Abstract
ATP-sensitive potassium channels (KATP channels) are hetero-octameric nucleotide-gated ion channels that couple cellular metabolism to excitability in various tissues. In the heart, KATP channels are activated during ischaemia and potentially during adrenergic stimulation. In the vasculature, they are normally active at a low level, reducing vascular tone, but the ubiquitous nature of these channels leads to complex and poorly understood channelopathies as a result of gain- or loss-of-function mutations. Zebrafish (ZF) models of these channelopathies may provide insights to the link between molecular dysfunction and complex pathophysiology, but this requires understanding the tissue dependence of channel activity and subunit specificity. Thus far, direct analysis of ZF KATP expression and functional properties has only been performed in pancreatic β-cells. Using a comprehensive combination of genetically modified fish, electrophysiology and gene expression analysis, we demonstrate that ZF cardiac myocytes (CM) and vascular smooth muscle (VSM) express functional KATP channels of similar subunit composition, structure and metabolic sensitivity to their mammalian counterparts. However, in contrast to mammalian cardiovascular KATP channels, ZF channels are insensitive to potassium channel opener drugs (pinacidil, minoxidil) in both chambers of the heart and in VSM. The results provide a first characterization of the molecular properties of fish KATP channels and validate the use of such genetically modified fish as models of human Cantú syndrome and ABCC9-related Intellectual Disability and Myopathy syndrome.
Keywords: ABCC9, Cantú syndrome, cardiomyocytes, cardiovascular, ion channels, KATP, KCNJ8, Kir6.2, SUR2, vascular smooth muscle cells, zebrafish
Graphical Abstract
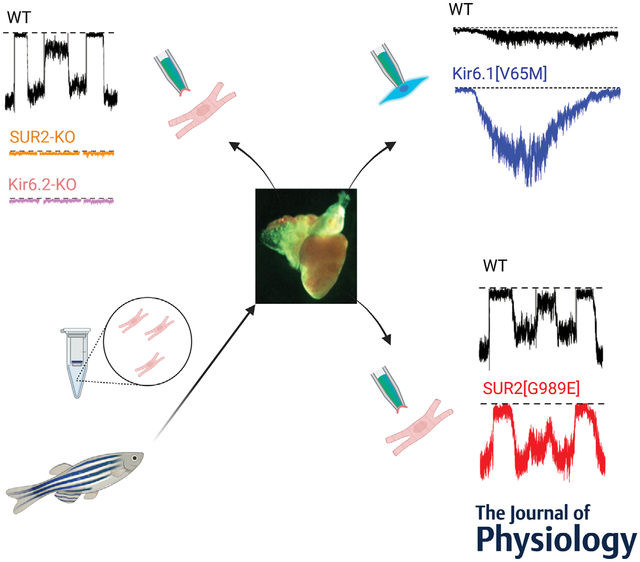
Patch-clamp analysis of atrial, ventricular, and vascular smooth muscle cells isolated from zebrafish reveal KATP channels of similar make-up and properties to those from mammalian cardiovascular tissues.
Introduction
Mammalian KATP channels are hetero-octameric potassium-selective ion channels composed of four pore-forming inwardly rectifying Kir6.x subunits (Kir6.1 or Kir6.2 encoded by KCNJ8 and KCNJ11, respectively) and four regulatory sulfonylurea receptor SURx subunits (SUR1, SUR2 encoded by ABCC8 and ABCC9), whose molecular heterogeneity is further increased by variable splicing of SUR2 into at least two distinct isoforms: SUR2A and SUR2B (Nichols, 2006). Regulated by intracellular nucleotides and membrane phospholipids, KATP channels serve as electrical transducers of the metabolic state of the cell by coupling cellular metabolism to the membrane potential (Nichols, 2006). KATP channels are widely expressed in plasma membranes throughout the body and serve a diverse range of functions such as ischaemic preconditioning in cardiomyocytes, protection against fibre damage in skeletal muscle, vasomotor control in vascular smooth muscle (VSM), regulation of insulin secretion in pancreatic β cells and determination of nerve-fibre excitability in the central nervous system (Cole et al. 1991; Suzuki et al. 2002; Li et al. 2013; Barzegar et al. 2014; Tinker et al. 2014). KATP channels in different tissues exhibit distinct nucleotide sensitivities as a result of distinct subunit compositions. In mammals, Kir6.2 is coupled with SUR1 in the pancreas and in neurons, Kir6.2 is coupled with SUR2A in striated muscles and Kir6.1 is coupled with SUR2B in VSM. The discovery of gain-of-function (GOF) or loss-of-function (LOF) mutations in KCNJ8 and ABCC9 as causes of Cantú Syndrome (CS) (van Bon et al. 2012; Harakalova et al. 2012a; Brownstein et al. 2013; Cooper et al. 2014, 2017; McClenaghan et al. 2018) or ABCC9-related Intellectual Disability and Myopathy syndrome (AIMS) (Smeland et al. 2019), respectively, provides a clear illustration of the pathologic potential of KATP channels.
Zebrafish (ZF) have long been a model organism in biology and, in recent years, have gained popularity as models to study human cardiovascular (CV) disease mechanisms and potential therapies (Yoong et al. 2007). Their highly conserved amenable genome (Howe et al. 2013; Hodgson et al. 2018), together with their rapid development and fecundity (Hodgson et al. 2018), offers potentially significant advantages for modelling congenital CV disease progression and high-throughput drug screening. Their nearly transparent larvae that survive at high ischaemic conditions for up to 5 days-post-fertilization (dpf) (Poss, 2007; Hodgson et al. 2018) are well suited for in vivo imaging studies. Several studies suggest a close similarity between the electrical activity of ZF and human cardiomyocytes (Nemtsas et al. 2010; Vornanen & Hassinen, 2016; van Opbergen et al. 2018), but electrophysiological studies of fish vascular myocytes in general are lacking and there has been no molecular dissection of fish cardiovascular KATP channels. Because of the early genome duplication that occurred in the teleost lineage (Vornanen & Hassinen, 2016), ZF contain at least two orthologues for many human genes, including a novel KATP subunit-Kir6.3 (encoded by kcnj11l) (Zhang et al. 2006), and there could be significant differences between the molecular basis of KATP in ZF and human CV system (Hassinen et al. 2015).
In this study, we have developed approaches for efficiently identifying and isolating cells from ZF CV tissues and for electrophysiological analysis of isolated cardiomyocytes and vascular smooth muscle (VSM) cells. Using a comprehensive combination of genetically modified fish, electrophysiology and gene expression analysis, we demonstrate that ZF cardiac and vascular smooth muscles express functional KATP channels of similar subunit composition, structure and metabolic sensitivity to their mammalian counterparts, validating the use of genetically modified ZF KATP to model human CV KATP channelopathies.
Methods
Ethical approval
All animal experiments were conducted under the guidelines of the animal welfare committee of the Royal Netherlands Academy of Arts and Sciences (KNAW) and the Washington University Institutional Animal Care and Use Committee (IACUC Protocol no. 20-0308) and conform to the principles and regulations as described in the Editorial by Grundy (Grundy, 2015).
Animal lines and maintenance
The Kir6.1 and SUR2-knockout (SUR2-KO) and Cantú GOF zebrafish were generated previously, as described (Tessadori et al. 2018). In brief, CRISPR-Cas9 genome editing was combined with a short template oligonucleotide to introduce a single nucleotide mutation into abcc9 (c.2969 GC>AA; Z.G983E and c.3176 G>A; Z.C1052Y) and kcnj8 (c.204 G>A; Z.V65M) to mimic the human disease-causing amino acid substitution, H.G989E and H.C1043Y in SUR2 and H.V65M in Kir6.1, respectively. We generated Kir6.2 (kcnj11) knockout fish using CRISPR-Cas9 genome editing. Two Cas9 target sites within the kcnj11 open reading frame were chosen using the UCSC Genome Browser. Alt-R CRISPR crRNA molecules (IDT DNA) targeting the sites CGAACAGGGACGGTTTCTAC and GAGTGGATGTCCGTTACGCA within the first third of the open reading frame of kcnj11 were produced and independently combined with Alt-R CRISPR tracRNA (IDT DNA) to generate functional gRNA molecules. Two nanolitres of solution containing both gRNA molecules (final concentration 25 pg/nl) and Cas9 protein (final concentration 322 pg/nl) were co-injected into one-cell stage zebrafish embryos of the wild type strain AB. Injected embryos were grown to adulthood to generate ‘founder’ fish (F0) and screened for large deletions by PCR across the kcnj11 locus with primers GTTGGCGGAGGATGTGTTAC and CAGTTATCACCGCGTGTTTG. Sequencing analysis confirmed a deletion of 129 bp, including portions of the pore-forming domain of the channel. The founder was outcrossed to wild-type AB to generate heterozygous carrier (F1) fish. Inbreeding of F1 fish generated homozygous (F2) fish, which were used for experiments.
SUR1 knockout fish were generated using ENU-mutagenesis at the Sanger Institute, as part of the Zebrafish Mutation Project, using N-ethyl-N-nitrosourea (ENU) mutagenesis to identify knockout alleles for all protein-coding regions in the zebrafish genome (https://www.sanger.ac.uk/resources/zebrafish/zmp/). This project outcrosses ENU-mutagenized F0 males to create a population of F1 fish heterozygous for ENU-induced mutations, which were then obtained through the Zebrafish International Research Consortium (ZIRC). The abcc8(sa15863) nonsense mutant allele (K499-STOP, TT CTGGCTCCRGTGCAGTACTTTGTGGCAACCAAGT TATCAGATGCACAG[A>T]AAAGCACATTGGTGAG CTACTTTATTTTGGTTAATGTCCTAATGAGGCCA) generated K499-STOP mutants that were in-crossed as heterozygotes to generate homozygous progeny, genotyped by Transnetyx using restriction digest with the inserted digestion site for HpyCHRIII which is inserted into the mutant allele (forward primer: TTGTTGTTGTCTGCTTTTTGC; reverse primer: TTTACAAGCACAGCGCTCAC) to identify homozygotes.
Fish were maintained in the WU zebrafish facility (http://zebrafishfacility.wustl.edu/documents.html). All procedures were approved by the Washington University in St Louis IACUC.
Reverse-transcriptase PCR
Adult wild-type zebrafish were anaesthetized by transfer into ice water before they were decapitated. After removal of the skin and opening of the pericardial sac, the hearts were harvested. Atrium, ventricle and bulbous arteriosus from 15 fish were pooled separately. RNA was isolated using Trizol (Ambion) and cDNA was prepared using the High-Capacity cDNA Reverse Transcription Kit (Thermo Scientific).
Isolation of zebrafish cardiomyocytes
Modified versions of published protocols (Brette et al. 2008; Louch et al. 2011; Sander et al. 2013) were used to isolate ZF ventricular and atrial cardiomyocytes (VCMs and ACMs). Briefly, fish were killed using cold-shock (8°C water immersion) followed by decapitation. The atria and ventricles were quickly excised, gently torn open using forceps to drain the blood and placed into separate 1.5 ml Eppendorf tubes containing perfusion buffer (PB; 10 mM HEPES, 30 mM taurine, 5.5 mM glucose, 10 mM BDM in PBS) and 5 ng/ml insulin. The PB in the tubes was then replaced by 750 μl of digestion buffer (DB; 5 mg/ml each of Collagenase Type II and Type IV (Worthington), 5 ng/ml insulin, 12.5 μM CaCl2 in PB) each, for four pooled atria and three pooled ventricles per tube. The tissues were digested at 32°C, on a shaking table (800 rpm) for 25 min (for atria) to 40 min (for ventricles). The digestion was ended by replacing the DB with stopping buffer (SB; 10% FBS, 10 mg/ml BSA, 5 ng/ml insulin, 12.5 μM CaCl2 in PB). The DB and SB were prepared freshly before each isolation using a fresh stock (<1 month) of PB. After 15 min of incubation at room temperature, the SB was replaced with PB, in which the tissue was gently triturated using a Pasteur pipette to disperse the cells, which were then viable for physiological measurements at room temperature for up to 12 h.
Isolation of zebrafish vascular smooth muscle cells
The bulbous arteriosus (BA) of teleost fish comprises vascular smooth muscle cells (VSMCs). By crossing with a previously reported Tg(tagln:egfp) smooth muscle cell transgenic reporter line (Liu et al. 2003; Seiler et al. 2010) and adapting existing protocols for isolation of mammalian arterial VSM (Huang et al. 2018), we successfully isolated and identified ZF VSMCs from BAs (Fig. 1A). BAs were excised from 4 ZF and placed in Solution 1 (S1; 0.1% BSA (w/v), 145 NaCl, 4 KCl, 10 HEPES, 10 glucose, 0.05 CaCl2, 1 MgCl2 (mM), adjusted to pH 7.4 using NaOH) on ice. S1 was then replaced by 400 μl Solution 2 (S2; 2 ml S1, 4 mg papain (Worthington), 2 mg DTT (Sigma)). The BA tissue was digested in S2 at 32°C on a shaking table (800 rpm) for 25 min. Following this, S2 was replaced by 500 μl Solution 3 (S3; 2 ml S1, 3 mg Collagenase Type H (Sigma), 2 mg trypsin inhibitor (Worthington), 1 mg elastase (Worthington)) and digested for additional 5 min. Digestion was terminated by replacing S3 with 500 μl S1. The tissue was triturated using Pasteur pipette and isolated cells were plated onto coverslips, then left to attach for at least 1 h before experiments and were used within 8 h.
Figure 1. Zebrafish cardiovascular cell types and KATP channel subunit expression.
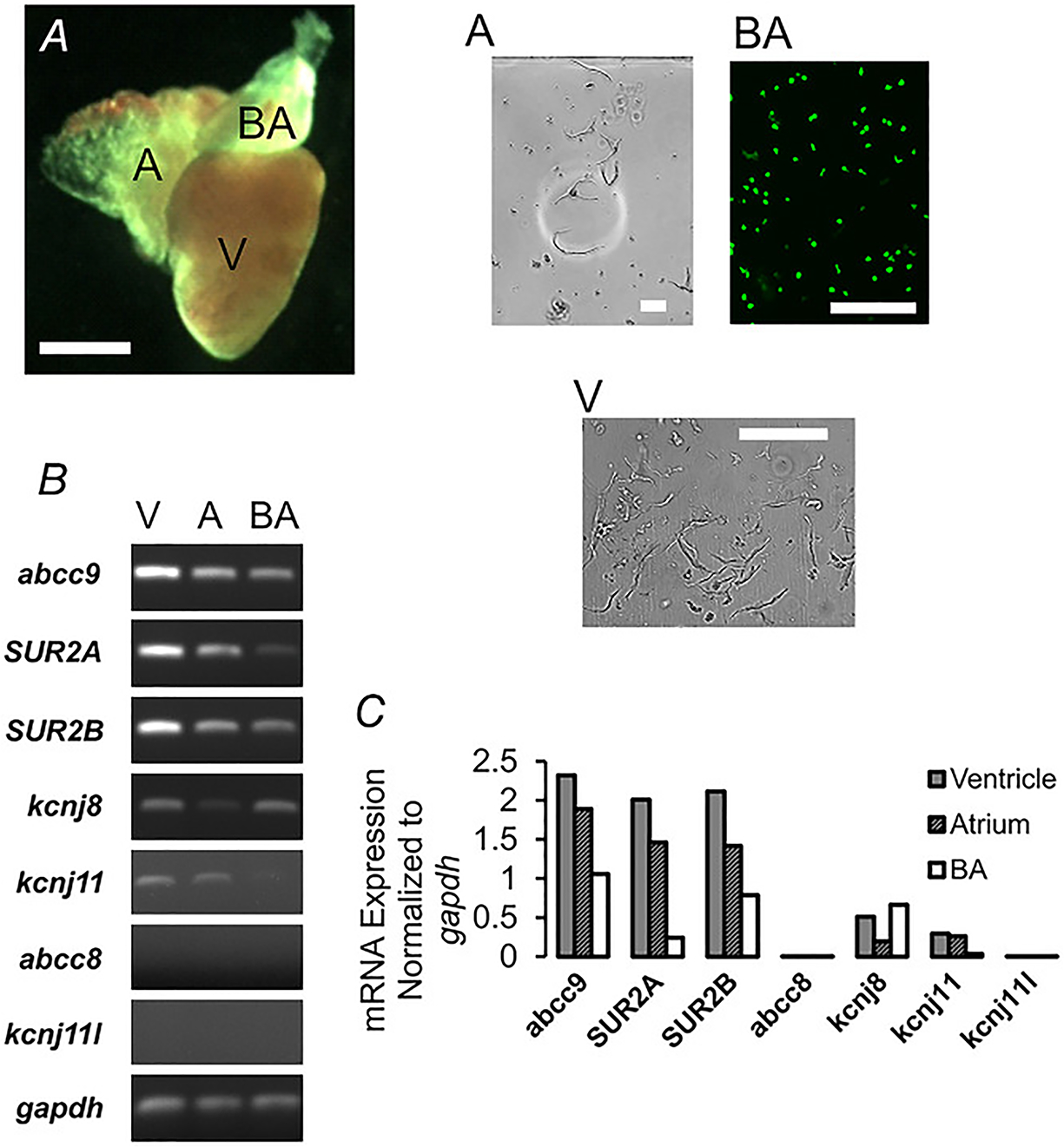
A, photomicrograph (left) of isolated zebrafish heart (green filter lens) reveals atrium (A), ventricle (V) and bulbous arteriosus (BA). Scale bar, 1 mm. Images of isolated cells (right) from each tissue. BA cells expressing GFP are shown under UV illumination. Scale bars, 100 μm. B, RT-PCR analysis of KATP channel subunit expression, including SUR2a and SUR2b isoforms and gapdh. C, quantification of data from B.
Whole-cell voltage-clamp and excised inside-out patch-clamp experiments
After successful isolation of the ZF CMs and VSMCs, inside-out excised patch-clamp experiments were performed to characterize KATP channel expression and activity, using a perfusion chamber equipped with oil gates and piezo-regulated flow control (Cannell & Lederer, 1986; Lederer & Nichols, 1989). The pipette solution for excised patch-clamp of CMs was KINT buffer (140 mM KCl, 10 mM HEPES and 1 mM EGTA at pH 7.4) and the bath solution was the same, with or without additional nucleotides/drugs/Mg2+, added depending on the study. For whole-cell patching, the bath solutions were either high-Na+ (136 NaCl, 6 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES and 10 glucose (mM) at pH 7.4 adjusted with NaOH) or high-K+ (140 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES and 10 glucose (mM) at pH 7.4 adjusted with KOH). The pipette solution for whole-cell patch clamp of VSMCs was internal cell solution (ICS; 110 potassium aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 10 HEPES, 0.5 CaCl2, 4 K2HPO4 and 5 EGTA (mM) at pH 7.2 adjusted with KOH) with or without 1 mM MgUTP or 0.1 mM ATP. The amount of MgCl2 needed to achieve 0.5 mM free Mg2+ concentration was calculated using Webmaxc Standard website (WEBMAXC STANDARD (ucdavis.edu)).
Micropipettes for patch-clamping were pulled from soda-lime glass microhematocrit tubes (Kimble-Chase 2502) using a P-97 puller (Sutter Instruments). Pipette tips were coated with a molten mix of Parafilm in mineral oil to reduce the capacitance and had resistances of 1—2 MΩ (for excised patching) or 3–4 MΩ (for whole-cell) when filled with pipette solution.
A characteristic feature of KATP channels is rapid inhibition by intracellular ATP. To measure KATP channel activity and sensitivity to ATP via inside-out excised patching, the patch was exposed to different concentrations of MgATP (0 mM, 5 mM, 10 μM and 100 μM). In the case of whole-cell patching, the cells were patched in high-Na+, mimicking the physiological extracellular solution, before switching the bath solution to high-K+ based buffers to shift the Nernst potential. Excised-patch membrane currents were recorded at a constant membrane potential of −50 mV and whole-cell currents at −70 mV, using an Axopatch-1D amplifier and Axon pCLAMP software (Molecular Devices, LLC). Experiments were performed at room temperature. A minimum of three recordings in each condition was obtained for statistical analysis. Excised-patch channel currents in solutions of varying nucleotide concentrations were normalized to the basal currents in the absence of nucleotides for respective recordings and the dose-response curve was plotted using a four-parameter Hill fit according to the equation:
| (1) |
where Imax is the maximum current (in zero ATP); Imin is the minimum current (in high, 5 mM ATP); [X] refers to the concentration of nucleotides/drugs; IC50 is the concentration of half-maximal inhibition; and H denotes the Hill coefficient.
Data analyses
All statistical analyses were performed using Microsoft Excel with the Real Statistics Resource Pack add-in (www.real-statistics.com). Significance values were calculated using the Kruskal-Wallis test and a subsequent post hoc Dunn’s test for pairwise comparison. The Mann-Whitney U test (for two independent samples) was used to compare two groups, with the Dunn/Sidak correction used when making multiple comparisons. All values are expressed as means ± SD and P values are provided in the figures.
Results
Zebrafish cardiac and vascular muscle express multiple KATP subunit transcripts
We performed PCR on cDNA generated from RNA isolated from the zebrafish CV system, to characterize Kir6 and SUR subunit expression in the zebrafish cardiac ventricle and atrium and from the bulbous arteriosus (Fig. 1A). SUR2A, SUR2B (abcc9), Kir6.1 (kcnj8) and Kir6.2 (kcnj11) were detected but not SUR1 (abcc8) or Kir6.3 (kcnj11l) (Fig. 1B). Notably, only Kir6.1 and SUR2B were detected in the bulbous arteriosus (Fig. 1C), which consists primarily of vascular smooth muscle cells (VSMCs), consistent with Kir6.1/SUR2B complexes forming vascular smooth muscle KATP channels, as in mammals. The additional presence of Kir6.2 and SUR2A expression in the heart (Fig. 1C) is consistent with cardiomyocyte KATP being formed of these subunits as in mammals, but the heart is innervated and permeated by capillaries and hence the presence of Kir6.1 and SUR2B transcripts in ventricle and atrium may reflect the presence of these other cell types.
Zebrafish cardiac muscle myocytes express functional KATP channels
As shown in Fig. 2A, both ventricular and atrial myocytes express robust ATP-sensitive potassium currents, with similar sensitivity to ATP in the absence or presence of Mg2+ (Fig. 2B), but at slightly higher density and lower sensitivity in the atrium (Fig. 2B). Similar to mammalian KATP channels, ADP in the presence of Mg2+ markedly activated atrial and ventricular KATP to a similar extent (Fig. 2C). As shown by ion replacement of K+ by Na+ (Fig. 3), the channels are highly K+ selective in both the atrium and ventricle, with single-channel conductance of ~80 pS in 140 mM KCl on either side of the membrane (Fig. 3), essentially the same as seen in mammalian myocytes (Babenko et al. 1998b).
Figure 2. KATP channel current in ventricular and atrial myocytes.
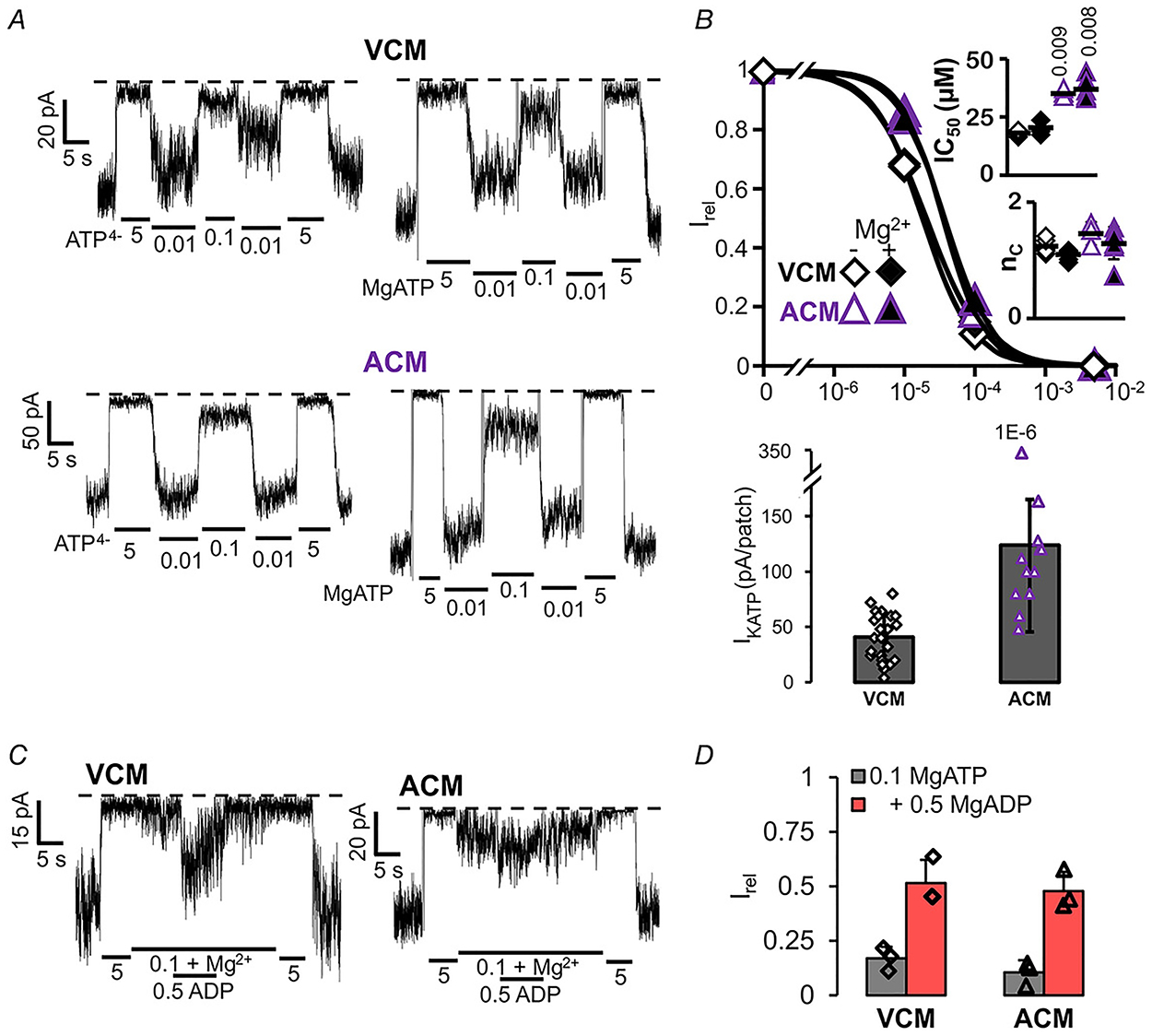
A, representative inside-out patch-clamp recordings from ventricular (VCM, above) and atrial (ACM, below) cardiac myocytes in the presence of differing [ATP], with (right) or without (left) Mg2+ ions. B, [ATP] dependence of channel activity (Irel) for VCM and ACM, from recordings as in A (n = 3–7 recordings from n = 3 preparations (three animals per preparation) in each case). Upper panel, dose-response relationships were fitted with Hill plots (eqn (1)). Graph shows fit to averaged data; inset shows individual data, mean and SD for fits to individual recordings. Lower panel, measured KATP density (current in zero ATP). C, representative inside-out patch-clamp recordings from VCM and ACM, in the presence of differing [ATP] and [ADP], with Mg2+ ions. D, currents in 0.1 mM ATP, with or without ADP, relative to current in zero ATP (Irel). Graph shows individual data, mean and SD.
Figure 3. Single channel KATP currents in ventricular and atrial myocytes.
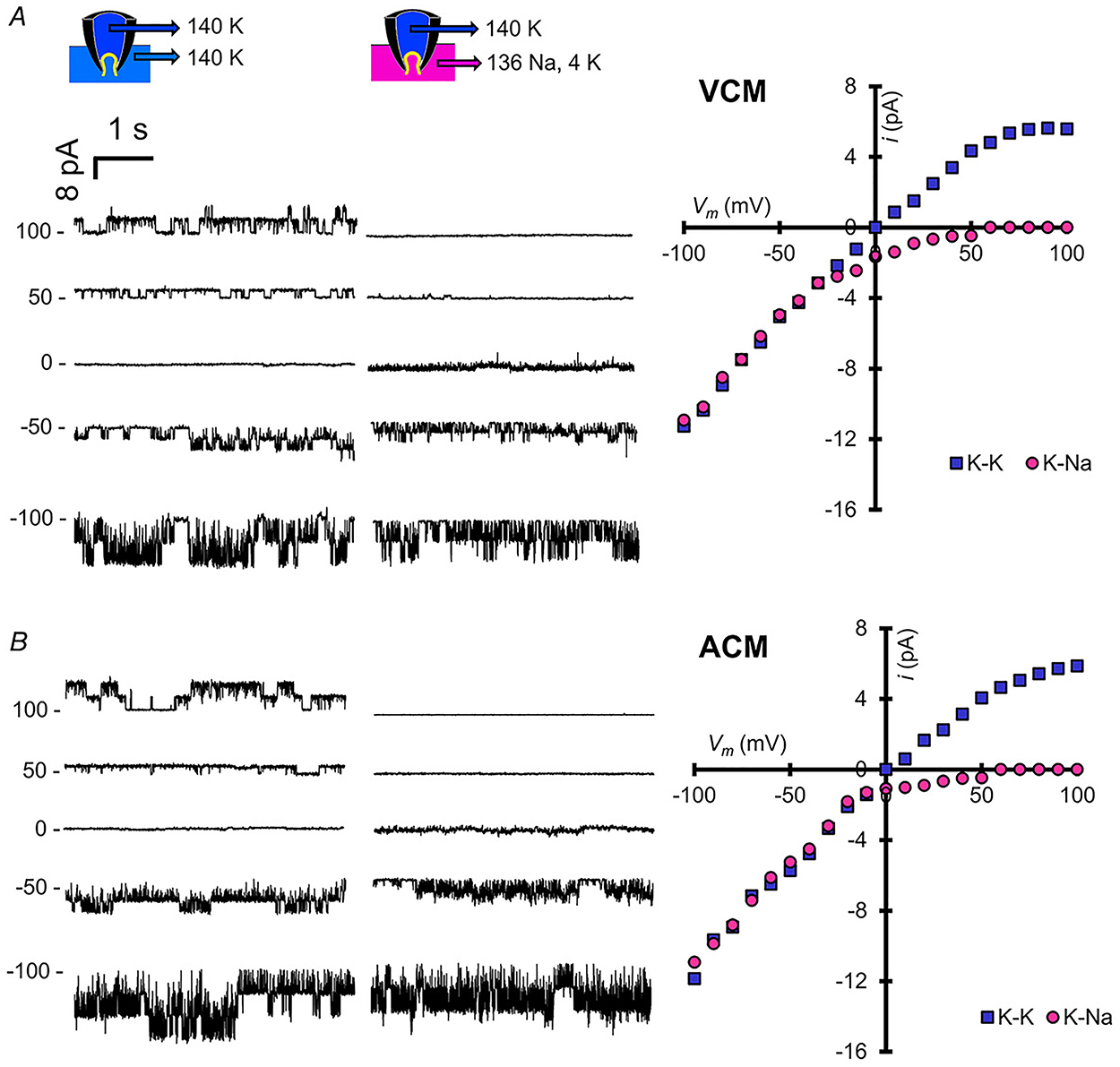
A and B, left: representative inside-out current recordings from VCM (A) and ACM (B) in 140 mM K on both sides of the membrane and after replacement of cytoplasmic solution with 4 mM K+/136 mM Na+. Markers indicate zero current. Right: single channel I-V relationships from each condition.
Ventricular and atrial KATP channels are generated by Kir6.2 and SUR2 subunits
To define the subunit composition of the functional KATP channels in each of the different tissues, we characterized currents from multiple genetically modified fish. We have previously generated zebrafish knockouts for each of SUR2 (abcc9) and Kir6.1 (kcnj8) (Tessadori et al. 2018) and have now acquired or generated knockouts for SUR1 (abcc8) and Kir6.2 (kcnj11) (see Methods). Both atrial and ventricular KATP currents were indistinguishable from control, in terms of ATP sensitivity in SUR1 and Kir6.1 knockouts, although the density was slightly lower in SUR1 knockouts, but currents were abolished in SUR2 and Kir6.2 knockouts (Figs 4A–C and 5A–C). These data confirm that SUR2 and Kir6.2 are essential components of functional KATP channels in both chambers and that neither SUR1 nor Kir6.1 contribute significantly. SUR2[G898E], SUR2[C1043Y] and Kir6.1[V65M] mutations are associated with CS (Grange et al. 2019) and have been shown to cause GOF in recombinant channels (Cooper et al. 2015, 2017; McClenaghan et al. 2018). Strikingly and essentially confirming the contribution of SUR2 but not Kir6.1 to active cardiac channels, ATP sensitivity was also markedly reduced in patches from SUR2[G898E] and SUR2[C1043Y], but not, Kir6.1[V65M], myocytes from both ventricle (Fig. 4B and D) and atrium (Fig. 5B and D).
Figure 4. Subunit dependence of KATP channels in ventricular myocytes.
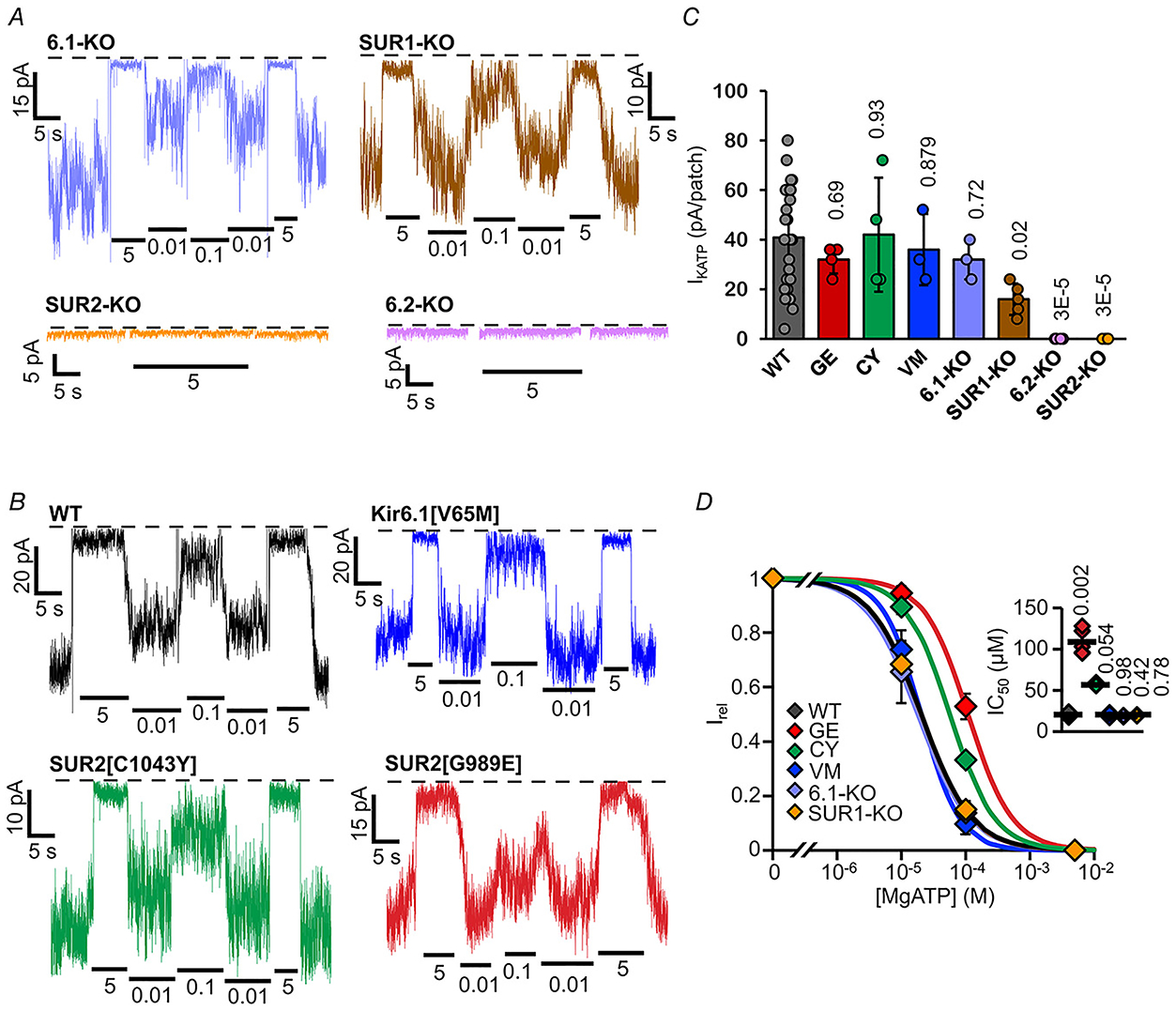
A and B, representative inside-out patch-clamp recordings from VCM in the presence of differing [ATP], as in Fig. 2A, from KATP subunit knockout fish (A) and Cantú mutant fish (B). with (right) or without (left) Mg2+ ions. C, measured KATP density (current in zero ATP) from individual experiments as in A and B (n = 3–24 recordings from 1–3 preparations (three animals per preparation), in each case). D, [ATP] dependence of channel activity (Irel) from recordings as in A,B. (above) Dose-response relationships were fitted with Hill plots (eqn (1)). Graph shows fit to averaged data; inset shows individual data, mean and SD for fits to individual recordings.
Figure 5. Subunit dependence of KATP channels in atrial myocytes.
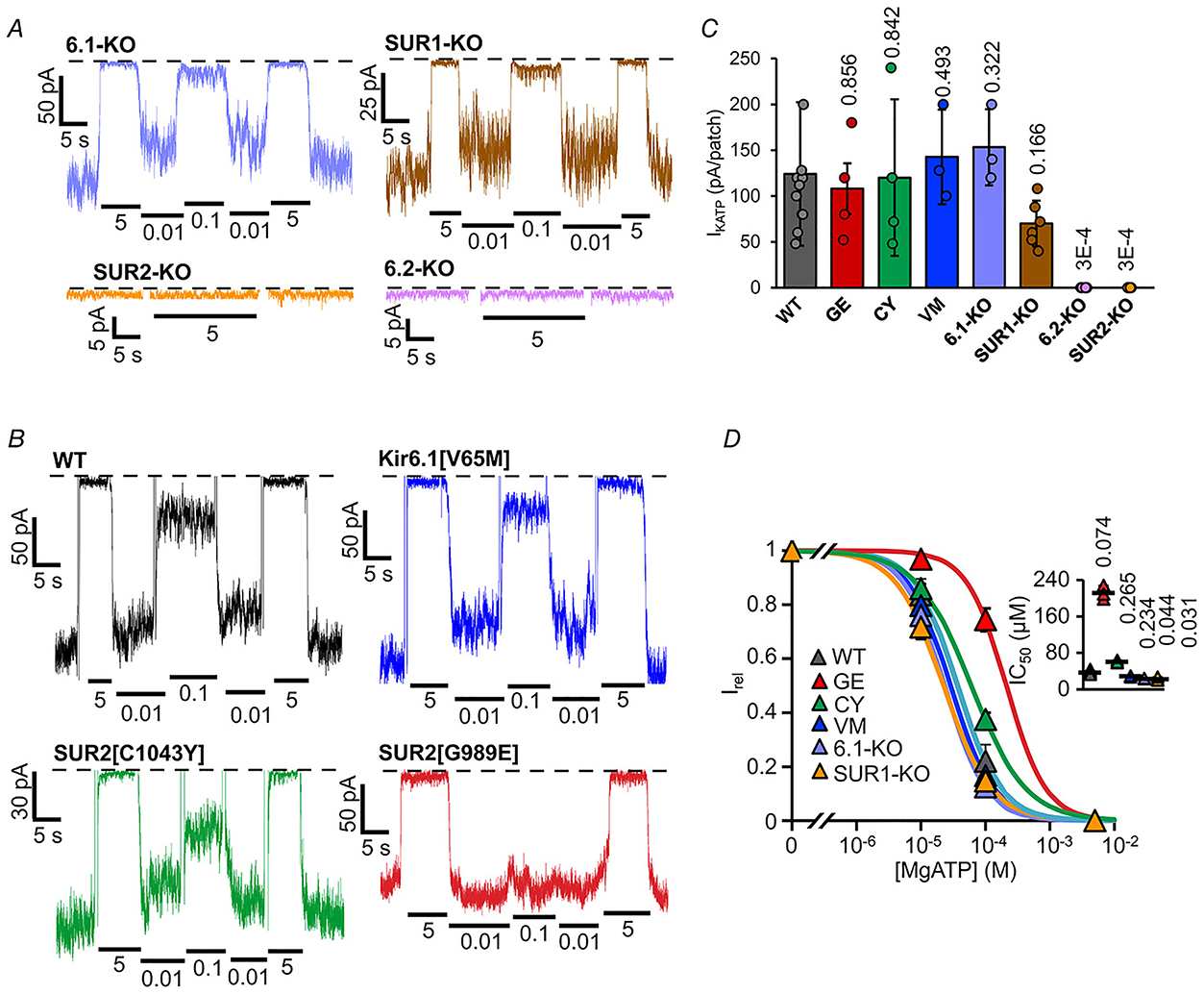
A and B, representative inside-out patch-clamp recordings from ACM in the presence of differing [ATP], as in Fig. 2A, from KATP subunit knockout fish (A) and Cantú mutant fish (B). with (right) or without (left) Mg2+ ions. C, measured KATP density (current in zero ATP) from individual experiments as in A and B (n = 3–12 recordings, from 1–3 preparations (four animals per preparation), in each case). D, [ATP] dependence of channel activity (Irel) from recordings as in A and B. (above) Dose-response relationships were fitted with Hill plots (eqn (1)). Graph shows fit to averaged data; inset shows individual data, mean and SD for fits to individual recordings.
Vascular smooth muscle KATP channels are generated by Kir6.1 and SUR2 subunits
We were not able to detect KATP channels in excised membrane patches from bulbous arteriosus (BA, not shown). However, in whole-cell voltage-clamp recordings, measurable K conductance was present with zero ATP in the pipette but absent when 100 μM ATP was included (Fig. 6A), similar to what is seen in mammalian vascular smooth muscle (Li et al. 2013; Huang et al. 2018). In marked contrast to cardiac myocytes, KATP currents were absent in Kir6.1 knockout vascular smooth muscle myocytes (Fig. 6B). In addition, ATP-sensitive K+ conductance was markedly increased in vascular smooth muscle myocytes from heterozygous Cantú SUR2[G898E] and Kir6.1[V65M] (Fig. 6B) and dramatically so in homozygous Kir6.1[V65M] (Fig. 6B) further confirming that VSM KATP channels are formed of SUR2 and Kir6.1 subunits, as in mammalian smooth muscle (Huang et al. 2018).
Figure 6. Subunit dependence of KATP channels in bulbous arteriosus myocytes.
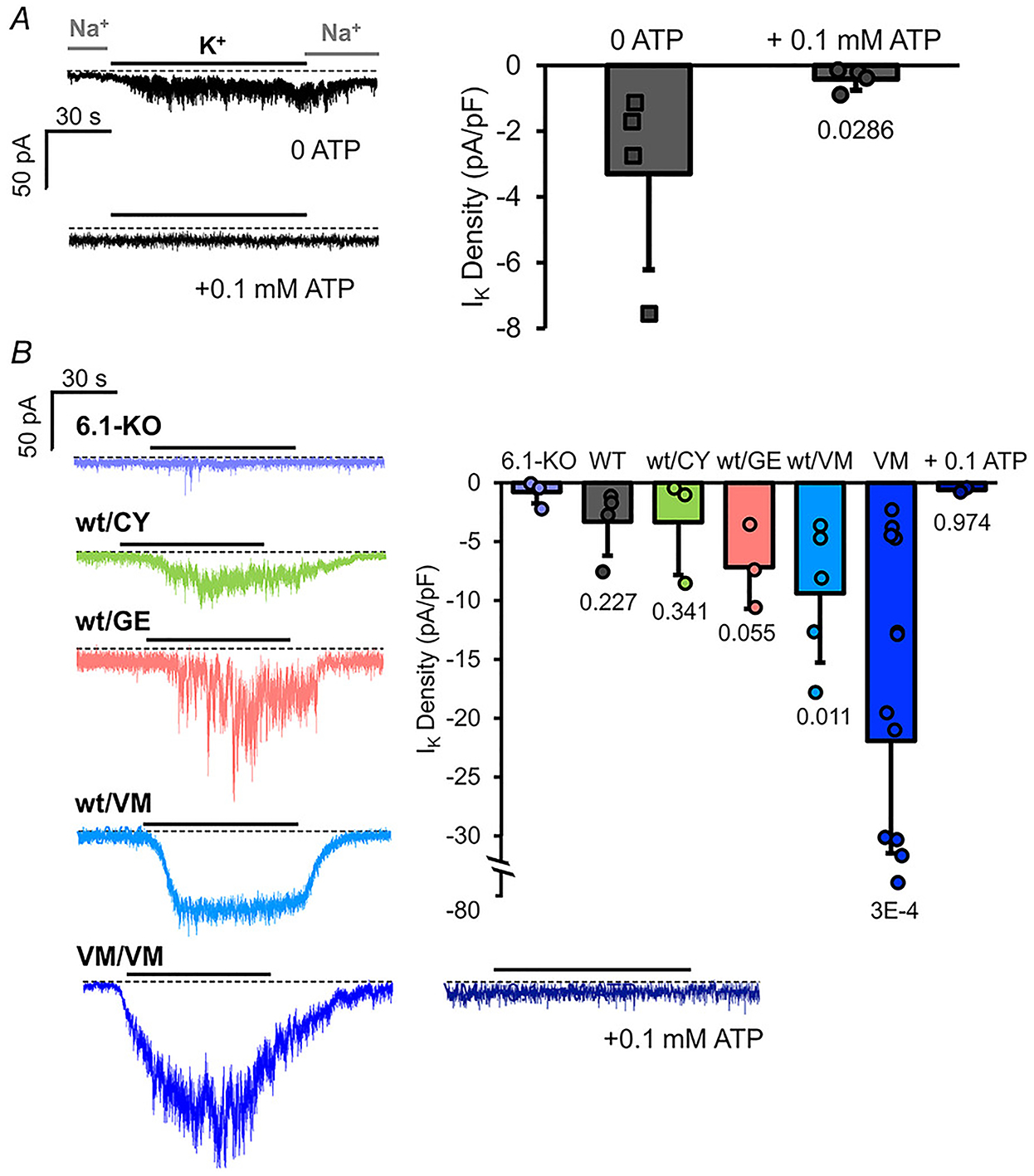
A, left: representative whole-cell voltage-clamp recordings from WT BA smooth muscle myocytes with zero 0.1 mM ATP in the recording pipette. Currents were recorded with 136 mM Na+/6 mM K+ (Na+) in the bath or 0 Na+/140 K+ (K+) as indicated. Right: measured K current density (current in K+ – current in Na+) from individual experiments as at left (n = 4 recordings from 2 preparations (five animals per preparation), in each case). B, left: representative whole-cell voltage-clamp recordings from Kir6.1 knockout and hetero- or homozygous Cantú mutant zebrafish as indicated. Right: measured K+ current density (current in K+ – current in Na+) from individual experiments as at left (n = 3–10 recordings from 1–3 preparations (five animals per preparation), in each case).
Zebrafish cardiac and vascular smooth muscle KATP channels are insensitive to K channel openers
Mammalian KATP channels are activated by a wide range of chemical K+ channel openers (KCOs), including pinacidil, minoxidil and diazoxide (Flagg et al. 2010). There are indirect indications that fish KATP channels may lack KCO sensitivity (Paajanen & Vornanen, 2002), but this has not been studied directly. As shown in Fig. 7A, zebrafish cardiac myocytes in both atrium and ventricle show no response to 100 μM of pinacidil or minoxidil, under conditions that cause marked activation of mouse cardiac KATP channels. Although diazoxide causes activation of SUR1-containing KATP channels from zebrafish pancreatic β-cells (Fig. 7A), diazoxide also has no effect on cardiac KATP, further arguing that SUR1 is not a significant component of the latter. Furthermore, while pinacidil causes marked activation of whole-cell KATP currents in mouse vascular smooth muscle myocytes, again there is no activation of zebrafish bulbous arteriosus channels (Fig. 7C).
Figure 7. K+ channel opener insensitivity in zebrafish cardiovascular myocytes.
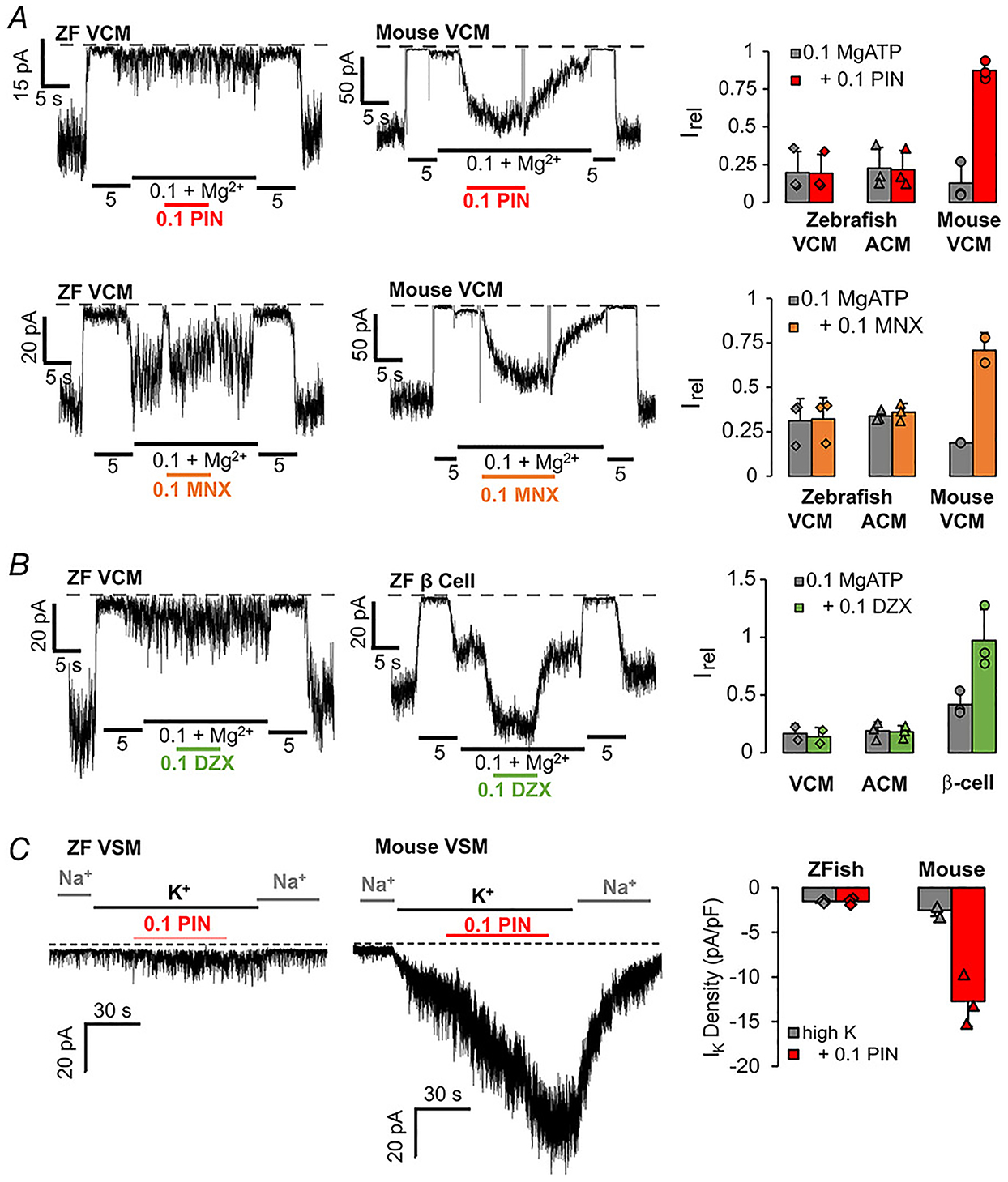
A, left: representative inside-out patch-clamp recordings from zebrafish and mouse VCM, as indicated, in the presence of differing [ATP], with or without pinacidil (0.1 mM, PIN) or minoxidil (0.1 mM MNX). Right: currents in 0.1 mM ATP with and without drug (Irel). Graph shows individual data, mean and SD (n = 3, in each case). B, left: representative inside-out patch-clamp recordings from zebrafish VCM and pancreatic β-cell, as indicated, in the presence of differing [ATP], with or without diazoxide (0.1 mM, DZX). Right: currents in 0.1 mM ATP with and without DZX (Irel). Graph shows individual data, mean and SD (n = 3 recordings from 1 preparation each), in each case). C, left: representative whole-cell voltage-clamp recordings from zebrafish BA (VSM) and mouse aortic VSM, as indicated, with or without addition of pinacidil (0.1 mM, PIN). Right: K+ current density in high K+ solution, with and without PIN. Graph shows individual data, mean and SD (n = 3 recordings from 1 preparation in each case).
Discussion
Structure and functional properties of cardiovascular KATP channels are conserved between zebrafish and mammals
Mammalian KATP channels are formed by several combinations of Kir6.1/2 and SUR1/2 subunits (Nichols, 2006). KATP subunit orthologues are found in all vertebrate genomes, but few studies have investigated the structural or functional properties of KATP channels in non-mammalian vertebrates, including fish. Emfinger et al. (2017) demonstrated that zebrafish islet β-cells express functional KATP channels of similar subunit composition, structure and metabolic sensitivity to their mammalian counterparts (Emfinger et al. 2017). Using PCR on cDNA isolated from various cardiovascular tissues, we show that zebrafish cardiac (CM) and vascular smooth muscle (VSM) cells express KATP channels with very similar composition to their mammalian counterparts, with Kir6.1/SUR2B in VSM and Kir6.2/SUR2A in cardiomyocytes (Figs 1–6). This makeup in functional KATP channels is supported by electrophysiological studies on isolated myocytes, with the exception that we are unable to distinguish SUR2A and SUR2B dependence of functional channels. Although the activity of SUR2A and SUR2B are readily differentiated in mammals via KCO pharmacology, as SUR2B, but not SUR2A, is activatable by diazoxide (Babenko et al. 1998a), this is not possible in the fish myocytes, given the lack of KCO activation in both cardiac and vascular KATP (see below).
Excised-patch-clamp techniques confirm that zebrafish ventricular (VCM) and atrial (ACM) myocyte sarcolemmal KATP channel properties are essentially indistinguishable from those in mammalian ventricles, with two significant exceptions. Firstly, although the prominent SUR subunit in rodent cardiac myocytes is SUR2 in the ventricles, but SUR1 in the atria (Flagg et al. 2007; Glukhov et al. 2010), both atrial and ventricular channels are SUR2-dependent in the fish (Figs 4 and 5). In this regard, it is notable that in humans there is evidence for both subunits in both atrial and ventricles (Fedorov et al. 2011). In addition, although KATP was still present in SUR1 knockout CM, there was a non-significant ~50% reduction of current density in both chambers (Figs 4 and 5). Secondly, while ZF VCM and ACM channels are inhibited by ATP and MgATP, with similar sensitivity to those in mammalian myocytes, both are insensitive to the KATP channel openers (KCOs) pinacidil and minoxidil (Fig. 7). Although this has not been directly demonstrated previously, these data are consistent with prior findings that these KCOs are ineffective at eliciting KATP conductance in whole-cell patch clamp on myocytes from other teleost fish myocytes (Paajanen & Vornanen, 2002), suggesting that KCO insensitivity may be common to SUR2-dependent channels in all fish. Prior studies identified L1249 and T1253 as key residues conferring opener sensitivity in rat SUR2a (Uhde et al. 1999; Moreau et al. 2000) and mutation of residue M1290 in hamster SUR1 to Thr (equivalent to residue 1253 in SUR2a) renders it fully activated by the other KCOs (Moreau et al. 2000). Alignment of the zebrafish and rat SUR2A sequence shows that the equivalent Leu and Thr residues are already present in ZF. Thus, it appears that additional unidentified residues may be involved in KCO binding.
Zebrafish as a model organism for studying cardiovascular KATP pathophysiology
Recent studies have shown that GOF and LOF in KCNJ8 (Kir6.1) and ABCC9 (SUR2) underlie human Cantú syndrome and ABCC9-related Intellectual Disability and Myopathy syndrome (AIMS), respectively (van Bon et al. 2012; Harakalova et al. 2012b; Smeland et al. 2019). In previous studies, we have shown that zebrafish carrying disease-causing mutations in the equivalent kcnj8 and abcc9 loci reiterate essential features of these syndromes (Tessadori et al. 2018; Smeland et al. 2019) and here we have shown that each of these CS mutations causes increased VSM KATP conductance, but cardiac channel properties are only affected in the SUR2 mutants. In mammals and in zebrafish, the cardiac and vascular Cantú syndrome phenotypes are similar for both the KCNJ8 (kcnj8) and ABCC9 (abcc9) mutations at the organismal level (Brownstein et al. 2013; Cooper et al. 2014). This has been explained in mouse studies by the finding that cardiac pathology is a secondary response to decreased vascular resistance (Huang et al. 2018; McClenaghan et al. 2020a,b). Our finding that the molecular effects of SUR2 mutations are present in CM and VSM, whereas those of Kir6.1 mutants are only present in VSM is important, suggesting that cardiac enlargement in each disease model arises from the same compensatory mechanisms in the fish as in the mouse. Confirming that the subunit composition of cardiac and vascular myocyte KATP channels is essentially identical to the mammalian counterparts in each case is thus an essential validation of the use of zebrafish as a model organism for studying the pathophysiological consequences of Cantú syndrome and of KATP activity changes in general.
Supplementary Material
Key points.
Zebrafish cardiac myocytes (CM) and vascular smooth muscle (VSM) express functional KATP channels of similar subunit composition, structure and metabolic sensitivity to their mammalian counterparts.
In contrast to mammalian cardiovascular KATP channels, zebrafish channels are insensitive to potassium channel opener drugs (pinacidil, minoxidil) in both chambers of the heart and in VSM.
We provide a first characterization of the molecular properties of fish KATP channels and validate the use of such genetically modified fish as models of human Cantú syndrome and ABCC9-related Intellectual Disability and Myopathy syndrome.
Acknowledgements
The authors would like to acknowledge the excellent technical assistance of the Washington University in St Louis Zebrafish Facility (http://zebrafishfacility.wustl.edu/).
Funding
These studies were supported by R01 grant HL140024 from the NIH (to C.G.N.), K99 grant HL150277 (to C.M.C.) and the E-Rare Joint Transnational Cantú Treat program I-2101-B26 (to G.v.H.).
Biography

Soma S. Singareddy is a PhD student in the Biomedical Engineering Program at Washington University in St Louis. His thesis work is focused on understanding the molecular make-up of cardiovascular KATP channels in zebrafish and their role in a fish model of Cantú syndrome, using electrophysiological and other techniques.
Footnotes
The peer review history is available in the Supporting Information section of this article (https://doi.org/10.1113/JP282157#support-information-section).
Competing interests
The authors declare no conflicts of interest.
Supporting information
Additional supporting information can be found online in the Supporting Information section at the end of the HTML view of the article. Supporting information files available:
Data availability statement
All original data has been uploaded to the following repositories:
https://osf.io/a83b7. and will be made available to interested parties on request.
References
- Babenko AP, Aguilar-Bryan L & Bryan J (1998a). A view of sur/KIR6.X, KATP channels. Annu Rev Physiol 60, 667–687. [DOI] [PubMed] [Google Scholar]
- Babenko AP, Gonzalez G, Aguilar-Bryan L & Bryan J (1998b). Reconstituted human cardiac KATP channels: functional identity with the native channels from the sarcolemma of human ventricular cells. Circ Res 83, 1132–1143. [DOI] [PubMed] [Google Scholar]
- Barzegar H, Azizi MH, Barzegar M & Hamidi-Esfahani Z (2014). Effect of potassium sorbate on antimicrobial and physical properties of starch-clay nanocomposite films. Carbohydr Polym 110, 26–31. [DOI] [PubMed] [Google Scholar]
- Brette F, Luxan G, Cros C, Dixey H, Wilson C & Shiels HA (2008). Characterization of isolated ventricular myocytes from adult zebrafish (Danio rerio). Biochem Biophys Res Commun 374, 143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein CA, Towne MC, Luquette LJ, Harris DJ, Marinakis NS, Meinecke P, Kutsche K, Campeau PM, Yu TW, Margulies DM, Agrawal PB & Beggs AH (2013). Mutation of KCNJ8 in a patient with Cantu syndrome with unique vascular abnormalities - support for the role of KATP channels in this condition. Eur J Med Genet 56, 678–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell MB & Lederer WJ (1986). A novel experimental chamber for single-cell voltage-clamp and patch-clamp applications with low electrical noise and excellent temperature and flow control. Pflugers Arch 406, 536–539. [DOI] [PubMed] [Google Scholar]
- Cole WC, McPherson CD & Sontag D (1991). ATP-regulated K+ channels protect the myocardium against ischemia/reperfusion damage. Circ Res 69, 571–581. [DOI] [PubMed] [Google Scholar]
- Cooper PE, McClenaghan C, Chen X, Stary-Weinzinger A & Nichols CG (2017). Conserved functional consequences of disease-associated mutations in the slide helix of Kir6.1 and Kir6.2 subunits of the ATP-sensitive potassium channel. J Biol Chem 292, 17387–17398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PE, Reutter H, Woelfle J, Engels H, Grange DK, van Haaften G, van Bon BW, Hoischen A & Nichols CG (2014). Cantu syndrome resulting from activating mutation in the KCNJ8 gene. Hum Mutat 35, 809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PE, Sala-Rabanal M, Lee SJ & Nichols CG (2015). Differential mechanisms of Cantu syndrome-associated gain of function mutations in the ABCC9 (SUR2) subunit of the KATP channel. J Gen Physiol 146, 527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emfinger CH, Welscher A, Yan Z, Wang Y, Conway H, Moss JB, Moss LG, Remedi MS & Nichols CG (2017). Expression and function of ATP-dependent potassium channels in zebrafish islet beta-cells. R Soc Open Sci 4, 160808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov VV, Glukhov AV, Ambrosi CM, Kostecki G, Chang R, Janks D, Schuessler RB, Moazami N, Nichols CG & Efimov IR (2011). Effects of KATP channel openers diazoxide and pinacidil in coronary-perfused atria and ventricles from failing and non-failing human hearts. J Mol Cell Cardiol 51, 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagg TP, Enkvetchakul D, Koster JC & Nichols CG (2010). Muscle KATP channels: recent insights to energy sensing and myoprotection. Physiol Rev 90, 799–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagg TP, Kurata HT, Masia R, Caputa G, Magnuson MA, Lefer DJ, Coetzee WA & Nichols CG (2008). Differential structure of atrial and ventricular K ATP. Circ Res 103, 1458–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhov AV, Flagg TP, Fedorov VV, Efimov IR & Nichols CG (2010). Differential KATP channel pharmacology in intact mouse heart. J Mol Cell Cardiol 48, 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange DK, Roessler HI, McClenaghan C, Duran K, Shields K, Remedi MS, Knoers N, Lee JM, Kirk EP, Scurr I, Smithson SF, Singh GK, van Haelst MM, Nichols CG & van Haaften G (2019). Cantu syndrome: findings from 74 patients in the International Cantu Syndrome Registry. Am J Med Genet C Semin Med Genet 181, 658–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harakalova M, van Harssel JJ, Terhal PA, van Lieshout S, Duran K, Renkens I, Amor DJ, Wilson LC, Kirk EP, Turner CL, Shears D, Garcia-Minaur S, Lees MM, Ross A, Venselaar H, Vriend G, Takanari H, Rook MB, van der Heyden MA, Asselbergs FW, Breur HM, Swinkels ME, Scurr IJ, Smithson SF, Knoers NV, van der Smagt JJ, Nijman IJ, Kloosterman WP, van Haelst MM, van Haaften G & Cuppen E (2012a). Dominant missense mutations in ABCC9 cause Cantu syndrome. Nat Genet 44, 793–796. [DOI] [PubMed] [Google Scholar]
- Harakalova M, van Harssel JJT, Terhal PA, van Lieshout S, Duran K, Renkens I, Amor DJ, Wilson LC, Kirk EP, Turner CLS, Shears D, Garcia-Minaur S, Lees MM, Ross A, Venselaar H, Vriend G, Takanari H, Rook MB, van der Heyden MAG, Asselbergs FW, Breur HM, Swinkels ME, Scurr IJ, Smithson SF, Knoers NV, van der Smagt JJ, Nijman IJ, Kloosterman WP, van Haelst MM, van Haaften G & Cuppen E (2012b). Dominant missense mutations in ABCC9 cause Cantú syndrome. Nat Genet 44, 793–796. [DOI] [PubMed] [Google Scholar]
- Hassinen M, Haverinen J, Hardy ME, Shiels HA & Vornanen M (2015). Inward rectifier potassium current (I K1) and Kir2 composition of the zebrafish (Danio rerio) heart. Pflugers Archiv 467, 2437–2446. [DOI] [PubMed] [Google Scholar]
- Hodgson P, Ireland J & Grunow B (2018). Fish, the better model in human heart research? Zebrafish Heart aggregates as a 3D spontaneously cardiomyogenic in vitro model system. Prog Biophys Mol Biol 138, 132–141. [DOI] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assuncao JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Threadgold G, Harden G, Ware D, Begum S, Mortimore B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Lloyd C, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Urun Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberlander M, Rudolph-Geiger S, Teucke M, Lanz C, Raddatz G, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Schuster SC, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nusslein-Volhard C, Hubbard TJ, Roest Crollius H, Rogers J & Stemple DL (2013). The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, McClenaghan C, Harter TM, Hinman K, Halabi CM, Matkovich SJ, Zhang H, Brown GS, Mecham RP, England SK, Kovacs A, Remedi MS & Nichols CG (2018). Cardiovascular consequences of KATP overactivity in Cantu syndrome. JCI Insight 3, e121153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer WJ & Nichols CG (1989). Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J Physiol 419, 193–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Knutsen RH, Zhang H, Osei-Owusu P, Moreno-Dominguez A, Harter TM, Uchida K, Remedi MS, Dietrich HH, Bernal-Mizrachi C, Blumer KJ, Mecham RP, Koster JC & Nichols CG (2013). Hypotension due to Kir6.1 gain-of-function in vascular smooth muscle. J Am Heart Assoc 2, e000365–e000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NA, Huang H, Yang Z, Herzog W, Hammerschmidt M, Lin S & Melmed S (2003). Pituitary corticotroph ontogeny and regulation in transgenic zebrafish. Mol Endocrinol 17, 959–966. [DOI] [PubMed] [Google Scholar]
- Louch WE, Sheehan KA & Wolska BM (2011). Methods in cardiomyocyte isolation, culture, and gene transfer. J Mol Cell Cardiol 51, 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClenaghan C, Hanson A, Sala-Rabanal M, Roessler HI, Josifova D, Grange DK, van Haaften G & Nichols CG (2018). Cantu syndrome-associated SUR2 (ABCC9) mutations in distinct structural domains result in KATP channel gain-of-function by differential mechanisms. J Biol Chem 293, 2041–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClenaghan C, Huang Y, Matkovich SJ, Kovacs A, Weinheimer CJ, Perez R, Broekelmann TJ, Harter TM, Lee J, Remedi MS & Nichols CG (2020). The mechanism of high-output cardiac hypertrophy arising from potassium channel gain-of-function in Cantú syndrome. Function 1(1), 10.1093/function/zqaa004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClenaghan C, Huang Y, Yan Z, Harter TM, Halabi CM, Chalk R, Kovacs A, van Haaften G, Remedi MS & Nichols CG (2020b). Glibenclamide reverses cardiovascular abnormalities of Cantu syndrome driven by KATP channel overactivity. J Clin Invest 130, 1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau C (2000). The molecular basis of the specificity of action of KATP channel openers. EMBO J 19, 6644–6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemtsas P, Wettwer E, Christ T, Weidinger G & Ravens U (2010). Adult zebrafish heart as a model for human heart? An electrophysiological study. J Mol Cell Cardiol 48, 161–171. [DOI] [PubMed] [Google Scholar]
- Nichols CG (2006). KATP channels as molecular sensors of cellular metabolism. Nature 440, 470–476. [DOI] [PubMed] [Google Scholar]
- Paajanen V & Vornanen M (2002). The induction of an ATP-sensitive K+ current in cardiac myocytes of air- and water-breathing vertebrates. Pflugers Arch 444, 760–770. [DOI] [PubMed] [Google Scholar]
- Poss KD (2007). Getting to the heart of regeneration in zebrafish. Semin Cell Dev Biol 18, 36–45. [DOI] [PubMed] [Google Scholar]
- Sander V, Sune G, Jopling C, Morera C & Izpisua Belmonte JC (2013). Isolation and in vitro culture of primary cardiomyocytes from adult zebrafish hearts. Nat Protoc 8, 800–809. [DOI] [PubMed] [Google Scholar]
- Seiler C, Abrams J & Pack M (2010). Characterization of zebrafish intestinal smooth muscle development using a novel sm22alpha-b promoter. Dev Dyn 239, 2806–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeland MF, McClenaghan C, Roessler HI, Savelberg S, Hansen GAM, Hjellnes H, Arntzen KA, Muller KI, Dybesland AR, Harter T, Sala-Rabanal M, Emfinger CH, Huang Y, Singareddy SS, Gunn J, Wozniak DF, Kovacs A, Massink M, Tessadori F, Kamel SM, Bakkers J, Remedi MS, Van Ghelue M, Nichols CG & van Haaften G (2019). ABCC9-related intellectual disability myopathy syndrome is a KATP channelopathy with loss-of-function mutations in ABCC9. Nat Commun 10, 4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E & Nakaya H (2002). Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest 109, 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessadori F, Roessler HI, Savelberg SMC, Chocron S, Kamel SM, Duran KJ, van Haelst MM, van Haaften G & Bakkers J (2018). Effective CRISPR/Cas9-based nucleotide editing in zebrafish to model human genetic cardiovascular disorders. Disease Models & Mechanisms 11, dmm035469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker A, Aziz Q & Thomas A (2014). The role of ATP-sensitive potassium channels in cellular function and protection in the cardiovascular system. Br J Pharmacol 171, 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhde I, Toman A, Gross I, Schwanstecher C & Schwanstecher M (1999). Identification of the potassium channel opener site on sulfonylurea receptors. J Biol Chem 274, 28079–28082. [DOI] [PubMed] [Google Scholar]
- van Bon BW, Gilissen C, Grange DK, Hennekam RC, Kayserili H, Engels H, Reutter H, Ostergaard JR, Morava E, Tsiakas K, Isidor B, Le Merrer M, Eser M, Wieskamp N, de Vries P, Steehouwer M, Veltman JA, Robertson SP, Brunner HG, de Vries BB & Hoischen A (2012). Cantu syndrome is caused by mutations in ABCC9. Am J Hum Genet 90, 1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Opbergen CJM, van der Voorn SM, Vos MA, de Boer TP & van Veen TAB (2018). Cardiac Ca2+ signalling in zebrafish: translation of findings to man. Prog Biophys Mol Biol 138, 45–58. [DOI] [PubMed] [Google Scholar]
- Vornanen M & Hassinen M (2016). Zebrafish heart as a model for human cardiac electrophysiology. Channels 10, 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoong S, O’Connell B, Soanes A, Crowhurst MO, Lieschke GJ & Ward AC (2007). Characterization of the zebrafish matrix metalloproteinase 9 gene and its developmental expression pattern. Gene Expr Patterns 7, 39–46. [DOI] [PubMed] [Google Scholar]
- Zhang C, Miki T, Shibasaki T, Yokokura M, Saraya A & Seino S (2006). Identification and characterization of a novel member of the ATP-sensitive K+ channel subunit family, Kir6.3, in zebrafish. Physiol Genomics 24, 290–297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All original data has been uploaded to the following repositories:
https://osf.io/a83b7. and will be made available to interested parties on request.


