI cohorts (cohorts 2 and 4). This was due to the need to exchange i.v. drug solution products two (ATM) to three (CZA) times daily within a 30-minute time frame, resulting in frequent dose interruptions.
FIG 1.
Disposition of study subjects.
Demographic and baseline characteristics of the safety population are shown in Table 2. There were equal numbers of male and female subjects, and most were not Hispanic or Latino (83%). Black or African American was the most common race (60%), followed by White (21%), Asian (15%), and multiracial (4%). The mean (SD [standard deviation]) age was 33.5 (6.2) years and the mean (SD) weight was 75.7 (12.1) kg. Most subjects (94%) had a prior or current medical condition of any system organ class (SOC) that was stable at time of enrollment. Nearly 70% (n = 33) had a mild cardiac abnormality, such as sinus bradycardia (n = 21) and/or sinus arrhythmia (n = 17), at enrollment. Additionally, 42% (n = 20) of subjects had a mildly abnormal clinical investigation finding within 1 month of enrollment (abnormality on screening electrocardiogram [ECG] [n = 18], decreased neutrophil/white blood cell count [n = 4], decreased hemoglobin [n = 2], or increased blood creatinine [n = 1]). All subjects were judged to be in good general health by the site principal investigator (PI) as determined by assessment of their medical history, vital signs, body mass index (BMI), body weight, clinical laboratory values, and physical examination. None of the pre-existing conditions were deemed by the site PI to put the subject at risk due to participation in the study, influence the results, or compromise the subject’s ability to participate in the study. The presence of these mild pre-existing conditions was consistent with prior healthy volunteer phase I studies conducted at the clinical site and reflects the site’s healthy volunteer population (24–27).
TABLE 2.
Demographic data by study groupa
| Characteristic | Cohort |
All subjects (n = 48) | |||||
|---|---|---|---|---|---|---|---|
| 1 (n = 8) | 2 (n = 8) | 3 (n = 8) | 4 (n = 8) | 5 (n = 8) | 6 (n = 8) | ||
| Sex, n (%) | |||||||
| Male | 4 (50) | 6 (75) | 3 (38) | 3 (38) | 4 (50) | 4 (50) | 24 (50) |
| Female | 4 (50) | 2 (25) | 5 (63) | 5 (63) | 4 (50) | 4 (50) | 24 (50) |
| Ethnicity, n (%) | |||||||
| Not Hispanic or Latino | 8 (100) | 7 (88) | 5 (63) | 7 (88) | 7 (88) | 6 (75) | 40 (83) |
| Hispanic or Latino | - | 1 (13) | 3 (38) | 1 (13) | 1 (13) | 2 (25) | 8 (17) |
| Race, n (%) | |||||||
| American Indian or Alaska Native | - | - | - | - | - | - | - |
| Asian | - | - | 3 (38) | - | 2 (25) | 2 (25) | 7 (15) |
| Black or African-American | 6 (75) | 5 (63) | 3 (38) | 7 (88) | 4 (50) | 4 (50) | 29 (60) |
| White | 2 (25) | 2 (25) | 2 (25) | 1 (13) | 1 (13) | 2 (25) | 10 (21) |
| Multiracial | - | 1 (13) | - | - | 1 (13) | - | 2 (4) |
| Age (yrs), mean (SD) | 37.4 (5.5) | 36.4 (7.0) | 34.5 (5.9) | 30.8 (6.5) | 28.5 (3.8) | 33.8 (4.6) | 33.5 (6.2) |
| BMI (kg/m2), mean (SD) | 25.74 (2.92) | 27.65 (3.44) | 26.16 (3.52) | 25.38 (4.09) | 25.55 (3.33) | 28.31 (4.58) | 26.46 (3.66) |
| Height (cm), mean (SD) | 171.38 (10.68) | 172.34 (9.03) | 165.39 (7.87) | 169.80 (7.80) | 168.64 (6.31) | 167.30 (11.36) | 169.14 (8.84) |
| Weight (kg), mean (SD) | 75.66 (11.31) | 81.98 (11.02) | 71.98 (14.95) | 72.75 (9.69) | 72.88 (12.39) | 78.90 (12.88) | 75.69 (12.07) |
SD, standard deviation; BMI, body mass index; -, indicates subjects without the condition/event in a column.
Safety.
Of the 48 subjects in the safety population, 46 (96%) experienced at least one adverse event. Based on the highest severity of the AE experienced, 6 subjects experienced a severe (grade 3) AE (13%), 19 experienced a moderate (grade 2) AE (40%), and 21 (44%) experienced a mild (grade 1) AE. There were no serious adverse events (SAEs) or deaths in any cohort. A summary of AEs by treatment-relatedness, severity, and cohort is shown in Table 3. Forty-one subjects (85%) had ≥1 related AEs; 3 had a severe (grade 3) related AE, 17 had a moderate (grade 2) related AE, and 36 had a mild (grade 1) related AE. Thirty-five subjects (73%) had ≥1 unrelated AEs; 3 had a severe (grade 3) unrelated AE, 10 had a moderate (grade 2) unrelated AE, and 32 had a mild (grade 1) unrelated AE. Additionally, 21 subjects had mild (grade 1) laboratory abnormalities that were deemed to be not clinically significant (NCS) by the site PI and not coded as AEs.
TABLE 3.
Summary of adverse events by cohorta
| AE profile (no. of subjects), n (%)b | Cohort |
All subjects (n = 48) | |||||
|---|---|---|---|---|---|---|---|
| 1 (n = 8) | 2 (n = 8) | 3 (n = 8) | 4 (n = 8) | 5 (n = 8) | 6 (n = 8) | ||
| At least one | 8 (100) | 6 (75) | 8 (100) | 8 (100) | 8 (100) | 8 (100) | 46 (96) |
| At least one related | 4 (50) | 6 (75) | 7 (88) | 8 (100) | 8 (100) | 8 (100) | 41 (85) |
| Mild (grade 1) | 3 (38) | 6 (75) | 6 (75) | 6 (75) | 8 (100) | 7 (88) | 36 (75) |
| Moderate (grade 2) | 2 (25) | 1 (13) | 3 (38) | 5 (63) | 2 (25) | 4 (50) | 17 (35) |
| Severe (grade 3) | - | - | 1 (13) | 2 (25) | - | - | 3 (6) |
| At least one unrelated | 7 (88) | 4 (50) | 6 (75) | 4 (50) | 7 (88) | 7 (88) | 35 (73) |
| Mild (grade 1) | 7 (88) | 4 (50) | 6 (75) | 3 (38) | 5 (63) | 7 (88) | 32 (67) |
| Moderate (grade 2) | 1 (13) | 1 (13) | 3 (38) | 1 (13) | 3 (38) | 1 (13) | 10 (21) |
| Severe (grade 3) | 1 (13) | - | - | 1 (13) | 1 (13) | - | 3 (6) |
| At least one mild (grade 1) NCS lab abnormality | 7 (88) | 4 (50) | 4 (50) | 5 (63) | 1 (13) | - | 21 (44) |
| At least one leading to early termination | - | - | - | - | - | - | - |
| At least one SAE | - | - | - | - | - | - | - |
| At least one related SAE | - | - | - | - | - | - | - |
AE, adverse event; NCS, not clinically significant; SAE, serious adverse event; -, indicates subjects without the condition/event in a column.
Subjects are counted once for each category regardless of the number of events.
A summary of AEs (any relatedness) and grade 1 NCS laboratory abnormalities which occurred in ≥10% of subjects by cohort is shown in Table 4. A list of investigation (per MedDRA definitions, an “investigation” is a clinical laboratory test concept, radiologic test concept, physical examination parameter, or physiologic test concept) AEs and NCS laboratory abnormalities by severity and cohort is displayed in Table 5. A summary of non-investigation AEs by severity and cohort is listed in Table 6.
TABLE 4.
Adverse events of any relatedness and grade 1 NCS laboratory abnormalities that occurred in ≥10% of safety population by cohort
| Adverse event, n (%) | Cohortc |
All subjects (n = 48) | |||||
|---|---|---|---|---|---|---|---|
| 1 (n = 8) | 2 (n = 8) | 3 (n = 8) | 4 (n = 8) | 5 (n = 8) | 6 (n = 8) | ||
| Hemoglobin decreased | 5 (63) | 4 (50) | 5 (63) | 4 (50) | 3 (38) | 3 (38) | 24 (50) |
| Aspartate aminotransferase increased | 1 (13) | 1 (13) | 5 (63) | 2 (25) | 4 (50) | 5 (63) | 18 (38) |
| Alanine aminotransferase increased | 1 (13) | - | 5 (63) | 3 (38) | 3 (38) | 5 (63) | 17 (35) |
| Prothrombin Time prolonged | - | 1 (13) | 1 (13) | 3 (38) | 4 (50) | 7 (88) | 16 (33) |
| Bradycardiaa | 2 (25) | 2 (25) | 4 (50) | 2 (25) | 4 (50) | 2 (25) | 16 (33) |
| Infusion site reactionb | 2 (25) | 2 (25) | 3 (38) | 2 (25) | 2 (25) | 4 (50) | 15 (31) |
| Neutrophil count decreased | 2 (25) | 1 (13) | 1 (13) | 2 (25) | 3 (38) | 2 (25) | 11 (23) |
| Abdominal discomfort or pain | 2 (25) | 1 (13) | 2 (25) | - | 2 (25) | 1 (13) | 8 (17) |
| Headache | 1 (13) | 1 (13) | 2 (25) | 1 (13) | 2 (25) | - | 7 (15) |
| Blood glucose increased | 3 (38) | - | 2 (25) | 2 (25) | - | - | 7 (15) |
| Electrocardiogram QT prolonged | 1 (13) | 2 (25) | - | 1 (13) | 1 (13) | - | 5 (10) |
| Phlebitis | 1 (13) | - | 1 (13) | - | 1 (13) | 2 (25) | 5 (10) |
Includes bradycardia or sinus bradycardia.
Includes infusion site extravasation, irritation, pain, or phlebitis.
-, indicates subjects without the condition/event in a column.
TABLE 5.
Investigation AEs (any relatedness) and grade 1 NCS laboratory abnormalities by severity and cohorta,b
| Investigation AE/NCS laboratory abnormality, n (%) | Cohorts |
|||||
|---|---|---|---|---|---|---|
| Single drug |
Combination |
|||||
| CZA-AVI |
ATM |
CZA-ATM |
||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Abdominal bruit | 1 (13) | - | - | - | - | - |
| Alanine aminotransferase increased | 1 (13) | - | 5 (63) | 3 (38) | 3 (38) | 5 (63) |
| Moderate AE | 1 (13) | - | 2 (25) | - | 1 (13) | 3 (38) |
| Severe AE | - | - | 1 (13) | 2 (25) | - | - |
| Alkaline phosphatase increased | - | 1 (13) | - | - | - | - |
| Aspartate aminotransferase increased | 1 (13) | 1 (13) | 5 (63) | 2 (25) | 4 (50) | 5 (63) |
| Moderate AE | - | - | 1 (13) | - | - | - |
| Severe AE | - | - | 2 (25) | - | - | |
| Blood bilirubin increased | - | 1 (13) | - | 1 (13) | - | - |
| Moderate AE | - | 1 (13) | - | - | - | - |
| Blood creatinine increased | 1 (13) | - | - | - | - | - |
| Blood glucose decreased | 1 (13) | 2 (25) | - | 1 (13) | - | - |
| Moderate AE | - | - | - | 1 (13) | - | - |
| Blood glucose increased | 3 (38) | - | 2 (25) | 2 (25) | - | - |
| Severe AE | - | - | - | 1 (13) | - | - |
| Blood lactate dehydrogenase increased | - | - | - | 2 (25) | 1 (13) | - |
| Moderate AE | - | - | - | 1 (13) | - | - |
| Blood potassium decreased | - | - | - | 2 (25) | - | - |
| Blood pressure systolic decreased | 1 (13) | - | - | 1 (13) | - | - |
| Blood pressure diastolic increased | - | - | - | - | - | 1 (13) |
| Urine culture positive | - | - | - | 1 (13) | - | - |
| Electrocardiogram QT prolonged | 1 (13) | 2 (25) | - | 1 (13) | 1 (13) | - |
| Hemoglobin decreased | 5 (63) | 4 (50) | 5 (63) | 4 (50) | 3 (38) | 3 (38) |
| Moderate AE | - | - | - | 1 (13) | 1 (13) | 2 (25) |
| Severe AE | 1 (13) | - | - | - | - | - |
| Neutrophil count decreased | 2 (25) | 1 (13) | 1 (13) | 2 (25) | 3 (38) | 2 (25) |
| Moderate AE | 1 (13) | 1 (13) | - | 1 (13) | 1 (13) | - |
| Severe AE | - | - | - | 1 (13) | - | |
| Platelets decreased | - | 1 (13) | - | - | - | - |
| Protein total decreased | - | - | 1 (13) | - | 1 (13) | 1 (13) |
| Protein urine present | - | 1 (13) | - | - | - | - |
| Prothrombin time prolonged | - | 1 (13) | 1 (13) | 3 (38) | 4 (50) | 7 (88) |
| Moderate AE | - | 1 (13) | - | 1 (13) | - | 1 (13) |
| White blood cell count decreased | - | - | - | - | 1 (13) | - |
AE, adverse event; NCS, not clinically significant; CZA, ceftazidime-avibactam; ATM, aztreonam; -, indicates subjects without the condition/event in a column. Per the Medical Dictionary for Regulatory Activities Terminology (MedDRA) definition, an “investigation” is a clinical laboratory test concept (including biopsies), radiologic test concept, physical examination parameter, or a physiologic test concept (e.g., pulmonary function test).
Unrelated grade 2 or higher AEs include: 1 severe AE in cohort 1 (hemoglobin decrease), 2 AEs (1 moderate and 1 severe) in cohort 5 (neutrophil count decrease), and 1 moderate AE in cohort 2 (prolonged prothrombin time).
TABLE 6.
| Non-investigation AEs, n (%)c | Cohorts |
|||||
|---|---|---|---|---|---|---|
| Single-drug |
Combination |
|||||
| CZA-AVI |
ATM |
CZA-ATM |
||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Cardiac disorders | ||||||
| Bradycardia | 2 (25) | 2 (25) | 4 (50) | 2 (25) | 4 (50) | 2 (25) |
| Moderate | 1 (13) | - | - | - | 1 (13) | - |
| Severe | - | - | - | 1 (13) | - | - |
| Palpitations | - | - | - | 1 (13) | - | - |
| Ventricular extrasystoles | 1 (13) | - | - | - | - | - |
| Eye disorders | ||||||
| Swelling of eyelid | - | - | - | 1 (13) | - | - |
| Moderate | - | - | - | 1 (13) | - | - |
| Gastrointestinal disorders | ||||||
| Abdominal discomfort or pain | 2 (25) | 1 (13) | 2 (25) | - | 2 (25) | 1 (13) |
| Diarrhea | - | - | - | - | 1 (13) | 2 (25) |
| Dyspepsia | 1 (13) | - | - | - | - | - |
| Infrequent bowl movements | - | - | - | - | 1 (13) | - |
| Nausea | 1 (13) | - | - | - | 1 (13) | - |
| General disorders and administration site conditions | ||||||
| Chest pain | - | - | 1 (13) | - | 1 (13) | - |
| Fatigue | - | - | - | - | - | 2 (25) |
| Infusion site reactionsd | 2 (25) | 2 (25) | 3 (28) | 2 (25) | 2 (25) | 4 (50) |
| Moderate | - | - | 1 (13) | - | - | - |
| Application site irritation | - | - | - | - | 1 (13) | - |
| Infections and infestations | ||||||
| Fungal infection | - | - | - | - | 1 (13) | - |
| Trichomoniasis | - | - | 1 (13) | - | - | - |
| Moderate | - | - | 1 (13) | - | - | - |
| Injury, poisoning and procedural complications | ||||||
| Contusion | - | - | - | - | 1 (13) | - |
| Tooth fracture | - | - | - | - | - | 1 (13) |
| Moderate | - | - | - | - | - | 1 (13) |
| Vascular access site bruising or swelling | - | - | 2 (25) | - | - | - |
| Musculoskeletal and connective tissue disorders | ||||||
| Joint or extremity pain | 1 (13) | 1 (13) | 1 (13) | - | - | - |
| Nervous system disorders | ||||||
| Headache | 1 (13) | 1 (13) | 2 (25) | 1 (13) | 2 (25) | - |
| Moderate | - | - | 1 (13) | 1 (13) | - | - |
| Renal and urinary disorders | ||||||
| Abnormal urine odor | - | - | - | - | - | 1 (13) |
| Respiratory, thoracic, and mediastinal disorders | ||||||
| Tachypnea | - | - | - | - | 1 (13) | - |
| Moderate | - | - | - | - | 1 (13) | - |
| Skin and subcutaneous tissue disorders | ||||||
| Rash | - | 1 (13) | - | - | - | - |
| Skin irritation | 1 (13) | - | - | - | - | - |
| Vascular disorders | ||||||
| Hypertension | - | 1 (13) | 2 (25) | - | - | - |
| Phlebitis | 1 (13) | 1 (13) | - | 1 (13) | 2 (25) | |
AE, adverse event; CZA, ceftazidime-avibactam; ATM, aztreonam; -, indicates subjects without the condition/event in a column.
Unrelated grade 2 or higher AEs include bradycardia, 1 severe in cohort 4 and 1 moderate in cohort 5; swelling of eyelid, 1 in cohort 4; infusion site reactions, 1 moderate in cohort 3; trichomoniasis: 1 moderate in cohort 3; tooth fracture: 1 moderate in cohort 6; headache, 1 moderate AE in cohort 3; and tachypnea, 1 moderate in cohort 5.
AEs are classified by MedDRA (Medical Dictionary for Regulatory Activities) system organ class.
Infusion site reactions include infusion site extravasation, infusion site irritation, infusion site pain, or infusion site phlebitis. Bradycardia includes bradycardia and sinus bradycardia.
Investigation AEs/NCS laboratory abnormalities: hepatic aminotransferase elevations.
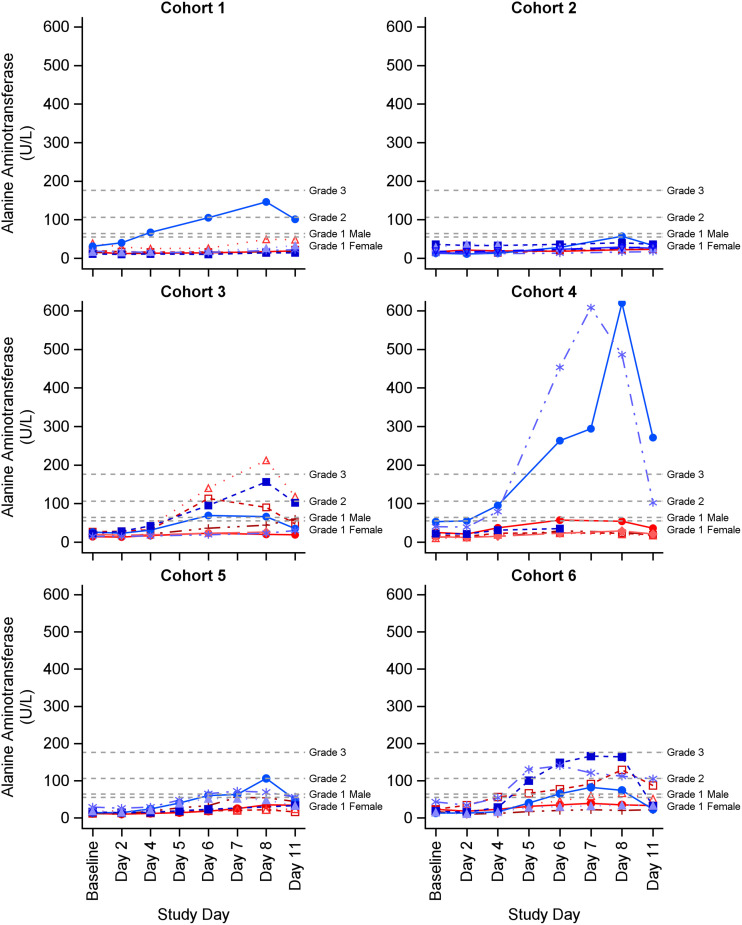
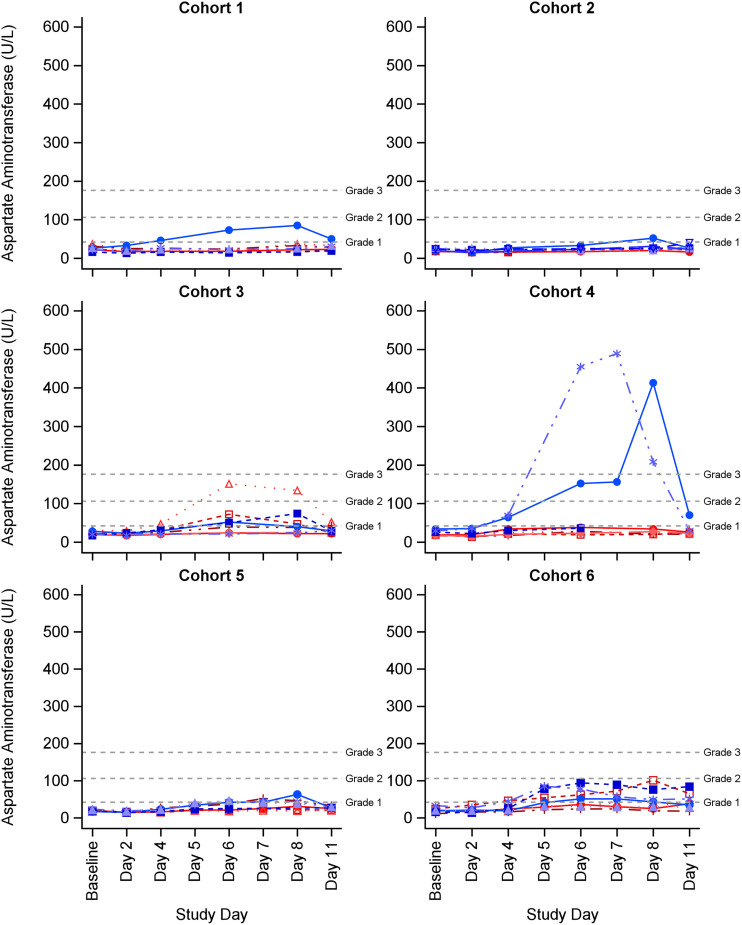
Hepatic aminotransferase elevations were the most frequently observed investigation abnormalities (Table 5). In total, 19 subjects (40%) experienced an alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) elevation (16 had ALT and AST elevations and 3 had an ALT or AST elevation). Eighteen of the 19 subjects experienced at least one related ALT or AST elevation AE, and 1 subject in cohort 2 experienced a grade 1 AST elevation that was deemed NCS by the site PI. The maximum severity of related ALT elevations was grade 3 in 3 subjects, grade 2 in 7 subjects, and grade 1 in 7 subjects. The maximum severity of related AST elevations was grade 3 in 2 subjects, grade 2 in 1 subject, and grade 1 in 14 subjects. Most ALT/AST elevations (n = 7) occurred in Black or African American male subjects (Appendix A in supplemental file 1). For subjects with ALT/AST elevations, ALT/AST values increased on or after study day 4, the highest observed ALT/AST values were on study day 7 or 8, and all ALT/AST elevations resolved after cessation of dosing (Fig. 2 and 3). All subjects who experienced ALT/AST elevations were asymptomatic, none had elevations in alkaline phosphatase (ALP) or bilirubin, and none had any clinical findings suggestive of acute liver failure (i.e., liver necrosis, cholestasis, or jaundice).
FIG 2.
Individual alanine aminotransaminase (ALT) values by study product cohort. ALT (U/L) reference range for male: 17–63. Grade 1 AE was >63 to 105 U/L; grade 2 AE was 106 to 175 U/L; grade 2 was >175 U/L. ALT (U/L) reference range for female: 14–54. Grade 1 AE was >54 to 105 U/L; grade 2 AE was 106 to 175 U/L; grade 2 was >175 U/L. Blue lines represent males and red lines represent females.
FIG 3.
Individual aspartate aminotransferase (AST) values by study product cohort. AST (U/L) reference range for all subjects: 15 to 41. Grade 1 AE was >42 to 105U/L; grade 2 AE was 106 to 175 U/L; grade 2 was >175 U/L. Blue lines represent males and red lines represent females.
Seventeen of the 19 subjects with ALT/AST elevations received ATM alone or in combination with CZA. In cohort 3 (2 g ATM every 6 h infused over 2 h), 5 subjects had asymptomatic ALT/AST elevations (ALT elevations: 1 subject with a grade 3 AE, 2 with grade 2 AEs, and 2 with grade 1 AEs; AST elevations: 1 subject with a grade 2 AE and 4 with grade 1 AEs). In cohort 4 (2 g ATM bolus, followed by 8 g daily CI), 3 subjects had asymptomatic ALT elevations (2 subjects with grade 3 AEs and 1 with grade 1 AE) and 2 had asymptomatic AST elevations (2 subjects with grade 3 AEs). One subject experienced maximum ALT and AST elevations of 620 U/L and 413 U/L, respectively, on study day 8, while a second subject experienced ALT and AST elevations of 608 U/L and 489 U/L, respectively, on study day 7. The two grade 2 ALT/AST increases in cohort 4 remained elevated for 1 to 2 days after ATM was discontinued, but trended down 2 to 4 days after ATM discontinuation and returned to baseline within 25 days. Daily AST/ALT monitoring was not performed as part of the study and 2 subjects in cohort 4 with grade 3 ALT/AST elevations were discharged from the study unit on day 7 and returned for two additional AE follow-up visits within 25 days after discontinuation of ATM.
The occurrence of two grade 3 ALT/AST AEs in cohort 4 halted the study (halting rule: 2 or more subjects experiencing a Grade 3-related AE of the same High-Level Term [HLT] type) and a Safety Monitoring Committee (SMC) was convened (see Materials and Methods). In response to the observed study halt, dosing of CZA-ATM in cohorts 5 and 6 was modified (Table 1) and additional safeguards were incorporated in the study protocol. In cohort 5, 3 subjects had asymptomatic increases in ALT (1 subject with a grade 2 AE and 2 with grade 1 AEs) and 4 had asymptomatic increases in AST (all Grade 1 AEs). In cohort 6, 5 subjects had asymptomatic elevations in ALT (3 subjects with grade 2 AEs and 2 with grade 1 AEs) and AST (5 subjects with grade 1 AEs) that resolved shortly after cessation of study products.
Other investigation AEs/NCS laboratory abnormalities.
Aberrant hematologic (i.e., hemoglobin decreased, neutrophil or white blood cell count decreased, platelet decreased) and coagulation values (i.e., prolonged thrombin time) were the second most frequently observed investigation abnormalities in the study population (Table 5). Most observed hematologic and coagulation AEs were of mild to moderate severity, and most mild (grade 1) laboratory abnormalities were classified as NCS by the site PI. One subject in cohort 5 had an unrelated grade 3 hemoglobin decrease and 1 subject in cohort 1 had an unrelated grade 3 neutrophil count decrease. These 2 unrelated grade 3 AEs were attributed to subjects with menstruation and African American ancestry, respectively. Similarly, most other investigation abnormalities were of mild severity. A grade 3-related increase in blood glucose was observed in 1 subject in cohort 4. One subject in cohort 2 had a grade 2 bilirubin increase, one subject in cohort 4 had a grade 2 blood lactate dehydrogenase increase, while another subject in cohort 4 had a grade 2 blood glucose decrease. All subjects with investigation abnormalities were asymptomatic, and all investigation AEs resolved or stabilized by end of study.
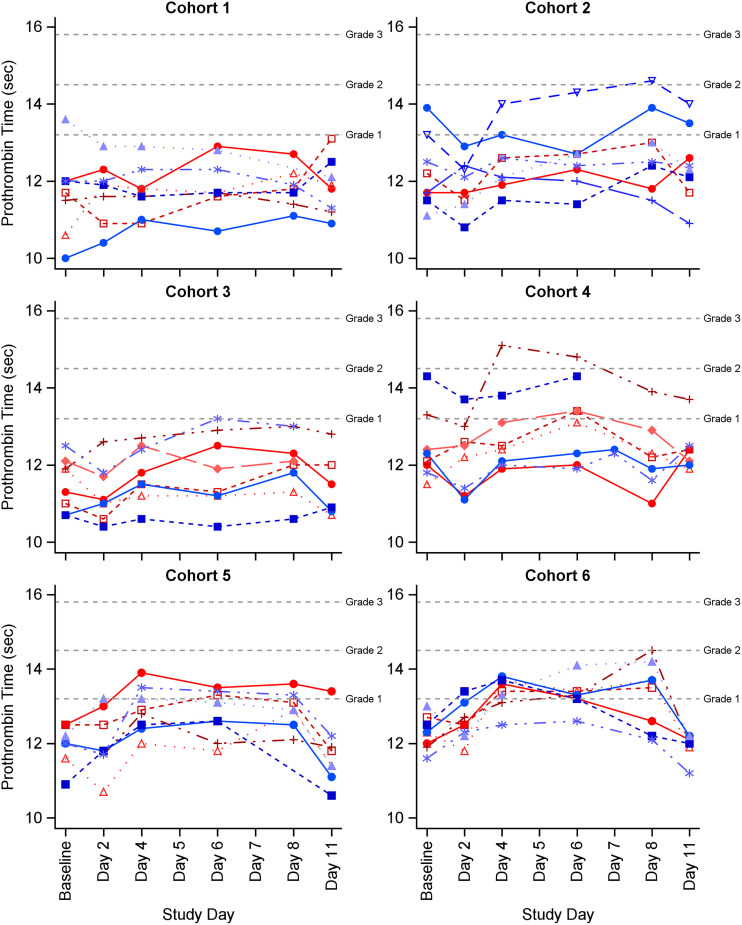
Most investigation abnormalities were similar across cohorts, except for prolonged prothrombin time, which was more pronounced in the combination cohorts (Fig. 4). Of the 16 subjects with prolonged prothrombin times (13 related AEs, 1 unrelated AE, and 2 grade 1 NCS laboratory abnormalities), 11 had received CZA-ATM. In cohort 5, 4 subjects had a prolonged prothrombin time (all grade 1 AEs) and 7 subjects in cohort 6 had a prolonged prothrombin time (1 grade 2 AE and 6 grade 1 AEs). Of the 11 prolonged prothrombin time AEs in the CZA-ATM cohorts, 6 (all grade 1) occurred in subjects with grade 1 or higher ALT/AST elevation AEs.
FIG 4.
Individual prothrombin values by study product cohort. Prothrombin time (PT, seconds) reference range for all subjects was 9.5 to 13.1 seconds. Grade 1 AE was >9.5 to 14.4 seconds; grade 2 was 14.5 to 15.7 seconds; grade 3 was >15.7 seconds. Blue lines represent males and red lines represent females.
Non-investigation AEs.
The frequencies of non-investigation AEs were similar across cohorts, and most were of mild severity and deemed to be unrelated to the study product(s) (Table 6). No subjects experienced a grade 3-related non-investigation AE, and one subject in cohort 4 had a grade 2 headache that was deemed related to the study product. One unrelated grade 3 bradycardia AE was experienced by a subject in cohort 4, and this was attributed to his high level of physical fitness. Unrelated grade 2 (moderate) AEs included bradycardia (n = 2), swelling of eyelid (n = 1), infusion site reactions (n = 1), trichomoniasis (n = 1), tooth fracture (n = 1), headache (n = 1), and tachypnea (n = 1). Fourteen subjects had unrelated infusion site AEs (13 grade 1 and 1 grade 2), and these were attributed to managing and changing peripheral i.v. access over the 7 days of dosing versus infusion site-reaction AEs related to the investigated study product(s).
DISCUSSION
Overall, co-administration of CZA-ATM as 2-h intermittent infusions was found to be generally safe. The most frequently observed related AEs in the CZA-ATM cohorts were hepatic aminotransferase elevations of mild to moderate severity. While aminotransferase AEs were commonplace in the CZA-ATM cohorts, no subjects in the CZA-ATM cohorts experienced aminotransferase elevations of ≥3 times the upper limit of normal, an indicator of drug-induced liver injury (28). Furthermore, no subjects with AST/ALT elevations were symptomatic, none had clinical findings suggestive of liver damage (e.g., jaundice or impaired synthetic function), and all AST/ALT AEs normalized after cessation of study products. The incidence of ALT/AST elevations in the CZA-ATM combination cohorts was found to be comparable to that in the ATM-alone cohorts, suggesting that coadministration of CZA with ATM as 2-h intermittent infusions does not augment ALT/AST elevations relative to ATM alone. Additionally, the asymptomatic ALT/AST elevations observed in subjects who received ATM, alone and in combination with CZA, are consistent with prior reports (22). Serum aminotransferase elevations are more common with ATM relative to other comparative β-lactam antibiotics, with ALT/AST elevation event rates ranging from 10% to 38% (22). To date, no individual case of frank liver injury and jaundice attributable to ATM has been reported, and ATM-induced hepatocellular injury appears to be marked only by asymptomatic serum liver enzyme elevations that normalize after cessation of dosing (22, 29).
The observed grade 3 AST/ALT elevations in two subjects in cohort 4 potentially suggest that CI administration of ATM at a dose of 8 g/day is associated with a more severe reversible hepatocellular pattern of liver injury. Notably, the 2 subjects with severe ALT/AST elevations in the ATM CI Cohort were African American males, who are at increased risk for more severe idiosyncratic drug-induced liver injury (30, 31). Furthermore, the observed degree of ALT/AST elevations for the 2 subjects in the ATM CI with severe ALT/AST elevations AEs were considerably higher than those observed in other study subjects with ALT/AST AEs (Fig. 2 and 3). This suggests that the pathogenesis for the drug-induced liver injury may have been potentially different for the two African-American subjects in the ATM CI cohort relative to other subjects in the ATM/CZA-ATM cohorts who experienced ALT/AST AEs (32, 33). The two severe ALT/AST elevations in the ATM CI cohort may potentially have been immune-mediated idiosyncratic drug-induced liver injuries, while the ALT/AST AEs in the 2-h intermittent infusion CZA-ATM cohorts were potentially due to the clinical adaptation process (32, 33). Further studies are needed to better understand the mechanism(s) responsible for ATM-induced hepatocellular injury and determine the patient populations at greatest risk for ALT/AST elevations with receipt of ATM. Until such studies are performed, some caution should be exercised with use of ATM CI at a dose of 8 g/day, especially in males of African American ancestry.
Other investigation AEs/mild NCS laboratory abnormalities that were frequently observed in the CZA-ATM cohorts were asymptomatic, self-limiting changes in hematologic and coagulation laboratory values of mild to moderate severity. Changes in hematologic and coagulation parameters are infrequent but well-described AEs associated with β-lactams, especially among patients receiving prolonged courses of high-dose therapy (34, 35). The mechanism for β-lactam-induced coagulation AEs is unknown but may be due, in part, to their interference with the action of vitamin K (36–38) or killing of vitamin K-producing intestinal bacteria (39, 40). Most hematologic and coagulation AEs/mild NCS laboratory abnormalities were similar across cohorts, except for prolonged prothrombin time, which was more pronounced in the combination cohorts. Due to the limited number of subjects across cohorts, it is unclear whether the observed prolonged prothrombin time AEs in the CZA-ATM cohorts were aberrant findings or represented additive toxicities from co-administration of two agents from the same drug class. Future studies are required to better ascertain whether relevant hematologic and coagulation AEs are more common with co-administration of CZA-ATM than with each agent administered alone.
From a clinical application perspective, the collective safety findings from this study suggest that clinicians should only consider using ATM in combination with CZA when the benefits outweigh the risk, and its use should be restricted to patients with limited or no alternative treatment options. With the limited number of approved agents with reliable microbiologic activity against MBL-producing Gram-negative bacterial infections (3, 6, 7), we believe CZA-ATM combination therapy still has a role, especially considering the real-world data suggesting that CZA-ATM may potentially confer better patient outcomes relative to other active regimens for adult patients with serious infections due to MBL-producing Enterobacterales (13). The Infectious Diseases Society of America guidance document on the treatment of antimicrobial-resistant infections list CZA-ATM as a preferred treatment for MBL-producing Enterobacterales infections, indicating that this regimen will continue to be used in clinical practice (41). If a clinician deems the use of CZA-ATM necessary in a patient with limited or no alternative treatment options, we recommend that the 2-h intermittent infusion CZA-ATM combination regimens evaluated in this study be prioritized given their observed safety profiles and performance (i.e., rapid bacterial killing and resistance suppression) in the HFIM study (19, 42). We recommend caution with use of ATM CI at a dose of 8 g/day with CZA, especially in males of African American ancestry, due to the two severe AST/AST elevations observed in cohort 4 (ATM CI alone). If one of the CZA-ATM regimens evaluated in this phase I study is prescribed, we suggest that selection of CZA with 6 or 8 g ATM daily is made on an individual patient basis. Although the two CZA-ATM combination regimens evaluated in this phase I study were found to be safe, more subjects in cohort 6 (CZA with ATM 8 g/daily) had asymptomatic ALT/AST and prolonged prothrombin time AEs relative to cohort 5 (CZA with 6 g ATM daily). However, 4 subjects in cohort 5 stopped ATM on day 6 (1 voluntarily withdrew from the study and 3 had missed ATM doses), while all subjects in cohort 6 completed dosing, and this may have contributed to the higher observed ALT/AST and prolonged prothrombin time AEs in subjects who received who received CZA with 2 g ATM i.v. over 2 h every 6 h (cohort 6). For more difficult-to-treat, deep-seated, or life-threatening infections, it may be reasonable to use 2.5 g CZA i.v. over 2 h every 8 h with 2 g ATM i.v. over 2 h every 6 h (8 g/day) if the perceived benefits outweigh the risks given its more rapid bactericidal activity relative to 2.5 g CZA i.v. over 2 h every 8 h with 1.5 g ATM i.v. over 2 h every 6 h (6 g/day) in the HFIM study (19). However, more efficacy and safety data are required to define the optimal roles of each CZA-ATM combination regimen evaluated in this phase I study for clinical practice. Irrespective of the CZA-ATM regimen selected, clinicians need to be vigilant in monitoring liver function tests and should have a low threshold for discontinuing therapy in patients with moderate to severe ALT/AST elevations, even among patients with asymptomatic serum aminotransferase elevations. We also encourage clinicians to conduct more frequent hematologic and coagulation laboratory testing as several subjects experienced study drug-related decreases in neutrophil counts and hemoglobin in the CZA-ATM cohorts, and prolonged prothrombin time AEs were more frequent in the CZA-ATM cohorts than in the single-agent cohorts.
Because this was a phase I study of healthy subjects, additional clinical studies are warranted to determine the safety and efficacy of the CZA-ATM regimens in patients with active infections, especially those who have unstable renal function or baseline renal impairment. Both CZA and ATM are renally eliminated (20, 21) and there is the potential for more pronounced liver enzyme elevations and other exposure-related AEs in patients with renal dysfunction due to reductions in the clearances of both CZA and ATM, resulting in increased daily and cumulative exposures. Furthermore, the results of the PK analyses suggested that ATM clearance may be reduced when it is used in combination with CZA, and this has the potential to result in higher-than-anticipated plasma exposure profiles in patients with reduced renal function and possibly more exposure-related AEs (23). More data are also needed on the safety of CZA-ATM combination regimens for >7 days, as patients often require >7 days of therapy to treat their infections. Finally, as with all agents evaluated in phase I studies, comparative Phase II-III randomized clinical trials (RCTs) are required to properly establish the safety and efficacy of the CZA-ATM combination regimens evaluated in this study. As part of these proposed Phase II to III RCTs, the efficacy and safety of CZA at either 6 or 8 g daily should be evaluated to better identify the optimal CZA-ATM combination regimen for patients with serious infections due to highly resistant Gram-negative infections. Additionally, plasma pharmacokinetic data should be collected, and PK/PD modeling should be performed to delineate the therapeutic window for CZA-ATM combination therapy (43).
In conclusion, co-administration of CZA-ATM as intermittent infusions over 2 h was found to be safe in this phase I study. Asymptomatic hepatic aminotransferase were the most common related AEs in the 2-h intermittent infusion CZA-ATM combination Cohorts, but the results suggest that co-administration of CZA-ATM does not exacerbate AST/ALT elevations or result in additive or sustained hepatic injury relative to ATM alone. Prolonged prothrombin time was the only AE that was more pronounced in the CZA-ATM Cohorts relative to the single drug Cohorts and this merit further investigation in future studies to determine if this is a clinically relevant safety concern with use of CZA-ATM. The CZA-ATM combination regimens evaluated in this study should only be used in the treatment of patients when the perceived benefits outweigh the risks because the combination is not FDA-approved for use in patients. Caution should be exercised with use of ATM CI with CZA, especially in males of males of African American ancestry, due to the two severe AST/AST elevations observed in cohort 4 (ATM CI 8 g/day). If CZA-ATM is prescribed, clinicians need to be vigilant in monitoring for hepatic injury and should have a low threshold for discontinuing therapy in patients with moderate to severe ALT/AST elevations. We also believe it is prudent to closely monitor hematologic and coagulation parameters as additional safety measures. Future controlled RCTs are required to better define the safety and efficacy of the CZA-ATM regimens evaluated in this study in patients with active infections.
MATERIALS AND METHODS
Study design and enrollment criteria.
The COMBINE study (ClinicalTrials.gov identifier: NCT03978091) was a phase I, open-label, single-center study that investigated the safety and PK of 7 days of CZA combined with ATM, CZA alone, and ATM alone in 48 healthy adult male and female volunteers aged 18 to 45 years (inclusive). Eligible subjects were admitted to the phase I study unit for 7 days to receive study product(s), and the final outpatient follow-up visit was day 11 + 3 post-day 1 of study product(s) administration. The study was approved by the Duke Health Institutional Review Board. Informed consent was obtained and documented prior to initiation of any study procedures. The study was conducted in accordance with Good Clinical Practice principles as established by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH).
Eligible subjects included healthy male and non-pregnant or non-breastfeeding female volunteers aged 18 to 45 years who were non-smokers with a body weight of ≥50 kg and BMI between 19 and 33 kg/m2. Sexually active female subjects were of non-childbearing potential or agreed to use a highly effective method of birth control, and sexually active male subjects capable of fathering children agreed to use barrier contraception (condom with spermicide) from the first dose of study product until 30 days following the last dose of study product. Subjects were excluded if they met any of the following criteria: history of any clinically significant disease or disorder, medical/surgical procedure, or trauma within 4 weeks prior to the first administration of study product(s); history or presence of gastrointestinal, hepatic, or renal disease, or any other condition known to interfere with absorption, distribution, metabolism, or excretion of drugs; known history of a clinically important allergy/hypersensitivity to AVI, any monobactam, or any β-lactam and/or l-arginine; creatinine clearance (CLCR) of ≤80 mL/minute (measured by Cockcroft-Gault method) (44); history of Clostridioides difficile infection in the past 90 days; receipt of any prescription medications (except for birth control pills or hormone replacement in females) within 14 days prior to study enrollment unless (in the opinion of the site investigator) the medication would not interfere with study procedures or affect subject safety; receipt of any herbal and dietary supplements within 14 days prior to study enrollment; ALT or AST laboratory value above the upper limit of normal; prolonged (>450 msec) or shortened QTcF (<340 msec), family history of long QT syndrome, or any clinically important abnormalities in rhythm, conduction, or morphology of resting ECG; a positive result on screening for HIV serum hepatitis B surface antigen or hepatitis C virus antibody; known or suspected current or history of drug or alcohol abuse; positive screen for drugs of abuse, cotinine (nicotine), or alcohol at screening and at admission to the study site prior to the first administration of the study product(s); receipt of a new chemical entity (compound not approved for marketing) or participation in a study that included drug treatment within 1 month of study enrollment; previous participation in current study; involvement in the planning and/or conduct of the study; known history of past or current epilepsy or seizure disorders, excluding febrile seizures of childhood; any ongoing/recent (during screening) medical complaints which may have interfered with analysis of study data; or being considered unlikely to comply with study procedures, restrictions, and requirements.
Safety monitoring.
Study safety was closely monitored using daily assessments of AEs, vital signs, and symptom-directed physical examinations. Serum chemistry, hematology, and coagulation laboratory tests were collected on days −1, 2, 4, 6, and 8 (prior to discharge from the study site). Liver function tests were obtained day −1, day 2, and daily beginning on day 4 until a subject completed dosing. A urinalysis was performed on days −1 and 4, an ECG was conducted on days −1 and 8, and a Coomb’s test was performed during the screening visit and on day 8. A comprehensive final safety evaluation, including clinical laboratory safety tests, was completed for each subject at the final study visit (day 11 + 3).
The type, incidence, relatedness, and severity of AEs and SAEs were recorded from start of infusion of the first dose of study product on day 1 through the final study visit. Adverse events were assessed by the investigator using a protocol-defined grading system based on FDA guidelines for industry for grading of AEs (Appendix B in supplemental file 1) (45). For events not included in the protocol-defined grading system, AEs were graded as mild (grade 1), moderate (grade 2), or severe (grade 3). The assessment of an AE’s relationship to the study product was performed by the licensed study physician as indicated on FDA Form 1572. An AE was considered related if there was a reasonable possibility that the administration of the study product caused the AE. An AE was considered unrelated if there was not a reasonable possibility that the administration of the study product caused the event. Mild (grade 1) abnormal laboratory values which were deemed to be NCS by the site PI were captured as part of the study but were not reported as AEs. All AEs and laboratory abnormalities reported as AEs were followed until resolution, return to pretreatment status, or stabilization even if the duration of follow-up went beyond the final study visit. Study and individual halting criteria were included to safeguard subject safety. Safety oversight was performed by an Independent Safety Monitor and a Safety Monitoring Committee.
Investigational drug cohorts.
Eligible subjects were assigned into one of six dosing cohorts (Table 1) and investigational product(s) were administered for 7 days. Four of the six cohorts (Cohorts 1 to 4) were single drug dosing Cohorts. These single drug dosing cohorts were included to collect baseline safety and PK data with CZA and ATM administered alone and were conducted prior to completion of the CZA-ATM combination cohorts (cohorts 5 and 6). In the CI cohorts, an initial loading dose was administered prior to starting the CI (20, 25, 26, 27, 28). Cohorts 5 and 6 were the CZA-ATM combination dosing cohorts. In both CZA-ATM combination cohorts, investigational products were prepared in separate IV bags and administered either via either a double lumen catheter connected to separate ports or a single lumen Y-site injection port (46). In the initial design, cohort 5 was 2.5 g CZA i.v. over 2 h every 8 h with 2 g ATM i.v. over 2 h every 6 h, and cohort 6 was 2.5 g CZA i.v. over 2 h × 1, then 7.5 g daily as a CI with 2 g ATM i.v. × 1, then 8 g/day as a CI. However, the study was temporarily halted due to the occurrence of severe AST/ALT-related AEs in two subjects in cohort 4 (see Results), and dosing in the combination cohorts was modified to 2.5 g CZA i.v. over 2 h every 8 h with 1.5 g ATM i.v. over 2 h every 6 h (cohort 5) and 2.5 g CZA i.v. over 2 h every 8 h with 2 g ATM i.v. over 2 h every 6 h (cohort 6). This CZA-ATM combination regimen was selected for evaluation because it was identified as being highly effective in the HFIM study which served as the basis for this phase I study (19). The SMC was in favor of escalating the ATM dose in combination with CZA in cohort 6 if no halting rules were met and there was no sign that administration of ATM with CZA exacerbated AST/ALT elevations in cohort 5 based on the results of the HFIM experiments, which demonstrated increased bacterial killing with 8 g/day versus 6 g/day combination regimens (19). The SMC did not recommend administering ATM as a CI in any subsequent cohorts because the safety of ATM CI was not established in this study. As an additional safeguard, the SMC recommended that more intensive liver function tests (measured daily from study days 4 to 8 versus every other day from study days 4 to 8) monitoring be performed. A SMC meeting was also scheduled after the completion of cohort 5 to review the safety data.
Safety assessment.
All subjects who received at least one dose of study product(s) were included in the safety analysis. Continuous safety variables were summarized using the following descriptive statistics: n (non-missing sample size), mean, standard deviation, median, maximum, and minimum. The frequency and percentages of observed levels were reported for all categorical measures. All AEs after the first dose of study product(s) were coded using Medical Dictionary for Regulatory Activities Terminology (MedDRA) dictionary version 23.1. The number of AEs and the number of subjects with an AE were summarized by MedDRA SOC (investigation versus non-investigation), preferred term (PT), maximum severity, relatedness to treatment, cohort, and investigational study product(s) received (CZA, ATM, and CZA-ATM). Per the MedDRA definition, an “investigation” is a clinical laboratory test concept (including biopsies), radiologic test concept, physical examination parameter, or physiologic test concept (e.g., pulmonary function test). Subjects were counted for each AE only once, at the highest grade observed. SAEs and AEs which led to study discontinuation were listed, and study withdrawal was summarized. Clinical laboratory data were compared to normal ranges as reported by the reference laboratory at each scheduled collection time point for each cohort, and frequency of abnormal laboratory values were tabulated.
Data availability.
Researchers interested in accessing the clinical trial data presented here are encouraged to submit a research proposal and publication plan. The proposal and plan will be reviewed by the ARLG publications committee and/or appropriate study team members. If approved, and upon receipt and approval of a signed data access/use agreement, individual participant data necessary to complete the proposed analysis will be made available. Related documents, including the study protocol, statistical analysis plan, and data dictionary, may also be shared. Access to data will only be granted to researchers who provide a methodologically and scientifically sound proposal. Proposed analyses that are duplicative of ongoing or proposed analyses may not be supported. To submit a proposal, please complete a proposal at the ARLG website (https://arlg.org/how-to-apply/protocol-concept). Alternatively, visit the Duke Clinical Research Institute website (https://dcri.org/our-work/analytics-and-data-science/data-sharing/). There may be costs associated with data sharing, which researchers are expected to cover.
ACKNOWLEDGMENTS
We like to acknowledge Robert Bonomo for his contributions to the conceptualization of this study.
This research was supported by the National Institute of Allergy and Infectious Diseases of the NIH under award no. UM1AI104681. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The Statistical and Data Coordinating Center (SDCC) was funded by the Division of Microbiology and Infectious Diseases of National Institute of Allergy and Infectious Diseases (contract no. 75N93021C00012).
T.P.L.: AbbVie (Consultant), BioFire Diagnostics (Grant/Research Support), Cidara (Advisor/Consultant), Entasis (Grant/Research Support), Ferring (Advisor/Consultant/Speaker), Genentech (Consultant), ICPD (Consultant), Johnson and Johnson (Consultant), Melinta (Advisor/Consultant), Merck (Advisor/Consultant, Grant/Research Support), Paratek (Advisor/Consultant), Roche (Consultant), Shionogi (Advisor/Consultant/Speaker), Spero (Advisor/Consultant), Wockhardt (Grant/Research Support), and Venatrox (Advisor/Consultant).
J.N.O.: Merck & Co., Inc., (Grant/Research Support), Paratek Pharmaceuticals (Grant/Research Support).
S.J.B.: NIH (grant/research support), FDA (grant/research support), the Patient-Centered Outcomes Research Institute (grant/research support), the Rheumatology Research Foundation’s Scientist Development Award (grant/research support), the Childhood Arthritis and Rheumatology Research Alliance (grant/research support), Purdue Pharma (grant/research support), and UCB (consultant).
V.G.F.: Affinergy (Grant/Research Support, Honoraria), Affinium (Honoraria), Amphliphi Biosciences (Honoraria), ArcBio (Stocks/Bonds), Basilea (Grant/Research Support, Honoraria), Bayer (Honoraria), C3J (Honoraria), Cerexa/Forest/Actavis/Allergan (Grant/Research Support), Contrafect (Grant/Research Support, Honoraria), Cubist/Merck (Grant/Research Support), Debiopharm (Grant/Research Support), Deep Blue (Grant/Research Support), Destiny (Honoraria), Genentech (Grant/Research Support, Honoraria), Integrated Biotherapeutics (Honoraria), Janssen (Grant/Research Support, Honoraria), Karius (Grant/Research Support), Medicines Co., (Honoraria), MedImmune (Grant/Research Support, Honoraria), NIH (Grant/Research Support), Novartis (Grant/Research Support, Honoraria), Pfizer (Grant/Research Support), Regeneron (Grant/Research Support, Honoraria), Sepsis Diagnostics (Sepsis Diagnostics patent pending), UpToDate (Royalties), Valanbio (Stocks/Bonds).
The remaining authors have no conflicts of interest to declare.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.WHO. 2021. Antimicrobial resistance fact sheet. WHO, Geneva, Switzerland. Available from http://www.who.int/mediacentre/factsheets/fs194/en/. Accessed 29 April 2015. [Google Scholar]
- 2.Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, Edwards JR, Sievert DM. 2016. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 37:1288–1301. 10.1017/ice.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. 2019. Antibiotic resistance threats in the United States, 2019. CDC, Atlanta, GA. Available from https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. [Google Scholar]
- 4.Walsh TR. 2005. The emergence and implications of metallo-beta-lactamases in Gram-negative bacteria. Clin Microbiol Infect 11(Suppl 6):2–9. 10.1111/j.1469-0691.2005.01264.x. [DOI] [PubMed] [Google Scholar]
- 5.Jacoby GA, Munoz-Price LS. 2005. The new beta-lactamases. N Engl J Med 352:380–391. 10.1056/NEJMra041359. [DOI] [PubMed] [Google Scholar]
- 6.Wright H, Bonomo RA, Paterson DL. 2017. New agents for the treatment of infections with Gram-negative bacteria: restoring the miracle or false dawn? Clin Microbiol Infect 23:704–712. 10.1016/j.cmi.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Tan X, Kim HS, Baugh K, Huang Y, Kadiyala N, Wences M, Singh N, Wenzler E, Bulman ZP. 2021. Therapeutic options for metallo-β-lactamase-producing Enterobacterales. Infect Drug Resist 14:125–142. 10.2147/IDR.S246174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall S, Hujer AM, Rojas LJ, Papp-Wallace KM, Humphries RM, Spellberg B, Hujer KM, Marshall EK, Rudin SD, Perez F, Wilson BM, Wasserman RB, Chikowski L, Paterson DL, Vila AJ, van Duin D, Kreiswirth BN, Chambers HF, Fowler VG, Jr., Jacobs MR, Pulse ME, Weiss WJ, Bonomo RA. 2017. Can ceftazidime-avibactam and aztreonam overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae? Antimicrob Agents Chemother 61:e02243-16. 10.1128/AAC.02243-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahme C, Butterfield JM, Nicasio AM, Lodise TP. 2014. Dual beta-lactam therapy for serious Gram-negative infections: is it time to revisit? Diagn Microbiol Infect Dis 80:239–259. 10.1016/j.diagmicrobio.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Davido B, Fellous L, Lawrence C, Maxime V, Rottman M, Dinh A. 2017. Ceftazidime-avibactam and aztreonam, an interesting strategy to overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 61:e01008-17. 10.1128/AAC.01008-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw E, Rombauts A, Tubau F, Padulles A, Camara J, Lozano T, Cobo-Sacristan S, Sabe N, Grau I, Rigo-Bonnin R, Dominguez MA, Carratala J. 2018. Clinical outcomes after combination treatment with ceftazidime/avibactam and aztreonam for NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae infection. J Antimicrob Chemother 73:1104–1106. 10.1093/jac/dkx496. [DOI] [PubMed] [Google Scholar]
- 12.Mojica MF, Ouellette CP, Leber A, Becknell MB, Ardura MI, Perez F, Shimamura M, Bonomo RA, Aitken SL, Shelburne SA. 2016. Successful treatment of bloodstream infection due to metallo-β-lactamase-producing Stenotrophomonas maltophilia in a renal transplant patient. Antimicrob Agents Chemother 60:5130–5134. 10.1128/AAC.00264-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falcone M, Daikos GL, Tiseo G, Bassoulis D, Giordano C, Galfo V, Leonildi A, Tagliaferri E, Barnini S, Sani S, Farcomeni A, Ghiadoni L, Menichetti F. 2021. Efficacy of ceftazidime-avibactam plus aztreonam in patients with bloodstream infections caused by metallo-β-lactamase-producing Enterobacterales. Clin Infect Dis 72:1871–1878. 10.1093/cid/ciaa586. [DOI] [PubMed] [Google Scholar]
- 14.Karlowsky JA, Kazmierczak KM, de Jonge BLM, Hackel MA, Sahm DF, Bradford PA. 2017. In vitro activity of aztreonam-avibactam against Enterobacteriaceae and Pseudomonas aeruginosa isolated by clinical laboratories in 40 countries from 2012 to 2015. Antimicrob Agents Chemother 61:e00472-17. 10.1128/AAC.00472-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazmierczak KM, Rabine S, Hackel M, McLaughlin RE, Biedenbach DJ, Bouchillon SK, Sahm DF, Bradford PA. 2016. Multiyear, multinational survey of the incidence and global distribution of metallo-β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:1067–1078. 10.1128/AAC.02379-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monogue ML, Abbo LM, Rosa R, Camargo JF, Martinez O, Bonomo RA, Nicolau DP. 2017. In vitro discordance with in vivo activity: humanized exposures of ceftazidime-avibactam, aztreonam, and tigecycline alone and in combination against New Delhi metallo-β-lactamase-producing Klebsiella pneumoniae in a murine lung infection model. Antimicrob Agents Chemother 61:e00486-17. 10.1128/AAC.00486-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh R, Kim A, Tanudra MA, Harris JJ, McLaughlin RE, Patey S, O’Donnell JP, Bradford PA, Eakin AE. 2015. Pharmacokinetics/pharmacodynamics of a β-lactam and β-lactamase inhibitor combination: a novel approach for aztreonam/avibactam. J Antimicrob Chemother 70:2618–2626. 10.1093/jac/dkv132. [DOI] [PubMed] [Google Scholar]
- 18.Wenzler E, Deraedt MF, Harrington AT, Danizger LH. 2017. Synergistic activity of ceftazidime-avibactam and aztreonam against serine and metallo-β-lactamase-producing Gram-negative pathogens. Diagn Microbiol Infect Dis 88:352–354. 10.1016/j.diagmicrobio.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Lodise TP, Smith NM, O’Donnell N, Eakin AE, Holden PN, Boissonneault KR, Zhou J, Tao X, Bulitta JB, Fowler VG, Chambers HF, Bonomo RA, Tsuji BT. 2020. Determining the optimal dosing of a novel combination regimen of ceftazidime/avibactam with aztreonam against NDM-1-producing Enterobacteriaceae using a hollow-fibre infection model. J Antimicrob Chemother 75:2622–2632. 10.1093/jac/dkaa197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bristol Myers Squibb. 2018. AZACTAM (aztreonam for injection, USP). Bristol Myers Squibb, Deerfield, IL. Package insert. https://packageinserts.bms.com/pi/pi_azactam.pdf. [Google Scholar]
- 21.Allergan USA. 2020. AVYCAZ (ceftazidime and avibactam) for Injection, for intravenous use. Allergan USA, Madison, NJ. Package insert. https://www.allergan.com/assets/pdf/avycaz_pi. [Google Scholar]
- 22.National Library of Medicine and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). 2022. LiverTox: clinical information and research information on drug-induced liver injury [Internet]. NIDDK, Bethesda, MD. Available from https://www.ncbi.nlm.nih.gov/books/NBK547852/. [PubMed] [Google Scholar]
- 23.Lodise TP, O’Donnell JN, Balevic S, Liu X, Gu K, George J, Raja S, Guptill JT, Zaharoff S, Schwager N, Fowler J, V G, Wall A, Wiegand K, Chambers HF, Group ARL . 2022. Pharmacokinetics of ceftazidime-avibactam in combination with aztreonam (COMBINE) in a phase 1 open, labeled study of healthy adults. Antimicrob Agents Chemother. 10.1128/aac.00936-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guptill JT, Raja SM, Juel VC, Walter EB, Cohen-Wolkowiez M, Hill H, Sendra E, Hauser B, Jackson P, Swamy GK. 2021. Safety, tolerability, and pharmacokinetics of NTM-1632, a novel mixture of three monoclonal antibodies against botulinum toxin B. Antimicrob Agents Chemother 65:e0232920. 10.1128/AAC.02329-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Eldik LJ, Sawaki L, Bowen K, Laskowitz DT, Noveck RJ, Hauser B, Jordan L, Spears TG, Wu H, Watt K, Raja S, Roy SM, Watterson DM, Guptill JT. 2021. First-in-human studies of MW01-6-189WH, a Brain-penetrant, antineuroinflammatory small-molecule drug candidate: phase 1 safety, tolerability, pharmacokinetic, and pharmacodynamic studies in healthy adult volunteers. Clin Pharmacol Drug Dev 10:131–143. 10.1002/cpdd.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guptill JT, Raja SM, Boakye-Agyeman F, Noveck R, Ramey S, Tu TM, Laskowitz DT. 2017. Phase 1 randomized, double-blind, placebo-controlled study to determine the safety, tolerability, and pharmacokinetics of a single escalating dose and repeated doses of CN-105 in healthy adult subjects. J Clin Pharmacol 57:770–776. 10.1002/jcph.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raja SM, G J, Juel VC, Walter EB, Cohen-Wolkowiez M, Hill H, Sendra E, Hauser B, Jackson P, Tomic M, Espinoza Y, Swamy GK. 2022. First-in-human clinical trial to assess the safety, tolerability and pharmacokinetics of single doses of NTM-1633, a novel mixture of monoclonal antibodies against botulinum toxin E. Antimicrob Agents Chemother 66:e01732-21. 10.1128/aac.01732-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.HHS, FDA, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). 2009. Guidance for industry drug-induced liver injury: premarketing clinical evaluation. FDA, Silver Spring, MD. Available from https://www.fda.gov/media/116737/download. [Google Scholar]
- 29.Lala V, Zubair M, Minter DA. 2022. Liver function tests. StatPearls Publishing, Treasure Island, FL. [PubMed] [Google Scholar]
- 30.Chalasani N, Reddy KRK, Fontana RJ, Barnhart H, Gu J, Hayashi PH, Ahmad J, Stolz A, Navarro V, Hoofnagle JH. 2017. Idiosyncratic drug induced liver injury in African-Americans Is associated with greater morbidity and mortality compared to Caucasians. Am J Gastroenterol 112:1382–1388. 10.1038/ajg.2017.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forde KA, Reddy KR, Troxel AB, Sanders CM, Lee WM, Acute Liver Failure SG, Acute Liver Failure Study Group . 2009. Racial and ethnic differences in presentation, etiology, and outcomes of acute liver failure in the United States. Clin Gastroenterol Hepatol 7:1121–1126. 10.1016/j.cgh.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devarbhavi H. 2012. An update on drug-induced liver injury. J Clin Exp Hepatol 2:247–259. 10.1016/j.jceh.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dara L, Liu ZX, Kaplowitz N. 2016. Mechanisms of adaptation and progression in idiosyncratic drug induced liver injury, clinical implications. Liver Int 36:158–165. 10.1111/liv.12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vardakas KZ, Kalimeris GD, Triarides NA, Falagas ME. 2018. An update on adverse drug reactions related to β-lactam antibiotics. Expert Opin Drug Saf 17:499–508. 10.1080/14740338.2018.1462334. [DOI] [PubMed] [Google Scholar]
- 35.Newman TJ, Dreslinski GR, Tadros SS. 1985. Safety profile of aztreonam in clinical trials. Rev Infect Dis 7(Suppl 4):S648–S655. 10.1093/clinids/7.supplement_4.s648. [DOI] [PubMed] [Google Scholar]
- 36.Tartaglione TA, Duma RJ, Qureshi GD. 1986. In vitro and in vivo studies of the effect of aztreonam on platelet function and coagulation in normal volunteers. Antimicrob Agents Chemother 30:73–77. 10.1128/AAC.30.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipsky JJ. 1984. Mechanism of the inhibition of the gamma-carboxylation of glutamic acid by N-methylthiotetrazole-containing antibiotics. Proc Natl Acad Sci USA 81:2893–2897. 10.1073/pnas.81.9.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipsky JJ. 1983. N-methyl-thio-tetrazole inhibition of the gamma carboxylation of glutamic acid: possible mechanism for antibiotic-associated hypoprothrombinaemia. Lancet 2:192–193. 10.1016/s0140-6736(83)90174-5. [DOI] [PubMed] [Google Scholar]
- 39.Conly JM, Ramotar K, Chubb H, Bow EJ, Louie TJ. 1984. Hypoprothrombinemia in febrile, neutropenic patients with cancer: association with antimicrobial suppression of intestinal microflora. J Infect Dis 150:202–212. 10.1093/infdis/150.2.202. [DOI] [PubMed] [Google Scholar]
- 40.Bang NU, Tessler SS, Heidenreich RO, Marks CA, Mattler LE. 1982. Effects of moxalactam on blood coagulation and platelet function. Rev Infect Dis 4(Suppl):S546–54. 10.1093/clinids/4.supplement_3.s546. [DOI] [PubMed] [Google Scholar]
- 41.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. 2022. Infectious Diseases Society of America 2022 Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin Infect Dis 75:187–212. 10.1093/cid/ciac268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.European Medicines Agency. 2016. Guideline on the use of pharmacokinetics and pharmacodynamics in the development of antimicrobial medicinal products (EMA/CHMP/594085/2015). Committee for Medicinal Products for Human Use, London, United Kingdom. [Google Scholar]
- 43.HHS, FDA, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). 2003. Guidance for industry exposure-response relationships: study design, data analysis, and regulatory applications. FDA, Silver Spring, MD. Available from https://www.fda.gov/media/71277/download. [Google Scholar]
- 44.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 45.HHS, FDA, CBER. 2007. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. HHS, Washington, DC. [DOI] [PubMed] [Google Scholar]
- 46.O’Donnell JN, Xu A, Lodise TP. 2020. Intravenous compatibility of ceftazidime-avibactam and aztreonam using simulated and actual Y-site administration. Clin Ther 42:1580–1586.e2. 10.1016/j.clinthera.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aac.00935-22-s0001.pdf, PDF file, 0.2 MB (209.5KB, pdf)
Data Availability Statement
Researchers interested in accessing the clinical trial data presented here are encouraged to submit a research proposal and publication plan. The proposal and plan will be reviewed by the ARLG publications committee and/or appropriate study team members. If approved, and upon receipt and approval of a signed data access/use agreement, individual participant data necessary to complete the proposed analysis will be made available. Related documents, including the study protocol, statistical analysis plan, and data dictionary, may also be shared. Access to data will only be granted to researchers who provide a methodologically and scientifically sound proposal. Proposed analyses that are duplicative of ongoing or proposed analyses may not be supported. To submit a proposal, please complete a proposal at the ARLG website (https://arlg.org/how-to-apply/protocol-concept). Alternatively, visit the Duke Clinical Research Institute website (https://dcri.org/our-work/analytics-and-data-science/data-sharing/). There may be costs associated with data sharing, which researchers are expected to cover.