Abstract
Background
Diabetic ketoacidosis (DKA) has been reported to be increasing in frequency during the COVID-19 pandemic. We aimed to examine the rates of DKA hospital admissions and the patient demographics associated with DKA during the pandemic compared with in prepandemic years.
Methods
Using a comprehensive, multiethnic, national dataset, the Secondary Uses Service repository, we extracted all emergency hospital admissions in England coded with DKA from March 1 to June 30, 2020 (first wave of the pandemic), July 1 to Oct 31, 2020 (post-first wave), and Nov 1, 2020, to Feb 28, 2021 (second wave), and compared these with DKA admissions in the equivalent periods in 2017–20. We also examined baseline characteristics, mortality, and trends in patients who were admitted with DKA.
Findings
There were 8553 admissions coded with DKA during the first wave, 8729 during the post-first wave, and 10 235 during the second wave. Compared with preceding years, DKA admissions were 6% (95% CI 4–9; p<0·0001) higher in the first wave of the pandemic (from n=8048), 6% (3–8; p<0·0001) higher in the post-first wave (from n=8260), and 7% (4–9; p<0·0001) higher in the second wave (from n=9610). In the first wave, DKA admissions reduced by 19% (95% CI 16–21) in those with pre-existing type 1 diabetes (from n=4965 to n=4041), increased by 41% (35–47) in those with pre-existing type 2 diabetes (from n=2010 to n=2831), and increased by 57% (48–66) in those with newly diagnosed diabetes (from n=1072 to n=1681). Compared with prepandemic, type 2 diabetes DKA admissions were similarly common in older individuals and men but were higher in those of non-White ethnicities during the first wave. The increase in newly diagnosed DKA admissions occurred across all age groups and these were significantly increased in men and people of non-White ethnicities. In the post-first wave, DKA admissions did not return to the baseline level of previous years; DKA admissions were 14% (11–17) lower in patients with type 1 diabetes (from n=5208 prepandemic to n=4491), 30% (24–36) higher in patients with type 2 diabetes (from n=2011 to n=2613), and 56% (47–66) higher in patients with newly diagnosed diabetes (from n=1041 to n=1625). During the second wave, DKA admissions were 25% (22–27) lower in patients with type 1 diabetes (from n=5769 prepandemic to n=4337), 50% (44–56) higher in patients with type 2 diabetes (from n=2608 to n=3912), and 61% (52–70) higher in patients with newly diagnosed diabetes (from n=1234 to n=1986).
Interpretation
Our results provide evidence for differences in the numbers and characteristics of people presenting with DKA during the COVID-19 pandemic compared with in the preceding 3 years. Greater awareness of risk factors for DKA in type 2 diabetes and vigilance for newly diagnosed diabetes presenting with DKA during the COVID-19 pandemic might help mitigate the increased impact of DKA.
Funding
None.
Introduction
Diabetic ketoacidosis (DKA) is a life-threatening consequence of the acute metabolic decompensation that can occur in people with established diabetes or at first presentation.1 DKA typically occurs in people with type 1 diabetes, and infection is one of the most common precipitants.1 However, DKA can occur in all types of diabetes, including type 2, and is not necessarily indicative of type 1 in people newly presenting with diabetes.
During the COVID-19 pandemic, older age, socioeconomic deprivation, male sex, non-White ethnicity, and chronic diseases including diabetes have been associated with worse outcomes from SARS-CoV-2 infection.2 An excess of people presenting with DKA has been reported in several studies during the pandemic.3, 4, 5, 6, 7, 8 These studies suggest that SARS-CoV-2 infection is associated with a higher risk of presenting with DKA,3 that the presentations disproportionately occur in people with type 2 diabetes8, 9, 10 and in children with new-onset type 1 diabetes,11, 12, 13 and that DKA in the presence of SARS-CoV-2 infection has a longer time to resolution with higher mortality.8, 14 These observations have led to the hypothesis that SARS-CoV-2 has a direct or indirect effect on the pancreas.15, 16
Major limitations of reports highlighting DKA cases during the pandemic are that they have either been single-centre, reporting non-systematically collected data on small cohorts, or have not compared with rates of prepandemic admissions with DKA or compared the demographic characteristics, such as ethnicity, of those affected.
Research in context.
Evidence before this study
We searched PubMed and medRxiv from Jan 1, 2020, to April 31, 2021, for publications in English, with search terms including “COVID-19”, “SARS-CoV-2”, “coronavirus”, “diabetes”, “hyperglycaemic emergencies” or “hyperglycemic emergencies”, and “diabetic ketoacidosis”. Case series from Wuhan, China and east Asia and, more recently, single-centre analyses and paediatric national datasets from Europe and North America have all reported an excess of hospital admissions with diabetic ketoacidosis (DKA) during the COVID-19 pandemic. The excess DKA cases have been reported in people with pre-existing type 1 diabetes, type 2 diabetes, and newly diagnosed diabetes; prompting some speculation as to whether the virus might have direct or indirect effects on pancreatic insulin production. However, a firm understanding about the excess DKA cases reported has been hindered by previous studies being small, often single-centre, restricted to paediatric cohorts, with no data on differences between ethnic groups, and not including comparison with prepandemic DKA incidence.
Added value of this study
To our knowledge, this is the largest analysis to date of DKA cases in a complete national dataset. We found a rise in all hospital admissions with DKA during the first wave, post-first wave, and second wave of the COVID-19 pandemic in England, compared with mean numbers for matched time periods over the preceding 3 years. The significant increases in DKA admissions in people with type 2 diabetes and people with newly diagnosed diabetes were offset by a concurrent significant reduction in people with type 1 diabetes presenting with DKA. These trends by diabetes type held true during the first wave, when testing for COVID-19 was restricted; post-first wave, when COVID-19 cases were very low; and second wave, when universal testing was available.
Implications of all the available evidence
The prompt recognition of new-onset diabetes and the features of those at risk of DKA in people with type 2 diabetes are key to mitigating the excess DKA admissions observed during the COVID-19 pandemic.
We aimed to examine DKA presentations during the COVID-19 pandemic to: determine whether numbers of DKA admissions had increased during the first wave, after the first wave, and during the second wave of the pandemic, compared with in preceding years; analyse the demographic characteristics associated with a presentation of DKA in those with pre-existing type 1 and type 2 diabetes and those with a new presentation of diabetes compared with in preceding years; and compare DKA admissions between the first wave, post-first wave, and second wave of the pandemic.
Methods
Study design and participants
We used data from the Secondary Uses Service (SUS), a comprehensive repository of health-care data from all hospitals in England, to identify emergency admissions to hospital with DKA, identified with a diagnosis code of E101, E111, E121, E131, or E141 in any diagnostic code position. Diabetes type was determined by linking SUS data to the National Diabetes Audit (NDA) for England. The NDA has collated data on people with diabetes registered with general practices in England since 2003, with almost complete participation of General Practices in England in recent years (99% in 2019–20, 98% in 2018–19, 98% in 2017–18, and 95% in 2016–17).17 Among the admissions with DKA identified, diagnostic codes for COVID-19 (U071 or U072) were further identified in any diagnostic code position.
To fulfil its statutory duties, National Health Service (NHS) England requires access to and linkage of a variety of national pseudonymised datasets, in line with the requirements of General Data Protection Regulation. Furthermore, in March 2020, the Secretary of State for Health and Social Care used powers under the UK Health Service (Control of Patient Information) Regulations 2002 to require organisations to process confidential patient information for the purposes of protecting public health, providing health-care services to the public, and monitoring and managing the COVID-19 outbreak and incidents of exposure.
Procedures
We studied three time periods during the COVID-19 pandemic: from March 1 to June 30, 2020, defined as the first wave; July 1 to Oct 31, 2020, the post-first wave; and Nov 1, 2020, to Feb 28, 2021, the second wave (until the latest date for which data was accessible, although the second wave did extend beyond this date). Emergency hospital admissions with DKA during each study period were compared with the mean in the corresponding time periods in the 3 preceding prepandemic years (March, 2017, to February, 2020).
Diabetes type, coded as type 1 or type 2, was determined by linking the emergency admissions data to NDA data, using the NHS number, a unique patient identifier. A small proportion of individuals were coded with other diabetes types (n=797, <1%) and were excluded from the analyses. Individuals with a date of diagnosis of diabetes after the date of admission to hospital with DKA, or who were admitted within 7 days of diagnosis, were classified as newly diagnosed.
Age, sex, ethnicity, and socioeconomic deprivation quintile were identified as potential confounding factors. Age was grouped as younger than 19 years, 19–29 years, 30–39 years, 40–49 years, 50–59 years, 60–69 years, and 70 years or older. Sex was recorded as female or male. Ethnicity was classified as White or non-White (Asian, Black, Mixed, or other ethnicity); non-White ethnicities were grouped together as we did not have sufficient numbers to examine these groups independently. Socioeconomic deprivation was defined by the English Index of Multiple Deprivation (IMD) 2019 associated with the Lower Layer Super Output Area derived from the patient's postcode and grouped into quintiles (from 1 [most deprived] to 5 [least deprived]).18 To account for geographical variation in exposure to SARS-CoV-2, region was included as a covariate, with allocation to one of the seven regions in England according to the location of the patient's general practice. All variables also had an unknown category.
Outcomes
The primary outcome was number of emergency admissions coded with DKA during the first wave, post-first wave, and second-wave of the COVID-19 pandemic, compared with the mean number of admissions coded with DKA for the corresponding time periods during the 3 prepandemic years. If there was more than one emergency admission with DKA for an individual during a time period, all admissions were counted, to assess whether proportions of multiple admissions changed during the pandemic. Secondary outcomes were numbers of DKA admissions by type of diabetes; numbers of DKA admissions within each type of diabetes by age, sex, ethnicity, and socioeconomic deprivation quintile; numbers of admissions with a diagnosis of COVID-19 by type of diabetes; and number of individuals with multiple admissions. In-hospital deaths were also analysed.
Statistical analysis
Numbers of admissions were calculated during the first wave, post-first wave, and second wave, and were compared with the mean admission numbers for the same 4-month periods over the 3 prepandemic years. Incidence rates were estimated using the number of admissions during the first wave, post-first wave, or second-wave, or the corresponding mean number of admissions prepandemic as the numerator. We used poisson regression analyses to test temporal differences in the numbers and incidence rates of DKA with time period (2017–20 or 2020–21) as the independent variable. For rates of admissions with type 1 diabetes and admissions with type 2 diabetes, the number of people with each diabetes type in England in the corresponding year was used as the denominator (2016–17 to 2019–20), and rates were expressed per 100 000 population with diabetes. For admission rates with newly diagnosed diabetes, mid-year estimates of the resident population in the corresponding years (2016–19) from the Office for National Statistics (ONS), with type 1 and type 2 diabetes populations subtracted, were used as the denominator,19 and rates were expressed per 100 000 general population.
A multivariable logistic regression was used to examine the association between in-hospital death and diabetes type for emergency admissions during the pandemic, adjusting for age, sex, ethnicity, deprivation quintile, region, and COVID-19 diagnosis. Records with missing values for age, sex, deprivation quintile, or region were not included in regression analyses. Data were missing for between less than 1% and 16% of variables.
Significance was defined as a p value of less than 0·05 and confidence intervals were set at 95%. All analyses were done with Stata version 16.
Role of the funding source
There was no funding source for this study.
Results
There were 8553 emergency hospital admissions coded with DKA during the first wave, 8729 during the post-first wave, and 10 235 during the second wave. Compared with the mean number in the equivalent time periods over the previous 3 years, DKA admissions were 6% (95% CI 4–9; p<0·0001) higher in the first wave, 6% (3–8; p<0·0001) higher in the post-first wave, and 7% (4–9; p<0·0001) higher in the second wave (table ).
Table.
Emergency hospital admissions coded with DKA during the first wave, post-first wave, and second wave of the COVID-19 pandemic and the mean in equivalent time periods in 2017–20, with associated percentage changes
|
First wave |
Post-first wave |
Second wave |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type 1 diabetes | Type 2 diabetes | Newly diagnosed diabetes | Total | Type 1 diabetes | Type 2 diabetes | Newly diagnosed diabetes | Total | Type 1 diabetes | Type 2 diabetes | Newly diagnosed diabetes | Total | |
| Overall | ||||||||||||
| During the COVID-19 pandemic | 4041 | 2831 | 1681 | 8553 | 4491 | 2613 | 1625 | 8729 | 4337 | 3912 | 1986 | 10 235 |
| Mean in equivalent time periods in 2017–20 | 4965 | 2010 | 1072 | 8048 | 5208 | 2011 | 1041 | 8260 | 5769 | 2608 | 1234 | 9610 |
| Percentage change | −19% | 41% | 57% | 6% | −14% | 30% | 56% | 6% | −25% | 50% | 61% | 7% |
| Age <19 years | ||||||||||||
| During the COVID-19 pandemic | 429 | 6 | 554 | 989 | 555 | 3 | 667 | 1225 | 529 | 5 | 628 | 1162 |
| Mean in equivalent time periods in 2017–20 | 683 | 9 | 371 | 1063 | 715 | 8 | 409 | 1132 | 784 | 9 | 451 | 1244 |
| Percentage change | −37% | −31% | 49% | −7% | −22% | −63% | 63% | 8% | −33% | −42% | 39% | −7% |
| Age 19–39 years | ||||||||||||
| During the COVID-19 pandemic | 1949 | 173 | 428 | 2550 | 2186 | 145 | 395 | 2726 | 2076 | 247 | 505 | 2828 |
| Mean in equivalent time periods in 2017–20 | 2555 | 172 | 279 | 3006 | 2676 | 190 | 269 | 3135 | 2874 | 180 | 309 | 3363 |
| Percentage change | −24% | 1% | 53% | −15% | −18% | −24% | 47% | −13% | −28% | 37% | 63% | −16% |
| Age 40–59 years | ||||||||||||
| During the COVID-19 pandemic | 990 | 972 | 405 | 2367 | 1065 | 863 | 340 | 2268 | 1023 | 1323 | 476 | 2822 |
| Mean in equivalent time periods in 2017–20 | 1054 | 649 | 227 | 1930 | 1109 | 640 | 189 | 1939 | 1313 | 857 | 237 | 2407 |
| Percentage change | −6% | 50% | 78% | 23% | −4% | 35% | 80% | 17% | −22% | 54% | 101% | 17% |
| Age ≥60 years | ||||||||||||
| During the COVID-19 pandemic | 673 | 1680 | 223 | 2576 | 685 | 1602 | 163 | 2450 | 709 | 2337 | 283 | 3329 |
| Mean in equivalent time periods in 2017–20 | 672 | 1180 | 137 | 1989 | 707 | 1171 | 117 | 1995 | 796 | 1559 | 163 | 2518 |
| Percentage change | 0% | 42% | 63% | 30% | −3% | 37% | 39% | 23% | −11% | 50% | 74% | 32% |
| White ethnicity | ||||||||||||
| During the COVID-19 pandemic | 3425 | 2033 | 1018 | 6476 | 3840 | 1966 | 1002 | 6808 | 3646 | 2711 | 1196 | 7553 |
| Mean in equivalent time periods in 2017–20 | 4278 | 1567 | 717 | 6563 | 4495 | 1562 | 689 | 6746 | 4964 | 2041 | 849 | 7854 |
| Percentage change | −20% | 30% | 42% | −1% | −15% | 26% | 45% | 1% | −27% | 33% | 41% | −4% |
| Non-White ethnicities | ||||||||||||
| During the COVID-19 pandemic | 386 | 535 | 394 | 1315 | 378 | 399 | 375 | 1152 | 443 | 801 | 463 | 1707 |
| Mean in equivalent time periods in 2017–20 | 428 | 290 | 208 | 927 | 439 | 293 | 219 | 950 | 477 | 356 | 240 | 1072 |
| Percentage change | −10% | 84% | 89% | 42% | −14% | 36% | 71% | 21% | −7% | 125% | 93% | 59% |
| IMD 1 (most deprived) | ||||||||||||
| During the COVID-19 pandemic | 1416 | 816 | 443 | 2675 | 1546 | 781 | 416 | 2743 | 1561 | 1192 | 551 | 3304 |
| Mean in equivalent time periods in 2017–20 | 1683 | 605 | 272 | 2561 | 1726 | 632 | 263 | 2622 | 1915 | 780 | 298 | 2993 |
| Percentage change | −16% | 35% | 63% | 4% | −10% | 24% | 58% | 5% | −18% | 53% | 85% | 10% |
| IMD 5 (least deprived) | ||||||||||||
| During the COVID-19 pandemic | 386 | 316 | 226 | 928 | 437 | 318 | 215 | 970 | 414 | 443 | 243 | 1100 |
| Mean in equivalent time periods in 2017–20 | 528 | 221 | 130 | 879 | 550 | 234 | 123 | 907 | 599 | 309 | 144 | 1053 |
| Percentage change | −27% | 43% | 74% | 6% | −20% | 36% | 75% | 7% | −31% | 43% | 68% | 4% |
Data are n unless otherwise stated. Means have been rounded to whole numbers so the sum of the row might not equal the total shown. The first wave was defined as from March 1 to June 30, 2020, the post-first wave was from July 1 to Oct 31, 2020, and the second wave was from Nov 1, 2020, to Feb 28, 2021. DKA=diabetic ketoacidosis. IMD=Index of Multiple Deprivation.
7474 individuals accounted for the 8553 admissions with DKA during the first wave, 7504 accounted for the 8729 admissions during the post-first wave, and 8926 accounted for the 10 235 admissions during the second wave. Of the individuals admitted in the first wave, 9% (95% CI 9–10) had more than one emergency admission with DKA during this period, which was significantly reduced from 11% (11–12) over the preceding 3 years (p<0·0001). 10% (9–11) of individuals in the post-first wave and 9% (8–9) in the second wave had more than one emergency admission; both significantly lower than in the corresponding time periods in the previous 3 years (12% [11–13] and 11% [11–12] respectively; both p<0·0001; appendix p 1).
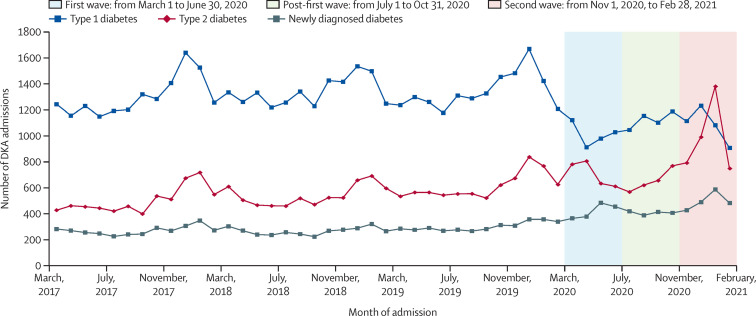
During the first wave, there were 4041 DKA admissions in people with a pre-existing diagnosis of type 1 diabetes; a 19% (95% CI 16–21; p<0·0001; from n=4965) reduction compared with preceding years (table, figure 1 ). However, DKA admissions in people with pre-existing type 2 diabetes increased by 41% (35–47; p<0·0001; from n=2010 to n=2831), and DKA admissions with newly diagnosed diabetes increased by 57% (48–66; p<0·0001; from n=1072 to n=1681). During the post-first wave, DKA admissions with type 1 diabetes were 14% (11–17; p<0·0001) lower than prepandemic levels, admissions with type 2 diabetes were 30% (24–36; p<0·0001) higher, and admissions with newly diagnosed diabetes were 56% (47–66; p<0·0001) higher. During the second wave, DKA admissions with type 1 diabetes were 25% (22–27; p<0·0001) lower than in the equivalent periods over the previous 3 years, admissions with type 2 diabetes were 50% (44–56; p<0·0001) higher, and admissions with newly diagnosed diabetes were 61% (52–70; p<0·0001) higher (table, figure 1).
Figure 1.
Number of emergency hospital admissions in England coded with DKA by type of diabetes from March, 2017, to February, 2021
DKA=diabetic ketoacidosis.
Of the 8553 admissions during the first wave, 1007 (12%) had a coded diagnosis of COVID-19. The proportions of each diabetes type with COVID-19 varied significantly; 6% (95% CI 5–7) of admissions with type 1 diabetes had a diagnosis of COVID-19, 23% (21–24) of admissions with type 2 diabetes, and 7% (6–9) of admissions with newly diagnosed diabetes. In the post-first wave, 391 (4%) of 8729 admissions also had a diagnosis of COVID-19. During the second wave, 2405 (23%) of 10 235 admissions also had a diagnosis of COVID-19, with significant differences by diabetes type: 12% of admissions with type 1 diabetes, 40% of admissions with type 2 diabetes, and 16% of admissions with newly diagnosed diabetes (appendix p 2).
Among people admitted with DKA with pre-existing type 1 diabetes, in the first wave, the median age was 34 years (IQR 24–52) compared with 30 years (21–48) in the prepandemic years (appendix p 3). Across study periods, the majority of admissions with type 1 diabetes occurred in people younger than 50 years (72% in the first wave vs 77% in prepandemic years; p<0·0001). However, there was a significant decrease in the proportion of admissions in people younger than 19 years (37% reduction) and in people aged 20–39 years (24% reduction) compared with in prepandemic years. Other characteristics of individuals admitted with type 1 diabetes, such as sex, ethnicity, and deprivation, were similar to in prepandemic years (appendix pp 4–6).
Among people with pre-existing type 2 diabetes admitted with DKA, there was no difference in the median age across study periods (64 years [IQR 52–76] in the first wave vs 64 years [51–76] in the prepandemic years; appendix p 3). During the pandemic and in the prepandemic years, the highest proportion of DKA admissions were in people older than 50 years, with a significant increase in DKA admissions in this age group in the first wave compared with prepandemic (82% vs 78%; p=0·0002). During the first wave, a significantly higher proportion of people of non-White ethnicities were admitted with DKA compared with in the prepandemic period (19% [95% CI 17–20] vs 14% [13–16]; p<0·0001; appendix pp 1–3).
Among people presenting with DKA with newly diagnosed diabetes, the median age was 30 years (IQR 13–51) in the first wave compared with 27 years (13–49) in the prepandemic period (appendix p 3). During the first wave, there were significantly higher proportions of men (64% [95% CI 61–66] vs 59% [56–62]; p=0·0009) and people of non-White ethnicities (23% [21–26] vs 19% [17–22]; p<0·0001) admitted with DKA than in the prepandemic period (appendix pp 4–6). No substantial differences were observed in the distribution of cases by age category in people with newly diagnosed diabetes.
The characteristics of people admitted during the second wave were broadly similar across people with type 1 diabetes, type 2 diabetes, and newly diagnosed diabetes (table).
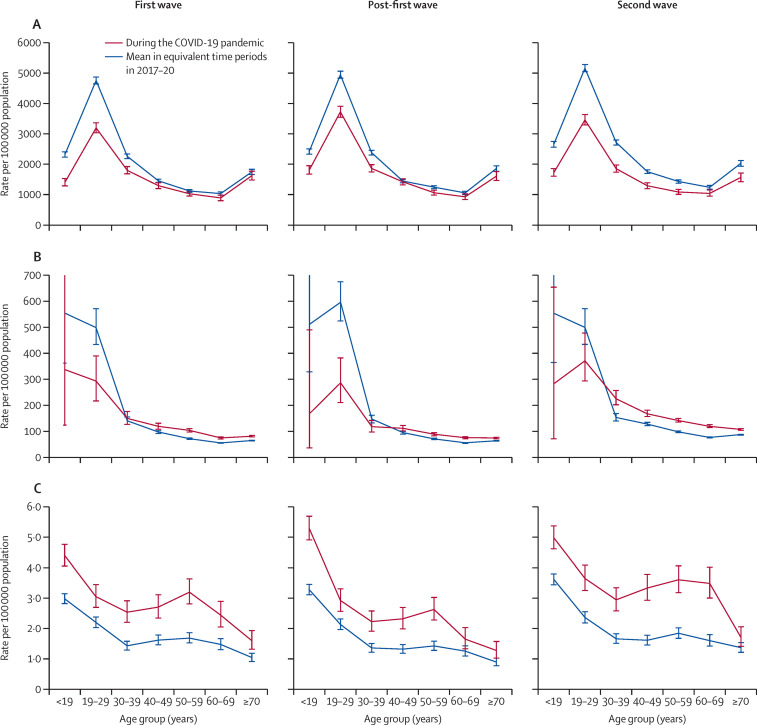
The 2019–20 NDA reported that there were 251 525 people with type 1 diabetes and 3 118 388 with type 2 diabetes in England. There were an estimated 52 917 048 individuals in England without (type 1 or type 2) diabetes according to the resident ONS population (appendix p 7). Incidence rates of DKA admissions per 100 000 people with diabetes during the first wave were 1607 (95% CI 1557–1657) for type 1 diabetes compared with 2092 (2059–2126) in preceding years, and 91 (87–94) for type 2 diabetes compared with 71 (69–73) in preceding years (appendix p 7). For newly diagnosed diabetes, the incidence rate of DKA admissions per 100 000 people was 3·2 (3·0–3·3) during the first wave compared with 2·0 (1·9–2·2) in previous years. The corresponding incidence rate ratios (IRR) for DKA admissions were 0·77 (95% CI 0·74–0·80) with type 1 diabetes, 1·28 (1·22–1·34) with type 2 diabetes, and 1·56 (1·47–1·65) with newly diagnosed diabetes in the first wave compared with in prepandemic years. IRRs for DKA admissions were 0·81 (0·79–0·84) with type 1 diabetes, 1·18 (1·13–1·23) with type 2 diabetes, and 1·55 (1·46–1·64) with newly diagnosed diabetes in the post-first wave and 0·71 (0·68–0·73), 1·36 (1·31–1·41), and 1·60 (1·51–1·68) during the second wave compared with in prepandemic years (appendix p 7). Compared with in previous years, incidence rates of DKA admission were lower in those younger than 40 years with type 1 diabetes across all study periods during the pandemic (figure 2 ). For type 2 diabetes, incidence rates were higher than in previous years, especially in those aged 40 years or older, during the first wave, post-first wave, and second wave. For people with newly diagnosed diabetes, incidence rates of DKA admission were higher across all age categories and periods of study during the pandemic compared with in prepandemic years.
Figure 2.
Incidence rates of emergency hospital admissions coded with DKA during the first wave, post-first wave, and second wave of the COVID-19 pandemic, and the mean in equivalent time periods in 2017–20 by age group and diabetes type
(A) Type 1 diabetes. (B) Type 2 diabetes. (C) Newly diagnosed diabetes. Incidence rates are per 100 000 population with diabetes for type 1 and type 2 diabetes and per 100 000 general population for newly diagnosed diabetes. The first wave was defined as from March 1 to June 30, 2020, the post-first wave was from July 1 to Oct 31, 2020, and the second wave was from Nov 1, 2020, to Feb 28, 2021. Error bars indicate 95% CI. DKA=diabetic ketoacidosis.
There were 658 (8%) in-hospital deaths for admissions coded with DKA during the first wave, 412 (5%) during the post-first wave, and 968 (9%) during the second wave; these were significantly higher than over the corresponding time periods in the previous years (4%, 3%, and 4% respectively; all p values p<0·0001) respectively (appendix p 8). Of those admitted with DKA who died in hospital, 319 (48%) had a diagnostic code of COVID-19 during the first wave, 96 (23%) during the post-first wave, and 607 (63%) during the second wave (p<0·0001). During the first wave, 3% of people admitted with DKA with type 1 diabetes died, compared with 17% of people with type 2 diabetes, and 3% of people with newly diagnosed diabetes (p<0·0001). During the first wave, in-hospital death occurred in less than 1% of people younger than 50 years at admission but in 24% of those aged 70 years or older (compared with 16% mortality in those aged ≥70 years in the prepandemic period). Higher proportions of deaths occurred in people aged 70 years or older with type 2 diabetes (29%) compared with those with type 1 diabetes (15%) or newly diagnosed diabetes (13%). Adjusted odds for in-hospital deaths showed rates of in-hospital death increased as age increased and were higher for people of non-White ethnicities and the most socioeconomically deprived compared with people of White ethnicity and the least socioeconomically deprived. People admitted with DKA with a diagnosis of COVID-19 had an odds ratio of 4·8 (95% CI 4·3–5·3) for in-hospital death compared with those with no COVID-19 diagnosis, and people aged 70 years or older had an odds ratio of 9·1 (7·3–11·5) compared with those aged 40–49 years. After adjustment for age, sex, ethnicity, deprivation, region, and time period, people with type 2 diabetes had an odds ratio of 1·9 (1·7–2·2; p<0·0001) and those with newly diagnosed diabetes had an odds ratio of 1·2 (1·0–1·6; p=0·059) for in-hospital death compared with those with type 1 diabetes (appendix p 9).
Discussion
In this study, to our knowledge the largest to date involving a comprehensive national dataset, we found that DKA admissions increased modestly during the COVID-19 pandemic compared with over the 3 preceding years. This trend was accounted for by a significant rise in DKA admissions in people with pre-existing type 2 diabetes and newly diagnosed diabetes despite a concurrent reduction in admissions in people with type 1 diabetes. DKA admissions were increased during the first wave, when testing for SARS-CoV-2 was relatively restricted, and during the second wave, when almost a quarter of admissions had COVID-19 and when universal testing for SARS-CoV-2 in those admitted to hospital had been established.
In both the first and second waves, the reduction in DKA admissions in people with type 1 diabetes was mainly in those younger than 30 years, with a reduction in multiple admissions. The traditional drivers of DKA in type 1 diabetes are insulin omission or concurrent illness, or both;1, 20 our findings therefore suggest that there were substantial modifications to these risk factors during both waves of the pandemic.
The excess DKA cases observed in people with type 2 diabetes during the first and second waves occurred in older people with similar demographic characteristics to those presenting prepandemic, which are similar to the characteristics associated with worse outcomes from COVID-19.2 Admissions with type 2 diabetes and DKA were more likely to have a concurrent diagnosis of COVID-19 and a higher odds ratio for in-hospital death compared with those with type 1 diabetes, even when adjusted for age. Our findings suggest excess DKA in individuals at risk of severe COVID-19 and in whom DKA most likely developed during critical illness. In a single-centre analysis of DKA admissions, only half of people admitted with type 2 diabetes and DKA were previously treated with insulin;10 nearly all had markedly elevated HbA1c levels, suggesting suboptimal glycaemic control predated the pandemic. The trend of higher type 2 diabetes and DKA admissions persisted in the post-first wave and was present in the second wave, which might reflect behavioural changes, reduced contact with health-care providers, a possible direct effect of SARS-CoV-2 on the pancreas, or extension of the yearly rise of DKA in type 2 diabetes which has been reported previously.21
We found a 57% increase in DKA admissions with newly diagnosed diabetes compared with preceding years, distributed across all age groups, during the first wave, and a 61% increase during the second wave. Between the two waves, when COVID-19 diagnoses were decreased, there was still a 56% increase in DKA admissions with newly diagnosed diabetes compared with preceding years, suggesting factors other than acute SARS-CoV-2 infection might have been driving DKA. It is also important to consider that the newly diagnosed admissions are those presenting with DKA, which might or might not reflect an increase in incidence of newly diagnosed diabetes.
A national paediatric registry study did not find an increase in incidence of new-onset type 1 diabetes during the pandemic,13 but two studies have shown an excess of DKA in children newly diagnosed with diabetes during the pandemic (presumed to be type 1 diabetes due to young age).12, 22 We observed an excess of DKA cases in children (<19 years) with newly diagnosed diabetes. Although we cannot conclude these individuals have type 1 diabetes, for people aged younger than 19 years one might reasonably speculate a new presentation of diabetes with DKA could potentially represent type 1 diabetes. However, we have concurrently shown a reduction in DKA in those with known type 1 diabetes. It is not possible in this analysis to draw conclusions as to the true incidence of type 1 diabetes.
Due to lag times in recording and extracting data, a proportion of cases of DKA assigned as newly diagnosed with diabetes using our method of acquisition might be identified in subsequent NDA data extracts as having had an established diagnosis of type 1 or type 2 diabetes or having been diagnosed in the same year as admission. A sensitivity analysis showed that the extent of changes introduced through these retrospective corrections decreased the estimate of the newly diagnosed population by 13%. Therefore, although the 57% increase during the first wave is likely to be an overestimation, the proportion that will be reclassified is relatively small.
It is currently not possible to be certain about diabetes type in adults who present with DKA with newly diagnosed diabetes. All patients with DKA, irrespective of diabetes type, are treated and discharged on insulin in line with national guidelines, and clarity will only emerge during the subsequent clinical course, sometimes many months later, when insulin has been successfully withdrawn in those with type 2 diabetes.
DKA is a preventable condition in those with established diabetes and has the potential to be mitigated in those with new-onset diabetes by earlier detection. Therefore, our findings in those with type 2 diabetes and newly diagnosed diabetes warrant careful attention.
These excess DKA admissions might reflect both direct and indirect effects of the pandemic, including: delays in presentation due to either health-care or patient factors, changes in behaviours that are permissive to presentations with DKA in susceptible individuals, the severity of acute illness with SARS-CoV-2 infection making acute metabolic decompensation of hyperglycaemia more likely, or a direct pancreatic effect of SARS-CoV-2.
Disruptions to delivery of health-care services during the COVID-19 pandemic have been noted internationally across care pathways for non-communicable diseases,23 with routine diabetes care being particularly affected.24 It is also possible that people delayed seeking routine medical care due to fear of contagion.25
Several behavioural changes could also affect glycaemia or induce metabolic decompensation. In a companion correspondence we showed significant differences in starting body weight during the pandemic observed in the National Health Service Diabetes Prevention Programme, with higher starting weights in particular in younger people, women, and those from more deprived areas.26
Delays in accessing care or behaviours that might contribute to DKA are likely to have differed between those with type 1 diabetes (who are insulin-treated and trained to titrate insulin according to glucose levels) and those with type 2 diabetes, who might not be insulin-treated or might not check glucose levels and will probably need support to address higher glucose levels.
One potential explanation for the excess of people with newly diagnosed diabetes presenting with DKA is a direct effect of SARS-CoV-2 on β cells inducing diabetes, as has been extensively speculated in the literature.27, 28, 29, 30, 31 However, although we acknowledge that in the first wave there was an under-ascertainment of COVID-19 infection, this was not the case in the second wave, by which point there was universal testing in those admitted to hospital, and it is noteworthy that many people presenting with DKA were COVID-19-negative.
In view of our analysis of routine data, we are unable to ascertain the mechanism of DKA in people with newly diagnosed diabetes. Longer-term follow-up of these individuals is required to determine diabetes type and ascertain whether the newly diagnosed cases are genuine diabetes or transient stress hyperglycaemia.
The high percentage of in-hospital deaths during the first and second waves compared with in preceding years is likely to be multifactorial and include concomitant COVID-19 and preponderance of type 2 diabetes admissions especially in older age groups. We found that older age groups had by far the highest adjusted odds ratios for in-hospital death. Notably, odds ratios for in-hospital death were also higher in those of non-White ethnicities and those from the highest deprivation quintile. The higher odds ratio for deaths in type 2 diabetes with DKA has not been shown in historical UK data previously,21 but has been observed in other COVID-19 studies.14, 32
This study has some limitations. Admissions due to DKA were based on hospital coding, which could not be biochemically confirmed in this analysis. Miscoding of DKA is a recognised issue;33 however, it is unlikely that the differences in DKA admission rates observed in this analysis were accounted for by differential proportions of coding errors occurring across the study periods. We were unable to confirm the diabetes type in people with newly diagnosed diabetes as all patients presenting with DKA are discharged on insulin therapy and clarity about diabetes type takes time to be determined, but will become visible and amenable to analysis in subsequent extracts of NDA data. Due to the nature of the datasets used, we were unable to examine additional clinical and biochemical features to assess differences in severity of DKA or relationship to other features, such as BMI or HbA1c levels. Finally, the diagnostic coding of concurrent COVID-19 during the first wave is likely to be an under-representation, as only after April 27, 2020 did SARS-CoV-2 testing criteria within the NHS in England become routine for all admissions, whereas before this there was symptomatic testing only. Our comparison with preceding years of the first wave DKA admissions goes some way to mitigate this potential bias and by the second wave, there was universal testing for SARS-CoV-2 with all hospital admissions.
In conclusion, in this systematic and comprehensive analysis of DKA admissions during the first and second waves of the COVID-19 pandemic in England, we observed reduced admissions of people with pre-existing type 1 diabetes and increased admissions of people with type 2 diabetes and those with newly diagnosed diabetes. The majority of the admissions were accounted for by men, older individuals, and those of non-White ethnicities with type 2 diabetes, the same demographic characteristics associated with poor outcomes from COVID-19 in the general population, which suggests the excess of cases occurred in those who were critically unwell from COVID-19. The excess of DKA admissions in people with newly diagnosed diabetes requires further study and must be interpreted in the context of overall diabetes incidence. The prompt recognition of new-onset diabetes and the features of those at risk of DKA in existing populations with diabetes are key to mitigating this excess of DKA in the context of the COVID-19 pandemic.
Data sharing
Data from the NDA can be requested through the NHS Digital Data Access Request Service process at: https://digital.nhs.uk/services/data-access-request-service-dars/data-access-request-service-dars-process.
Declaration of interests
SM, EB, PK, BY, KK, and JV are members of clinical advisory groups to the NDA. SM is a trustee of the Diabetes Research and Wellness Foundation and has a grant in support of an investigator-initiated study from DexCom. PK, BY, KK, and JV are members of the NDA research committee. ST has received honoraria for speaking from Lilly and Sanofi-Aventis. KD has received honoraria for speaking and travel grants from Novo Nordisk, Sanofi-Aventis, Lilly, and Boehringer Ingelheim, and consulting fees from Sanofi-Aventis; is Chair of the Joint British Diabetes Societies for Inpatient Care; and is committee member of the Association of British Clinical Diabetologists. PK is National Specialty Adviser for Diabetes and Obesity at NHS England and NHS Improvement. BY is clinical lead for the NDA and a trustee of Diabetes UK. KK has been a consultant and speaker for Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, and Merck Sharp & Dohme; has received grants in support of investigator-initiated studies from Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Pfizer, and Boehringer Ingelheim; has served on advisory boards for Novo Nordisk, Sanofi-Aventis, Lilly, and Merck Sharp & Dohme; and is Chair of the Ethnicity Subgroup of Scientific Advisory Group for Emergencies (SAGE) and member of SAGE. JV is the National Clinical Director for Diabetes and Obesity at NHS England and NHS Improvement. EV declares no competing interests.
Acknowledgments
Acknowledgments
NHS England and NHS Improvement provided the infrastructures for the data repository and data linkages for these analyses and the salaries for the data analysts who contributed to this work. SM is supported by a Future Leaders Mentorship award from the European Federation for the Study of Diabetes and by the National Institute for Health Research (NIHR) Biomedical Research Centre at Imperial College Healthcare NHS Trust. KK is supported by the UK NIHR Applied Research Collaboration East Midlands and the NIHR Leicester Biomedical Research Centre.
Contributors
SM, EB, EV, ST, KD, PK, BY, KK, and JV conceived the study. SM, EB, and EV managed the data and did the statistical analyses. SM, EB, and JV had full access to the data and verified the data presented. All authors contributed to drafting of each version of the manuscript. All authors had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Dhatariya K. Glaser, Codner E, Umpierrez GE. Diabetic ketoacidosis. Nat Rev Dis Primers. 2020;6:41. doi: 10.1038/s41572-020-0165-1. [DOI] [PubMed] [Google Scholar]
- 2.Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8:823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22:1935–1941. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heaney AI, Griffin GD, Simon EL. Newly diagnosed diabetes and diabetic ketoacidosis precipitated by COVID-19 infection. Am J Emerg Med. 2020;38:2491.e3–2491.e4. doi: 10.1016/j.ajem.2020.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chee YJ, Ng SJH, Yeoh E. Diabetic ketoacidosis precipitated by COVID-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;164 doi: 10.1016/j.diabres.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoe Chan K, Thimmareddygari D, Ramahi A, Atallah L, Baranetsky NG, Slim J. Clinical characteristics and outcome in patients with combined diabetic ketoacidosis and hyperosmolar hyperglycemic state associated with COVID-19: a retrospective, hospital-based observational case series. Diabetes Res Clin Pract. 2020;166 doi: 10.1016/j.diabres.2020.108279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim NY, Ha E, Moon JS, Lee YH, Choi EY. Acute hyperglycemic crises with coronavirus disease-19: case reports. Diabetes Metab J. 2020;44:349–353. doi: 10.4093/dmj.2020.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armeni E, Aziz U, Qamar S, et al. Protracted ketonaemia in hyperglycaemic emergencies in COVID-19: a retrospective case series. Lancet Diabetes Endocrinol. 2020;8:660–663. doi: 10.1016/S2213-8587(20)30221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croft A, Bucca A, Jansen JH, et al. First-time diabetic ketoacidosis in type 2 diabetics with COVID-19 infection: A novel case series. J Emerg Med. 2020;59:e193–e197. doi: 10.1016/j.jemermed.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misra S, Khozoee B, Huang J, et al. Comparison of diabetic ketoacidosis in adults during the SARS-CoV-2 outbreak and over the same time period for the preceding 3 years. Diabetes Care. 2021;44:e29–e31. doi: 10.2337/dc20-2062. [DOI] [PubMed] [Google Scholar]
- 11.Unsworth R, Wallace S, Oliver NS, et al. New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K. Diabetes Care. 2020;43:e170–e171. doi: 10.2337/dc20-1551. [DOI] [PubMed] [Google Scholar]
- 12.Kamrath C, Mönkemöller K, Biester T, et al. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID-19 pandemic in Germany. JAMA. 2020;324:801–804. doi: 10.1001/jama.2020.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tittel SR, Rosenbauer J, Kamrath C, et al. Did the COVID-19 lockdown affect the incidence of pediatric type 1 diabetes in Germany? Diabetes Care. 2020;43:e172–e173. doi: 10.2337/dc20-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasquel FJ, Messler J, Booth R, et al. Characteristics of and mortality associated with diabetic ketoacidosis among US patients hospitalized with or without COVID-19. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F, Wang H, Fan J, Zhang Y, Wang H, Zhao Q. Pancreatic injury patterns in patients with coronavirus disease 19 pneumonia. Gastroenterology. 2020;159:367–370. doi: 10.1053/j.gastro.2020.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bornstein SR, Dalan R, Hopkins D, Mingrone G, Boehm BO. Endocrine and metabolic link to coronavirus infection. Nat Rev Endocrinol. 2020;16:297–298. doi: 10.1038/s41574-020-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NHS Digital National Diabetes Audit Data Collection. 2021. https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-audit
- 18.Ministry of Housing. Communities & Local Government National statistics: English indices of deprivation 2019. Sept 26, 2019. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019
- 19.Office for National Statistics Lower layer Super Output Area population estimates. Sept 9, 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/lowersuperoutputareamidyearpopulationestimates
- 20.Umpierrez GE, Kitabchi AE. Diabetic ketoacidosis: risk factors and management strategies. Treat Endocrinol. 2003;2:95–108. doi: 10.2165/00024677-200302020-00003. [DOI] [PubMed] [Google Scholar]
- 21.Zhong VW, Juhaeri J, Mayer-Davis EJ. Trends in hospital admission for diabetic ketoacidosis in adults with type 1 and type 2 diabetes in England, 1998–2013: a retrospective cohort study. Diabetes Care. 2018;41:1870–1877. doi: 10.2337/dc17-1583. [DOI] [PubMed] [Google Scholar]
- 22.Ho J, Rosolowsky E, Pacaud D, et al. Diabetic ketoacidosis at type 1 diabetes diagnosis in children during the COVID-19 pandemic. Pediatr Diabetes. 2021;22:552–557. doi: 10.1111/pedi.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO COVID-19 significantly impacts health services for noncommunicable diseases. June 1, 2020. https://www.who.int/news/item/01-06-2020-covid-19-significantly-impacts-health-services-for-noncommunicable-diseases
- 24.Chudasama YV, Gillies CL, Zaccardi F, et al. Impact of COVID-19 on routine care for chronic diseases: a global survey of views from healthcare professionals. Diabetes Metab Syndr. 2020;14:965–967. doi: 10.1016/j.dsx.2020.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazzerini M, Barbi E, Apicella A, Marchetti F, Cardinale F, Trobia G. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health. 2020;4:e10–e11. doi: 10.1016/S2352-4642(20)30108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valabhji J, Barron E, Bradley D, Bakhai C, Khunti K, Jebb S. Impact of the COVID-19 pandemic on body weight in people at high risk of type 2 diabetes referred to the English National Health Service Diabetes Prevention Programme. Lancet Diabetes Endocrinol. 2021 doi: 10.1016/S2213-8587(21)00218-7. published online Sept 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubino F, Amiel SA, Zimmet P, et al. New-onset diabetes in COVID-19. N Engl J Med. 2020;383:789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol. 2020;18:2128. doi: 10.1016/j.cgh.2020.04.040. 30.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller JA, Groß R, Conzelmann C, et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3:149–165. doi: 10.1038/s42255-021-00347-1. [DOI] [PubMed] [Google Scholar]
- 31.Kusmartseva I, Wu W, Syed F, et al. Expression of SARS-CoV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metab. 2020;32:1041. doi: 10.1016/j.cmet.2020.11.005. 51.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pal R, Banerjee M, Yadav U, Bhattacharjee S. Clinical profile and outcomes in COVID-19 patients with diabetic ketoacidosis: a systematic review of literature. Diabetes Metab Syndr. 2020;14:1563–1569. doi: 10.1016/j.dsx.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.VanderWeele J, Pollack T, Oakes DJ, et al. Validation of data from electronic data warehouse in diabetic ketoacidosis: caution is needed. J Diabetes Complications. 2018;32:650–654. doi: 10.1016/j.jdiacomp.2018.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the NDA can be requested through the NHS Digital Data Access Request Service process at: https://digital.nhs.uk/services/data-access-request-service-dars/data-access-request-service-dars-process.