Abstract
Background
The SARS-CoV-2 omicron (B.1.1.529 BA.1) lineage was first detected in November, 2021, and is associated with reduced vaccine effectiveness. By March, 2022, BA.1 had been replaced by sub-lineage BA.2 in the USA. As new variants evolve, vaccine performance must be continually assessed. We aimed to evaluate the effectiveness and durability of BNT162b2 (Pfizer–BioNTech) against hospital and emergency department admissions for BA.1 and BA.2.
Methods
In this test-negative, case-control study, we sourced data from the electronic health records of adult (aged ≥18 years) members of Kaiser Permanente Southern California (KPSC), which is a health-care system in the USA, who were admitted to one of 15 KPSC hospitals or emergency departments (without subsequent hospitalisation) between Dec 27, 2021, and June 4, 2022, with an acute respiratory infection and were tested for SARS-CoV-2 by RT-PCR. Omicron sub-lineage was determined by use of sequencing, spike gene target failure, and the predominance of variants in certain time periods. Our main outcome was the effectiveness of two or three doses of BNT162b2 in preventing emergency department or hospital admission. Variant-specific vaccine effectiveness was evaluated by comparing the odds ratios from logistic regression models of vaccination between test-positive cases and test-negative controls, adjusting for the month of admission, age, sex, race and ethnicity, body-mass index, Charlson Comorbidity Index, previous influenza or pneumococcal vaccines, and previous SARS-CoV-2 infection. We also assessed effectiveness by the time since vaccination. This study is registered at ClinicalTrials.gov, NCT04848584, and is ongoing.
Findings
Of 65 813 total admissions during the study period, we included 16 994 in our analyses, of which 7435 were due to BA.1, 1056 were due to BA.2, and 8503 were not due to SARS-CoV-2. In adjusted analyses, two-dose vaccine effectiveness was 40% (95% CI 27 to 50) for hospitalisation and 29% (18 to 38) for emergency department admission against BA.1 and 56% (31 to 72) for hospitalisation and 16% (−5 to 33) for emergency department admission against BA.2. Three-dose vaccine effectiveness was 79% (74 to 83) for hospitalisation and 72% (67 to 77) for emergency department admission against BA.1 and 71% (55 to 81) for hospitalisation and 21% (1 to 37) for emergency department admission against BA.2. Less than 3 months after the third dose, vaccine effectiveness was 80% (74 to 84) for hospitalisation and 74% (69 to 78) for emergency department admission against BA.1. Vaccine effectiveness 3 months or more after the third dose was 76% (69 to 82) against BA.1-related hospitalisation and 65% (56 to 73) against BA.1-related emergency department admission. Against BA.2, vaccine effectiveness was 74% (47 to 87) for hospitalisation and 59% (40 to 72) for emergency department admission at less than 3 months after the third dose and 70% (53 to 81) for hospitalisation and 5% (−21 to 25) for emergency department admission at 3 months or more after the third dose.
Interpretation
Two doses of BNT162b2 provided only partial protection against BA.1-related and BA.2-related hospital and emergency department admission, which underscores the need for booster doses against omicron. Although three doses offered high levels of protection (≥70%) against hospitalisation, variant-adapted vaccines are probably needed to improve protection against less severe endpoints, like emergency department admission, especially for BA.2.
Funding
Pfizer.
Introduction
Since the identification of SARS-CoV-2 in Wuhan, China, in December, 2019, new variants of concern have emerged.1 The most recent variant of concern, omicron (B.1.1.529), was originally identified in South Africa in November, 2021, and quickly outcompeted previous variants. Subsequently, large waves of COVID-19 that surpassed the levels recorded at any other time during the pandemic occurred in essentially every region across the globe.1 The omicron variant has numerous mutations in its spike protein, including multiple mutations in the receptor-binding domain, one of the main targets for neutralising antibodies.2, 3, 4, 5 Omicron has been associated with higher transmissibility6, 7, 8, 9 and lower antibody neutralisation6, 10 compared with older variants such as delta (B.1.617.2).7, 8, 9
Research in context.
Evidence before this study
We searched PubMed, medRxiv, and press coverage without language restrictions for preprints and peer-reviewed articles published between Jan 1, 2020, and June 15, 2022, using the search terms “BNT162b2” AND “COVID-19” OR “SARS-CoV-2” AND “vaccin*” AND “effective*” OR “outcome” AND “omicron” OR “BA*”. We found that the omicron variant (B.1.1.529) of SARS-CoV-2 emerged in November, 2021, and has been associated with higher transmissibility and lower antibody neutralisation compared with older variants, including delta (B.1.617.2). Within months of the emergence of omicron, sub-lineage BA.2 became the dominant strain around the world. There are sparse data describing vaccine effectiveness against BA.2. A Swedish study showed that three doses of COVID-19 vaccines had a similar effectiveness against severe COVID-19 caused by BA.1 versus BA.2, but vaccine effectiveness after two doses decreased once BA.2 became the predominant circulating variant. Furthermore, a Danish household contact study reported increased transmissibility and reduced vaccine protection for BA.2 compared with BA.1. By contrast, studies done in the UK and Qatar have described similar vaccine effectiveness against severe COVID-19 for BA.1 and BA.2, without any differential waning between the two sub-lineages.
Added value of this study
Our study compares the effectiveness of two and three doses of BNT162b2 (Pfizer–BioNTech) against hospital and emergency department admissions for BA.1 and BA.2. Overall, two doses provided only partial protection (16–56%) against hospitalisation and emergency department admission due to BA.1 or BA.2. Three doses offered high levels of protection (≥70%) against hospitalisation for BA.1 or BA.2 and emergency department admission for BA.1, but only partial protection (21%) against BA.2-related emergency department admission.
Implications of all the available evidence
A minimum of three doses of BNT162b2 is needed to protect against omicron-related hospitalisations. Variant-adapted vaccines are probably needed to protect against less severe endpoints, like emergency department admission, especially for BA.2.
Within months of the emergence of omicron, sub-lineage BA.2 became a dominant global variant and was classified as a new variant of concern. As of June 11, 2022, BA.2 and BA.2.12.1 accounted for 78% of all SARS-CoV-2 infections sequenced worldwide.1 BA.2 shares several mutations with sub-lineage BA.1, but also has eight unique mutations present in its spike protein that are thought to be associated with differences in receptor binding affinity, transmissibility, and glycosylation.11, 12, 13
Previous studies have shown that the COVID-19 vaccines BNT162b2 (Pfizer–BioNTech) and mRNA-1273 (Moderna) are less effective against omicron BA.1 than against older variants of concern; however, protection against severe omicron-related COVID-19 in the general population after three vaccine doses has remained high.14, 15, 16, 17, 18, 19, 20, 21 Nevertheless, data describing COVID-19 vaccine effectiveness against omicron BA.2 are sparse. A Swedish study showed that three doses of COVID-19 vaccines had a similar effectiveness against severe COVID-19 caused by BA.1 versus BA.2, but vaccine effectiveness after two doses decreased once BA.2 became the predominant circulating variant.22 Furthermore, a Danish household contact study reported increased transmissibility and reduced vaccine protection for BA.2 compared with BA.1.20 By contrast, studies done in the UK and Qatar have described similar vaccine effectiveness against severe COVID-19 for BA.1 and BA.2, without any differential waning between the two sub-lineages.23, 24
As new SARS-CoV-2 variants evolve, vaccine effectiveness must be continually assessed against a range of outcomes. We aimed to evaluate the effectiveness and durability of two and three doses of BNT162b2 against hospital and emergency department admissions for BA.1 and BA.2 SARS-CoV-2 infection.
Methods
Study design and participants
In this test-negative, case-control study, we included adult (aged ≥18 years) members of Kaiser Permanente Southern California (KPSC), which is a large, integrated health-care system with more than 4·7 million members in southern California, USA.25 Eligible patients were admitted to one of 15 KPSC hospitals or emergency departments (without subsequent hospitalisation) between Dec 27, 2021, (a date when 90% of SARS-CoV-2 infections at KPSC were omicron; data not shown) and June 4, 2022, with a diagnosis of acute respiratory infection based on International Classification of Diseases (tenth revision) codes (appendix pp 1–3) and were tested for SARS-CoV-2 by RT-PCR (a criterion that helps to reduce health-care seeking and testing bias).26 Members younger than 18 years were not included because the timings of recommendations for mRNA vaccine doses differed by age and vaccine effectiveness might also vary for younger age groups. Participants were required to have at least 1 year of health plan membership so that we could determine comorbidities and medical history. A 45-day gap in membership was allowed to account for any delays in renewal of membership. COVID-19-vaccinated individuals must have received either two or three doses of BNT162b2; individuals who had received partial (ie, one dose) or heterologous vaccination were excluded. This study received ethics approval from the KPSC Institutional Review Board, which granted a waiver of informed consent.
Procedures
During the study period (Dec 27, 2021–June 4, 2022), all people admitted to KPSC hospitals were tested for SARS-CoV-2 and those with COVID-19 who presented to hospital for non-emergency conditions (eg, non-emergency surgery and inpatient diagnostic procedures) had their appointments rescheduled to a later date. Patients tested outside of the KPSC system before their admission were re-tested within the KPSC system. Omicron sub-lineage (BA.1 vs BA.2) was determined by use of a combination of sequencing, spike gene target failure (SGTF), and the predominance of variants in certain time periods (appendix p 6). Specimens that underwent whole-genome sequencing (NovaSeq 6000 Sequencing System S1 Flow Cell, which included the NovaSeq 6000 Sequencing System S1 Reagent Kit version 1.5 [300 cycles]; Illumina, San Diego, CA, USA) or were tested with the Thermo Fisher TaqPath COVID-19 Combo Kit (Waltham, MA, USA), which can distinguish SGTF among SARS-CoV-2-positive specimens, were included in the analysis, as previously described.14 Viral sequences were assigned a Pango lineage by use of pangoLEARN. Mutations in the spike protein of omicron BA.1-positive specimens cause PCR probes targeting the spike gene to fail, but SGTF is rare for the BA.2 omicron sub-lineage.27, 28 For samples tested with the Thermo Fisher kit, those with SGTF were characterised as BA.1 between Dec 27, 2021, (study start) and May 8, 2022 (the date of nadir, when 13 [2%] of 611 samples had SGTF, with the subsequent increase suggesting the arrival of BA.4 or BA.5 sub-lineages). Therefore, samples with SGTF after May 8, 2022, were assumed to be BA.4 or BA.5 and were excluded from our analysis. Samples without SGTF were categorised as BA.2 from Jan 27, 2022, onwards (the date when BA.2 accounted for ≥90% of samples without SGTF; data not shown) and were excluded before this date (when samples without SGTF were presumably delta). Specimens that did not undergo whole-genome sequencing and were tested with RT-PCR but not by use of the Thermo Fisher kit specifically (ie, SGTF could not readily be determined) were assigned to BA.1 from Dec 27, 2021, to Feb 28, 2022, and to BA.2 from April 12, 2022, to May 25, 2022, when 90% or more of known COVID-19 cases were due to these variants (data not shown). Specimens not assigned to a variant by one of these methods were excluded from our analyses. On the basis of the study period and the conditions we have specified, we assumed that all SARS-CoV-2 infections were due to omicron. We did an internal validation study of isolates that had SGTF designation by Thermo Fisher and had undergone whole-genome sequencing to confirm the accuracy of sub-lineage identification by SGTF.
KPSC has an integrated electronic health record system that includes data, such as previous COVID-19 test results, for members across all settings of care. All KPSC members were eligible for COVID-19 vaccines at no cost on the basis of US Food and Drug Administration authorisation. Vaccination data were captured within the electronic health record system if provided at a KPSC site or were sourced from the California Immunization Registry. Patients were categorised as having received two doses of BNT162b2 if the emergency department or hospital admission was at least 14 days after the receipt of the second dose and they had not received three doses. Patients were categorised as having received three doses of BNT162b2 if they received a third dose of BNT162b2 at least 28 days after receiving two doses of BNT162b2, the hospital or emergency department admission occurred at least 14 days after the receipt of the third dose,14, 29, 30, 31 and they had not received four doses. Individuals were considered unvaccinated if they had never received BNT162b2 or any other COVID-19 vaccine.
Outcomes and statistical analysis
We compared patient and clinical characteristics by vaccination status and variant using the χ2 test for categorical variables and Fisher's exact test for binary variables. Our main outcome was the effectiveness of two or three doses of BNT162b2 in preventing emergency department or hospital admission. Unadjusted and adjusted vaccine effectiveness were evaluated by comparing the odds ratios (ORs) from logistic regression models of vaccination between test-positive cases and test-negative controls. Cases were patients with a hospital or emergency department admission for acute respiratory infection and a positive KPSC laboratory-confirmed SARS-CoV-2 RT-PCR test from a sample collected from 14 days before the initial admission date to 3 days after the admission. Controls were patients with a hospital or emergency department admission for acute respiratory infection and a KPSC laboratory-confirmed negative SARS-CoV-2 RT-PCR test collected from 14 days before admission to 3 days after admission and no positive COVID-19 tests within 90 days of admission. If a patient met the inclusion criteria for either a case or a control and had multiple admissions that were more than 30 days apart, the patient could contribute more than one event to the study.
Vaccine effectiveness was calculated as 1−OR multiplied by 100%, with corresponding 95% CIs calculated by use of the Wald method. Adjusted ORs and 95% CIs were estimated in multivariable logistic regression models by adjusting for the month of emergency department or hospital admission, age (18−49 years, 50−64 years, or ≥65 years), sex (male or female), race and ethnicity (Hispanic, non-Hispanic White, non-Hispanic Black, non-Hispanic Asian or Pacific Islander, or other or unknown), body-mass index (<18·5 kg/m2, 18·5−24·9 kg/m2, 25·0−29·9 kg/m2, 30·0−34·9 kg/m2, ≥35·0 kg/m2, or unknown), Charlson Comorbidity Index (0, 1, 2, 3, or ≥4), receipt of an influenza vaccine in the year before admission (yes or no), receipt of a pneumococcal vaccine in the 5 years before admission (to adjust for health-care seeking behaviour; yes or no), and documentation (PCR or lateral flow test) of previous SARS-CoV-2 infection (ever or never). Analyses were done separately for hospital and emergency department admission. Analyses were further stratified by variant (BA.1 vs BA.2) and immunocompetent status. By use of a previously published methodology,30 immunocompetence was defined as the absence of any immunocompromising conditions, including leukaemia, lymphoma, congenital immunodeficiencies, asplenia or hyposplenia, or HIV or AIDS; no history of haematopoietic stem-cell or solid organ transplantation; and no receipt of immunocompromising medication.30 We also assessed effectiveness by the time since vaccination (admission <6 months vs ≥6 months since completion of two-dose [only] vaccination or <3 months vs ≥3 months since completion of three-dose vaccination).
Because secular trends might affect vaccine effectiveness estimates, we did additional analyses to explore the impact of different methods of accounting for secular trends. In addition to the primary analysis, which adjusted for the month of admission as a categorical variable, we fit models that (1) adjusted for date as a linear trend, (2) matched test-positive cases to test-negative controls on test date (closest match within 2 weeks), and (3) matched test-positive cases to test-negative controls on test date (closest match within 2 weeks), age, sex, race and ethnicity, and the number of vaccine doses received. Matching was 1:1 for BA.1 and BA.2 and two test-negative controls were matched to each test-positive case (where lower positivity rates allowed for more controls). Conditional logistic regression was used for modelling matched data. We also explored adjusting for the week of admission to allow for finer changes in secular effects than were allowed when adjusting for month. All analyses were done by use of SAS Enterprise Guide statistical software, version 7.1. This study is registered with ClinicalTrials.gov, NCT04848584.
Role of the funding source
This study was sponsored by Pfizer. The study design was developed by KPSC but approved by Pfizer. Pfizer had no role in data collection or data analysis. Pfizer had a role in data interpretation, writing of the report, and in the decision to submit for publication.
Results
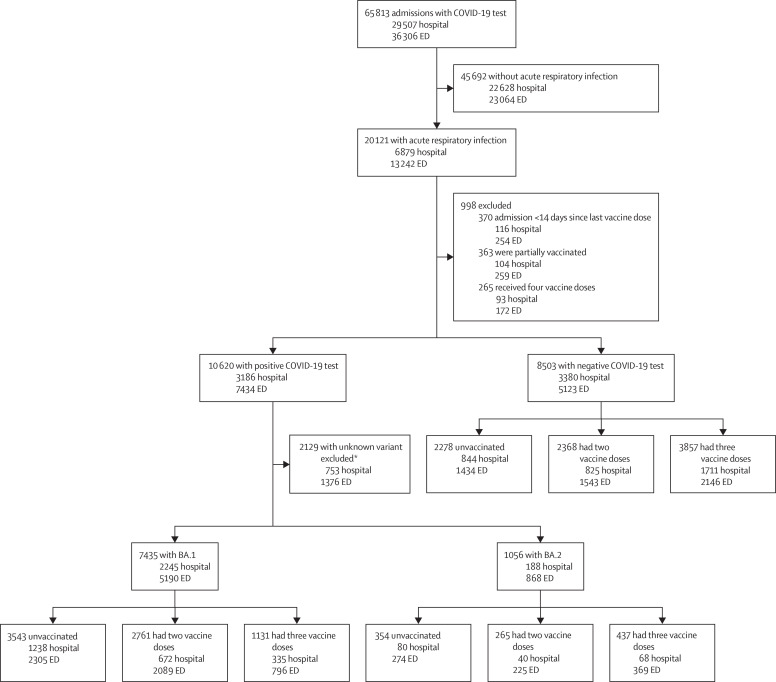
Between Dec 27, 2021, and June 4, 2022, there were 29 507 hospital admissions and 36 306 emergency department admissions with a documented SARS-CoV-2 RT-PCR test across 15 KPSC hospitals in southern California (figure 1 ). 6879 (23·3%) of 29 507 hospital admissions and 13 242 (36·5%) of 36 306 emergency department admissions were for acute respiratory infection (figure 1). The final study population consisted of 16 994 admissions. Patients were either SARS-CoV-2-negative or SARS-CoV-2-positive, for which variant sub-lineage could be designated by the Thermo Fisher TaqPath COVID-19 Combo Kit (and thus SGTF could be determined), whole-genome sequencing, or the time when the infection occurred (ie, when ≥90% of all SARS-CoV-2 infections were due to a particular variant). 8491 (50·0%) of 16 994 admissions had a positive SARS-CoV-2 test, with 7435 (87·6%) having BA.1 and 1056 (12·4%) having BA.2. Of 16 994 admissions, 6175 (36·3%) were unvaccinated, 5394 (31·7%) were vaccinated with only two doses, and 5425 (31·9%) were vaccinated with three doses. The median age of the study population was 55 years (IQR 36−73). Compared with those who tested positive for SARS-CoV-2, those who tested negative were more likely to be older and White and have comorbidities and evidence of previous SARS-CoV-2 infection (table 1 ). Compared with participants who had received three vaccine doses, unvaccinated participants were more likely to be younger or Black or Hispanic and less likely to have comorbidities or have received a previous influenza or pneumococcal vaccine (table 2 ). One patient who was negative for SARS-CoV-2 had an unknown sex and was excluded from our adjusted analyses.
Figure 1.
Trial profile
ED=emergency department. *Samples for which the SARS-COV-2 variant could not be identified included those that were not tested with the Thermo Fisher TaqPath COVID-19 Combo Kit (and thus spike gene target failure could not be determined), those that did not undergo whole-genome sequencing, and those that could not be assigned a variant lineage on the basis of the time when the infection occurred.
Table 1.
Characteristics of test-positive cases and test-negative controls
| Negative (n=8503) | BA.1 (n=7435) | BA.2 (n=1056) | Total (n=16 994) | p value* | ||
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age group | ||||||
| 18–49 years | 3208 (37·7%) | 3570 (48·0%) | 541 (51·2%) | 7319 (43·1%) | <0·0001 | |
| 50–64 years | 1541 (18·1%) | 1664 (22·4%) | 215 (20·4%) | 3420 (20·1%) | .. | |
| ≥65 years | 3754 (44·1%) | 2201 (29·6%) | 300 (28·4%) | 6255 (36·8%) | .. | |
| Sex | ||||||
| Female | 4901/8502 (57·6%)† | 4282 (57·6%) | 640 (60·6%) | 9823/16 993 (57·8%) | 0·14 | |
| Male | 3601/8502 (42·4%)† | 3153 (42·4%) | 416 (39·4%) | 7170/16 993 (42·2%) | .. | |
| Race and ethnicity | ||||||
| Non-Hispanic Asian or Pacific Islander | 702 (8·3%) | 605 (8·1%) | 130 (12·3%) | 1437 (8·5%) | <0·0001 | |
| Non-Hispanic Black | 1129 (13·3%) | 996 (13·4%) | 126 (11·9%) | 2251 (13·2%) | .. | |
| Hispanic | 3378 (39·7%) | 3581 (48·2%) | 425 (40·2%) | 7384 (43·5%) | .. | |
| Other or unknown | 314 (3·7%) | 283 (3·8%) | 55 (5·2%) | 652 (3·8%) | .. | |
| Non-Hispanic White | 2980 (35·0%) | 1970 (26·5%) | 320 (30·3%) | 5270 (31·0%) | .. | |
| Body-mass index | ||||||
| Underweight (<18·5 kg/m2) | 321 (3·8%) | 154 (2·1%) | 19 (1·8%) | 494 (2·9%) | <0·0001 | |
| Normal or healthy weight (18·5–24·9 kg/m2) | 2164 (25·4%) | 1582 (21·3%) | 271 (25·7%) | 4017 (23·6%) | .. | |
| Overweight (25·0–29·9 kg/m2) | 2383 (28·0%) | 2097 (28·2%) | 317 (30·0%) | 4797 (28·2%) | .. | |
| Obese, class 1 (30·0–34·9 kg/m2) | 1725 (20·3%) | 1730 (23·3%) | 241 (22·8%) | 3696 (21·7%) | .. | |
| Obese, class 2–3 (≥35·0 kg/m2) | 1822 (21·4%) | 1780 (23·9%) | 202 (19·1%) | 3804 (22·4%) | .. | |
| Unknown | 88 (1·0%) | 92 (1·2%) | 6 (0·6%) | 186 (1·1%) | .. | |
| Comorbidities | ||||||
| Hypertension | 4051 (47·6%) | 2531 (34·0%) | 336 (31·8%) | 6918 (40·7%) | <0·0001 | |
| Congestive heart failure | 1522 (17·9%) | 574 (7·7%) | 77 (7·3%) | 2173 (12·8%) | <0·0001 | |
| Myocardial infarction | 587 (6·9%) | 256 (3·4%) | 33 (3·1%) | 876 (5·2%) | <0·0001 | |
| Peripheral vascular disease | 2806 (33·0%) | 1381 (18·6%) | 195 (18·5%) | 4382 (25·8%) | <0·0001 | |
| Cerebrovascular disease | 674 (7·9%) | 335 (4·5%) | 47 (4·5%) | 1056 (6·2%) | <0·0001 | |
| Diabetes status | ||||||
| Diabetes with unknown glycated haemoglobin | 138 (1·6%) | 102 (1·4%) | 10 (0·9%) | 250 (1·5%) | <0·0001 | |
| Diabetes with glycated haemoglobin <7·5% | 1489 (17·5%) | 897 (12·1%) | 116 (11·0%) | 2502 (14·7%) | .. | |
| Diabetes with glycated haemoglobin ≥7·5% | 934 (11·0%) | 740 (10·0%) | 97 (9·2%) | 1771 (10·4%) | .. | |
| Chronic obstructive pulmonary disease | 2453 (28·8%) | 1188 (16·0%) | 176 (16·7%) | 3817 (22·5%) | <0·0001 | |
| Renal disease | 1835 (21·6%) | 984 (13·2%) | 129 (12·2%) | 2948 (17·3%) | <0·0001 | |
| Malignancy | 919 (10·8%) | 384 (5·2%) | 59 (5·6%) | 1362 (8·0%) | <0·0001 | |
| Organ transplant | 55 (0·6%) | 128 (1·7%) | 9 (0·9%) | 192 (1·1%) | <0·0001 | |
| Charlson Comorbidity Index | ||||||
| 0 | 3131 (36·8%) | 3868 (52·0%) | 589 (55·8%) | 7588 (44·7%) | <0·0001 | |
| 1 | 1327 (15·6%) | 1337 (18·0%) | 165 (15·6%) | 2829 (16·6%) | .. | |
| 2 | 857 (10·1%) | 655 (8·8%) | 91 (8·6%) | 1603 (9·4%) | .. | |
| 3 | 594 (7·0%) | 401 (5·4%) | 52 (4·9%) | 1047 (6·2%) | .. | |
| ≥4 | 2594 (30·5%) | 1174 (15·8%) | 159 (15·1%) | 3927 (23·1%) | .. | |
| Vaccine and infection history | ||||||
| Influenza vaccine in the year before admission | 4917 (57·8%) | 3010 (40·5%) | 545 (51·6%) | 8472 (49·9%) | <0·0001 | |
| Pneumococcal vaccine in the 5 years before admission | 2085 (24·5%) | 1504 (20·2%) | 188 (17·8%) | 3777 (22·2%) | <0·0001 | |
| Previous positive SARS-CoV-2 PCR test | 1922 (22·6%) | 552 (7·4%) | 117 (11·1%) | 2591 (15·2%) | <0·0001 | |
| BNT162b2 vaccination status | ||||||
| Unvaccinated | 2278 (26·8%) | 3543 (47·7%) | 354 (33·5%) | 6175 (36·3%) | <0·0001 | |
| 2 doses (only) <6 months ago | 659 (7·8%) | 846 (11·4%) | 41 (3·9%) | 1546 (9·1%) | .. | |
| 2 doses (only) ≥6 months ago | 1709 (20·1%) | 1915 (25·8%) | 224 (21·2%) | 3848 (22·6%) | .. | |
| 3 doses <3 months ago | 1517 (17·8%) | 788 (10·6%) | 50 (4·7%) | 2355 (13·9%) | .. | |
| 3 doses ≥3 months ago | 2340 (27·5%) | 343 (4·6%) | 387 (36·6%) | 3070 (18·1%) | .. | |
Data are n (%) or n/N (%), unless otherwise specified.
χ2 test comparing negative, BA.1, and BA.2 groups.
One patient had an unknown sex and was excluded.
Table 2.
Patient characteristics by vaccination status
| Unvaccinated (n=6175) | Two doses only (n=5394) | Three doses (n=5425) | Total (n=16 994) | p value* | ||
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age group | ||||||
| 18–49 years | 3133 (50·7%) | 2800 (51·9%) | 1386 (25·5%) | 7319 (43·1%) | <0·0001 | |
| 50–64 years | 1326 (21·5%) | 1090 (20·2%) | 1004 (18·5%) | 3420 (20·1%) | .. | |
| ≥65 years | 1716 (27·8%) | 1504 (27·9%) | 3035 (55·9%) | 6255 (36·8%) | .. | |
| Sex | ||||||
| Female | 3494 (56·6%) | 3284/5393 (60·9%)† | 3045 (56·1%) | 9823/16 993 (57·8%) | <0·0001 | |
| Male | 2681 (43·4%) | 2109/5393 (39·1%)† | 2380 (43·9%) | 7170/16 993 (42·2%) | .. | |
| Race and ethnicity | ||||||
| Non-Hispanic Asian or Pacific Islander | 301 (4·9%) | 445 (8·2%) | 691 (12·7%) | 1437 (8·5%) | <0·0001 | |
| Non-Hispanic Black | 996 (16·1%) | 738 (13·7%) | 517 (9·5%) | 2251 (13·2%) | .. | |
| Hispanic | 2666 (43·2%) | 2650 (49·1%) | 2068 (38·1%) | 7384 (43·5%) | .. | |
| Other or unknown | 254 (4·1%) | 213 (3·9%) | 185 (3·4%) | 652 (3·8%) | .. | |
| Non-Hispanic White | 1958 (31·7%) | 1348 (25·0%) | 1964 (36·2%) | 5270 (31·0%) | .. | |
| Body-mass index | ||||||
| Underweight (<18·5 kg/m2) | 181 (2·9%) | 147 (2·7%) | 166 (3·1%) | 494 (2·9%) | <0·0001 | |
| Normal or healthy weight (18·5–24·9 kg/m2) | 1421 (23·0%) | 1198 (22·2%) | 1398 (25·8%) | 4017 (23·6%) | .. | |
| Overweight (25·0–29·9 kg/m2) | 1646 (26·7%) | 1535 (28·5%) | 1616 (29·8%) | 4797 (28·2%) | .. | |
| Obese, class 1 (30·0–34·9 kg/m2) | 1388 (22·5%) | 1155 (21·4%) | 1153 (21·3%) | 3696 (21·7%) | .. | |
| Obese, class 2–3 (≥35·0 kg/m2) | 1440 (23·3%) | 1288 (23·9%) | 1076 (19·8%) | 3804 (22·4%) | .. | |
| Unknown | 99 (1·6%) | 71 (1·3%) | 16 (0·3%) | 186 (1·1%) | .. | |
| Comorbidities | ||||||
| Hypertension | 1921 (31·1%) | 1961 (36·4%) | 3036 (56·0%) | 6918 (40·7%) | <0·0001 | |
| Congestive heart failure | 525 (8·5%) | 633 (11·7%) | 1015 (18·7%) | 2173 (12·8%) | <0·0001 | |
| Myocardial infarction | 225 (3·6%) | 245 (4·5%) | 406 (7·5%) | 876 (5·2%) | <0·0001 | |
| Peripheral vascular disease | 1065 (17·2%) | 1113 (20·6%) | 2204 (40·6%) | 4382 (25·8%) | <0·0001 | |
| Cerebrovascular disease | 298 (4·8%) | 298 (5·5%) | 460 (8·5%) | 1056 (6·2%) | <0·0001 | |
| Diabetes status | ||||||
| Diabetes with unknown glycated haemoglobin | 111 (1·8%) | 70 (1·3%) | 69 (1·3%) | 250 (1·5%) | <0·0001 | |
| Diabetes with glycated haemoglobin <7·5% | 609 (9·9%) | 679 (12·6%) | 1214 (22·4%) | 2502 (14·7%) | .. | |
| Diabetes with glycated haemoglobin ≥7·5% | 539 (8·7%) | 551 (10·2%) | 681 (12·6%) | 1771 (10·4%) | .. | |
| Chronic obstructive pulmonary disease | 1142 (18·5%) | 1081 (20·0%) | 1594 (29·4%) | 3817 (22·5%) | <0·0001 | |
| Renal disease | 740 (12·0%) | 809 (15·0%) | 1399 (25·8%) | 2948 (17·3%) | <0·0001 | |
| Malignancy | 343 (5·6%) | 341 (6·3%) | 678 (12·5%) | 1362 (8·0%) | <0·0001 | |
| Organ transplant | 29 (0·5%) | 58 (1·1%) | 105 (1·9%) | 192 (1·1%) | <0·0001 | |
| Charlson Comorbidity Index | ||||||
| 0 | 3346 (54·2%) | 2665 (49·4%) | 1577 (29·1%) | 7588 (44·7%) | <0·0001 | |
| 1 | 1078 (17·5%) | 925 (17·1%) | 826 (15·2%) | 2829 (16·6%) | .. | |
| 2 | 481 (7·8%) | 459 (8·5%) | 663 (12·2%) | 1603 (9·4%) | .. | |
| 3 | 297 (4·8%) | 291 (5·4%) | 459 (8·5%) | 1047 (6·2%) | .. | |
| ≥4 | 973 (15·8%) | 1054 (19·5%) | 1900 (35·0%) | 3927 (23·1%) | .. | |
| Vaccine and infection history | ||||||
| Influenza vaccine in the year before admission | 1311 (21·2%) | 2651 (49·1%) | 4510 (83·1%) | 8472 (49·9%) | <0·0001 | |
| Pneumococcal vaccine in the 5 years before admission | 1050 (17·0%) | 1120 (20·8%) | 1607 (29·6%) | 3777 (22·2%) | <0·0001 | |
| Previous positive SARS-CoV-2 PCR test | 935 (15·1%) | 900 (16·7%) | 756 (13·9%) | 2591 (15·2%) | 0·0004 | |
Data are n (%) or n/N (%), unless otherwise specified.
χ2 test comparing unvaccinated, two vaccine doses, and three vaccine doses groups.
One patient had an unknown sex and was excluded.
Of the 16 994 admissions in the study population, 5813 (34·2%) were hospital admissions and 11 181 (69·5%) were emergency department admissions (without subsequent hospital admission). Of 2433 hospital admissions with positive SARS-CoV-2 tests, 2245 (92·3%) infections were designated BA.1 and 188 (7·7%) infections were designated BA.2 (figure 1). 5190 (85·7%) of 6058 emergency department admissions with positive SARS-CoV-2 tests were due to BA.1 and 868 (14·3%) were due to BA.2 (figure 1). Of the 1056 infections identified as BA.2, 14 (1·3%) were sequenced, 233 (22·1%) were run on Thermo Fisher and identified on the basis of SGTF status, and 809 (76·6%) were categorised by use of the date. Of the 7435 infections identified as BA.1, 1210 (16·3%) were sequenced, 2048 (27·5%) were run on Thermo Fisher and identified on the basis of SGTF status, and 4177 (56·2%) were categorised by use of the date. 40 patients had multiple events in the study. Nine patients had multiple events included in the BA.1 analysis and two patients had multiple events included in the BA.2 analysis. The remaining 29 patients had one BA.1 event and one BA.2 event. We did an internal validation study of isolates that had SGTF designation by Thermo Fisher and had undergone whole-genome sequencing to confirm the accuracy of sub-lineage identification by SGTF. BA.1 sub-lineage was confirmed by whole-genome sequencing in 1250 (99·4%) of 1258 samples and BA.2 sub-lineage was confirmed in 461 (100·0%) of 461 samples.
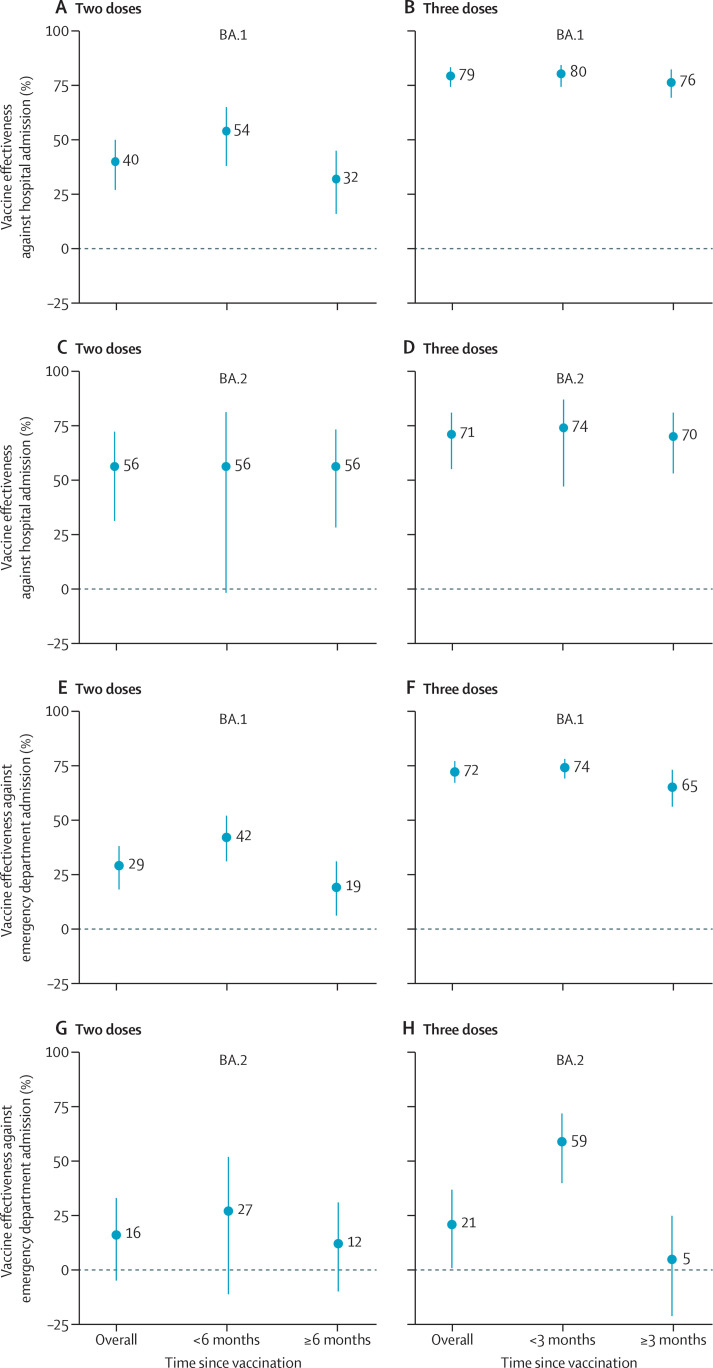
In adjusted analyses, vaccine effectiveness against hospital admission due to BA.1 was 40% (95% CI 27 to 50) after two doses and 79% (74 to 83) after three doses (figure 2 ; appendix p 3). Vaccine effectiveness against emergency department admission due to BA.1 was 29% (18 to 38) after two doses and 72% (67 to 77) after three doses. For BA.2, vaccine effectiveness against hospital admission was 56% (31 to 72) after two doses and 71% (55 to 81) after three doses and effectiveness against emergency department admission was 16% (−5 to 33) after two doses and 21% (1 to 37) after three doses. Results for our unadjusted analysis can be found in the appendix (p 5).
Figure 2.
Adjusted effectiveness of BNT162b2 against omicron-related hospital and emergency department admissions by sub-lineage
Vaccine effectiveness against hospital admission after two doses (A) and three doses (B) for BA.1 and after two doses (C) and three doses (D) for BA.2. Vaccine effectiveness against emergency department admission after two doses (E) and three doses (F) for BA.1 and after two doses (G) and three doses (H) for BA.2. Estimates were adjusted for the month of admission, age, sex, race and ethnicity, body-mass index, Charlson Comorbidity Index, previous influenza vaccination, previous pneumococcal vaccination, and previous SARS-CoV-2 infection.
Vaccine effectiveness after two doses against hospitalisation due to BA.1 waned from 54% (95% CI 38 to 65) at less than 6 months after the second dose to 32% (16 to 45) at 6 months or more after the second dose (figure 2; appendix p 3), although 95% CIs between the two timepoints overlapped. Waning of vaccine effectiveness after two doses was also observed against BA.1-related emergency department admissions (42% [95% CI 31 to 52] at <6 months after the second dose vs 19% [6 to 31] at ≥6 months after the second dose). Vaccine effectiveness after two doses did not wane against hospital admission due to BA.2 (56% [95% CI –2 to 81] at <6 months after the second dose vs 56% [28 to 73] at ≥6 months after the second dose), although wide 95% CIs preclude strong conclusions about durability between the two timepoints. Vaccine effectiveness against emergency department admission for BA.2 was low, equalling 27% (95% CI –11 to 52) at less than 6 months after the second dose and 12% (−10 to 31) at 6 months or more after the second dose. Among 5394 admissions with only two doses of vaccine, 1546 (28·7%) occurred less than 6 months after the second dose. By variant, 846 (30·6%) of 2761 post-second dose BA.1 admissions occurred less than 6 months after the second dose, compared with 41 (15·5%) of 265 admissions for BA.2.
A third dose substantially increased vaccine effectiveness against hospitalisation to 80% (95% CI 74 to 84) for BA.1 and 74% (47 to 87) for BA.2 at less than 3 months after the third dose (appendix p 3). 3 months or more after the third dose, no considerable waning of vaccine effectiveness against hospitalisation was observed for BA.1 (76% [69 to 82]) or BA.2 (70% [53 to 81]). Three doses also increased vaccine effectiveness against emergency department admission for BA.1 to 74% (69 to 78) at less than 3 months after the third dose, with some waning to 65% (56 to 73) at 3 months or more after the third dose, although 95% CIs between the two timepoints overlapped. Vaccine effectiveness against emergency department admission for BA.2 increased to 59% (40 to 72) at less than 3 months after the third dose but decreased to 5% (−21 to 25) at 3 months or more after the third dose. Among 5425 admissions with three vaccine doses, 2355 (43·4%) occurred less than 3 months after the third dose. By variant, 788 (69·7%) of 1131 post-third dose BA.1 admissions occurred less than 3 months after the third dose, compared with 50 (11·4%) of 437 admissions for BA.2.
In the subset of immunocompetent patients (n=16 098), vaccine effectiveness was slightly higher than that in the total population (albeit with overlapping 95% CIs) for both outcomes after three doses (appendix p 3), but showed similar trends overall (appendix p 7). Only a small number of patients (896 [5·3%] of 16 994) were categorised as immunocompromised in this analysis and as such there are no results for this subgroup. In additional analyses considering alternative methods to address secular confounding, overall vaccine effectiveness estimates and patterns of waning were very similar to those in our main analysis (appendix pp 4–5). We also explored adjusting for the week of admission, but, because small numbers of events in some weeks led to convergence issues, we do not report these data.
Discussion
Two doses of BNT162b2 provided only partial protection against BA.1-related and BA.2-related hospital and emergency department admission. Adjusted vaccine effectiveness against hospital admissions decreased to 32% for BA.1 and to 56% for BA.2 at 6 months or more after the second dose. Against emergency department admission, vaccine effectiveness was 19% for BA.1 and 12% for BA.2 at 6 months or more after the second dose. Three doses of BNT162b2 provided high levels of protection (≥70%) against both BA.1-related and BA.2-related hospitalisation. These findings, consistent with previous evidence,20, 32 underscore the need for booster doses, particularly against omicron. For the milder outcome of emergency department admissions, vaccine effectiveness against BA.2 was only 59% at less than 3 months after the third dose, decreasing substantially at 3 months or more after the third dose (5%), whereas vaccine effectiveness against BA.1 remained high (74%) at less than 3 months after dose three and was 65% at 3 months or more. These results could be explained by differences in the virological characteristics of BA.2 versus BA.1, including its higher transmissibility and increased immune evasion.11, 20, 33 Supplementing our findings, current data suggest that variant-adapted vaccines are probably needed to improve protection against less severe endpoints, like emergency department admission, especially for BA.2.
Our findings are consistent with those of a 2022 study evaluating breakthrough BA.2 infections, in which people receiving three doses of BNT162b2 had a lower incidence of breakthrough SARS-CoV-2 infections (16·6%) than did people receiving only two doses (49·2%).34 Although a previous study34 showed that neutralising antibody titres were lower for BA.2 than for BA.1, it also showed that three doses of BNT162b2 enhanced neutralising activity against omicron and better activated spike protein-specific memory B cells compared with only two doses.34 Other reports have also shown similar findings for the effectiveness of mRNA vaccines against severe BA.2-related outcomes, whereby two doses offer only partial protection that is meaningfully bolstered by a booster dose.22, 23, 24 A US study35 of immunocompetent adults showed lower mRNA vaccine effectiveness against moderate and severe COVID-19-associated illness for BA.2 compared with BA.1. The study also showed that booster doses improved vaccine effectiveness against omicron and its sub-lineages.35 To date, only one study22 done in Sweden has shown differences in vaccine effectiveness against severe COVID-19 between the BA.1 and BA.2 sub-lineages. Based on how the BA.1 period was defined in this study, however, the delta variant could have accounted for as much as 25% of all infections designated BA.1. Given that vaccine effectiveness against delta is higher than against BA.1, incorrect designation could have biased vaccine effectiveness estimates upwards for BA.1.22 Similar to findings from Qatar,36 we observed only marginal differences in vaccine effectiveness against hospitalisation for BA.1 versus BA.2. Disagreement between our findings and those of the Swedish study might be due to variation in how severe outcomes were defined, the timing of the two studies (and thus the prevalence of BA.2.12.1), hospitalisation criteria in the USA versus Sweden, population seroprevalence, or other factors.
We have previously shown that waning vaccine effectiveness against hospital and emergency department admissions is modified by immunocompromised status.15 In this analysis, vaccine effectiveness was only minimally different among immunocompetent participants compared with the overall population. However, this result was probably due to the small number of immunocompromised people in our population, which occurred, in part, because we excluded participants who had received four vaccine doses.
Our study is not without limitations. Because this study was observational and retrospective, there could have been residual confounding that was not controlled for in the analysis. However, the test-negative study design, which required patients to have an acute respiratory infection-related admission, helped to reduce bias related to differential health care-seeking behaviour and to ensure that cases were truly admitted for COVID-19 rather than with COVID-19.37 Furthermore, during the study period, all people admitted to KPSC hospitals were tested for SARS-CoV-2 and those with COVID-19 who presented for non-emergency appointments had their appointment rescheduled for a later date; this approach does not eliminate all admissions with (rather than for) COVID-19, but does reduce their number. These hospital policies probably helped to prevent incidental SARS-CoV-2 infections from being included in our analyses. Nonetheless, future studies evaluating additional markers of COVID-19 severity, including the use of intensive care or mechanical ventilation, might better prevent the inclusion of patients who are hospitalised for non-COVID-19-related conditions but test positive for SARS-CoV-2, which might engender the production of less biased estimates of vaccine effectiveness against severe COVID-19.37
In the test-negative study design, differences in health care-seeking behaviour by vaccination status can still lead to bias. This bias is of particular concern for milder outcomes (eg, certain symptoms) that might or might not be medically attended. Our focus in this study on more severe outcomes (admission to the emergency department or hospital for acute respiratory infection) minimises this potential. Furthermore, we adjusted our analyses for previous pneumococcal and influenza vaccination to account for differences in health care-seeking behaviour and particularly in the proclivity to vaccinate. The misclassification of cases and controls is unlikely in our study design because the patients in our analyses were systematically tested for SARS-CoV-2 by RT-PCR. Patients tested outside of the KPSC system before their admission were re-tested within the KPSC system. Ascertainment of previous SARS-CoV-2 infection status was limited to infections recorded in patients' medical records. Because COVID-19 testing can be done at facilities outside of the KPSC system, including at home, it is likely that not all previous infections were documented in patients' medical records. Under-reporting of previous infection, especially among the unvaccinated, could lead to underestimation of vaccine effectiveness or overestimation of the degree of waning with time. Specifically, the number of unvaccinated controls with at least some level of natural immunity to SARS-CoV-2 probably increased during our study period, which would bias vaccine effectiveness estimates downwards.38, 39 Whether increased natural immunity in the unvaccinated population stemming from the large BA.1 wave contributed to the dampening of vaccine effectiveness estimates during the subsequent BA.2 wave needs further investigation and could partially explain our findings. We distinguished BA.1 and BA.2 using several methods, including sequencing, SGTF, and the predominance of variants in certain time periods. Although this approach could have led to the misclassification of some variants, an internal validation study showed that the BA.1 sub-lineage was confirmed in 99·4% of samples and the BA.2 sub-lineage was confirmed in 100% of samples. A descendent lineage, BA.2.12.1, rapidly emerged during our study period and accounted for 27% of all sequenced specimens in Los Angeles County, CA, USA, for the week ending April 30, 2022.40 We could not distinguish BA.2 from BA.2.12.1 in our analysis. However, in most studies, immune escape by BA.2.12.1 appears to be only moderately greater than that by BA.2 and differences in clinical severity by these descendant lineages are not well described.32, 41, 42 Thus, how the descendent lineage impacted our vaccine effectiveness estimates for BA.2 is unknown. By contrast, studies evaluating neutralising antibody titres against BA.4 and BA.5 suggest that these variants are likely to have an even greater escape potential against current vaccines than BA.1 or BA.2.41, 42, 43, 44 Although we did not have sufficient data to assess vaccine effectiveness against BA.4 or BA.5, it will be important to evaluate the effectiveness of current and updated vaccines against these variants, which have become globally predominant, when feasible.
In conclusion, our data suggest that two doses of BNT162b2 provided only partial protection against BA.1-related and BA.2-related hospital and emergency department admission, which underscores the need for booster doses against omicron. Although three doses of BNT162b2 offered high levels of protection (≥70%) against BA.1-related and BA.2-related hospitalisation, variant-adapted vaccines are probably needed to improve protection against less severe endpoints, like emergency department admission, especially for BA.2.
Data sharing
Anonymised data that support the findings of this study can be made available from the investigative team, so long as the following conditions are met: (1) agreement to collaborate with the study team on all publications; (2) provision of external funding for the time of administrators and investigators necessary for this collaboration; (3) demonstration that the external investigative team is qualified and has documented evidence of training for human participant protections; and (4) agreement to abide by the terms outlined in data use agreements between institutions. Reasonable inquiries can be sent to the corresponding author and there are no date restrictions on data availability.
Declaration of interests
SRV, LJ, LP, and JMM are employees of, and hold stock, stock options, or both in, Pfizer. SYT, TBF, JMS, VH, and BKA received research support from Pfizer during the conduct of this study, which was paid directly to KPSC. BKA received research support for work unrelated to this study from Pfizer, Moderna, Dynavax, Seqirus, and GlaxoSmithKline. JMS received research support from ALK, Dynavax, and Novavax for work unrelated to this study. TBF previously owned stock in Pfizer. SYT received research support from Genentech and funds from the Centers for Disease Control and Prevention for work unrelated to this study. LP owns stock in Merck & Co. FX declares no competing interests.
Acknowledgments
Acknowledgments
This study was sponsored by Pfizer. We thank Harpreet S Takhar, Oluwaseye A Ogun, Donald McCarthy, Errol Lopez, Joann M Zamparo, Kaije Pan, and Sharon Gray for assistance with management and data support on this study. We thank Michael Aragones, Soon Kyu Choi, Lee Childs, Julie Stern, Kourtney Kottman, Jonathan Arguello, Raul Calderon, Charanjot Singh, Jose Rodriguez, Jared Davis, Joanna Truong, Samuel Payan, Katy Taylor, Vanessa Pan, Sarbjit Kaur-Chand, Samantha Quinones, Samantha Baluyot, and Elmer Ayala for their technical and laboratory support processing SARS-CoV-2 specimens.
Contributors
SYT, JMS, LP, LJ, and JMM conceived this study. JMS, TBF, VH, and FX did the analysis, accessed and verified the raw data, and were the only authors with access to all the data in the study. SYT, JMS, JMM, and LP wrote the first draft of the protocol. SYT, LP, and JMM wrote the first draft of the manuscript. All authors contributed to designing the study, drafting the protocol, and editing the manuscript for important intellectual content. All authors provided final approval of the manuscript version to be published and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.WHO Weekly epidemiological update on COVID-19—25 May 2022. Edition 93. May 25, 2022. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19-25-may-2022
- 2.Evans JP, Zeng C, Qu P, et al. Neutralization of SARS-CoV-2 omicron sub-lineages BA.1, BA.1.1, and BA.2. Cell Host Microbe. 2022;30:1093. doi: 10.1016/j.chom.2022.04.014. 102.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iketani S, Liu L, Guo Y, et al. Antibody evasion properties of SARS-CoV-2 omicron sublineages. Nature. 2022;604:553–556. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marks KJ, Whitaker M, Anglin O, et al. Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:271–278. doi: 10.15585/mmwr.mm7107e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Tang W, Gao H, et al. Structural and functional characteristics of SARS-CoV-2 omicron subvariant BA.2 spike. bioRxiv. 2022 doi: 10.1101/2022.04.28.489772. published online April 28. (preprint). [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Yu Y, Zhao Y, He D. Reduction in the infection fatality rate of omicron variant compared with previous variants in South Africa. Int J Infect Dis. 2022;120:146–149. doi: 10.1016/j.ijid.2022.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viana R, Moyo S, Amoako DG, et al. Rapid epidemic expansion of the SARS-CoV-2 omicron variant in southern Africa. Nature. 2022;603:679–686. doi: 10.1038/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lino A, Cardoso MA, Martins-Lopes P, Gonçalves HMR. Omicron—the new SARS-CoV-2 challenge? Rev Med Virol. 2022;32 doi: 10.1002/rmv.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araf Y, Akter F, Tang YD, et al. Omicron variant of SARS-CoV-2: genomics, transmissibility, and responses to current COVID-19 vaccines. J Med Virol. 2022;94:1825–1832. doi: 10.1002/jmv.27588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruell H, Vanshylla K, Korenkov M, et al. Delineating antibody escape from omicron sublineages. bioRxiv. 2022 doi: 10.1101/2022.04.06.487257. published online May 31. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamasoba D, Kimura I, Nasser H, et al. Virological characteristics of the SARS-CoV-2 omicron BA.2 spike. Cell. 2022;185:2103. doi: 10.1016/j.cell.2022.04.035. 15.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonager J, Bennedbæk M, Bager P, et al. Molecular epidemiology of the SARS-CoV-2 variant omicron BA.2 sub-lineage in Denmark, 29 November 2021 to 2 January 2022. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.10.2200181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott P, Eales O, Steyn N, et al. Twin peaks: the Omicron SARS-CoV-2 BA.1 and BA.2 epidemics in England. Science. 2022;376 doi: 10.1126/science.abq4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tartof SY, Slezak JM, Puzniak L, et al. Durability of BNT162b2 vaccine against hospital and emergency department admissions due to the omicron and delta variants in a large health system in the USA: a test-negative case-control study. Lancet Respir Med. 2022;10:689–699. doi: 10.1016/S2213-2600(22)00101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tartof SY, Slezak JM, Puzniak L, et al. Immunocompromise and durability of BNT162b2 vaccine against severe outcomes due to omicron and delta variants. Lancet Respir Med. 2022;10:e61–e62. doi: 10.1016/S2213-2600(22)00170-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar. N Engl J Med. 2022;386:1804–1816. doi: 10.1056/NEJMoa2200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Accorsi EK, Britton A, Fleming-Dutra KE, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA. 2022;327:639–651. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collie S, Champion J, Moultrie H, Bekker LG, Gray G. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N Engl J Med. 2022;386:494–496. doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyngse FP, Mølbak K, Denwood M, et al. Effect of vaccination on household transmission of SARS-CoV-2 delta variant of concern. Nat Commun. 2022;13 doi: 10.1038/s41467-022-31494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauring AS, Tenforde MW, Chappell JD, et al. Clinical severity of, and effectiveness of mRNA vaccines against, Covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376 doi: 10.1136/bmj-2021-069761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Björk J, Bonander C, Moghaddassi M, et al. COVID-19 vaccine effectiveness against severe disease from SARS-CoV-2 omicron BA.1 and BA.2 subvariants—surveillance results from southern Sweden, December 2021 to March 2022. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.18.2200322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirsebom FCM, Andrews N, Stowe J, et al. COVID-19 vaccine effectiveness against the omicron (BA.2) variant in England. Lancet Infect Dis. 2022;22:931–933. doi: 10.1016/S1473-3099(22)00309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chemaitelly H, Ayoub HH, AlMukdad S, et al. Duration of mRNA vaccine protection against SARS-CoV-2 omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun. 2022;13 doi: 10.1038/s41467-022-30895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16:37–41. doi: 10.7812/tpp/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozasa K, Fukushima W. Commentary: test-negative design reduces confounding by healthcare-seeking attitude in case-control studies. J Epidemiol. 2019;29:279–281. doi: 10.2188/jea.JE20180177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399:437–446. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott L, Hsiao NY, Moyo S, et al. Track omicron's spread with molecular data. Science. 2021;374:1454–1455. doi: 10.1126/science.abn4543. [DOI] [PubMed] [Google Scholar]
- 29.Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tartof SY, Slezak JM, Puzniak L, et al. Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: A retrospective cohort study. Lancet Reg Health Am. 2022;9 doi: 10.1016/j.lana.2022.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowen JE, Addetia A, Dang HV, et al. Omicron spike function and neutralizing activity elicited by a comprehensive panel of vaccines. Science. 2022;377:890–894. doi: 10.1126/science.abq0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hui KPY, Ho JCW, Cheung MC, et al. SARS-CoV-2 omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603:715–720. doi: 10.1038/s41586-022-04479-6. [DOI] [PubMed] [Google Scholar]
- 34.Zhou R, Liu N, Li X, et al. Three-dose vaccination-induced immune responses protect against SARS-CoV-2 omicron-BA.2. bioRxiv. 2022 doi: 10.1101/2022.05.09.491254. published online May 11. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Link-Gelles R, Levy ME, Gaglani M, et al. Effectiveness of 2, 3, and 4 COVID-19 mRNA vaccine doses among immunocompetent adults during periods when SARS-CoV-2 omicron BA.1 and BA.2/BA.2.12.1 sublineages predominated—VISION Network, 10 states, December 2021–June 2022. MMWR Morb Mortal Wkly Rep. 2022;71:931–939. doi: 10.15585/mmwr.mm7129e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med. 2022;387:21–34. doi: 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stowe J, Andrews N, Kirsebom F, Ramsay M, Lopez Bernal J. Effectiveness of COVID-19 vaccines against omicron and delta hospitalisation: test negative case-control study. medRxiv. 2022 doi: 10.1101/2022.04.01.22273281. published online April 1. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipsitch M. Challenges of vaccine effectiveness and waning studies. Clin Infect Dis. 2019;68:1631–1633. doi: 10.1093/cid/ciy773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahn R, Schrag SJ, Verani JR, Lipsitch M. Identifying and alleviating bias due to differential depletion of susceptible people in postmarketing evaluations of COVID-19 vaccines. Am J Epidemiol. 2022;191:800–811. doi: 10.1093/aje/kwac015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.County of Los Angeles Public Health With significant increases in transmission and outbreaks at high-risk settings, getting boosted should be a high priority for residents and workers—12,378 new positive cases and 14 new deaths due to COVID-19 in Los Angeles county since Saturday. May 23, 2022. http://publichealth.lacounty.gov/phcommon/public/media/mediapubhpdetail.cfm?prid=3867
- 41.Hachmann NP, Miller J, Collier A-RY, et al. Neutralization escape by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med. 2022;387:86–88. doi: 10.1056/NEJMc2206576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao Y, Yisimayi A, Jian F, et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by omicron infection. Nature. 2022;608:593–602. doi: 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qu P, Faraone J, Evans JP, et al. Neutralization of the SARS-CoV-2 omicron BA.4/5 and BA.2.12.1 subvariants. N Engl J Med. 2022;386:2526–2528. doi: 10.1056/NEJMc2206725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tuekprakhon A, Nutalai R, Dijokaite-Guraliuc A, et al. Antibody escape of SARS-CoV-2 omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. 2022;185:2422. doi: 10.1016/j.cell.2022.06.005. 33.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymised data that support the findings of this study can be made available from the investigative team, so long as the following conditions are met: (1) agreement to collaborate with the study team on all publications; (2) provision of external funding for the time of administrators and investigators necessary for this collaboration; (3) demonstration that the external investigative team is qualified and has documented evidence of training for human participant protections; and (4) agreement to abide by the terms outlined in data use agreements between institutions. Reasonable inquiries can be sent to the corresponding author and there are no date restrictions on data availability.