Abstract
Chlamydia pneumoniae infection has been associated with asthma and atherosclerosis. Smooth muscle cells represent host cells for chlamydiae during chronic infection. In this study we demonstrated that C. pneumoniae infection of human smooth muscle cells in vitro increased production of interleukin 6 (IL-6) and basic fibroblast growth factor (bFGF) as shown by reverse transcription-PCR, immunoblotting, and enzyme-linked immunosorbent assay. In contrast, levels of platelet-derived growth factor A-chain mRNA were not affected after infection. The stimulation of bFGF and IL-6 production was most effective when viable chlamydiae were used as inoculum. Furthermore, inhibition of bacterial protein synthesis with chloramphenicol prevented up-regulation of IL-6 and bFGF in infected cells. Addition of IL-6 antibody to infected cultures diminished bFGF expression, indicating involvement of produced IL-6. These findings suggest that chlamydial infection of smooth muscle cells elicits a cytokine response that may contribute to structural remodeling of the airway wall in chronic asthma and to fibrous plaque formation in atherosclerosis.
Chlamydia pneumoniae (in a recent paper renamed Chlamydophila pneumoniae) is an obligate intracellular bacterial pathogen that causes acute respiratory infections (9). Moreover, chronic or recurrent chlamydial infections have been associated with asthma and atherosclerosis. C. pneumoniae has a biphasic growth cycle. Infectious elementary bodies (EBs) enter the host cell and differentiate into reticulate bodies (RBs). These RBs divide by binary fission within the expanding endosome, resulting in development of an intracellular inclusion. After a period of growth, RBs reorganize into new EBs that are released by host cell lysis or exocytosis. Chronic infections are obviously associated with lytic and nonlytic phases in which chlamydiae do not replicate.
Evidence for C. pneumoniae in asthma comes from serodiagnostic studies and culture (3, 13, 14). The association of asthma with elevated specific immunoglobulin G (IgG) antibodies seems to be strongest for nonatopic long-standing asthma (37). These studies suggest an important role for chronic infection as a promoting factor that would produce a tendency to severe chronic asthma. It is possible that chlamydiae amplify the inflammation in patients with early mild asthma, leading to permanent changes in the airways (37). Furthermore, C. pneumoniae can probably initiate adult-onset asthma (15). Activation of a synthetic phenotype of smooth muscle cells (SMC) plays an important role in the pathogenesis of asthma (17). Chronic inflammation and cycles of repair in chronic asthma lead to structural remodeling of the airway wall. This process is characterized by smooth muscle hyperplasia and hypertrophy and by thickening of the basement membrane with deposition of collagen types III and V (31, 33). The increase in the amount of SMC results in an enhanced contractile response and in irreversible airflow obstruction.
The pathogenesis of atherosclerosis also involves an abnormal proliferation of SMC within the arterial wall (32). An association of Chlamydia infection with atherosclerosis has been demonstrated by seroepidemiological studies in which raised titers of IgG and IgA antibodies to C. pneumoniae were found in patients with coronary arterial disease (reviewed in reference 11). The organism has been detected in atherosclerotic lesions and could be cultured from plaques from patients with severe coronary heart disease (24, 29, 39).
Several growth factors and cytokines, such as basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), and interleukin 6 (IL-6), have been implicated in the processes of airway wall thickening and subepithelial fibrosis in asthma as well as fibrous plaque formation in atherosclerosis (17, 32, 40).
SMC are a cell type that is infected by C. pneumoniae during chronic infection. In atheromatous plaques, chlamydiae were prominently observed in SMC by immunohistochemical staining (39). C. pneumoniae is capable of infecting SMC in vitro (20). However, the effects of chlamydial infection on SMC have been little examined. Therefore, we studied the production of IL-6, bFGF, and PDGF by human SMC in response to infection with C. pneumoniae.
MATERIALS AND METHODS
Chlamydia propagation.
High-titer stocks of C. pneumoniae strain TW-183 and Chlamydia trachomatis serovar D strain IC Cal 8 (hereafter called C. trachomatis D/IC Cal 8; obtained from the Institute of Ophthalmology, London, United Kingdom) were propagated in buffalo green monkey (BGM) cells. Chlamydiae were inoculated onto cell monolayers in 25-cm2 flasks and centrifuged at 2,000 × g for 45 min at 37°C. The inoculum was removed and replaced with serum-free SF-3 medium (Cytogen, Lohmar, Germany). Infected cells were collected in phosphate-buffered saline (PBS) with 0.2 M sucrose and 2% fetal bovine serum (FBS) 72 h after infection and lysed by sonication. The chlamydial suspension was centrifuged at 800 × g to remove cellular debris. Supernatants were stored at −70°C. Infectivity titers of chlamydial stocks were quantified by titrating the number of inclusion-forming units (IFU) per milliliter in BGM cells. These titers were used to determine the infectious doses for SMC. Cell cultures and chlamydial stocks were checked for Mycoplasma contamination by culture and PCR by the Bundesinstitut für Gesundheitlichen Verbraucherschutz und Veterinärmedizin Jena, Jena, Germany.
Cell culture.
Human bronchial SMC (BSMC; Clonetics CC-2576) were purchased from BioWhittaker Europe (Verviers, Belgium). The cells were subcultured in modified MCDB 131 basal medium (Clonetics, BioWhittaker), containing 5% FBS and the following supplements (Clonetics, BioWhittaker): human recombinant epidermal growth factor (0.5 ng/ml), human recombinant bFGF (2 ng/ml), insulin (5 μg/ml), gentamicin (50 μg/ml), and amphotericin B (50 ng/ml). The cells stained positively with antibodies to smooth muscle α-actin (monoclonal mouse IgG, clone 1A4; Dako, Hamburg, Germany). BSMC at passages 4 to 6 were used in the experiments.
Infection of SMC.
BSMC were seeded into 35-mm-diameter culture wells or into 11-mm-diameter culture tubes containing a glass coverslip. Cells grew to confluence and were then made quiescent by maintenance in basal medium containing 0.5% FBS but no antibiotics for 72 h.
Chlamydial stocks were diluted in basal medium without supplements and inoculated onto cell monolayers. BSMC (4 × 105 to 7 × 105 per 35-mm-diameter well, 6 × 104 to 8 × 104 per 11-mm-diameter tube) were infected by centrifugation at 2,000 × g at 37°C for 45 min at different infectious doses. After the inoculum was decanted, the cells were washed in medium to remove nonadsorbed chlamydiae and further incubated with basal medium containing 0.5% FBS but no antibiotics. For mock-infected cultures, BSMC were centrifuged with a harvest of uninfected BGM cells.
For determination of chlamydial inclusions, coverslips were removed from the culture tubes at 72 h after infection, fixed with methanol, and stained with fluorescein isothiocyanate-conjugated antibody to chlamydial lipopolysaccharide (LPS) (Imagen; Dako). The number of inclusions per coverslip was calculated from determination of inclusions in 20 randomly selected ×400 microscopic fields.
For heat inactivation, chlamydial suspensions were held at 75°C for 10 min prior inoculation onto cell monolayers. For UV inactivation, chlamydial suspensions were placed under a UV lamp (15 W at 30 cm) for 15 min. In some experiments as indicated, chlamydial protein synthesis was inhibited with chloramphenicol (100 μg/ml; Sigma, Deisenhofen, Germany). Cell cultures were treated with chloramphenicol from 90 min prior to infection to 18 h after infection. Rabbit anti-human IL-6 (Sigma) was used to neutralize IL-6 activity in infected cultures.
RNA extraction and reverse transcription-PCR (RT-PCR) analysis.
Total RNA was prepared from cell monolayers using the RNAgents total RNA isolation system (Promega, Mannheim, Germany) according to the manufacturer's instructions. First-strand cDNA was reverse transcribed from 1 μg of RNA in a total reaction volume of 20 μl with 15 U of avian myeloblastosis virus reverse transcriptase and 0.5 μg of oligo(dT)15 primer, using the Promega reverse transcription system as instructed by the manufacturer.
Each 25 μl of PCR mixture contained 1 μl of cDNA (corresponding to 50 ng of RNA), 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 0.2 mM each deoxynucleoside triphosphate, 1.5 mM MgCl2, 0.4 μM specific primers (Table 1), and 1.25 U of Taq DNA polymerase (Promega). As an internal control, cDNA was also amplified for pyruvate dehydrogenase (PDH) mRNA. Thirty cycles of amplification were carried out in a TRIO-Thermoblock (Biometra, Göttingen, Germany). Reactions consisted of an initial incubation at 95°C for 7 min and then cycling at 95°C for 30 s, 60°C (PDH, bFGF, and PDGF-A primers) or 65°C (IL-6 primers) for 1 min, and 72°C for 1 min, with a final incubation at 72°C for 10 min. Negative controls were performed by omitting RNA from cDNA synthesis and PCR amplification. Products were electrophoresed on a 2% agarose gel containing SYBR Green I (Molecular Probes; purchased from MoBiTec, Göttingen, Germany) and visualized on a UV transilluminator. For comparison of the bands, photographs of the products were scanned and the volumes (optical density times millimeters squared) of the band images were quantitated with Multi Analyst software (Bio-Rad, Munich, Germany). All IL-6, bFGF, and PDGF-A signals were normalized against the PDH signal from the same sample. All amplification results shown are representative of three separate experiments.
TABLE 1.
Sequences of primers used for RT-PCR
| Specificity | Primer paira | Product size (bp) | Reference |
|---|---|---|---|
| IL-6 | 5′-AGCTCAGCTATGAACTCCTTCTC-3′ | 338 | 22 |
| 5′-GTCTCCTCATTGAATCCAGATTGG-3′ | |||
| bFGF | 5′-TCACCACGCTGCCCGCCTTGC-3′ | 342 | 23 |
| 5′-CAGTTCGTTTCAGTGCCACAT-3′ | |||
| PDGF-A | 5′-CCTGCCCATTCGGAGGAAGAG-3′ | 225 | 38 |
| 5′-TTGGCCACCTTGACGCTGCG-3′ | |||
| PDH | 5′-GGTATGGATGAGGACCTGGA-3′ | 105 | 35 |
| 5′-CTTCCACAGCCCTCGACTAA-3′ |
Top row, sense strand; bottom row, antisense strand.
The specificity of the PCR products was confirmed by sequencing of the amplified bands. Sequences were determined by the dideoxy-chain termination technique using a BigDye terminator kit (Applied Biosystems, Weiterstadt, Germany) according to the supplier's protocol. Automated sequencing was performed with an ABI Prism 310 genetic analyzer (Perkin-Elmer, Applied Biosystems).
Immunoblotting.
Cell monolayers were washed twice with cold PBS followed by adding 100 μl of radioimmunoassay buffer (0.15 M NaCl, 50 mM Tris-HCl, 1% deoxycholic acid, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS]) with phenylmethylsulfonyl fluoride (100 μg/ml; Serva, Heidelberg, Germany), leupeptin (2 μg/ml; Serva), and aprotinin (50 μg/ml; Sigma). The cells were scraped, and the suspensions were incubated on ice for 30 min. The cell lysates were centrifuged in a microcentrifuge, and the supernatants were mixed with an equal volume of Laemmli sample buffer. The samples were electrophoresed on 15% polyacrylamide–SDS gels. Transfer of fractionated proteins to nitrocellulose membranes was carried out with a semidry transblot system (Bio-Rad) using Bjerrum and Schafer-Nielsen transfer buffer (48 mM Tris, 39 mM glycine, 1.3 mM SDS, 20% methanol [pH ∼9]). Blots were blocked with 4% bovine serum albumin (BSA; Sigma) in Tris-buffered saline (TBS) containing 0.05% Tween for 4 h. For identification of bFGF, blots were sequentially incubated with a 1:500 dilution of mouse monoclonal antibody to human bFGF (clone FB-8; Sigma) and with a 1:2,000 dilution of alkaline phosphatase-conjugated goat anti-mouse IgG (Dianova, Hamburg, Germany). Primary and secondary antibodies were diluted in TBS-Tween with 4% BSA. Blots were incubated with each antibody for 1 h at room temperature. Washes between antibody addition were performed with TBS-Tween three times for 5 min. Blots were developed with 5-bromo-4-chloro-3-indolylphosphate toluidine salt–p-nitroblue tetrazolium chloride (Sigma Fast; Sigma). All results shown are representative of three experiments.
IL-6 and bFGF immunoassays.
For enzyme immunoassays, supernatants of infected and mock-infected cultures were centrifuged at 14,000 × g for 5 min and stored at −70°C until assayed. Levels of IL-6 were measured by IL-6 CytoSet enzyme-linked immunosorbent assay (ELISA) (Biosource, Ratingen, Germany) according to the manufacturer's protocol.
To quantify bFGF levels in culture supernatants, plastic wells (Maxisorp ImmunoModuls; Nunc, Wiesbaden, Germany) were coated with 200 μl of samples or human recombinant bFGF standard (Biochrom, Berlin, Germany) per well overnight at 4°C. The plates were then washed three times with TBS-Tween and blocked with 4% BSA in TBS-Tween for 3 h at room temperature. Following a further three washes, plates were sequentially incubated with mouse monoclonal antibody to human bFGF (clone FB-8; Sigma) diluted 1:1,000 in 4% BSA-TBS-Tween (200 μl/well) for 2 h, biotinylated F(ab′)2 fragment of rabbit anti-mouse IgG (Dako) at a dilution of 1:5,000 (200 μl/well) for 1 h, and streptavidin-peroxidase conjugate (Dako) diluted 1:5,000 (200 μl/well) for 1 h. After each incubation, the plates were washed three times with TBS-Tween. For signal development, the wells were incubated with tetramethylbenzidine substrate (Sigma). The reaction was stopped with 1.5 M H2SO4. Absorbance was measured by optical density reading at 450 nm with an SLT Rainbow spectrophotometer (Tecan, Crailsheim, Germany). The amounts of bFGF were determined by reference curves obtained using known quantities of bFGF standard.
RESULTS
Growth of C. pneumoniae in BSMC.
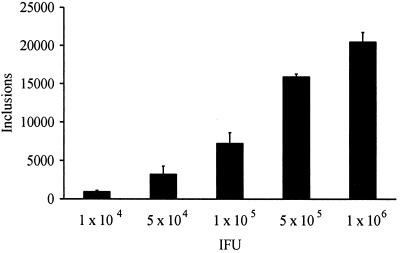
First experiments confirmed earlier observations that C. pneumoniae is capable of infecting SMC (20). Infection of BSMC with C. pneumoniae TW-183 resulted in intracellular growth characterized by the development of typical inclusion bodies. Increasing the number of IFU caused a concomitant increase in inclusion-containing cells (Fig. 1). An infectious dose of 106 IFU as titrated in BGM cells produced an average of about 2 × 104 inclusions per culture tube in BSMC.
FIG. 1.
Infectivity of C. pneumoniae TW-183 for BSMC. Intracellular inclusions were stained with fluorescein isothiocyanate-conjugated antibody to LPS (Imagen; Dako). Data are expressed as mean numbers (with standard deviations) of inclusions per 11-mm-diameter coverslip.
Production of bFGF, IL-6, and PDGF-A in BSMC exposed to C. pneumoniae.
The levels of mRNAs were assessed by RT-PCR. After serum starvation, mock-infected cells produced only small amounts of bFGF, IL-6, and PDGF-A mRNA over a 48-h incubation (Fig. 2). Exposure to C. pneumoniae enhanced the amounts of IL-6 and bFGF mRNA in a dose-dependent manner (Fig. 2A). Levels of IL-6 and bFGF mRNA in infected cells increased over the 48-h period of the experiments in comparison to mock-infected cells (Fig. 2B). In contrast, mRNA levels for PDGF-A and the housekeeping gene PDH were not affected by chlamydial infection of BSMC (Fig. 2).
FIG. 2.
C. pneumoniae infection increases levels of IL-6 and bFGF mRNA in cultured BSMC. (A) Amplification of cDNA from RNA preparations of cells infected with various doses of TW-183. Lane 1, mock-infected cells; lane 2, 5 × 106 IFU/well; lane 3, 107 IFU/well; lane 4, 2 × 107 IFU/well. Total RNA was extracted at 18 h after infection. (B) Time course of mRNA expression. Lanes 5, 7, and 9, mock-infected cells; lanes 6, 8, and 10, 107 IFU/well.
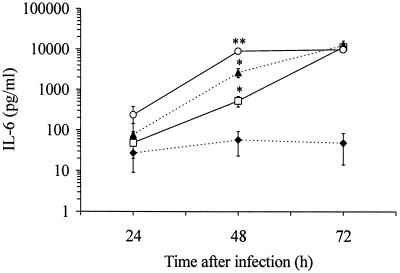
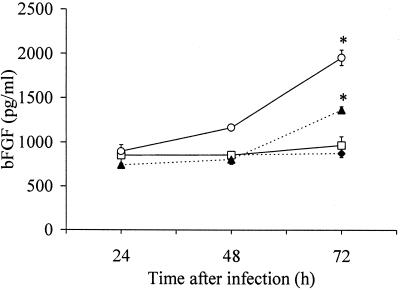
Further experiments were performed to determine the production of IL-6 and bFGF protein. Cells infected with C. pneumoniae secreted increased quantities of IL-6 in a time-dependent fashion (Fig. 3). Following 48 h of infection, significant differences in IL-6 release were observed in response to increasing infectious doses (Fig. 3). The time course of IL-6 secretion was dependent on the infectious dose. At the highest inoculum used, IL-6 production peaked at 48 h after infection, whereas at lower infectious doses IL-6 levels increased over a 72-h period of time. Infected cells released 200-fold more IL-6 than mock-infected cells. A feature of bFGF is that it lacks a signal peptide for secretion and is cell associated rather than secreted (1). Therefore, production of bFGF protein was examined by immunoblot analysis of total cell lysates. Immunoblots of BSMC showed a bFGF band of approximately 21 to 24 kDa. The amount of bFGF in infected cultures increased with larger amounts of the infectious dose as well as over a 72-h period of time (Fig. 4). Immunoblots of mock-infected cells indicated no substantial differences during the same incubation time. Additionally, amounts of bFGF in culture supernatants were slightly greater in infected cultures than in mock-infected cultures at 72 h after infection (Fig. 5). The bFGF level in infected cultures was increased about twofold.
FIG. 3.
Release of IL-6 by C. pneumoniae-infected BSMC in comparison to mock-infected cells. IL-6 was determined by ELISA. ⧫, mock infected; □, 5 × 105 IFU; ▴, 106 IFU; ○, 2 × 106 IFU. Values are means (with standard deviations) of three experiments. ∗, P ≤ 0.04 compared to mock-infected cells; ∗∗, P ≤ 0.01 compared to mock-infected cells (Welch t test).
FIG. 4.
Immunoblot analysis of bFGF protein in C. pneumoniae-infected BSMC. (A) bFGF levels in cells infected with various doses of chlamydiae. Lane 1, mock-infected cells; lane 2, 2 × 106 IFU/well; lane 3, 5 × 106 IFU/well; lane 4, 2 × 107 IFU/well. Total cell lysates were prepared at 48 h postinfection. (B) Time course of bFGF production. Lanes 1, 3, and 5, mock-infected cells; lanes 2, 4, and 6, 107 IFU/well.
FIG. 5.
Detection of bFGF in culture supernatants of BSMC by ELISA. ⧫, mock infected; □, 5 × 105 IFU; ▴, 106 IFU; ○, 2 × 106 IFU. Values are means (with standard deviations) of three experiments. ∗, P ≤ 0.03 compared to mock-infected cells (Welch t test).
Requirement for live chlamydiae in bFGF and IL-6 response of BSMC to C. pneumoniae.
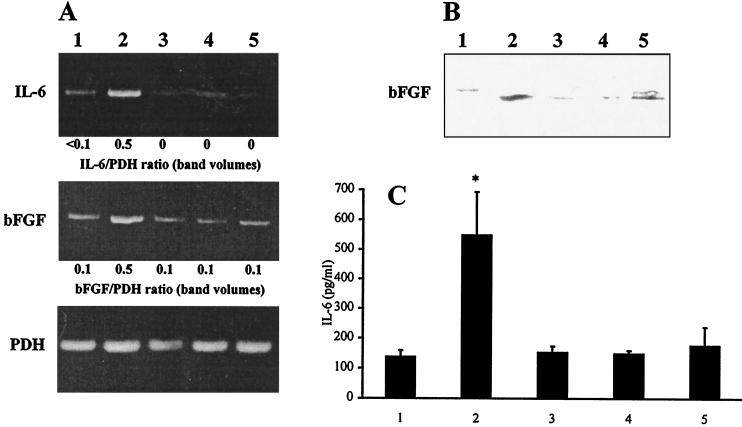
The increased levels of IL-6 and bFGF in infected cultures could be caused by the extracellular presence of chlamydiae or by events associated with the invasion process and intracellular growth. Heat and UV treatment of the chlamydial inoculum reduced the levels of IL-6 and bFGF to that of mock-infected cultures (Fig. 6). Heat inactivation of chlamydiae can block their uptake by host cells but does not affect chlamydial LPS. UV-treated chlamydiae may still be able to invade cells (25, 30). Inactivated chlamydiae formed no inclusions in BSMC or in BGM cells which were used for titration of chlamydial stocks.
FIG. 6.
Effects of heat and UV inactivation of chlamydiae, and of chloramphenicol treatment, on IL-6 and bFGF levels in BSMC cultures. (A) Detection of IL-6 and bFGF mRNA by RT-PCR. Total RNA was isolated at 18 h postinfection. (B) Immunoblotting of bFGF protein. Cell lysates were prepared at 48 h postinfection. (A and B) Cells grown in 35-mm-diameter culture wells were infected with 107 IFU/well or centrifuged with inactivated chlamydiae (corresponding to 107 IFU). (C) Detection of IL-6 in culture supernatants by ELISA. IL-6 concentrations were measured at 48 h postinfection. Cells grown in 11-mm-diameter culture tubes were infected with 5 × 105 IFU or centrifuged with inactivated chlamydiae (corresponding to 5 × 105 IFU). Lane 1, mock-infected cells; lane 2, infected cells; lane 3, cells inoculated with heat-inactivated chlamydiae; lane 4, cells inoculated with UV-inactivated chlamydiae; lane 5, infected cells treated with chloramphenicol. ∗, P ≤ 0.01 compared to lanes 1, 3, 4, and 5 (Welch t test).
To determine whether bacterial protein synthesis is required for the host cell response, infected cells were treated with chloramphenicol at 100 μg/ml, a concentration which completely inhibits chlamydial protein synthesis. In chloramphenicol-treated cultures, no inclusion bodies of C. pneumoniae could be found. The addition of chloramphenicol to infected cultures prevented the stimulation of IL-6 and bFGF production (Fig. 6).
Effect of IL-6 antibody on bFGF levels.
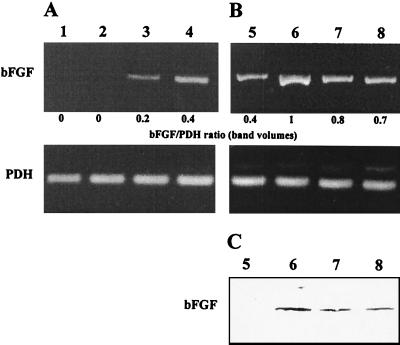
IL-6 stimulated expression of bFGF mRNA in uninfected cells (Fig. 7A). We tested whether the release of IL-6 contributed to the increased amounts of bFGF in Chlamydia-infected cultures. Neutralization of IL-6 resulted in less intense bands of bFGF in RT-PCR assays and immunoblots of infected cells (Fig. 7B and C). These findings suggest that bFGF levels were mediated by the release of IL-6.
FIG. 7.
Role of IL-6 in mediating bFGF production in BSMC following C. pneumoniae infection. (A) Effect of IL-6 on bFGF mRNA levels in uninfected cells. RT-PCR analysis was conducted on total RNA extracted from BSMC incubated with IL-6 at 0 (lane 1), 1 (lane 2), 5 (lane 3), and 20 (lane 4) ng/ml for 6 h. (B and C) Effect of IL-6 antibody on C. pneumoniae-stimulated bFGF synthesis as determined by RT-PCR analysis (B) and immunoblotting (C). Lanes 5, mock-infected cells; lanes 6, cells infected with 107 IFU/well; lanes 7 and 8, infected cells incubated in the presence of 1,000 and 4,000 nU of IL-6 antibody per ml, respectively.
Infection of BSMC with C. trachomatis.
To examine whether other Chlamydia species are able to stimulate IL-6 and bFGF production from BSMC, we performed some experiments with C. trachomatis D/IC Cal 8, a strain which readily infects freshly isolated human synovial fibroblasts (34). BSMC supported replication of C. trachomatis. In comparison to C. pneumoniae TW-183, C. trachomatis D/IC Cal 8 produced inclusions that were larger in size but fewer in number (Fig. 8; Table 2). Increased IL-6 production was observed in response to C. trachomatis compared to mock-infected cells, although IL-6 levels were significantly higher in C. pneumoniae-infected cultures (Table 2). Additionally, C. trachomatis infection caused an increase in bFGF protein levels in BSMC (Fig. 9).
FIG. 8.
Morphology of C. pneumoniae and C. trachomatis inclusions in BSMC. Monolayers were stained with fluorescein isothiocyanate-conjugated antibody to chlamydial LPS (Imagen; Dako). (A) C. pneumoniae TW-183 at 72 h postinfection; (B) C. trachomatis D/IC Cal 8 at 48 h postinfection. Magnification, ×400.
TABLE 2.
Comparison of C. trachomatis and C. pneumoniae infection of BSMC
| Treatmenta | No. of inclusionsb | IL-6 secreted (pg/ml)c |
|---|---|---|
| Mock | 150 (40) | |
| C. trachomatis D/IC Cal 8 | 4,096 (1,006)d | 697 (187)e |
| C. pneumoniae TW-183 | 17,241 (3,138) | 6,217 (385) |
BSMC monolayers grown in 11-mm-diameter culture tubes were infected with 106 IFU of C. trachomatis D/IC Cal 8 or C. pneumoniae TW-183. Mock-infected cells were inoculated with a suspension of lysed BGM cells.
Inclusions per coverslip were counted after immunofluorescence staining at 72 h after infection. Values are means (with standard deviations) of three experiments.
IL-6 concentrations in culture supernatants were determined by ELISA at 48 h after infection. Results are means (with standard deviations) of three experiments.
Significantly different from values for C. pneumoniae-infected cells (Welch t test, P ≤ 0.03).
Significantly different from values for C. pneumoniae-infected cells (Welch t test, P ≤ 0.001) and mock-infected cells (Welch t test, P ≤ 0.04).
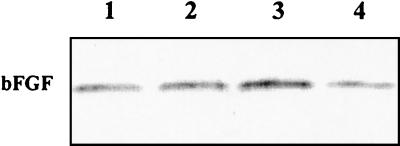
FIG. 9.
Immunoblot analysis of bFGF protein in BSMC infected with C. trachomatis serotype D. Lane 1, 5 × 106 IFU/well; lane 2, 107 IFU/well; lane 3, 2 × 107 IFU/well; lane 4, mock-infected cells. Total cell lysates were prepared at 48 h postinfection.
DISCUSSION
Interactions between SMC and chlamydiae are probably involved in the pathogenesis of chronic infections with C. pneumoniae. Our study demonstrates significant production of bFGF and IL-6 from SMC in response to C. pneumoniae infection. IL-6, an important cytokine in inflammation, has been implicated in instability of atherosclerotic plaques (40). It can increase platelet activity and fibrinogen levels, which would lead to increased blood viscosity and endothelial damage (27). Increased basal levels of IL-6 were found in the airways of asthmatic patients compared with normal control subjects (41). In addition, IL-6 concentrations in bronchoalveolar fluid are higher in patients with intrinsic asthma than in patients with allergic asthma (36). A mitogenic role of IL-6 for rat vascular and guinea pig airway SMC has been reported (7, 18). While this work was in progress, studies that describe the enhanced production of IL-6 by C. pneumoniae-infected endothelial cells and by SMC exposed to chlamydial heat shock protein 60 have been published (8, 21). Furthermore, it is known that monocytic cells secrete IL-6 in response to chlamydial infection (16). These in vitro findings suggest that overexpression of IL-6 may be an important factor in the immunopathogenesis of infections with C. pneumoniae.
Our study shows that chlamydial infection of BSMC stimulates bFGF production. bFGF is a multifunctional cytokine involved in proliferation and differentiation of many cell types. bFGF is thought to function as a key mitogen of SMC replication in atherosclerosis and asthma (6, 17, 32). The factor lacks a signal peptide for secretion and resides in an intracellular pool. It could be liberated from infected cells during host cell lysis at the end of the chlamydial growth cycle. Released bFGF may act as a paracrine regulator that stimulates adjoining viable SMC to proliferate. An important role of this factor for medial SMC replication in atherosclerosis has been suggested (32). bFGF can up-regulate expression of interstitial collagenase which mediates the turnover of the extracellular matrix (19). This turnover may support migration and proliferation of SMC.
In contrast to IL-6 and bFGF, levels of PDGF-A mRNA in SMC were not increased after chlamydial infection. PDGF, a growth factor for several cell types, consists of two peptide chains, so that AA, BB, or AB dimers are produced by different cell types. SMC are a primary source of PDGF-AA (1). Levels of PDGF-AA are not raised in asthma (2). However, PDGF has been implicated in proliferation and migration of SMC in atherosclerosis (5).
The time course of IL-6 and bFGF production confirmed that chlamydiae are slow inducers of cellular cytokine responses in contrast to other invasive bacterial pathogens (30). IL-6 and bFGF production was not examined later than 72 h after infection because cytopathic effects characterized by cell lysis began to occur at this time. The detection of increased bFGF levels in culture supernatants after 3 days of infection may be explained by beginning host cell lysis. Heat- and UV-inactivated chlamydiae failed to enhance production of IL-6 and bFGF. These results suggest that a heat-labile component or invasion of host cells may be important for stimulating synthesis of these factors. The results obtained with heat-inactivated chlamydiae support the conclusion that chlamydial LPS at an extracellular stage is not responsible for eliciting production of IL-6 and bFGF. The absence of increased production of both factors by Chlamydia-infected cells treated with chloramphenicol indicates a role of early metabolism of TW-183 in induction of the cellular response. These findings correspond to studies of the cytokine response of epithelial cells to C. trachomatis infection and to studies of IL-8 and monocyte chemotactic protein 1 production by C. pneumoniae-infected endothelial cells (25, 26, 30). However, it cannot be excluded that the release of LPS from the chlamydial envelope once organisms are intracellular might provide a source of bioactive LPS for induction of signaling pathways.
Coombs and Mahony recently reported that conditioned medium from C. pneumoniae-infected endothelial cell cultures stimulates replication of SMC (4). Whether conditioned medium from Chlamydia-infected SMC is mitogenic for uninfected SMC in vitro remains to be investigated. It cannot be excluded that infected SMC also release growth inhibitory cytokines, such as beta interferon (28).
The ability to stimulate bFGF and IL-6 production by SMC was not a unique property of C. pneumoniae. C. trachomatis serotype D also caused an up-regulation of both factors, although not at the same extent as C. pneumoniae TW-183. However, C. trachomatis has not been associated with chronic respiratory diseases and was not detected in atherosclerotic tissue as revealed by immunocytochemistry (12, 39). The possibility exists that C. trachomatis strains which cause ocular and genital infections are not easily disseminated to multiple organs, in contrast to respiratory C. pneumoniae infections. A recent study has shown that pulmonary infection with C. trachomatis mouse pneumonitis strain can induce a cardiovascular pathology in mice (10). However, these inflammatory and fibrotic changes were different from the pathology of atherosclerosis (10). In comparison to C. pneumoniae TW-183, C. trachomatis serovar D formed fewer but larger inclusions in BSMC and caused only a slight increase in IL-6 levels. The lesser ability of C. trachomatis to activate SMC would provide a strong argument for a specific role of C. pneumoniae in atherosclerosis. Therefore, a greater number of C. pneumoniae and C. trachomatis strains should be compared to determine whether fundamental differences between the two species in infection and activation of SMC exist.
In conclusion, this report demonstrates that C. pneumoniae activates SMC to produce factors that are obviously involved in the pathogenesis of atherosclerosis and airway remodeling in asthma. Since C. pneumoniae is an obligate intracellular parasite, it is not probable that this pathogen acts as an innocent bystander in these diseases. However, the pathological significance of Chlamydia in chronic inflammatory diseases is not well understood. Examination of interactions of chlamydiae with their host cells may contribute to elucidating a potential role of C. pneumoniae as a causative or modulatory factor in nonatopic asthma and atherosclerosis.
ACKNOWLEDGMENTS
This work was supported by grant BMBF 01ZZ9602 from the Bundesministerium für Bildung und Forschung.
We thank the collaborators of the Bundesinstitut für Gesundheitlichen Verbraucherschutz und Veterinärmedizin Jena for performing Mycoplasma testing, C. Kroegel (University Clinic, Pneumology, Jena, Germany) for helpful discussion, and E. Birch-Hirschfeld (Institute of Virology, University of Jena) for providing oligonucleotide primers.
REFERENCES
- 1.Bonner J C, Badgett A, Lindroos P M, Coin P G. Basic fibroblast growth factor induces expression of the PDGF receptor-α on human bronchial smooth muscle cells. Am J Physiol. 1996;271:L880–L888. doi: 10.1152/ajplung.1996.271.6.L880. [DOI] [PubMed] [Google Scholar]
- 2.Chanez P, Vignola M, Stenger R, Vic P, Michel F B, Bousquet J. Platelet-derived growth factor in asthma. Allergy. 1995;50:878–883. doi: 10.1111/j.1398-9995.1995.tb02493.x. [DOI] [PubMed] [Google Scholar]
- 3.Cook P J, Davies P, Tunnicliffe W, Ayres J G, Honeybourne D, Wise R. Chlamydia pneumoniae and asthma. Thorax. 1998;53:254–259. doi: 10.1136/thx.53.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coombs B K, Mahony J B. Chlamydia pneumoniae infection of human endothelial cells induces proliferation of smooth muscle cells via an endothelial-derived soluble factor(s) Infect Immun. 1999;67:2909–2915. doi: 10.1128/iai.67.6.2909-2915.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cucina A, Pagliei S, Borrelli V, Corvino V, Stipa F, Cavallaro A, Sterpetti A V. Oxidised LDL (OxLDL) induces production of platelet derived growth factor AA (PDGF AA) from aortic smooth muscle cells. Eur J Vasc Endovasc Surg. 1998;16:197–202. doi: 10.1016/s1078-5884(98)80220-7. [DOI] [PubMed] [Google Scholar]
- 6.Davis M G, Zhou M, Ali S, Coffin J D, Doetschman T, Dorn G W., II Intracrine and autocrine effects of basic fibroblast growth factor in vascular smooth muscle cells. J Mol Cell Cardiol. 1997;29:1061–1072. doi: 10.1006/jmcc.1997.0383. [DOI] [PubMed] [Google Scholar]
- 7.De S, Zelazny E T, Souhrada J F, Souhrada M. IL-1β and IL-6 induce hyperplasia and hypertrophy of cultured guinea pig airway smooth muscle cells. J Appl Physiol. 1995;78:1555–1563. doi: 10.1152/jappl.1995.78.4.1555. [DOI] [PubMed] [Google Scholar]
- 8.Dechend R, Maass M, Gieffers J, Dietz R, Scheidereit C, Leutz A, Gulba D C. Chlamydia pneumoniae infection of vascular smooth muscle cells and endothelial cells activates NF-κB and induces tissue factor and PAI-1 expression. Circulation. 1999;100:1369–1373. doi: 10.1161/01.cir.100.13.1369. [DOI] [PubMed] [Google Scholar]
- 9.Everett K D E, Bush R M, Andersen A A. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov., and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int J Syst Bacteriol. 1999;49:415–440. doi: 10.1099/00207713-49-2-415. [DOI] [PubMed] [Google Scholar]
- 10.Fan Y, Wang S, Yang X. Chlamydia trachomatis (mouse pneumonitis strain) induces cardiovascular pathology following respiratory tract infection. Infect Immun. 1999;67:6145–6151. doi: 10.1128/iai.67.11.6145-6151.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbs R G J, Carey N, Davies A H. Chlamydia pneumoniae and vascular disease. Br J Surg. 1998;85:1191–1197. doi: 10.1046/j.1365-2168.1998.00861.x. [DOI] [PubMed] [Google Scholar]
- 12.Grayston J T, Campbell L A. The role of Chlamydia pneumoniae in atherosclerosis. Clin Infect Dis. 1999;28:993–994. doi: 10.1086/514764. [DOI] [PubMed] [Google Scholar]
- 13.Hahn D L. Intracellular pathogens and their role in asthma: Chlamydia pneumoniae in adult patients. Eur Respir Rev. 1996;6:224–230. [Google Scholar]
- 14.Hahn D L, Bukstein D, Luskin A, Zeitz H. Evidence for Chlamydia pneumoniae infection in steroid-dependent asthma. Ann Allergy Asthma Immunol. 1998;80:45–49. doi: 10.1016/S1081-1206(10)62938-9. [DOI] [PubMed] [Google Scholar]
- 15.Hahn D L, McDonald R. Can acute Chlamydia pneumoniae respiratory tract infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81:339–344. doi: 10.1016/S1081-1206(10)63126-2. [DOI] [PubMed] [Google Scholar]
- 16.Heinemann M, Susa M, Simnacher U, Marre R, Essig A. Growth of Chlamydia pneumoniae induces cytokine production and expression of CD14 in a human monocytic cell line. Infect Immun. 1996;64:4872–4875. doi: 10.1128/iai.64.11.4872-4875.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirst S J. Airway smooth muscle cell culture: application to studies of airway remodelling and phenotype plasticity. Eur Respir J. 1996;9:808–820. doi: 10.1183/09031936.96.09040808. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda U, Ikeda M, Oahara T, Oguchi A, Kamitani T, Tsuruya Y, Kano S. Interleukin 6 stimulates growth of vascular smooth muscle cells in a PDGF-dependent manner. Am J Physiol. 1991;260:H1713–H1717. doi: 10.1152/ajpheart.1991.260.5.H1713. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy S H, Ronda S, Qin H, Aho S, Selber J, Tan E M L. Basic FGF regulates interstitial collagenase gene expression in human smooth muscle cells. J Cell Biochem. 1997;65:32–41. [PubMed] [Google Scholar]
- 20.Knoebel E, Vijayagopal P, Figueroa II J E, Martin D H. In vitro infection of smooth muscle cells by Chlamydia pneumoniae. Infect Immun. 1997;65:503–506. doi: 10.1128/iai.65.2.503-506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kol A, Bourcier T, Lichtmann A H, Libby P. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J Clin Investig. 1999;103:571–577. doi: 10.1172/JCI5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotake S, Schumacher H R, Jr, Wilder R L. A simple nested RT-PCR method for quantitation of the relative amounts of multiple cytokine mRNAs in small tissue samples. J Immunol Methods. 1996;199:193–203. doi: 10.1016/s0022-1759(96)00184-6. [DOI] [PubMed] [Google Scholar]
- 23.Lambert D, Eaton C L, Harrison B J. Fibroblast growth factors and their receptors in parathyroid disease. World J Surg. 1998;22:520–525. doi: 10.1007/s002689900429. [DOI] [PubMed] [Google Scholar]
- 24.Maass M, Bartels C, Engel P M, Mamat U, Sievers H H. Endovascular presence of viable Chlamydia pneumoniae is a common phenomenon in coronary artery disease. J Am Coll Cardiol. 1998;31:827–832. doi: 10.1016/s0735-1097(98)00016-3. [DOI] [PubMed] [Google Scholar]
- 25.Molestina R E, Dean D, Miller R D, Ramirez J A, Summersgill J T. Characterization of a strain of Chlamydia pneumoniae isolated from a coronary atheroma by analysis of the omp1 gene and biological activity in human endothelial cells. Infect Immun. 1998;66:1370–1376. doi: 10.1128/iai.66.4.1370-1376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molestina R E, Miller R D, Ramirez J A, Summersgill J T. Infection of human endothelial cells with Chlamydia pneumoniae stimulates transendothelial migration of neutrophils and monocytes. Infect Immun. 1999;67:1323–1330. doi: 10.1128/iai.67.3.1323-1330.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pai J, Knoop F C, Hunter W J., III Chlamydia pneumoniae and occlusive vascular disease: identification and characterization. J Pharmacol Toxicol Methods. 1998;39:51–61. doi: 10.1016/s1056-8719(98)00002-1. [DOI] [PubMed] [Google Scholar]
- 28.Palmer H, Libby P. Interferon-β: a potential autocrine regulator of human vascular smooth muscle cell growth. Lab Investig. 1992;66:715–721. [PubMed] [Google Scholar]
- 29.Ramirez J A. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with coronary atherosclerosis. Ann Intern Med. 1996;125:979–982. doi: 10.7326/0003-4819-125-12-199612150-00008. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen S J, Eckmann L, Quayle A J, Schen L, Zhang Y-X, Anderson D J, Fierer J, Stephens R S, Kagnoff M F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Investig. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redington A E, Howarth P H. Airway remodelling in asthma. Thorax. 1997;52:310–312. doi: 10.1136/thx.52.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reidy M A. Growth factors and arterial smooth muscle cell proliferation. Ann N Y Acad Sci. 1994;714:225–230. doi: 10.1111/j.1749-6632.1994.tb12047.x. [DOI] [PubMed] [Google Scholar]
- 33.Roberts C R. Is asthma a fibrotic disease? Chest Suppl. 1995;107(Suppl.):111S–117S. doi: 10.1378/chest.107.3_supplement.111s. [DOI] [PubMed] [Google Scholar]
- 34.Rödel J, Groh A, Hartmann M, Schmidt K-H, Lehmann M, Lungershausen W, Straube E. Expression of interferon regulatory factors and indoleamine 2,3-dioxygenase in Chlamydia trachomatis-infected synovial fibroblasts. Med Microbiol Immunol. 1999;187:205–212. doi: 10.1007/s004300050094. [DOI] [PubMed] [Google Scholar]
- 35.Rolfs A, Schuller I, Finckh U, Weber-Rolfs I. PCR: clinical diagnostics and research. Berlin, Germany: Springer-Verlag; 1992. pp. 102–107. [Google Scholar]
- 36.Virchow J C, Jr, Kroegel C, Walker C, Matthys H. Inflammatory determinants of asthma severity: mediator and cellular changes in bronchoalveolar lavage fluid of patients with severe asthma. J Allergy Clin Immunol. 1996;98:S27–S33. [PubMed] [Google Scholar]
- 37.von Hertzen L, Töyrylä M, Gimishanov A, Bloigu A, Leinonen M, Saikku P, Haahtela T. Asthma, atopy and Chlamydia pneumoniae antibodies in adults. Clin Exp Allergy. 1999;29:522–528. doi: 10.1046/j.1365-2222.1999.00504.x. [DOI] [PubMed] [Google Scholar]
- 38.Wang A M, Doyle M V, Mark D F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci USA. 1989;86:9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamashita K, Ouchi K, Shirai M, Gondo T, Nakazawa T, Ito H. Distribution of Chlamydia pneumoniae infection in the atherosclerotic carotid artery. Stroke. 1998;29:773–778. doi: 10.1161/01.str.29.4.773. [DOI] [PubMed] [Google Scholar]
- 40.Yazdani S, Simon A D, Vidhun R, Gulotta C, Schwartz A, Rabbani L E. Inflammatory profile in unstable angina versus stable angina in patients undergoing percutaneous interventions. Am Heart J. 1998;136:357–361. doi: 10.1053/hj.1998.v136.90408. [DOI] [PubMed] [Google Scholar]
- 41.Yokoyama A, Kohno N, Fujino S, Hamada H, Inone Y, Fujioka S, Ishida S, Hiwada K. Circulating interleukin-6 levels in patients with bronchial asthma. Am J Respir Crit Care Med. 1995;151:1354–1358. doi: 10.1164/ajrccm.151.5.7735584. [DOI] [PubMed] [Google Scholar]