Abstract
Normal myofibroblast differentiation is critical for proper skin wound healing. Neoexpression of α-smooth muscle actin (α-SMA), a marker for myofibroblast differentiation, is driven by transforming growth factor (TGF)-β receptor–mediated signaling. Hyaluronan and its three synthesizing enzymes, hyaluronan synthases (Has 1, 2, and 3), also participate in this process. Closure of skin wounds is significantly accelerated in Has1/3 double-knockout (Has1/3-null) mice. Herein, TGF-β activity and dermal collagen maturation were increased in Has1/3-null healing skin. Cultures of primary skin fibroblasts isolated from Has1/3-null mice had higher levels of TGF-β activity, α-SMA expression, and phosphorylation of p38 mitogen-activated protein kinase at Thr180/Tyr182, compared with wild-type fibroblasts. p38α mitogen-activated protein kinase was a necessary element in a noncanonical TGF-β receptor signaling pathway driving α-SMA expression in Has1/3-null fibroblasts. Myocardin-related transcription factor (MRTF), a cofactor that binds to the transcription factor serum response factor (SRF), was also critical. Nuclear localization of MRTF was increased, and MRTF binding to SRF was enhanced in Has1/3-null fibroblasts. Inhibition of MRTF or SRF expression by RNA interference suppresses α-SMA expression at baseline and diminished its overexpression in Has1/3-null fibroblasts. Interestingly, total matrix metalloproteinase activity was increased in healing skin and fibroblasts from Has1/3-null mice, possibly explaining the increased TGF-β activation.
Skin wound healing is a complex event that involves various types of cells working collaboratively at different stages of the process. Disruption of the skin initiates hemostasis, in which platelets aggregate within the wound site to stop the bleeding, followed by inflammation, in which leukocytes, monocytes, and macrophages migrate to the wound site to destroy invading pathogens and produce a variety of inflammatory and profibrotic cytokines.1 In response to these stimuli, epidermal keratinocytes migrate from the wound edges toward the center to help seal the open defect. Fibroblasts are also mobilized, first migrating to the wound edges and then into the provisional matrix.1,2 A subset of fibroblasts transforms into myofibroblasts in later stages of proliferation. Myofibroblast differentiation is critical for functional fibroblast maturation, allowing the cells to produce key components of granulation tissues, such as collagen and fibronectin.2 Nascent expression of α-smooth muscle actin (α-SMA) is a well-established hallmark for myofibroblast differentiation.3 Incorporation of α-SMA into the actin cytoskeleton allows myofibroblasts to form stress fibers that connect with the extracellular matrix via focal adhesions, and to undergo contraction, which is important during wound closure, scar formation, and tissue remodeling.3
Transforming growth factor (TGF)-β receptor signaling is the most potent pathway in regulating α-SMA gene expression and myofibroblast differentiation.4 TGF-β1 is the predominant isoform among the three TGF-β ligands (TGF-β1, TGF-β2, and TGF-β3) that bind to the TGF-β receptor II. On ligand binding, the TGF-β receptor II homodimer forms a heterotetrameric complex with the TGF-β receptor I homodimer, resulting in the phosphorylation of the GS domain of TGF-β receptor I and the activation of its kinase activity, which, in turn, phosphorylates receptor-regulated Smads, including Smad2 and Smad3. Phosphorylated Smad2 or Smad3 dissociates from TGF-β receptor I and forms a complex with Smad4, which translocates to the nucleus and activates transcription of target genes.4 In addition to this well-recognized Smad-dependent canonical pathway, the activation of the TGF-β receptor may also activate several other Smad-independent signaling pathways, such as mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinase (ERK) 1/2, c-Jun N-terminal kinase (JNK), p38 MAPK, phosphatidylinositol 3-kinase/AKT, and RhoA.5 Among all these so-called noncanonical pathways, p38 MAPK has been shown to have a crucial role in regulating tissue fibrosis in various organs, including kidney,6 eye,7 and cardiac muscle.8
One mechanism by which p38 MAPK regulates fibroblast activation and fibrotic processes is via phosphorylation of transcription factors, such as serum response factor (SRF) and myocardin-related transcription factor A (MRTF-A), and subsequent modulation of their functions.9,10 SRF is a mammalian transcription factor that binds to a consensus sequence CArG box [CC(A/T)6GG], also known as serum response element, that is found in smooth muscle cell–specific contractile genes.11 Studies showed that p38 MAPK mediates TGF-β–induced SRF expression.12 The full transcriptional activity of SRF requires its binding with various cofactors, including MRTF-A.13 At baseline, MRTF-A is sequestered in the cytoplasm as a result of its binding to monomeric globular actin (G-actin). In response to activation of Rho/Rho-associated protein kinase (ROCK) signaling or growth factor stimulation, G-actin polymerizes into filamentous actin and decreases in amount, thereby releasing MRTF from cytosolic sequestration. MRTF then relocates to the nucleus, where it binds to SRF, which triggers serum response element–dependent transcription of the α-SMA gene.14,15 In addition, MRTF can be phosphorylated at certain residues by kinases, such as ERK and p38 MAPK, which affects the subcellular localization of MRTF.16
Hyaluronan (HA) is a linear glycosaminoglycan composed of repeating disaccharide units of d-glucuronic acid and N-acetyl-d-glucosamine.17 HA is the only glycosaminoglycan that is not sulfated, not linked to a core protein, and not synthesized by the Golgi pathway. Instead, HA is normally synthesized on the cytoplasmic surface of the plasma membrane by three HA synthases (Has1, Has2, and Has3), and extruded into the extracellular space.18 Has2 is the predominant HAS in most tissues, including the skin, and the only one for which genetic depletion results in embryonic lethality because of defects in cardiac development.19 Has1 and Has3 synthesize HA with an estimated molecular mass of 2 × 105 to ∼2 × 106 Da. On the other hand, large HA molecules (>2 × 106 Da) are synthesized by Has2.20 HA is the most abundant glycosaminoglycan in the skin,17 and the roles of HA and hyaluronan synthases in regulating tissue injury and repair have been extensively studied. A previous study from our laboratory showed that wound closure was accelerated in Has1 and Has3 double-knockout (Has1/3-null) mice.21 A further in vitro study found that Has2 was overexpressed in Has1/3-null fibroblasts (possibly as a compensatory response for loss of the other two Has enzymes), which resulted in an increase in HA synthesis and size of the pericellular HA coat.22 In addition, Has1/3-null fibroblasts were more resistant to apoptosis induced by environmental stress, such as serum starvation or UVB irradiation. The enhanced apoptosis resistance appeared to be Has2 dependent because it could be significantly abrogated by inhibition of Has2 expression.22 These findings suggest that myofibroblasts may have a slower turnover rate in Has1/3-null mice, leading to their accumulation and acceleration of wound closure in Has1/3-null skin. An unanswered question is whether fibroblast-to-myofibroblast conversion might be dysregulated in Has1/3-null wounds (perhaps affecting the production of extracellular matrix) and what role HA and Has2 may have in this dysregulation. Studies in other tissues, such as the lung, showed that HA and Has2 have an important regulatory effect on pulmonary fibroblast differentiation and function.23 This was the motivation behind seeking a better mechanistic understanding about what happens to myofibroblast differentiation in the healing skin wounds of Has1/3-null mice. The present study shows an enhanced myofibroblast differentiation in Has1/3-null fibroblasts (as monitored by α-SMA expression). This enhancement occurred via a noncanonical TGF-β receptor signaling pathway (that extends from the cell membrane to the nucleus) mediated by p38 MAPK and resulting in enhanced function of MRTF/SRF. Interestingly, the enhanced myofibroblast differentiation observed in Has1/3-null mice appeared to be independent of HA or Has2. The findings from this study provide insight into an interesting phenotype in which constitutive up-regulation of a p38 MAPK-mediated, noncanonical TGF-β receptor signaling has a critical role in myofibroblast differentiation and in Has1/3-null skin wounds.
Materials and Methods
Animals, Wound Healing Experiments, and Tissue Harvesting
C57BL/6J mice were obtained from JAX Laboratories (Bar Harbor, ME). Has1 and Has3 double-knockout mice (Has1/3 null) were generated by intercrossing Has1−/− and Has3−/− mice to generate mice nullizygous for both alleles, as previously described.21 All mice were maintained in accordance with guidelines of the American Association for the Accreditation of Laboratory Animal Care. All wounding and tissue harvesting procedures were preapproved by our Institutional Animal Care and Use Committee. C57BL/6J wild-type (WT) mice or Has1/3-null mice (aged 8 to 10 weeks) were anesthetized with ketamine/xylazine, and the fur was shaved from the upper back. Then, 5-mm full-thickness excisional wounds (two wounds per mouse) were generated down to the fascia using sterile biopsy punches. At designated time points after wounding (day 0, day 1, day 3, day 5, day 7, and day 10), mice were anesthetized, and wounds were harvested along with unwounded skin for further preservation, processing, and analysis.
Primary Cell Culture
Primary mouse dermal fibroblasts were isolated from the skin of 2- to 3-day–old pups from WT C57BL/6J mice, per our established protocol.22 Briefly, the entire trunk skin was removed and incubated overnight with 0.25% trypsin without EDTA, followed by mechanical separation of epidermis from dermis. To isolate fibroblasts, the dermis was finely diced and incubated with 400 U/mL of collagenase type I (Worthington Biochemical, Lakewood, NJ) for 30 minutes and with DNAase-I (100 U/mL) for 10 minutes at 37°C. The digested tissue suspension was passed through a 100-μm cell strainer to remove undigested tissue pieces. Hair follicles were removed by centrifugation at 9 × g for 3 minutes. The fibroblasts were collected and cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS; Life Technologies, Carlsbad, CA), 1% penicillin/streptomycin, and 1.0 g/L of glucose. The cells were maintained at 37°C in a humidified incubator with 5% CO2, and fresh growth medium was added every other day. For experiments, cells from passage 1 or 2 were used at subconfluent density. Recombinant human TGF-β1 was purchased from R&D Systems (Minneapolis, MN). TGF-β receptor inhibitor SB431542 (catalog number 1614), p38 MAPK inhibitor SB202190 (catalog number 1264), ERK1/2 inhibitor U0126 (catalog number 1144), and the broad-spectrum matrix metalloproteinase (MMP) inhibitor marimastat (catalog number 2631) were purchased from TOCRIS Bioscience (Bristol, UK). Epidermal growth factor receptor (EGFR) inhibitor AG1478 (catalog number 658548) was purchased Calbiochem/Millipore-Sigma (San Diego, CA). Mink lung epithelial cells stably expressing a plasminogen activator inhibitor-1 promoter-luciferase construct [a kind gift from Daniel Rifkin (Department of Cell Biology, NYU Grossman School of Medicine, New York, NY)] were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS, 1% penicillin/streptomycin, and 200 μg/mL geneticin (G418; Life Technologies), and passaged every 2 to 3 days.
Inhibition of p38 MAPK, MRTF, SRF, and Has2 by RNA Interference
ON-TARGETplus SMARTpool siRNAs and nontargeted scrambled siRNA were purchased from Dharmacon/Horizon Discovery (Cambridge, UK): p38-αMAPK (Mapk14; catalog number L-040125-00-0005), p38-βMAPK (Mapk11; catalog number L-050928-00-0005), MRTF (catalog number L-054350-00-0005), SRF (catalog number L-009800-00-0005), Has2 (catalog number L-042589-01-0005), and nontargeted scrambled siRNA (catalog number D-001810-01-05). Reverse transfection was used to transfect siRNA into the primary mouse skin fibroblasts, as published previously.22 Briefly, for a 6-well tissue culture plate format, 30 pmol of siRNA was mixed with 8 μL of Lipofectamine RNAiMAX (Life Technologies) in 500 mL of Opti-MEM. The final duplex concentration was 100 nmol/L. The siRNA/transfection reagent mixture was added to the plates first, followed by fibroblasts seeded at a density of 2.2 × 105 per well. The cells were refed with fresh antibiotic-free medium at 24 hours after transfection. The knockdown efficacy of targeted gene expression by RNA interference (RNAi) was confirmed by either quantitative real-time PCR analysis of mRNA levels or Western blot analysis of protein abundances.
Histology and Immunohistochemistry
For collagen imaging, Histochoice-fixed paraffin sections (5 μm thick) were stained using a Masson trichrome staining kit (Thermo Fisher Scientific, Pittsburgh, PA), according to the manufacturer's protocol. For visualization of α-SMA, sections were incubated overnight at 4°C with a rabbit anti-mouse α-SMA antibody (1:200; Abcam, Cambridge, MA) and processed using the rabbit ABC staining system (Santa Cruz Biotechnology, Dallas, TX) following the manufacturer's instructions. All treated sections were mounted in Vectamount Permanent Mounting Medium (Vector Laboratories, Inc., Burlingame, CA). To quantify the expression of newly synthesized collagen or α-SMA, histologic skin sections (stained with either Masson trichrome or anti–α-SMA antibody) were scanned at ×20 or ×10 on a Leica (Wetzlar, Germany) slide scanner. The digitized images were analyzed using IPLab Spectrum software version 3.1.2 (Vienna, VA). Specifically, a software drawing tool was used to paint over all positive areas of interest (blue stain for new collagen, or brown areas for α-SMA immunostain) within the dermis. These painted areas were then summed and expressed as a percentage of total dermal area.
Western Blot Analysis
The primary antibodies for Western blot analysis purchased from Cell Signaling Technology (Danvers, MA) were as follows: rabbit anti–phosphorylated p38 MAPK specific for epitopes Thr180/Tyr182 (catalog number 4511), total p38 MAPK (catalog number 9212), rabbit anti–megakaryoblastic leukemia 1 (anti-MKL1)/MRTF-A (catalog number 14760), rabbit anti–poly (ADP-ribose) polymerase (catalog number 9542), anti–glyceraldehyde-3-phosphate dehydrogenase (catalog number 2118), and rabbit anti-SRF (catalog number 5147). Rabbit anti–α-smooth muscle actin antibody was purchased from Abcam (catalog number 5694). Secondary antibodies, including goat anti-rabbit and goat anti-mouse IgG conjugated with horseradish peroxidase, were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Following treatment, cells were scraped off the culture plates and lysed in radioimmunoprecipitation assay buffer supplemented with protease inhibitor cocktails (Millipore, Burlington, MA). They were diluted in 4X NuPAGE LDS sample buffer (Life Technologies) and boiled at 70°C for 10 minutes. Equal amounts of proteins were separated by electrophoresis on a 4% to 12% gradient polyacrylamide gel (Life Technologies), followed by transfer to polyvinylidene difluoride membranes (Immobilon-P; Millipore). They were then blocked in 5% nonfat milk in Tris-buffered saline with 0.05% Tween-20 for 1 hour at room temperature, and incubated with primary antibodies at 4°C overnight, followed by incubation with the appropriate secondary antibody for 1 hour at room temperature. Signals were developed using an enhanced chemiluminescence lighting Western blotting detection reagent kit (GE Health Care, Piscataway, NJ). Protein bands of interest were imaged and digitally quantified using one-dimensional analysis software (Gel Logic 5.0; Carestream Health, Inc., Rochester, NY). The membranes were stripped and reprobed for glyceraldehyde-3-phosphate dehydrogenase as loading controls.
RNA Isolation and Quantitative Real-Time RT-PCR
RNA was prepared from treated fibroblasts using TRIzol reagent (Life Technologies), per the manufacturer's instruction. The extracted RNA was then reverse transcribed into cDNA using a random primer and Superscript III Reverse Transcriptase (Life Technologies). For real-time PCR, assay reagents and the following TaqMan gene expression probes were purchased from Applied Biosystems/Life Technologies (Foster City, CA): acta2 (reference number Mm01546133_m1), SRF (reference number Mm00491032_m1), Mkl1 (MRTF-A; reference number Mm00461840_m1), and eukaryotic 18S ribosomal RNA endogenous control (FAM/MGB probe; catalog number 4332641). The mRNA expression levels were measured in triplicate and calculated using the 2–ΔΔCT method, and levels were presented as a fold difference relative to the untreated normal control.
TGF-β Activity Assay
Mink lung epithelial cells, permanently transfected with a truncated plasminogen activator inhibitor-1 promoter fused to a firefly luciferase reporter construct, were employed for this assay.24 Mink lung epithelial cells were plated at a density of 4.0 × 105 cells/mL in a 96-well plate; 4 hours later, the media were removed and replaced with conditioned media from primary murine dermal fibroblasts, or with protein lysates from skin tissues. After a 16-hour incubation, samples/standards were removed and the mink lung epithelial cells were washed 2× with phosphate-buffered saline. Then, 25 μL of 1× lysis buffer (reference number E397A; Promega, Madison, WI) was added to each well for 10 minutes, followed by 100 μL luciferase assay substrate (reference number E151A; Promega) in luciferase assay buffer (reference number E152A; Promega) for 5 minutes at room temperature with rotation. Luciferase activity was read using a spectrophotometer and quantified by luminescence (relative light units).
Matrix Metalloproteinase Activity Assay
Total MMP activities of skin fibroblasts or healing skin tissues from either WT or Has1/3-null mice were assayed using an MMP activity kit (catalog number ab112147; Abcam), according to the manufacturer's instruction. Briefly, equal amounts of protein lysate from cells or skin were added to 96-well plates (50 μL final volume; 25 μL assayed in duplicate) followed by 4-amino phenyl mercuric acetate. The sample/4-amino phenyl mercuric acetate mixtures were incubated at 37°C for up to 2 hours and transferred to a new 96-well plate, and MMP red substrate working solution was added in a 1:1 ratio. The mixture was incubated in the dark for 30 minutes, and the fluorescent intensity was read at excitation/emission wavelengths of 540/590 nm.
Immunoprecipitation Assay
Fibroblasts were scraped and lysed in radioimmunoprecipitation assay buffer25 supplemented with a protease inhibitor cocktail (EMD/Millipore, Gibbstown, NJ). A 10 μL aliquot of anti-SRF antibody (catalog number 5147; Cell Signaling Technology), or of IgG control (catalog number 2729; Cell Signaling Technology), was added to 500 μg/500 μL of each lysate and incubated at 4°C overnight. After incubation, 20 μL of pre-equilibrated protein A/G agarose beads (number SC2003; Santa Cruz Biotechnology) was added to each sample and incubated with rotation at 4°C for 2 hours, followed by washing three times with radioimmunoprecipitation assay buffer. Proteins were eluted by heating the beads in 45 μL of 5× Laemmli sample buffer at 95°C for 5 minutes, followed by centrifugation at 20,000 × g for 15 seconds. The supernatant containing eluted proteins was transferred to a new tube, and eluted proteins were probed for SRF and MRTF using Western blot analysis (see above).
Statistical Analysis
The results were expressed as means ± SD or SEM. Data were analyzed using GraphPad Prism 9 software (GraphPad Software, San Diego, CA). Statistical comparisons between two groups were done by two-tailed unpaired t-test, with a significance level of P < 0.05.
Results
Facilitated Dermal Maturation and Increased TGF-β Activity in the Healing Skin of Has1/3-Null Mice
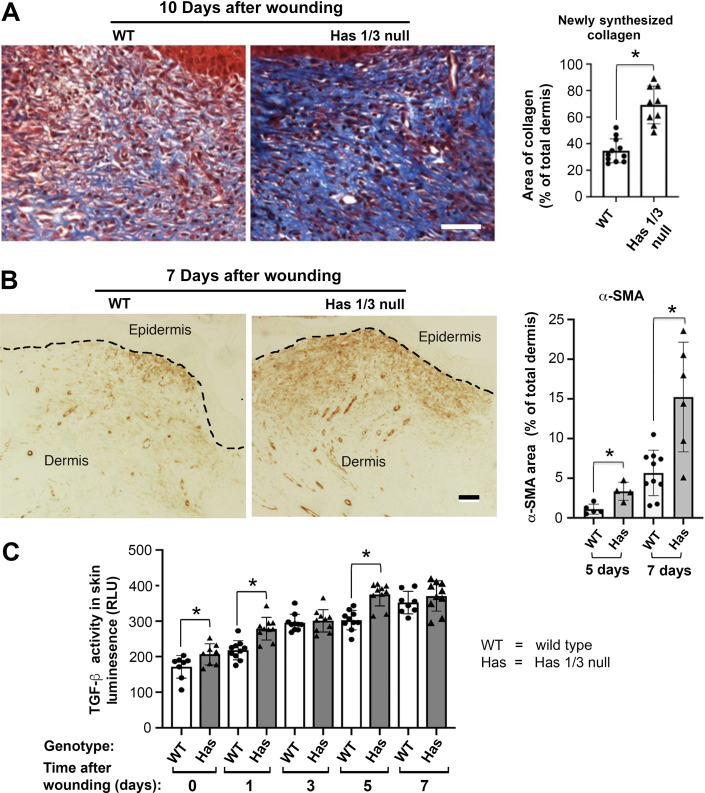
The Has1/3-null mice appear to be grossly normal, with good fertility and normal life spans. Skin wounds of Has1/3-null mice close faster than in WT mice, and the early inflammatory phase (neutrophil/macrophage infiltration) was accelerated.21 To characterize changes that occur later in the process (tissue fibrosis and remodeling), excisional wounds were generated in WT or Has1/3-null mice using 5-mm skin biopsy punches, and the healing skin was harvested 10 days after wounding. Collagen deposition, a marker for dermal repair and maturation, was visualized by Masson trichrome staining and analyzed semiquantitatively. As shown in Figure 1A, new (blue-stained) collagen was significantly more abundant in 10-day healing skin of Has1/3-null mice than in WT mice, providing evidence that dermal maturation is facilitated in Has1/3-null healing skin. In Figure 1B, myofibroblasts (identified by immunohistochemistry as α-SMA–expressing cells) were induced in the healing wounds of both WT and Has1/3-null mice at day 5 and day 7 after injury, and were found to be significantly more abundant in Has1/3-null wounds. Because the TGF-β receptor pathway is arguably the most potent signaling system for regulating fibroblast function (including fibroblast-to-myofibroblast conversion) and skin fibrosis,4 the TGF-β activity in healing skin was assessed and compared between Has1/3-null and WT mice. As shown in Figure 1C, TGF-β activity was significantly higher in the healing skin of Has1/3-null mice than in their WT counterparts, at baseline and at day 1 and day 5 after wounding.
Figure 1.
Accelerated dermal maturation, increased α-smooth muscle actin (α-SMA) expression, and up-regulated transforming growth factor (TGF)-β activity in hyaluronan synthase (Has) 1/3–null healing skin. A: Masson trichrome–stained sections at 10 days after wounding and quantitative analyses of newly synthesized collagen are shown. Area of new collagen was measured using the image processing technique described in Materials and Methods. B: Immunostaining of α-SMA–positive cells in the dermis shows earlier myofibroblast differentiation in Has1/3-null wounds at days 5 and 7 after wounding, relative to wild-type (WT) wounds. Dashed lines indicate the junction between epidermis and dermis. Area of α-SMA staining was measured using the image processing technique described in Materials and Methods. C: TGF-β activity assays show that TGF-β activity (measured via luciferase assay) is significantly up-regulated at day 0, day 1, and day 5 after excisional wounding in Has1/3-null healing skin compared with WT. Data shown are relative light units (RLUs). Data are given as means ± SD (A–C). ∗P < 0.05. Scale bars = 50 μm (A and B).
Increased α-SMA Expression and Up-Regulated TGF-β Activity in Has1/3-Null Mouse Skin Fibroblasts
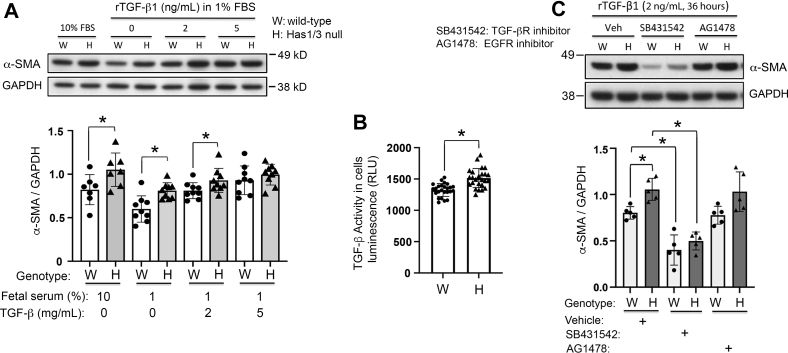
To better understand the cellular and molecular mechanism(s) for the facilitated dermal maturation and increased TGF-β activity in Has1/3-null healing skin, primary skin fibroblasts were isolated from either WT or Has1/3-null mouse pups and cultured in vitro. The protein abundance of α-SMA, the established marker for myofibroblast differentiation, was found to be significantly increased in Has1/3-null skin fibroblasts compared with WT fibroblasts when cultured in Dulbecco's modified Eagle's medium plus 10% FBS or in Dulbecco's modified Eagle's medium plus 1% FBS; these media contain relatively high or low amounts of exogenous TGF-β, respectively (Figure 2A). When supplemented with increasing amounts of recombinant human TGF-β1 (rTGF-β1), this phenotypic difference in α-SMA protein abundance between Has1/3-null and WT fibroblasts was blunted, especially with 5 ng/mL of exogenous rTGF-β1 (Figure 2A). This suggests that the phenotypic difference in α-SMA gene expression depends on a difference in intrinsic TGF-β signaling activity between Has1/3-null and WT fibroblasts, and that this intrinsic difference can be overwhelmed by exogenous addition of TGF-β1 ligands. To address this, TGF-β activity in conditioned media from primary mouse skin fibroblasts cultured in vitro was assessed and was found to be significantly higher in Has1/3-null skin fibroblasts compared with that in WT fibroblasts (Figure 2B).
Figure 2.
Up-regulated α-smooth muscle actin (α-SMA) expression and increased transforming growth factor (TGF)-β activity in hyaluronan synthase (Has) 1/3–null primary mouse skin fibroblasts. A: Wild-type (WT) or Has1/3-null fibroblasts grown in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) were synchronized by switching the cultures to 1% FBS media for 24 hours. At time 0, fresh DMEM supplemented with 10% FBS, or with 1% FBS in the presence of 0, 2, or 5 ng/mL of exogenous recombinant human TGF-β1 (rTGF- β1), was added for 36 hours before harvest. Top panel: Representative Western blot analysis. Bottom panel: Densitometric analyses of the α-SMA bands normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from multiple experiments. B: TGF-β activity assay showing higher TGF-β activity in primary Has1/3-null fibroblasts than in WT fibroblasts; data are presented as relative light units (RLUs). C: Blockade of the TGF-β receptor (TGF-βR; using a specific chemical inhibitor, SB431542), but not blockade of epidermal growth factor receptor (EGFR; using inhibitor AG1478), suppresses α-SMA expression at baseline and diminishes the difference in α-SMA expression between WT versus Has1/3-null skin fibroblasts. Mouse skin fibroblasts were cultured with DMEM supplemented with 2 ng/mL rTGF-β1, then treated with either the TGF-βR inhibitor or the EGFR inhibitor for 36 hours; 0.1% dimethyl sulfoxide was used as the vehicle control (Veh). Western blot analyses were reprobed with GAPDH as a loading control. Top panel: Representative Western blot analysis. Bottom panel: Densitometric analyses of the α-SMA band. Data are given as means ± SD (A–C). ∗P < 0.05.
To assess whether the increases in α-SMA expression after exogenous TGF-β addition, and whether the differences in α-SMA expression between Has1/3-null and WT fibroblasts, after adding TGF-β might be mediated via the TGF-β receptor, fibroblasts were treated with a TGF-β receptor antagonist (Figure 2C). Fibroblasts were treated with 2 ng/mL of rTGF-β1 for 36 hours to induce myofibroblast differentiation, in either the absence or the presence of SB431542, a chemical inhibitor of TGF-β receptor, or of AG1478, a chemical inhibitor of EGFR. The rationale for inhibiting EGFR in parallel to TGF-β receptor inhibition is that EGFR was previously found to colocalize with CD44, the principal receptor for HA, in lipid rafts in the plasma membrane of oral fibroblasts. Thus, those two receptors have a synergistic effect in potentiating signal transduction through MAPK/ERK, to regulate fibroblast proliferation.26 As shown in Figure 2C, inhibition of TGF-β receptor, but not inhibition of EGFR, suppressed α-SMA expression and abrogated the difference in α-SMA expression between Has1/3-null and WT fibroblasts, suggesting that this phenotypic difference is at least partially mediated by TGF-β receptor signaling, whereas EGFR has a dispensable role herein.
Increased α-SMA Expression in Has1/3-Null Mouse Skin Fibroblasts Requires p38α MAPK-Dependent Signaling
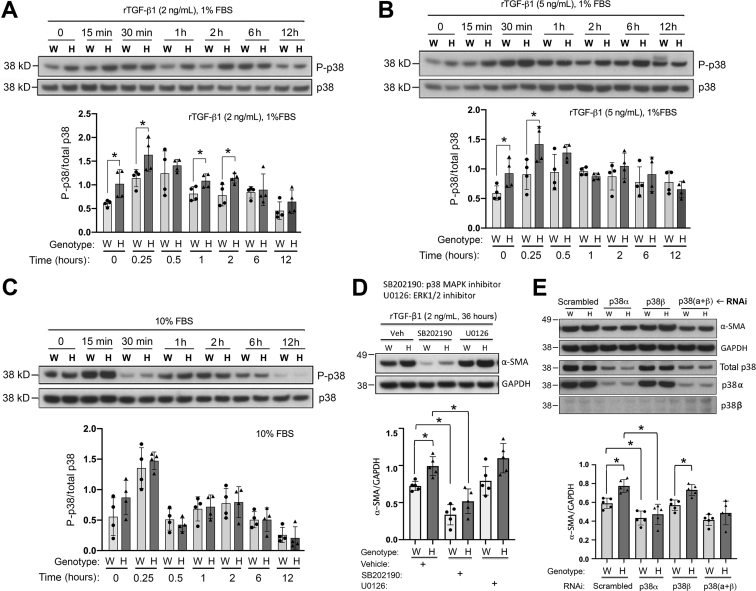
p38 MAPK-mediated noncanonical TGF-β receptor signaling has a critical role in regulating myofibroblast differentiation in primary murine skin fibroblasts.25 The current study further examined and compared the activation level of p38 MAPK in WT and Has1/3-null skin fibroblasts. The cells were cultured in media containing various concentration of fetal bovine serum and recombinant TGF-β1 (10% FBS; 1% FBS supplemented with 2 ng/mL of rTGF-β1; or 1% FBS supplemented with 5 ng/mL of rTGF-β1), and the phosphorylation of p38 MAPK at Thr180/Tyr182 was analyzed by Western blot analysis during a 12-hour time window, which is typically the time frame most critical for most receptor-mediated p38 MAPK-dependent activation events.27, 28, 29 As shown in Figure 3, A–C, changes in levels of p38 MAPK phosphorylation were time-dependent, with the maximum increase in phosphorylation observed at 15 to 30 minutes after exposure to rTGF-β1, followed by a return to baseline by 12 hours. More importantly, in the absence of any exogenous TGF-β1 (except for trace amounts present in 1% FBS in the culture medium), the level of phosphorylated p38 was constitutively higher in Has1/3-null fibroblasts compared with WT fibroblasts (Figure 3, A and B). This phenotypic difference (higher p38 phosphorylation in Has1/3 null than in WT) was preserved at 15 minutes after TGF-β1 stimulation, regardless of the amount of TGF-β1 added. However, at 30 minutes after stimulation, the p38 phosphorylation levels in WT cells appeared to catch up to those in Has1/3-null cells, so that there was no longer any significant difference between them. This observation is consistent with a situation in which p38 signaling, already prestimulated in the Has1/3-null fibroblasts, reaches saturation earlier, and no longer responds, whereas WT signaling continues to climb. With the lowest TGF-β1 stimulation (2 ng/mL), p38 phosphorylation declines rapidly, and a significant difference between Has1/3 and WT cells reappears at 1 and 2 hours (Figure 3A). However, in fibroblasts stimulated with 5 ng/mL of TGF-β1 (Figure 3B), or cultured in 10% FBS, which contains not only abundant TGF-β1 but also other growth factors and cytokines (Figure 3C), the phenotypic difference in phosphorylated p38 levels between Has1/3 and WT cells does not recur, perhaps because p38-regulated signaling is maximally stimulated in the latter situations. To avoid these apparent saturation effects, the lowest dose of TGF-β1 (2 ng/mL) was selected for subsequent experiments.
Figure 3.
Enhanced myofibroblast differentiation in hyaluronan synthase (Has) 1/3–null (H) fibroblasts is driven by up-regulated p38 mitogen-activated protein kinase (MAPK) activation in Has1/3-null fibroblasts. A–C: Wild-type (WT or W) or Has1/3-null fibroblasts grown in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) were synchronized by switching the cultures to 1% FBS medium for 24 hours. A and B: At time 0, fresh medium with 1% FBS containing either 2 ng/mL of recombinant human transforming growth factor-β1 (rTGF-β1; A) or 5 ng/mL of rTGF-β1 (B) was added, and then maintained until harvest at the indicated time points. C: A third group of cultures received 10% FBS medium with no additional factors. A–C: A representative Western blot analysis [phosphorylated p38 (P-p38) MAPK and total p38 MAPK) is shown (top panels), with quantitation of four experiments (bottom panels). D: Chemical inhibition of p38 MAPK, but not inhibition of extracellular signal-regulated kinase (ERK) 1/2, suppresses α-smooth muscle actin (α-SMA) expression at baseline and abrogates the phenotypic difference in α-SMA expression between WT versus Has1/3-null fibroblasts. Primary mouse skin fibroblasts cultured in DMEM with 2 ng/mL rTGF-β1 were treated with p38 MAPK inhibitor (SB202190) or with epidermal growth factor receptor inhibitor (U0126) for 36 hours. Dimethyl sulfoxide 0.1% was used as the vehicle control (Veh). E: Inhibition of p38α, but not p38β, by RNA interference (RNAi) abrogates the phenotypic difference in α-SMA expression between WT versus Has1/3-null fibroblasts. Primary mouse skin fibroblasts, cultured as above, were treated with RNAi directed against p38 MAPK-α or p38 MAPK-β. Nontargeted scrambled siRNA was used as the control (Scrambled). Data are given as means ± SD (A–E). ∗P < 0.05. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Another member of the MAPK superfamily, ERK1/2, has been shown to have overlapping roles with p38 MAPK in mediating TGF-β receptor signaling and regulating cell functions.26,30,31 To further determine the role of both p38 MAPK and ERK1/2 in mediating the increased α-SMA expression in Has1/3-null fibroblasts, fibroblasts were treated with 2 ng/mL of rTGF-β1 for 36 hours to induce myofibroblast differentiation in the presence or absence of either p38 MAPK inhibitor, SB202190, or ERK1/2 inhibitor, U0126. Inhibition of p38 MAPK, but not inhibition of ERK1/2, suppressed α-SMA expression at baseline and abrogated the difference in α-SMA expression between Has1/3-null and WT fibroblasts (Figure 3D), suggesting that the phenotypic difference in α-SMA expression is at least partially mediated by p38 MAPK. Smad2-mediated canonical TGF-β receptor signaling has a dispensable role in regulating α-SMA expression in WT mouse skin fibroblasts.25 In the current study, inhibiting Smad2 gene expression by RNAi did not have any effect on the phenotypic difference in α-SMA gene expression between Has1/3-null and WT mouse skin fibroblasts (data not shown), suggesting that Smad2-mediated signaling is dispensable for the α-SMA expression phenotype.
There are four p38 MAPK isoforms identified so far, including p38α (MAPK14/SAPK2a), p38β (MAPK11/SAPK2b), p38γ (MAPK12/SAPK3), and p38δ (MAPK13/SAPK4), which share structural homology and substrate similarities.12 Although the α-isoform is the only p38 MAPK that is essential for mouse embryo development and the most well-characterized isoform, both p38α and p38β are ubiquitously expressed.32 Therefore, to further elucidate the role of these two isoforms of p38 MAPK in regulating α-SMA gene expression in primary mouse skin fibroblasts, the expression of the α- and/or the β-isoform of p38 MAPK was inhibited by RNAi (Figure 3E). p38β MAPK was barely detectable in primary mouse skin fibroblasts; and knockdown of the α-isoform but not the β-isoform by RNAi suppressed the expression of α-SMA in primary mouse fibroblasts and abrogated the phenotypic difference between WT and Has1/3-null cells. This finding provides evidence that the α-isoform is the predominant form of p38 MAPK in controlling α-SMA expression in primary mouse skin fibroblasts, and its enhanced activation is responsible for the increased α-SMA expression in Has1/3-null fibroblasts.
Increased α-SMA Gene Expression in Has1/3-Null Fibroblasts Is Mediated by Enhanced Function of the MRTF/SRF Complex
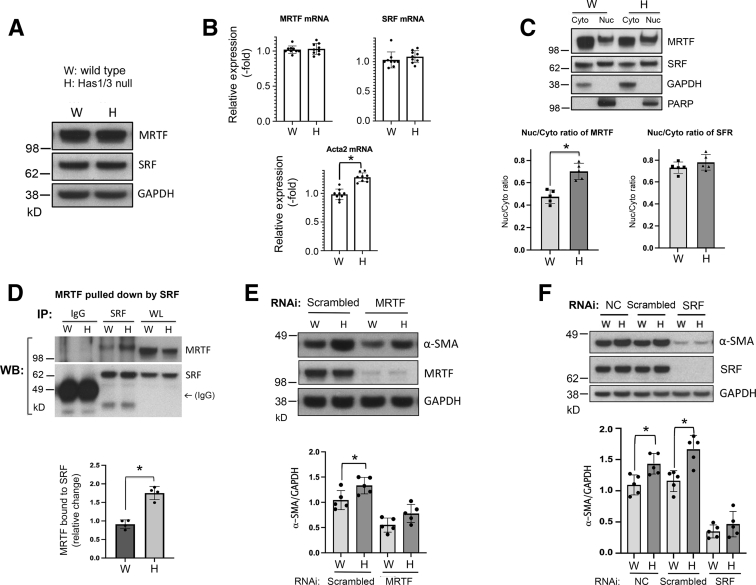
It has been well demonstrated that the transcription factor, SRF, together with its cofactor, MRTF, has crucial roles in governing the expression of contractile genes in smooth muscle cells and myofibroblasts.13,25,33 In addition, p38 MAPK has a role in regulating the expression or function of these proteins.9,10 Therefore the expression, subcellular localization, and the binding of these two transcription factors was examined in both WT and Has1/3-null primary mouse skin fibroblasts. As shown in Figure 4, A and B, the expression of both SRF and MRTF did not differ between WT and Has1/3-null fibroblasts, as determined at the protein level by Western blot analysis (Figure 4A) or at the mRNA level by reverse quantitative PCR (Figure 4B). The level of Acta2 mRNA was significantly higher in Has1/3-null fibroblasts (Figure 4B). Further Western blot analysis of SRF and MRTF in subcellular fractions (cytoplasmic versus nuclear) showed that nuclear localization of MRTF (but not SRF) was significantly increased in Has1/3-null fibroblast lysates compared with WT cells (Figure 4C). Taken together with the prior result of up-regulated phosphorylated p38 MAPK (Figure 3), these observations are consistent with the previously published findings that the nuclear localization of MRTF depends on p38 MAPK activity.25 Furthermore, increased nuclear accumulation of MRTF leads to significantly increased binding of MRTF with SRF in Has1/3-null fibroblasts compared with WT cells, as determined by an immunoprecipitation assay (Figure 4D).
Figure 4.
Up-regulation of α-smooth muscle actin (α-SMA) expression in hyaluronan synthase (Has) 1/3–null fibroblasts is mediated by increased nuclear accumulation of myocardin-related transcription factor (MRTF) and its binding to serum response factor (SRF). A: Representative Western blot analysis images are shown of SRF and MRTF in primary mouse skin fibroblasts. B: Quantitative analyses of mRNA levels by RT-PCR show up-regulated expression of the α-SMA gene (Acta2), but not of SRF or MRTF, in Has1/3-null fibroblasts relative to wild-type fibroblasts. C: Increased nuclear localization of MRTF in Has1/3-null fibroblasts is shown. Top panel: Western blot analyses are shown of MRTF and SRF from cytoplasmic (Cyto) or nuclear (Nuc) fractions in primary mouse skin fibroblasts, along with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and poly (ADP-ribose) polymerase (PARP) as controls for purity of the cytoplasmic and nuclear fractions, respectively. Bottom panels: Nuc/Cyto ratios of MRTF (left panel) or SRF (right panel), determined from densitometric scanning of bands in five independent experiments. D: Immunoprecipitation (IP) assay shows increased binding between MRTF and SRF in Has1/3-null fibroblasts. An anti-SRF antibody (SRF) or normal rabbit Ig (IgG) was added to the cell lysates to pull down protein complexes, and the membrane was probed with anti-MRTF or anti-SRF antibodies. Top panel: A representative Western blot analysis, showing MRTF and SRF pulled down by the anti-SRF antibody. Bottom panel: A quantitative analysis of the SRF-bound MRTF bound to SRF in four independent experiments. E: Knockdown of MRTF partially abrogates the α-SMA expression phenotype in Has1/3-null fibroblasts. Lysates were prepared from fibroblasts transfected with MRTF siRNA or nontargeted Scrambled siRNA, and Western blot analyses of α-SMA and MRTF were run. Top panel: A representative Western blot analysis of both α-SMA and MRTF, along with GAPDH, in cell lysates from fibroblasts transfected with MRTF siRNA or nontargeted Scrambled siRNA. Bottom panel: Densitometric analyses of the specific bands on Western blot (WB) analyses from five independent experiments (α-SMA normalized to GAPDH). F: Knockdown of SRF completely abrogates the α-SMA expression phenotype in Has1/3-null fibroblasts. Lysates of fibroblasts were transfected with SRF siRNA or nontargeted Scrambled siRNA, and Western blot analyses of α-SMA and MRTF were prepared. The graph shows densitometric analyses of Western blot analyses from five independent experiments (α-SMA normalized to GAPDH). Data are given as means ± SD (B–F). ∗P < 0.05. NC, untreated normal control; RNAi, RNA interference; WL, whole lysate as input control.
Next, the regulatory roles of MRTF and SRF in generating the phenotypic difference in α-SMA expression between Has1/3-null and WT fibroblasts were explored by knocking down the gene expression of each factor in primary mouse skin fibroblasts using RNAi. As shown in Figure 4E, knockdown of MRTF suppresses the α-SMA gene expression at baseline and partially abrogates the phenotypic difference in α-SMA expression between WT and Has1/3-null fibroblasts. Knockdown of SRF generated a more significant suppressive effect on α-SMA gene expression and completely abrogated the difference between WT and Has1/3-null fibroblasts (Figure 4F). These observations suggest that enhanced function of SRF/MRTF has a crucial role in mediating the increased α-SMA expression in Has1/3-null primary mouse skin fibroblasts.
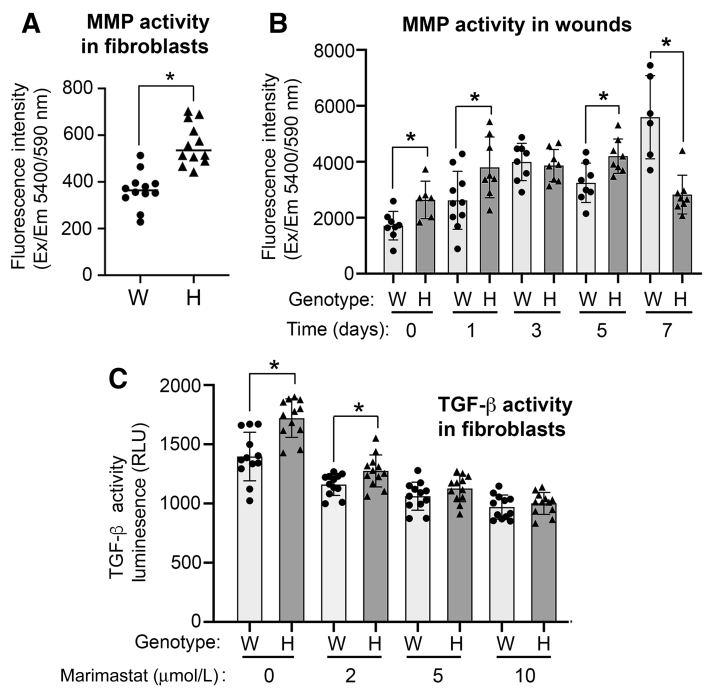
MMP Activity Is Up-Regulated in Has1/3-Null Mouse Skin Fibroblasts and Skin, and Inhibition of MMP Activity Diminishes TGF-β Activity in Has1/3-Null Fibroblasts
It is well known that TGF-β exists in the extracellular matrix in the form of a latent complex, wherein the TGF-β ligands are bound to several other proteins, and that full activation of TGF-β requires the coordination of integrins or other plasma membrane proteins with various MMPs to cleave the latent complex and release the active TGF-β ligands.34 Because increased TGF-β activity and up-regulated α-SMA expression were observed in Has1/3-null skin fibroblasts, whether there might be any difference in MMP activity between WT and Has1/3-null fibroblasts was explored further. Indeed, as shown in Figure 5A, MMP activity was significantly higher in Has1/3-null fibroblasts than in WT cells. Interestingly, when examined in vivo, the changes in MMP activity appeared to be a bit complicated and involved time-dependent behavior [ie, the healing skin of Has1/3-null mice showed higher MMP activity at specific early time points (including day 0, day 1, and day 5) but a significantly lower MMP activity at day 7], compared with that of WT littermates (Figure 5B). To further validate the role of MMP activity in mediating the phenotypic difference in TGF-β activity between Has1/3-null and WT fibroblasts, both types of cells were treated with a broad-spectrum MMP inhibitor, marimastat, at different dosages (2, 5, and 10 μmol/L) for 24 hours. A dose-dependent suppressive effect on TGF-β activity was observed in both type of cells, and the phenotypic difference was diminished starting at a dosage of 5 μmol/L and higher (Figure 5C).
Figure 5.
Up-regulated matrix metalloproteinase (MMP) activity in fibroblasts and healing skin of hyaluronan synthase (Has) 1/3–null (H) mice. A: MMP activities in primary mouse skin fibroblasts [wild type (WT or W) versus Has1/3 null] cultured in vitro are shown. B: MMP activities are shown in healing mouse skin at different time points after excisional wounding, in wild-type versus Has1/3-null fibroblasts. C: Transforming growth factor (TGF)-β activity assay shows higher TGF-β activity in primary Has1/3-null fibroblasts than in WT fibroblasts without marimastat treatment or with marimastat at a concentration of 2 μmol/L. Data are presented as means ± SD (A–C). ∗P < 0.05. Em, emission; Ex, excitation; RLU, relative light unit.
Increased α-SMA Gene Expression Is Independent of Extracellular HA or HAS2 Expression
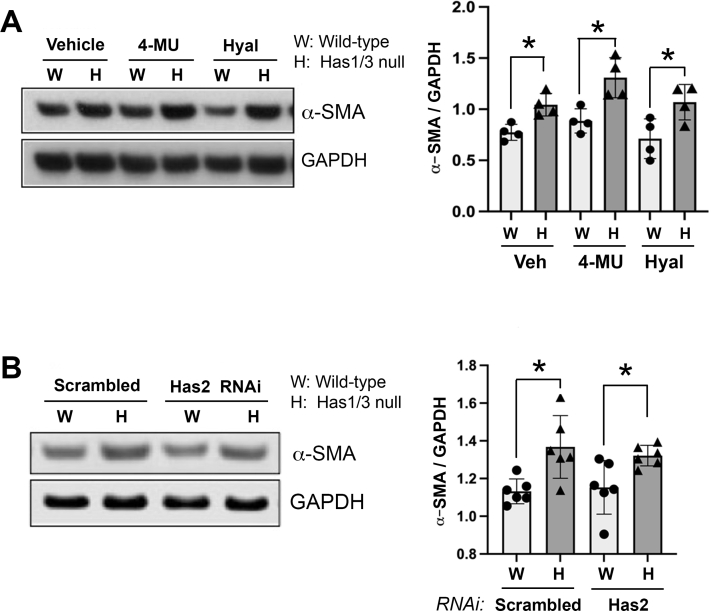
Both Has2 and extracellular HA are dispensable for regulating α-SMA expression in primary WT mouse skin fibroblasts.25 Herein, to explore whether Has2 or hyaluronan is involved in mediating the α-SMA expression phenotype in Has1/3-null fibroblasts, Has2 gene expression was inhibited by RNAi in WT and Has1/3-null fibroblasts. Alternatively, both types of cells were treated with either hyaluronidase or 4-methylumbelliferone. The protein abundance of α-SMA was then examined by Western blot analysis. However, no changes in α-SMA expression in Has1/3-null mouse skin fibroblasts were detected after these treatments (Supplemental Figure S1), providing evidence that the phenotypic difference in α-SMA expression between Has1/3-null and WT mouse skin fibroblasts is independent of either extracellular HA levels or Has2 expression.
Discussion
The present study demonstrated that dermal maturation is accelerated and TGF-β activity is up-regulated in healing wounds of Has1/3-null mice. Consistently, enhanced myofibroblast differentiation and elevated TGF-β activity were found in primary Has1/3-null fibroblasts cultured in vitro. Further exploration of the molecular mechanism revealed that p38 MAPK activation is up-regulated in Has1/3-null fibroblasts, especially when they are cultured with a low concentration of exogenous TGF-β ligands. As a consequence, nuclear localization of MRTF is increased, and its binding to SRF is enhanced in Has1/3-null skin fibroblasts. In addition, the increased α-SMA expression in Has1/3-null fibroblasts could be abrogated by chemical inhibition of p38 MAPK or of the TGF-β receptor, or by inhibition of MRTF or SRF gene expression. Furthermore, matrix metalloprotease activity was elevated significantly in Has1/3-null skin fibroblasts in vitro and in the healing skin of Has1/3-null mice, compared with WT counterparts. In addition, inhibition of MMP activity abrogated the increased TGF-β activity in Has1/3-null fibroblasts, suggesting that the up-regulated MMP activity may contribute to the increased TGF-β signaling and enhanced myofibroblast differentiation observed in Has1/3-null skin and cells.
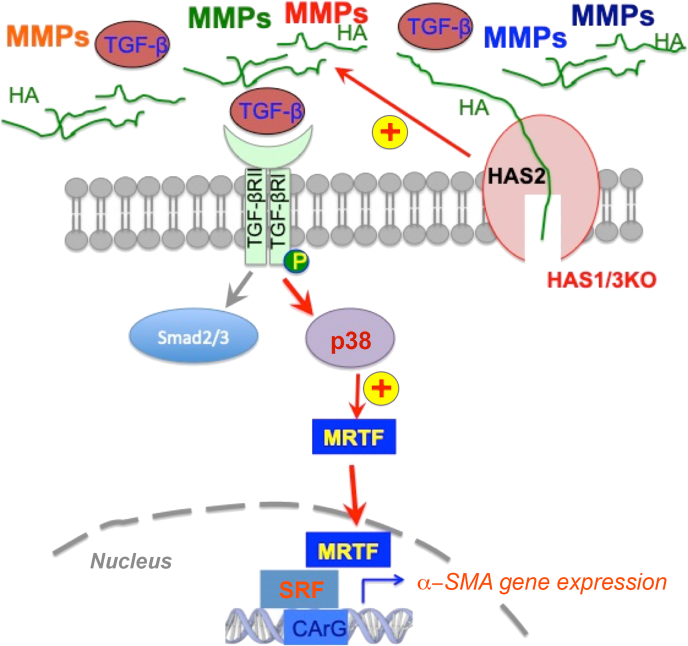
As illustrated in Figure 6, collective findings from this study support a working model in which changes occurring at the cell membrane are connected to increased nuclear transcription of the fibrosis-related gene, α-SMA, via activation of a noncanonical signaling pathway involving the TGF-β receptor, p38/MAPK, MRTF, and SRF. Somewhat surprisingly, these changes appear to be independent of levels of Has2 and/or of hyaluronan in the extracellular matrix. Instead, in this particular mouse model, the up-regulated activity of metalloproteases through an as-yet unknown mechanism appears to have a role in activation of TGF-β ligands, which then stimulates the downstream profibrotic pathway.
Figure 6.
Schematic summary and working model. Matrix metalloproteinase (MMP) activity is up-regulated in hyaluronan synthase (Has) 1/3–null fibroblasts and skin, leading to increased release of transforming growth factor (TGF)-β ligands that bind to TGF-β receptor, which, in turn, enhances the p38 mitogen-activated protein kinase–mediated, noncanonical TGF-β receptor (TGF-βR) signaling pathway. As a consequence, nuclear accumulation of myocardin-related transcription factor (MRTF) is increased, and binding between MRTF/serum response factor (SRF) is also increased. This results in up-regulated expression of α-smooth muscle actin and enhanced myofibroblast differentiation, which facilitates dermal maturation in Has1/3-null healing skin. CArG, [CC(A/T)6GG] DNA [consensus] sequences; HA, hyaluronan; KO, knockout.
The Complex Role of Hyaluronan and Hyaluronan Synthases in Regulating Myofibroblast Differentiation and Tissue Fibrosis
The present study represents the first report to demonstrate an enhanced myofibroblast differentiation and increased fibrosis phenotype in the healing skin wounds of mice lacking Has1 and Has3. A previous study showed that wound closure was accelerated in the healing skin of Has1/3-null mice.21 In addition, there was an increased inflammatory cell infiltrate at wound sites of Has1/3-null healing skin, strongly suggesting that enhanced inflammation may contribute to the facilitated wound closure.21 A great deal of evidence shows that inflammatory cells, such as macrophages,35 or epidermal keratinocytes,36 secrete various proinflammatory and profibrotic cytokines that regulate fibroblast function via a paracrine effect. This paracrine effect is believed to have an important role in the profibrotic phenotype observed in vivo in Has1/3-null healing skin. We plan to further investigate this point in the future. On the other hand, an autocrine effect is implicated in the current study based on our observations of increased TGF-β activity, α-SMA expression, and increased MMP activity in Has1/3-null fibroblasts cultured in vitro, and this may also be important for determining the myofibroblastic phenotype in vivo.
In the literature, there are conflicting reports on the role of hyaluronan and Has2 in regulating fibroblast function and tissue fibrosis. Meran et al37 showed the following: myofibroblast differentiation of human dermal fibroblasts induced by TGF-β1 was associated with an induction of Has1 and Has2 gene expression and with assembly of pericellular hyaluronan coats; and inhibition of hyaluronan synthesis in dermal fibroblasts significantly attenuated TGF-β1–mediated differentiation, suggesting HA and Has2 have a positive role in promoting human myofibroblast differentiation.38 In addition, HA facilitated the TGF-β1–induced proliferation of human dermal fibroblasts in a CD44-EGFR–dependent manner.26 Li et al23 demonstrated the following: targeted overexpression of Has2 in pulmonary fibroblasts produced an aggressive fibrosis phenotype in the lung on bleomycin treatment; and, conversely, conditional deletion of Has2 in mesenchymal cells abrogated the invasive fibroblast phenotype and alleviated the lung fibrosis. These findings suggest a positive role of HA and Has2 in promoting fibroblast function and tissue fibrosis. On the other hand, Evanko et al39 showed that prolonged inhibition of HA synthesis by 4-methylumbelliferone or removal of extracellular HA by hyaluronidase treatment enhanced the differentiation of human lung fibroblasts to myofibroblasts, and increased deposition of fibronectin and type I collagen in the extracellular matrix. This suggests that in this situation HA has a negative regulatory effect on α-SMA gene expression and myofibroblast differentiation. In fibroblasts from wild-type25 or Has1/3-null mice (this study), we showed that HA and Has2 appear to be dispensable for mediating enhanced myofibroblast differentiation driven by TGF-β. The reason for discrepancies between these studies remains to be elucidated, but differences in species, anatomic tissue type, and cell culture conditions should all be considered. For example, in our case with primary mouse skin fibroblasts cultured in plastic dishes, abnormally enhanced integrin/extracellular matrix signaling generated by high mechanical tension on plastic surfaces40,41 may have an overpowering effect that counteracts the signaling mediated by HA/Has2 in regulating myofibroblast differentiation.
To date, there are a few other examples of studies that have examined tissue fibrosis associated with inflammation in mice that lack one or more Has enzymes. For example, deficiency of Has1 resulted in chronic joint inflammation and widespread intra-articular fibrosis in a murine model of knee cartilage damage.42 Has3-deficient mice exposed to carbon tetrachloride showed greater hepatic accumulation of fibrosis-associated transcripts than their wild-type counterparts.43 For Has2-deficient mice after cardiac ischemia/reperfusion, the number of α-SMA (+) cells within the infarcted myocardium and the surrounding border zone was reduced, and the fibroblasts showed impaired contractility.44 Finally, kidneys from Has1/Has3 double-null mice were predisposed to profibrotic renal injury.45 Our current report provides another addition to the limited knowledge base of how hyaluronan synthases regulate tissue fibrosis following injury and inflammation. The discrepancy between an increased fibrosis phenotype in vivo and a dispensable role of HA and Has2 in fibroblast cultures in vitro appears to be puzzling. But the difference is always a topic of discussion and has been attributed to various reasons, including off-target effects of gene depletion methods, and genetic compensation, which may occur during embryonic development.46 In addition, studies show that hyaluronan synthases, such Has147 and Has2,48,49 have a crucial role in processes, including inflammation and epithelial-mesenchymal transition, while not necessarily correlating to their enzyme activity, suggesting that these proteins may have other HA-independent functions.
The Key Role of p38 MAPK/TGF-β Receptor Signaling in Regulating α-SMA Gene Expression in Primary Mouse Skin Fibroblasts
An often-reported signaling pathway in fibrosis involves Smad2/3, which is often considered the canonical pathway that mediates profibrotic signaling. For instance, a series of studies from Cardiff University established the role of the canonical Smad2/3-dependent pathway in mediating myofibroblast differentiation driven by TGF-β in human lung fibroblasts, wherein knockdown of Smad2 or Smad3 by RNAi effectively abrogated the TGF-β–driven phenotype.38,50 In contrast, Molkentin et al8 showed that targeted depletion of p38 MAPK in fibroblasts blocked the differentiation of cardiac fibroblasts into myofibroblasts and reduced fibrosis after ischemic injury or chronic neurohumoral stimulation. Similarly, the current study shows that depletion of p38 MAPK inhibited myofibroblast differentiation in vitro. As another example, transgenic mice with fibroblast-specific activation of p38 MAPK developed interstitial and perivascular fibrosis in the heart, lung, and kidney as a result of enhanced myofibroblast accumulation.8 The above findings strongly suggest that p38 MAPK has a central role in regulating fibroblast function and fibrosis development in certain tissue types, such as heart and skin. A previous study also showed that p38 MAPK regulates α-SMA expression in primary mouse skin fibroblasts.25 The current study showed that phosphorylation of p38 MAPK at residues Thr180/Tyr182 is increased in Has1/3-null primary mouse skin fibroblasts, and that blockade of p38 MAPK diminishes the differences in levels of expression of α-SMA in Has1/3-null versus WT cells. These findings suggest that noncanonical p38 MAPK-mediated TGF-β receptor signaling has a crucial role in mediating increased α-SMA expression in this system. In addition, the α-isoform of p38 MAPK is predominant in regulating α-SMA expression. These observations are in harmony with findings from many other groups showing that p38 MAPK has a crucial role in mediating TGF-β–induced myofibroblast differentiation and tissue fibrosis.6, 7, 8
The Central Role of MRTF/SRF in Governing α-SMA Expression and How These Factors Are Regulated by p38 MAPK
SRF, as first characterized and named by Treisman51 in 1986, is a transcription factor that binds to the serum response element associated with a variety of genes, including immediate early response genes, such as c-fos, and muscle contractile genes, such as actin and myosin.52 The core sequence of the serum response element is a CC A/T-rich GG sequence or a CArG box. The intrinsic transcriptional activity of SRF alone is weak unless SRF is bound to various cofactors, such as MRTF.53 As one of the major co-activators of SRF, MRTF associates with SRF through a basic region (B1) and an adjacent Glu-rich domain.14,54 Its N-terminal domain contains three Arg-Pro-X-X-X-Glu-Leu motifs that mediate MRTF's binding to monomeric globular G-actin. This binding sequesters MRTF from entering the nucleus and binding to SRF. On stimulation with growth factors and cytokines, actin polymerization is induced within cells (ie, globular G-actin is converted into filamentous F-actin), which frees MRTF from G-actin binding, allowing it to enter the nucleus and bind to SRF.15 The subcellular localization and function of MRTF is also regulated by phosphorylation on certain residues that can be phosphorylated by ERK and p38 MAPK. MRTF-A phosphorylation at serine 454 by ERK inhibits serum-induced nuclear localization.55 Nuclear accumulation of MRTF, and α-SMA promoter activity induced by cell-cell contact disassembly in porcine kidney epithelial cells, is aborted by inhibition of p38 MAPK.56 In a previous study, chemical inhibition of p38 MAPK sequestered MRTF in the cytoplasm and reversed the nuclear accumulation of MRTF induced by treatment with either rTGF-β1 or CD44 RNAi, suggesting that p38 MAPK has a central role in regulating subcellular localization of MRTF in primary mouse skin fibroblasts.25
The current study showed that nuclear MRTF levels are increased as a consequence of heightened p38 MAPK activation and of MRTF binding to SRF in Has1/3-null fibroblasts relative to WT fibroblasts. In contrast, no changes in levels of SRF (the principal binding partner of MRTF) are observed. Control of myofibroblast differentiation by MRTF and SRF has been well documented in various types of cells.57 TGF-β1–induced expressions of smooth muscle–specific proteins (eg, SMαA and SM22-α) were effectively reduced by knockdown of MRTF-A/B. Conversely, the expressions of these contractile-related proteins were significantly increased by the forced expression of a constitutively active MRTF-A construct.58 A previous study showed that MRTF is crucial for α-SMA expression in primary murine dermal fibroblasts, either at baseline or for mediating CD44's regulatory effects.25 The current study showed that knocking down gene expression of either MRTF or SRF effectively eliminated any differences in α-SMA expression between Has1/3-null and WT fibroblasts, suggesting that MRTF and SRF are critical in mediating increased α-SMA expression in Has1/3-null skin fibroblasts.
Up-Regulated MMP Activity in Fibroblasts and Healing Skin of Has1/3-Null Mice
MMPs are calcium- or zinc-dependent endopeptidases.59 Functions of MMPs are crucial for all stages of skin wound healing, by modifying the wound matrix and allowing cytokine release, and by cell migration and tissue remodeling. In addition, the functions of MMPs are tightly regulated at specific stages of wound repair, and specific MMPs are confined to particular locations in the wounds.59 For instance, fibroblasts that utilize MMPs are able to migrate into the wound area, remodel fibrin clots, and facilitate replacement with new extracellular matrix. MMP-9 can be recruited to the cell surface of fibroblasts by binding of a fibronectin-like domain in MMP-9 to lysyl hydroxylase 3 in the cellular plasma membrane, and this recruitment leads to TGF-β activation and myofibroblast differentiation.60 In the skin, various types of cells express MMPs, including fibroblasts, keratinocytes, endothelial cells, and inflammatory cells (eg, monocytes and macrophages).59 Various MMPs, induced in response to signals, including cytokines, hormones, and cell-to-cell and cell-to-matrix contacts, are secreted by fibroblasts in an autocrine manner, and they become membrane bound to the extracellular matrix or to the surface of cells, where they critically regulate fibroblast functions.61 To acquire further insight into mechanisms that lead to increased TGF-β activity in Has1/3-null fibroblasts, the overall MMP activity in mouse skin fibroblasts was examined and found to be up-regulated in Has1/3-null skin fibroblasts. Inhibition of MMP activity diminished the increased TGF-β activity in Has1/3-null fibroblasts. This provides evidence that increased MMP activity may regulate TGF-β activation of fibroblasts in an autocrine manner. An overall increase in MMP activity was shown in early states of wound healing in Has1/3-null healing skin. This indicated that a transient increase in MMP activity may be sufficient to drive increased TGF-β activity and enhance dermal maturation. However, the long-term consequences of such changes in MMP levels are unknown, and it remains an untested possibility that prolonged MMP activity might actually contribute to dysregulated inflammation and poor-quality wound healing.62
In conclusion, the present study shows that in Has1/3-null primary murine dermal fibroblasts, α-SMA gene expression is increased via a noncanonical pathway that involves TGF-β receptor, p38 MAPK, and MRTF/SRF transcription factors. Activation of this TGF-β receptor–mediated pathway may be attributable to increased MMP activity that is detected in both primary skin fibroblasts and in healing skin of Has1/3-null mice. Interestingly, the described changes appear to be independent of extracellular HA levels or Has2 expression. The link between loss of Has1/Has3 and activation of the noncanonical TGF-β–mediated pathway remains to be defined, but theoretically could involve an interaction between MMPs and other molecules that are indirectly influenced by the presence/absence of the Has proteins at the level of the cell membrane. Loss of the Has genes could then lead to constitutive up-regulation of MMP activity and enhanced cleavage of pro–TGF-β with resultant TGF-β ligand release. This phenomenon is apparently independent of the classic HA-synthetic functions of Has1 and Has3, and therefore might be indirect (perhaps due to alterations in molecular pathways acquired during embryonic development). Although these basic questions remain, the current findings provide novel insights into how the presence of hyaluronan synthases may regulate profibrotic signaling and myofibroblast differentiation during wound healing in a p38 MAPK–SRF–MRTF dependent manner.
Acknowledgments
We thank Dr. Judy Drazba and other personnel in the Digital Imaging Core for assistance with tissue histology and imaging.
Footnotes
Supported by federal grants NIHP01 HL107147 (E.V.M.) and NIH1K12HL14195 (V.C.H.) and by a National Scleroderma Foundation New Investigator Award (Y.W.).
Disclosures: None declared.
Supplemental material for this article can be found at http://doi.org/10.1016/j.ajpath.2022.08.003.
Contributor Information
Yan Wang, Email: wangy5@ccf.org.
Edward V. Maytin, Email: maytine@ccf.org.
Supplemental Data
Supplemental Figure S1.
Removal of extracellular hyaluronan or hyaluronan synthase (HAS) 2 RNA interference (RNAi) has no effect on increased α-smooth muscle actin (α-SMA) expression in Has1/3-null skin fibroblasts. A: The effect of 4-methylumbelliferone (4-MU) or hyaluronidase treatment on α-SMA expression. Left panel: Representative Western blot analysis images of α-SMA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from lysates of fibroblasts [wild type (WT) versus Has1/3 null] treated with 4-MU (1 mmol/L), Streptococcus dysgalactiae hyaluronidase (Hyal; 0.3 U/mL), or 0.1% dimethyl sulfoxide as vehicle control (Veh). Right panel: Densitometric analyses of the specific bands on Western blot analyses from four independent experiments (α-SMA normalized to GAPDH). B: The effect of Has2 RNAi on increased α-SMA expression in Has1/3-null fibroblasts. Left panel: Representative Western blot analysis images of α-SMA and GAPDH from lysates of fibroblasts (WT versus Has1/3 null) transfected with Has2 siRNA or nontargeted Scrambled siRNA. Right panel: Densitometric analyses of the specific bands on Western blot analyses from six independent experiments (α-SMA normalized to GAPDH). Data are presented as means ± SD (A and B). ∗P < 0.05.
References
- 1.Singer A.J., Clark R.A. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 2.Rodrigues M., Kosaric N., Bonham C.A., Gurtner G.C. Wound healing: a cellular perspective. Physiol Rev. 2019;99:665–706. doi: 10.1152/physrev.00067.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 4.Akhurst R.J., Hata A. Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S.I., Choi M.E. TGF-beta-activated kinase-1: new insights into the mechanism of TGF-beta signaling and kidney disease. Kidney Res Clin Pract. 2012;31:94–105. doi: 10.1016/j.krcp.2012.04.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stambe C., Nikolic-Paterson D.J., Hill P.A., Dowling J., Atkins R.C. p38 Mitogen-activated protein kinase activation and cell localization in human glomerulonephritis: correlation with renal injury. J Am Soc Nephrol. 2004;15:326–336. doi: 10.1097/01.asn.0000108520.63445.e0. [DOI] [PubMed] [Google Scholar]
- 7.Meyer-ter-Vehn T., Han H., Grehn F., Schlunck G. Extracellular matrix elasticity modulates TGF-beta-induced p38 activation and myofibroblast transdifferentiation in human tenon fibroblasts. Invest Ophthalmol Vis Sci. 2011;52:9149–9155. doi: 10.1167/iovs.10-6679. [DOI] [PubMed] [Google Scholar]
- 8.Molkentin J.D., Bugg D., Ghearing N., Dorn L.E., Kim P., Sargent M.A., Gunaje J., Otsu K., Davis J. Fibroblast-specific genetic manipulation of p38 mitogen-activated protein kinase in vivo reveals its central regulatory role in fibrosis. Circulation. 2017;136:549–561. doi: 10.1161/CIRCULATIONAHA.116.026238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronkina N., Lafera J., Kotlyarov A., Gaestel M. Stress-dependent phosphorylation of myocardin-related transcription factor A (MRTF-A) by the p38(MAPK)/MK2 axis. Sci Rep. 2016;6:31219. doi: 10.1038/srep31219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin-Garrido A., Brown D.I., Lyle A.N., Dikalova A., Seidel-Rogol B., Lassegue B., San Martin A., Griendling K.K. NADPH oxidase 4 mediates TGF-beta-induced smooth muscle alpha-actin via p38MAPK and serum response factor. Free Radic Biol Med. 2011;50:354–362. doi: 10.1016/j.freeradbiomed.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strauch A.R., Hariharan S. Dynamic interplay of smooth muscle alpha-actin gene-regulatory proteins reflects the biological complexity of myofibroblast differentiation. Biology (Basel) 2013;2:555–586. doi: 10.3390/biology2020555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner N.A., Blythe N.M. Cardiac fibroblast p38 MAPK: a critical regulator of myocardial remodeling. J Cardiovasc Dev Dis. 2019;6:27. doi: 10.3390/jcdd6030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miano J.M., Long X., Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol. 2007;292:C70–C81. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- 14.Olson E.N., Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11:353–365. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miralles F., Posern G., Zaromytidou A.I., Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 16.Panayiotou R., Miralles F., Pawlowski R., Diring J., Flynn H.R., Skehel M., Treisman R. Phosphorylation acts positively and negatively to regulate MRTF-A subcellular localisation and activity. Elife. 2016;5:e15460. doi: 10.7554/eLife.15460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser J.R., Laurent T.C., Laurent U.B. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 18.Itano N., Kimata K. Mammalian hyaluronan synthases. IUBMB Life. 2002;54:195–199. doi: 10.1080/15216540214929. [DOI] [PubMed] [Google Scholar]
- 19.Camenisch T.D., Spicer A.P., Brehm-Gibson T., Biesterfeldt J., Augustine M.L., Calabro A., Jr., Kubalak S., Klewer S.E., McDonald J.A. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itano N., Sawai T., Yoshida M., Lenas P., Yamada Y., Imagawa M., Shinomura T., Hamaguchi M., Yoshida Y., Ohnuki Y., Miyauchi S., Spicer A.P., McDonald J.A., Kimata K. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999;274:25085–25092. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- 21.Mack J.A., Feldman R.J., Itano N., Kimata K., Lauer M., Hascall V.C., Maytin E.V. Enhanced inflammation and accelerated wound closure following tetraphorbol ester application or full-thickness wounding in mice lacking hyaluronan synthases Has1 and Has3. J Invest Dermatol. 2012;132:198–207. doi: 10.1038/jid.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y., Lauer M.E., Anand S., Mack J.A., Maytin E.V. Hyaluronan synthase 2 protects skin fibroblasts against apoptosis induced by environmental stress. J Biol Chem. 2014;289:32253–32265. doi: 10.1074/jbc.M114.578377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y., Jiang D., Liang J., Meltzer E.B., Gray A., Miura R., Wogensen L., Yamaguchi Y., Noble P.W. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med. 2011;208:1459–1471. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jurukovski V., Dabovic B., Todorovic V., Chen Y., Rifkin D.B. Methods for measuring TGF-b using antibodies, cells, and mice. Methods Mol Med. 2005;117:161–175. doi: 10.1385/1-59259-940-0:161. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., Mack J.A., Maytin E.V. CD44 inhibits alpha-SMA gene expression via a novel G-actin/MRTF-mediated pathway that intersects with TGFbetaR/p38MAPK signaling in murine skin fibroblasts. J Biol Chem. 2019;294:12779–12794. doi: 10.1074/jbc.RA119.007834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meran S., Luo D.D., Simpson R., Martin J., Wells A., Steadman R., Phillips A.O. Hyaluronan facilitates transforming growth factor-beta1-dependent proliferation via CD44 and epidermal growth factor receptor interaction. J Biol Chem. 2011;286:17618–17630. doi: 10.1074/jbc.M111.226563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takekawa M., Tatebayashi K., Itoh F., Adachi M., Imai K., Saito H. Smad-dependent GADD45beta expression mediates delayed activation of p38 MAP kinase by TGF-beta. EMBO J. 2002;21:6473–6482. doi: 10.1093/emboj/cdf643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters I., Tossidou I., Achenbach J., Woroniecki R., Mengel M., Park J.K., Paschy M., de Groot K., Haller H., Schiffer M. IGF-binding protein-3 modulates TGF-beta/BMP-signaling in glomerular podocytes. J Am Soc Nephrol. 2006;17:1644–1656. doi: 10.1681/ASN.2005111209. [DOI] [PubMed] [Google Scholar]
- 29.Sapkota G.P. The TGFbeta-induced phosphorylation and activation of p38 mitogen-activated protein kinase is mediated by MAP3K4 and MAP3K10 but not TAK1. Open Biol. 2013;3:130067. doi: 10.1098/rsob.130067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roux P.P., Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakagawa T., Lan H.Y., Glushakova O., Zhu H.J., Kang D.H., Schreiner G.F., Bottinger E.P., Johnson R.J., Sautin Y.Y. Role of ERK1/2 and p38 mitogen-activated protein kinases in the regulation of thrombospondin-1 by TGF-beta1 in rat proximal tubular cells and mouse fibroblasts. J Am Soc Nephrol. 2005;16:899–904. doi: 10.1681/ASN.2004080689. [DOI] [PubMed] [Google Scholar]
- 32.Canovas B., Nebreda A.R. Diversity and versatility of p38 kinase signalling in health and disease. Nat Rev Mol Cell Biol. 2021;22:346–366. doi: 10.1038/s41580-020-00322-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Posern G., Treisman R. Actin' together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol. 2006;16:588–596. doi: 10.1016/j.tcb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Wipff P.J., Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol. 2008;87:601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Krzyszczyk P., Schloss R., Palmer A., Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol. 2018;9:419. doi: 10.3389/fphys.2018.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Werner S., Krieg T., Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 37.Meran S., Thomas D., Stephens P., Martin J., Bowen T., Phillips A., Steadman R. Involvement of hyaluronan in regulation of fibroblast phenotype. J Biol Chem. 2007;282:25687–25697. doi: 10.1074/jbc.M700773200. [DOI] [PubMed] [Google Scholar]
- 38.Webber J., Meran S., Steadman R., Phillips A. Hyaluronan orchestrates transforming growth factor-beta1-dependent maintenance of myofibroblast phenotype. J Biol Chem. 2009;284:9083–9092. doi: 10.1074/jbc.M806989200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evanko S.P., Potter-Perigo S., Petty L.J., Workman G.A., Wight T.N. Hyaluronan controls the deposition of fibronectin and collagen and modulates TGF-beta1 induction of lung myofibroblasts. Matrix Biol. 2015;42:74–92. doi: 10.1016/j.matbio.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43:146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 41.Jones C., Ehrlich H.P. Fibroblast expression of alpha-smooth muscle actin, alpha2beta1 integrin and alphavbeta3 integrin: influence of surface rigidity. Exp Mol Pathol. 2011;91:394–399. doi: 10.1016/j.yexmp.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan D.D., Xiao W.F., Li J., de la Motte C.A., Sandy J.D., Plaas A. Deficiency of hyaluronan synthase 1 (Has1) results in chronic joint inflammation and widespread intra-articular fibrosis in a murine model of knee joint cartilage damage. Osteoarthritis Cartilage. 2015;23:1879–1889. doi: 10.1016/j.joca.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCracken J.M., Jiang L., Deshpande K.T., O'Neil M.F., Pritchard M.T. Differential effects of hyaluronan synthase 3 deficiency after acute vs chronic liver injury in mice. Fibrogenesis Tissue Repair. 2016;9:4. doi: 10.1186/s13069-016-0041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petz A., Grandoch M., Gorski D.J., Abrams M., Piroth M., Schneckmann R., Homann S., Muller J., Hartwig S., Lehr S., Yamaguchi Y., Wight T.N., Gorressen S., Ding Z., Kotter S., Kruger M., Heinen A., Kelm M., Godecke A., Flogel U., Fischer J.W. Cardiac hyaluronan synthesis is critically involved in the cardiac macrophage response and promotes healing after ischemia reperfusion injury. Circ Res. 2019;124:1433–1447. doi: 10.1161/CIRCRESAHA.118.313285. [DOI] [PubMed] [Google Scholar]
- 45.Williams A.P., Midgley A.C., Brown C.V., Roberts T.H., Morris N.G., Steadman R., Philips A.O., Meran S. The hyaluronan synthase-1 isoenzyme promotes differentiation to a distinct subset of myofibroblasts that limit fibrosis progression. J Am Soc Nephrol. 2018;29(Suppl):3. Abstract TH-OR011. [Google Scholar]
- 46.El-Brolosy M.A., Stainier D.Y.R. Genetic compensation: a phenomenon in search of mechanisms. PLoS Genet. 2017;13:e1006780. doi: 10.1371/journal.pgen.1006780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siiskonen H., Oikari S., Pasonen-Seppanen S., Rilla K. Hyaluronan synthase 1: a mysterious enzyme with unexpected functions. Front Immunol. 2015;6:43. doi: 10.3389/fimmu.2015.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Porsch H., Bernert B., Mehic M., Theocharis A.D., Heldin C.H., Heldin P. Efficient TGFbeta-induced epithelial-mesenchymal transition depends on hyaluronan synthase HAS2. Oncogene. 2013;32:4355–4365. doi: 10.1038/onc.2012.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim Y.H., Lee S.B., Shim S., Kim A., Park J.H., Jang W.S., Lee S.J., Myung J.K., Park S., Lee S.J., Kim M.J. Hyaluronic acid synthase 2 promotes malignant phenotypes of colorectal cancer cells through transforming growth factor beta signaling. Cancer Sci. 2019;110:2226–2236. doi: 10.1111/cas.14070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Webber J., Jenkins R.H., Meran S., Phillips A., Steadman R. Modulation of TGFbeta1-dependent myofibroblast differentiation by hyaluronan. Am J Pathol. 2009;175:148–160. doi: 10.2353/ajpath.2009.080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986;46:567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- 52.Chai J., Tarnawski A.S. Serum response factor: discovery, biochemistry, biological roles and implications for tissue injury healing. J Physiol Pharmacol. 2002;53:147–157. [PubMed] [Google Scholar]
- 53.Miano J.M. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol. 2003;35:577–593. doi: 10.1016/s0022-2828(03)00110-x. [DOI] [PubMed] [Google Scholar]
- 54.Gasparics A., Sebe A. MRTFs- master regulators of EMT. Dev Dyn. 2018;247:396–404. doi: 10.1002/dvdy.24544. [DOI] [PubMed] [Google Scholar]
- 55.Muehlich S., Wang R., Lee S.M., Lewis T.C., Dai C., Prywes R. Serum-induced phosphorylation of the serum response factor coactivator MKL1 by the extracellular signal-regulated kinase 1/2 pathway inhibits its nuclear localization. Mol Cell Biol. 2008;28:6302–6313. doi: 10.1128/MCB.00427-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sebe A., Masszi A., Zulys M., Yeung T., Speight P., Rotstein O.D., Nakano H., Mucsi I., Szaszi K., Kapus A. Rac, PAK and p38 regulate cell contact-dependent nuclear translocation of myocardin-related transcription factor. FEBS Lett. 2008;582:291–298. doi: 10.1016/j.febslet.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Small E.M. The actin-MRTF-SRF gene regulatory axis and myofibroblast differentiation. J Cardiovasc Transl Res. 2012;5:794–804. doi: 10.1007/s12265-012-9397-0. [DOI] [PubMed] [Google Scholar]
- 58.Crider B.J., Risinger G.M., Jr., Haaksma C.J., Howard E.W., Tomasek J.J. Myocardin-related transcription factors A and B are key regulators of TGF-beta1-induced fibroblast to myofibroblast differentiation. J Invest Dermatol. 2011;131:2378–2385. doi: 10.1038/jid.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caley M.P., Martins V.L., O'Toole E.A. Metalloproteinases and wound healing. Adv Wound Care. 2015;4:225–234. doi: 10.1089/wound.2014.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dayer C., Stamenkovic I. Recruitment of matrix metalloproteinase-9 (MMP-9) to the fibroblast cell surface by lysyl hydroxylase 3 (LH3) triggers transforming growth factor-beta (TGF-beta) activation and fibroblast differentiation. J Biol Chem. 2015;290:13763–13778. doi: 10.1074/jbc.M114.622274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kobayashi T., Kim H., Liu X., Sugiura H., Kohyama T., Fang Q., Wen F.Q., Abe S., Wang X., Atkinson J.J., Shipley J.M., Senior R.M., Rennard S.I. Matrix metalloproteinase-9 activates TGF-beta and stimulates fibroblast contraction of collagen gels. Am J Physiol Lung Cell Mol Physiol. 2014;306:L1006–L1015. doi: 10.1152/ajplung.00015.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gill S.E., Parks W.C. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol. 2008;40:1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]