Abstract
Staphylococcus aureus and Streptococcus pyogenes express pyrogenic toxin superantigens (PTSAgs) that are associated with toxic shock syndrome (TSS) and staphylococcal food poisoning (SFP). Most PTSAgs cause TSS in deep-tissue infections, whereas only TSS toxin 1 (TSST-1) is associated with menstrual, vaginal TSS. In contrast, SFP has been linked only with staphylococcal enterotoxins (SEs). Because of the differential abilities of PTSAgs to cause systemic or localized symptoms in a site-dependent manner, the present study was undertaken to assess the toxins' abilities to cross mucosal barriers. The activity of three PTSAgs when delivered orally, vaginally, or intravenously to rabbits and orally to monkeys was investigated. TSST-1 induced shock via all three routes in rabbits. Although active when administered intravenously, SEC1 and streptococcal pyrogenic exotoxin A (SPEA) did not cause symptoms when administered orally or vaginally. Only SEC1 induced emesis in the monkey feeding assay. TSST-1, albeit less stable than SEC1 and SPEA to pepsin, induced diarrhea in monkeys. Our results may explain the unique association of TSST-1 with menstrual TSS and why SPEA is only rarely associated with TSS after pharyngitis, despite being highly associated with TSS after subcutaneous infections. Finally, our studies indicate that enterotoxicity in SFP is not the result of superantigenicity.
Coagulase-positive staphylococci and Streptococcus pyogenes express a family of pyrogenic toxin superantigens (PTSAgs) (6) responsible for several human and animal diseases. The toxins in this family include several staphylococcal enterotoxin (SE) variants (SEA, SEB, SEC1, SEC2, SEC3, SEC-ovine, SEC-bovine, SEC-canine, SED, SEE, SEG, SEH, and SEI) (5, 6, 14), toxic shock syndrome toxin 1 (TSST-1) (3, 30), TSST-ovine (18), streptococcal pyrogenic exotoxins (SPEA, SPEB, SPEC, and SPEF) (5, 6, 24), and streptococcal superantigen (21). The PTSAgs are confirmed virulence factors in several human and animal diseases caused by these two genera. Although the pathogenesis of toxic shock syndrome (TSS) has not been completely elucidated, systemic toxicity in this disease results in part from the immunomodulatory superantigen activity of the toxins (20). The massive T-cell proliferation of the toxins results in excessive release of cytokines which, at abnormally high levels, affects the cardiovascular system, resulting in shock. In addition, a multitude of other organ systems (i.e., renal, hepatic, gastrointestinal, and nervous) are affected, producing less-specific symptoms such as diarrhea, emesis, and reduced kidney and liver function.
Another human illness, staphylococcal food poisoning (SFP), is acquired by ingestion of preformed toxin and has been linked only to the SEs (14). The hallmark symptom, emesis, is often accompanied by diarrhea and abdominal cramping, but the absence of a fever suggests that toxemia is minimal. One other toxin, TSST-1, was originally classified as an enterotoxin variant, SEF (3). However, this activity was later proposed to be the result of contamination with other toxins; since TSST-1 was less stable than SEs in the presence of pepsin and lacks cysteine residues, it was later reclassified (4), and it is now generally accepted not to be a SE.
One major issue that remains to be addressed concerns whether or not there is a difference in the ability of PTSAgs to cross mucosal barriers. This is an issue that could have several important consequences for the pathogenesis of PTSAg-associated diseases. For example, of the PTSAgs, only TSST-1 is associated with TSS during infection or colonization at the mucosal surface (i.e., menstrual TSS). In contrast, many PTSAgs, including TSST-1, SEs, and SPEs, are associated with TSS in cases of deep tissue infection or bacteremia (i.e., nonmenstrual staphylococcal or streptococcal TSS). We undertook the present study to compare the abilities of three staphylococcal and streptococcal PTSAgs to induce local or systemic symptoms when the toxins were delivered intravenously, orally, or vaginally to Dutch Belted rabbits or orally to pigtail monkeys (Macaca nemestrina). Representative toxins included TSST-1, SEC1, and SPEA.
MATERIALS AND METHODS
Purification of toxins.
TSST-1 and SEC1 were purified from Staphylococcus aureus RN4220 or Escherichia coli LE294 carrying tst or sec cloned in plasmids pCE104 (22) or pMIN164 (13), respectively. SPEA was obtained from S. pyogenes strain 594 (23). Bacterial strains were grown in dialyzed beef heart medium completed with buffered glucose supplement (15, 26). Staphylococcal cultures were grown aerobically with agitation; streptococcal cultures were incubated in 7% CO2 with moderate stirring. Recombinant S. aureus RN4220 strains were maintained in the presence of erythromycin selection. Bacterial cultures were precipitated with 4 volumes of cold absolute ethanol and then resuspended in minimal volumes of pyrogen-free water (TSST-1 and SEC1) or pyrogen-free acetate saline buffer, pH 4.5 (SPEA). Cell debris was separated by centrifugation at 10,000 × g for 30 min, and supernatants were dialyzed against pyrogen-free water. Proteins were separated by successive flat-bed isoelectric focusing in pH gradients of 3.5 to 10 and 6 to 8 (TSST-1), 3.5 to 10 and 4 to 6 (SPEA), or 3.5 to 10 and 7 to 9 (SEC1). Fractions containing the toxins, as determined by double-immunodiffusion assays, were pooled, extensively dialyzed against pyrogen-free water, and stored lyophylized until used.
Rabbit model experiments.
The rabbit model described originally by Kim and Watson (15) and Schlievert (26) was used to compare enhancement of endotoxin shock in vivo by TSST-1, SEC1, and SPEA. Each animal (young adult American Dutch Belted; 1.5 to 2.0 kg) was given either an initial intravenous dose (100, 10, 1.0, 0.1, or 0.01 μg/kg) or an oral or vaginal dose (100, 10, 1.0, 0.1, or 0.01 μg/animal) containing a selected toxin dissolved in pyrogen-free phosphate-buffered saline (PBS; pH 7.2). To accomplish the oral dosing, toxins were administered by delivering the solutions in the back of the animal mouths using a syringe. In other experiments, toxins were administered in 0.1-ml volumes into the vaginas of rabbits which had been anesthetized with ketamine (25 mg/kg of body weight; Phoenix Pharmaceuticals, Inc., St. Joseph, Mo.) and xylazine (20 mg/kg; Phoenix Pharmaceuticals, Inc.). In these animals, the toxins were administered through catheters inserted into the rabbit vaginas by way of the vestibule. After 4 h, an intravenous injection of endotoxin from Salmonella enterica serovar Typhimurium was administered in the marginal ear vein at a dose of 10 μg/kg. Mortality was monitored for 48 h.
Emesis assays.
To compare the emetic capability of TSST-1, SEC1, and SPEA, the toxins were administered using modifications of the standard monkey feeding assay for SEs (2). The toxins were dissolved in artificially flavored fruit punch (Lyons-Magnus, Clovis, Calif.) and administered to young adult pigtail monkeys (M. nemestrina) by use of a sterile syringe. Prior to use in experiments, all animals were trained to accept fluid from the syringe.
Proteolytic lability studies.
We compared the general stability of TSST-1, SEC1, and SPEA to degradation in the presence of pepsin as an indication of their potential lability in the gastrointestinal tract. Purified preparations of each (1 mg/ml) were incubated at 25°C with pepsin (500 μg/ml; Sigma Chemical Co., St. Louis, Mo.) for various periods of time in 200 mM sodium acetate buffer (pH 4.5). After incubation, the digestions were terminated by boiling for 5 min in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. The extent of proteolysis was assessed by SDS-PAGE according to standard techniques (17).
RESULTS
In initial experiments the effects of each toxin given intravenously were compared to the effects of identical doses applied to the animals' vaginal mucosa. For either route of application, TSST-1 was extremely toxic to rabbits. The endpoint for TSST-1-induced lethality through potentiation of endotoxin was 0.1 μg by either route, suggesting that TSST-1 efficiently crossed the vaginal mucosa (Table 1). Unlike TSST-1, SEC1 and SPEA were essentially inactive when applied to the vaginal mucosa. Only one death was noted at the highest dose of 100 μg of SEC1; no deaths occurred in animals receiving lower doses applied to the vagina. SEC1 and SPEA were both highly lethal when administered intravenously with an endpoint similar to that for TSST-1.
TABLE 1.
Activity of vaginal, intravenous, or oral PTSAgs in rabbits when coadministered with endotoxin
| Dose of PTSAgsa (μg) | No. of animals killed/total no. of animals testedb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| TSST-1
|
SEC1
|
SPEA
|
|||||||
| Vaginal | i.v. | Oral | Vaginal | i.v. | Oral | Vaginal | i.v. | Oral | |
| 100 | 2/2 | 2/2 | 3/3 | 1/2 | 2/2 | 0/3 | 0/2 | ND | 0/3 |
| 10 | 2/2 | 2/2 | 3/3 | 0/2 | 2/2 | 0/3 | 0/2 | 2/2 | 0/3 |
| 1 | 2/2 | 2/2 | 1/2 | 0/2 | 2/2 | ND | 0/2 | 2/2 | ND |
| 0.1 | 1/2 | 2/2 | 0/2 | ND | 2/2 | ND | ND | 1/2 | ND |
| 0.01 | 0/2 | 0/2 | 0/2 | ND | 0/2 | ND | ND | 0/2 | ND |
Toxins, dissolved in pyrogen-free PBS (pH 7.2), were delivered (i) vaginally into rabbits via catheterization thorugh the vestibule, (ii) intravenously or (iii) orally as indicated, and the animals were monitored for symptoms. After 4 h, a sublethal dose of endotoxin (10 μg/kg, 1/50 50% lethal dose) was administered in the marginal ear vein. Animals were monitored an additional 48 h for symptoms and lethality.
ND, not determined; i.v., intravenous.
To determine whether the effects seen in the rabbit model were unique to the vaginal mucosa, experiments were conducted to evaluate the activity of orally administered PTSAgs followed by endotoxin administered intravenously. The results of these experiments, summarized in Table 1, indicate that TSST-1 crossed the gastric mucosa. The toxin enhanced endotoxin shock and induced lethality when given orally at the two highest doses used of 100 and 10 μg/rabbit. SEC1 and SPEA failed to induce systemic symptoms when administered orally. Although TSST-1 induced systemic toxicity if applied either vaginally or orally, it was less potent via the oral route. This reduced albeit significant activity for orally administered TSST-1 was of interest, since only the SEs have been implicated previously in an illness (SFP) associated with ingestion of toxins.
To investigate potential enterotoxicity due to delivery of PTSAgs to the gastrointestinal mucosa, PTSAgs were compared in a monkey feeding assay (2). This is the preferred model for demonstrating SE-induced emesis and diarrhea, the two hallmark symptoms of human SFP (14). Only TSST-1 and SEC1 exhibited activity when fed to monkeys, although each induced a unique set of symptoms with some overlap (Table 2). The minimal emetic dose for SEC1 in M. nemestrina was 1.0 μg/kg. Two of four animals given an oral bolus of SEC1 at this dose demonstrated emesis which was routinely associated with diarrhea. Although TSST-1 did not induce emesis at any dose given, the majority of animals given an oral dose of 1.0 μg/kg were afflicted with diarrhea within 8 h. To rule out the possibility that residual diarrheagenic activity of TSST-1 was caused by a previously undetected but copurifying SE expressed by S. aureus, two animals were given an equivalent dose of TSST-1 purified from recombinant E. coli harboring and expressing tst. Animals receiving the E. coli-derived toxin reacted identically to those receiving TSST-1 from S. aureus and exhibited diarrhea but not emesis. Although SEC1 and SPEA share nearly 50% amino acid identity (6), monkeys fed extremely high doses of SPEA (250 μg/kg) displayed no observable symptoms.
TABLE 2.
Activity of orally-administered PTSAgs without endotoxin in monkeys
| Dose (μg/kg) | No. of animals with emesis (or diarrhea)/total no. of animals after an oral dose ofa:
|
||
|---|---|---|---|
| TSST-1 | SEC1 | SPEA | |
| 250 | ND | ND | 0/2 (0/2) |
| 100 | ND | ND | 0/2 (0/2) |
| 10 | 0/4 (4/4)b | 2/2 (2/2) | 0/2 (0/2) |
| 1.0 | 0/2 (1/2) | 2/4 (4/4) | ND |
| 0.1 | 0/2 (0/2) | 0/2 (0/2) | ND |
ND, not determined. Numbers in parentheses refer to the total number of animals with diarrhea/total number of animals.
Two sources of recombinant TSST-1 were used for this dose. Two animals received recombinant TSST-1 from S. aureus RN4220 and two additional animals received E. coli-derived recombinant TSST-1. No differences in symptoms were observed.
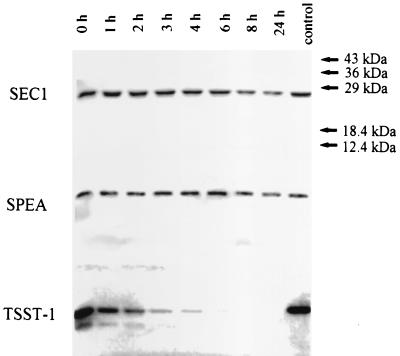
The unique stability of SEs to peptic hydrolysis is correlated with their ability to cause symptoms when orally ingested. Interestingly, SEC1 and SPEA were both resistant to peptic digestion (Fig. 1), suggesting that lability in the gastrointestinal tract is not likely to be responsible for SPEA's lack of enteric activity. Furthermore, SEC1 and SPEA are both potent superantigens and yet differ in emetic ability, providing additional evidence that superantigen activity is not directly involved in the symptoms of SFP. Although TSST-1 was nearly completely degraded by pepsin after 4 h, a small portion resisted degradation, even during prolonged incubation. These observations are consistent with reduced oral activity in rabbits compared to vaginally administered toxin.
FIG. 1.
Susceptibility of TSST-1, SEC1, and SPEA to pepsin as an indication of their potential lability in the gastrointestinal tract. Purified preparations of each (each 1 mg/ml) were incubated (37°C, pH 4.5) with pepsin (500 μg/ml; Sigma). Aliquots were removed at the times indicated, mixed with SDS-PAGE sample buffer, boiled, and resolved by SDS-PAGE (17) to determine the extent of proteolysis.
DISCUSSION
The goal of this study was to compare the biological activities of three representative staphylococcal and streptococcal PTSAgs when intravenously, orally, or vaginally administered. Several significant differences were observed in our comparison of TSST-1, SEC1, and SPEA. First, TSST-1 caused systemic toxicity in rabbits following oral feeding or vaginal exposure. Taken together and consistent with the results of others (8), these results suggest that TSST-1 is unique among these three toxins and is capable of crossing epithelial cell barriers and subsequently inducing systemic toxicity. It is important to note that serum levels of administered toxins were not measured, and thus these conclusions regarding differential abilities to cross mucosal surfaces are only inferred from biological effects seen in the animals. Our data provide an explanation for the association of TSST-1 with TSS following mucosal colonization (i.e., menstrual TSS) and a lack of association of other PTSAgs with this type of staphylococcal infection. S. aureus strains isolated from menstrual vaginal associated cases of TSS nearly always produce TSST-1 either alone or together with SEs, whereas isolates from these patients rarely, if ever, express SEs alone (27). During menstruation, S. aureus strains, including those making TSST-1, grow to relatively high numbers and comprise approximately 60% of the aerobic microflora (29). In contrast, at times other than menstruation, S. aureus comprises less than 1% of the aerobic flora.
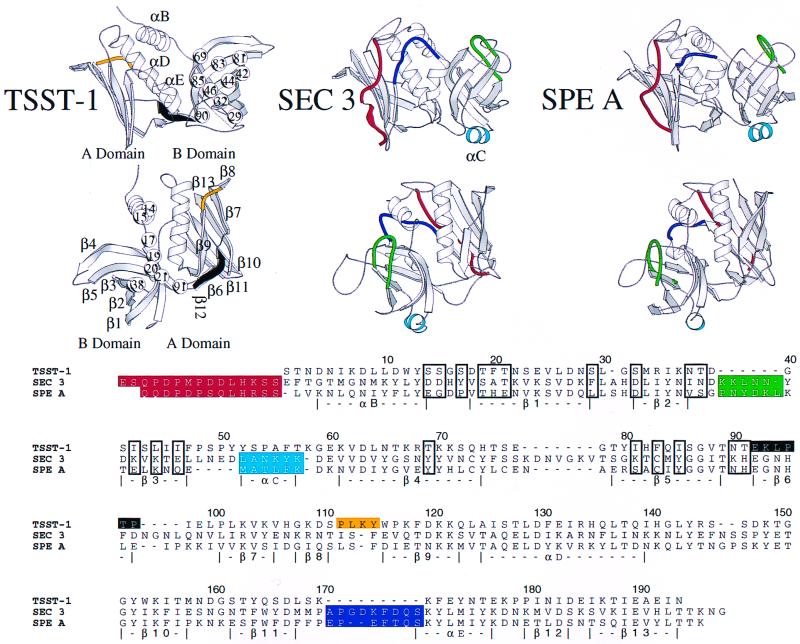
We hypothesize that the reason for the TSST-1 association with menstrual vaginal illness may reside in unique structural features of TSST-1 not shared with SEC1 and SPEA (Fig. 2) which facilitate its ability to cross mucous membranes. Incidentally, Fig. 2 shows the structure of SEC3 rather than the SEC1 that was used in bioassays. The structure of SEC1 has not been determined, but these two toxins share 93% homology and are thus likely to have highly similar structures. The mechanism by which TSST-1 transverses epithelial cells could include either passive diffusion or the involvement of a cellular receptor. The involvement of a receptor specific for TSST-1 is more likely and would presumably require a recognizable surface shape or the presence of large hydrophobic patches on the surface of the protein. The most pronounced structural differences between TSST-1, SEC, and SPEA that affect the shape of the molecular surface include the long amino-terminal extension (red) and presence of α-helix C (cyan) in the latter two toxins (Fig. 2). Also, the flaps surrounding α-helix D in SEC and SPEA are high in the front (navy) and low in the back. Only the rear flap (yellow) covers TSST-1 α-helix D, and experiments have demonstrated that Gln 136 can impact lethality and the conformation of the rear flap (β8-β9) region (22). In the B domain, the secondary structure elements of TSST-1 are longer and more complete than the analogous regions of SEC1 and SPEA. Note the angular difference between β strands 1, 2, and 3 and a vertical axis in the B domain of TSST-1 compared to SEC and SPEA. The longer loops and turns of SEC and SPEA (green) may also mask or blur surface features that could interact with epithelial cell receptors. While all three toxins are largely hydrophilic, TSST-1 has unique patches of hydrophobic and neutral residues on the front and rear of the B-domain β-barrel. The first patch is on the front of the β-barrel and includes Ser 29, Ser 32, Ile 42, Leu 44, Leu 46, Thr 69, Ile 81, Phe 83, Ile 85, and Asn 90. The second patch is comprised of Ser 14, Ser 15, Ser 17, Thr 19, Phe 20, Thr 21, Asn 37, Thr 38, and Thr 91.
FIG. 2.
Shared and unique structural features of TSST-1, SEC3, and SPEA. All three toxins share a bilobal tertiary fold that begins and ends in the A domain. In the figure, front (top) and rear (middle) views of TSST-1 (25), SEC 3 (12), and a molecular model of SPEA (6) are shown with the α-helices lettered and β strands numbered for TSST-1. At the bottom, a structure-based amino acid sequence alignment is shown. Numbers at the top of the alignment refer to the TSST-1 sequence. Structural features of SEC3 and SPEA absent or significantly altered in the tertiary fold of TSST-1 are red, green, cyan, yellow, and navy, while β-strand 6, present only in TSST-1, is black. Residues in TSST-1 making up hydrophobic stretches are shown as spheres (top) or are boxed in the amino acid sequence alignment. SEC1 used in the present study has 93% primary sequence identity to SEC3.
It is possible that the presence of the hydrophobic patches on TSST-1 that are absent in SEC1 and SPEA explains the susceptibility of TSST-1 to pepsin versus the resistance observed for the other two toxins. It is less clear why there are differences between SEC1 and SPEA in ability to cause emesis. However, fine structural differences are likely to provide the explanation.
It is also interesting to note that streptococcal TSS associated with throat infections is less common than illness associated with infection of skin breaks, despite the presence of the organism and SPEA production (28). For example, in a recent outbreak of streptococcal TSS in southeast Minnesota (7), a large number of children attending the local school had the causative SPEA-positive M3 streptococci in the throat, yet the children did not develop TSS as a consequence of the infection. However, TSS cases did occur in individuals who acquired the same organism in wounds. Similarly, the major risk factor in children for streptococcal TSS is superinfection of chickenpox lesions rather than infection of the throat (19).
A second major difference among the toxins studied was that SPEA is nonemetic in monkeys, despite its high degree of relatedness to the SEs and its resistance to degradation by pepsin. In addition, it is incapable of inducing systemic toxicity when fed to rabbits or when given vaginally. SEC1, one of the classical SEs and a confirmed cause of SFP, efficiently induced emesis in monkeys as expected. Since both SPEA and SEC1 are potent superantigens and resistant to pepsin, it is unlikely that emesis after oral administration of SEs, as in SFP, results from their action as superantigens. This is consistent with previous findings Betley and colleagues (9–11) and Alber et al. (1), who studied amino acid requirements for both emesis and superantigenicity of SEA and SEB, respectively. Thus, for example, Hoffman et al. (11) showed that His 61 of SEA appears to be an important residue for emetic but not superantigenic activity. In addition, Alber and colleagues (1) also provided evidence for separation of these two activities in that carboxymethylation of SEB to modify histidine residues resulted in a toxin that lost its enterotoxic but not superantigenic activity. It is interesting that SPEA does not have an analogous histidine residue to SEA His 61. However, neither do SEB and SEC, but these latter two toxins are emetic. It is therefore more likely that the orientation of the loop (green in Fig. 2) in the SEs is more important in emetic activity than the actual residue present. Additional studies between the SPEA and the SEs, especially SEB and SEC, should yield important information regarding additional functional requirements for inducing emesis. These studies would be facilitated by the large degree of sequence similarity between these related toxins. For example, SPEA and SEC1 share 49% sequence identity at the amino acid levels.
The diarrheagenic activity for TSST-1 observed in this study is interesting and the mechanism involved is currently under investigation. One possibility is that TSST-1 is a true SE and is improperly classified. Although Bergdoll et al. previously reported that TSST-1 induces emesis and initially proposed the designation SEF for this toxin (3), this activity was later attributed to SE contamination. Although the toxin was significantly less stable than SEC1 in the presence of pepsin, a small amount of the toxin remained intact for an extended period of time. Although it is possible that sufficient levels of TSST-1 persisted in the gastrointestinal tract to allow induction of symptoms, there are several reasons that we do not propose that TSST-1 is an SE. First, it has no significant sequence similarity to currently known SEs. Further, crucial structural features, such as a disulfide bond, presumed to be required for emesis are absent from the structure of TSST-1. Finally, TSST-1 has not been associated with cases of SFP. An alternative possibility is that, prior to degradation, some TSST-1 is able to cross the gut mucosa into circulation and induce TSS. Evidence that this could occur was provided in the rabbit feeding experiment. TSST-1, but not SPEA or SEC1, induced systemic symptoms in rabbits following oral administration. SEC1 which causes emesis directly in the gastrointestinal tract is not believed to enter circulation in high concentrations. The small amount that does enter circulation in orally fed rats is rapidly removed by the kidneys (8). Although TSST-1 has been shown to have the potential to be internalized by endothelial cells (16), it is unclear whether internalization is a prerequisite to passing through cells or whether SEs or SPEs lack this ability.
ACKNOWLEDGMENTS
This work was supported by U.S. Public Health Service grants HL36611, AI22159, GM54384, and AI28401 and U.S. Department of Agriculture grant 9402399.
We thank Cynthia Stauffacher for providing the atomic coordinates of SEC3 used in Fig. 2. Monkey feeding assays were performed at the Washington Regional Primate Research Facility (Seattle, Wash.) with the assistance of Ray Colby and Debra Glanister. Melodie Bahan is gratefully acknowledged for typing the manuscript.
P.M.S., L.M.J., M.R., and G.A.B. contributed equally to this work.
REFERENCES
- 1.Alber G, Hammer D K, Fleischer B. Relationship between enterotoxic and T lymphocyte-stimulating activity of staphylococcal enterotoxin B. J Immunol. 1990;144:4501–4506. [PubMed] [Google Scholar]
- 2.Bergdoll M S. Monkey feeding test for staphylococcal enterotoxins. Methods Enzymol. 1988;165:324–333. doi: 10.1016/s0076-6879(88)65048-8. [DOI] [PubMed] [Google Scholar]
- 3.Bergdoll M S, Crass B A, Reiser R F, Robbins R N, Davis J P. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic shock syndrome Staphylococcus aureus isolates. Lancet. 1981;i:1017–1021. doi: 10.1016/s0140-6736(81)92186-3. [DOI] [PubMed] [Google Scholar]
- 4.Bergdoll M S, Schlievert P M. Toxic-shock syndrome toxin. Lancet. 1984;ii:691. [Google Scholar]
- 5.Bohach G A, Fast D J, Nelson R D, Schlievert P M. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. CRC Crit Rev Microbiol. 1989;17:251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 6.Bohach G A, Stauffacher C V, Ohlendorf D H, Chi Y-I, Vath G M, Schlievert P M. The staphylococcal and streptococcal pyrogenic toxin family. In: Singh B R, Tu A T, editors. Natural toxins II. New York, N.Y: Plenum Publishing Corp.; 1995. pp. 131–154. [DOI] [PubMed] [Google Scholar]
- 7.Cockerill F R, III, MacDonald K L, Thompson R L, Roberson F, Kohner P C, Besser-Wiek J, Manahan J M, Musser J M, Schlievert P M, Talbot J, Frankfort B, Steckelberg J M, Wilson W R, Osterholm M T the Investigative Team. A outbreak of severe invasive group A streptococcal disease associated with high carriage rates of the invasive clone among community school-age children. JAMA. 1997;277:38–43. [PubMed] [Google Scholar]
- 8.Hamad A R, Marrack P, Kappler J W. Transcytosis of staphylococcal superantigen toxins. J Exp Med. 1997;185:1447–1454. doi: 10.1084/jem.185.8.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris T O, Betley M J. Biological activities of staphylococcal enterotoxin type A mutants with N-terminal substitutions. Infect Immun. 1995;63:2133–2140. doi: 10.1128/iai.63.6.2133-2140.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris T O, Grossman D, Kappler J W, Marrack P, Rich R R, Betley M J. Lack of complete correlation between emetic and T-cell stimulatory activities of staphylococcal enterotoxins. Infect Immun. 1993;61:3175–3183. doi: 10.1128/iai.61.8.3175-3183.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman M, Tremain M, Mansfield J, Betley M. Biochemical and mutational analysis of the histidine residues of staphylococcal enterotoxin A. Infect Immun. 1996;64:885–890. doi: 10.1128/iai.64.3.885-890.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann M L, Jablonski L M, Crum K K, Hackett S P, Chi Y-I, Stauffacher C V, Stevens D L, Bohach G A. Predictions of T-cell receptor- and major histocompatibility complex-binding sites on staphylococcal enterotoxin C1. Infect Immun. 1994;62:3396–3407. doi: 10.1128/iai.62.8.3396-3407.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hovde C J, Hackett S P, Bohach G A. Nucleotide sequence of the staphylococcal enterotoxin C3 gene: sequence comparison of all three type C staphylococcal enterotoxins. Mol Gen Genet. 1990;220:329–333. doi: 10.1007/BF00260504. [DOI] [PubMed] [Google Scholar]
- 14.Jablonski L M, Bohach G A. Staphylococcus aureus. In: Beuchat M, Doyle M, Montville T, editors. Fundamentals of food microbiology. Washington, D.C.: ASM Press; 1996. pp. 353–375. [Google Scholar]
- 15.Kim Y B, Watson D W. A purified group A streptococcal pyrogenic exotoxin. Physicochemical and biological properties including the enhancement of susceptibility to endotoxin shock. J Exp Med. 1970;131:611–628. doi: 10.1084/jem.131.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kushnaryov V M, MacDonald H S, Reiser R, Bergdoll M S. Staphylococcal toxic shock syndrome toxin specifically binds to cultured human epithelial cells and is rapidly internalized. Infect Immun. 1984;45:566–571. doi: 10.1128/iai.45.3.566-571.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Lee P K, Kreiswirth B N, Deringer J R, Projan S J, Eisner W, Smith B L, Carlson E, Novick R P, Schlievert P M. Nucleotide sequences and biological properties of toxic shock syndrome toxin-1 from ovine and bovine-associated Staphylococcus aureus. J Infect Dis. 1992;165:1056–1063. doi: 10.1093/infdis/165.6.1056. [DOI] [PubMed] [Google Scholar]
- 19.Leggiadro R J, Bugnitz M C, Peck B A, Luedtke G S, Kim M H, Kaplan E L, Schlievert P M. Group A streptococcal bacteremia in a Mid-South children's hospital. South Med J. 1993;86:615–618. doi: 10.1097/00007611-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 21.Mollick J A, Miller G G, Musser J M, Cook R G, Grossman D, Rich R R. A novel superantigen isolated from pathogenic strains of Streptococcus pyogenes with aminoterminal homology to staphylococcal enterotoxins B and C. J Clin Investig. 1993;92:710–719. doi: 10.1172/JCI116641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray D L, Earhart C A, Mitchell D T, Ohlendorf D H, Novick R P, Schlievert P M. Localization of biologically important regions on toxic shock syndrome toxin-1. Infect Immun. 1996;64:371–374. doi: 10.1128/iai.64.1.371-374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nauciel C, Blass J, Mangalo R, Raynaud M. Evidence for two molecular forms of streptococcal erythrogenic toxin. Conversion to a single form by 2-mercaptoethanol. Eur J Biochem. 1969;11:160–164. doi: 10.1111/j.1432-1033.1969.tb00754.x. [DOI] [PubMed] [Google Scholar]
- 24.Norrby-Teglund A, Newton D, Kotb M, Holm S E, Norgren M. Superantigenic properties of the group A streptococcal exotoxin SpeF (MF) Infect Immun. 1994;62:5227–5233. doi: 10.1128/iai.62.12.5227-5233.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prasad G S, Earhart C A, Murray D L, Novick R P, Schlievert P M, Ohlendorf D H. Structure of toxic shock syndrome toxin-1. Biochemistry. 1993;32:13761–13766. doi: 10.1021/bi00213a001. [DOI] [PubMed] [Google Scholar]
- 26.Schlievert P M. Enhancement of host susceptibility to lethal endotoxin shock by staphylococcal pyrogenic exotoxin type C. Infect Immun. 1982;36:123–128. doi: 10.1128/iai.36.1.123-128.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlievert P M. Staphylococcal enterotoxin B and toxic-shock syndrome toxin-1 are significantly associated with nonmenstrual TSS. Lancet. 1986;i:1149–1150. doi: 10.1016/s0140-6736(86)91859-3. [DOI] [PubMed] [Google Scholar]
- 28.Schlievert P M, Assimacopoulos A P, Cleary P P. Severe invasive group A streptococcal disease: clinical description and mechanisms of pathogenesis. J Lab Clin Med. 1996;127:13–22. doi: 10.1016/s0022-2143(96)90161-4. [DOI] [PubMed] [Google Scholar]
- 29.Schlievert P M, Osterholm M T, Kelly J A, Nishimura R D. Toxin and enzyme characterization of Staphylococcus aureus isolates from patients with and without toxic-shock syndrome. Ann Intern Med. 1982;96:937–940. doi: 10.7326/0003-4819-96-6-937. [DOI] [PubMed] [Google Scholar]
- 30.Schlievert P M, Shands K N, Dan B B, Schmid G P, Nishimura R D. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981;143:509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]