Abstract
Antimicrobial resistance is an increasing public health problem worldwide. The interest of a focus on antimicrobial resistance in acne lies on the facts that acne vulgaris (acne) is the most common skin disease worldwide, that the bacterium Cutibacterium acnes (C. acnes, formerly Propionibacterium acnes) plays a key role in the pathogenesis of acne, while at the same time being part of the skin flora, and that antibiotics are commonly recommended for acne treatment. The overuse of topical and/or systemic antibiotics, the long treatment courses used for acne, and the availability of over-the-counter antibiotic preparations, have led to the worldwide emergence of resistant strains in acne patients. In this review, we discuss the epidemiological trends of antimicrobial resistance in acne, the need to avoid the perturbation of the skin microbiome caused by anti-acne antibiotics, and the clinical practice considerations related to the emergence of resistant strains in acne patients. In light of the increasing risk of antimicrobial resistance, raising concerns over the misuse of antibiotics, prescribing patterns can be a critical target for antibiotic stewardship efforts. Also, the selection of non-antibiotic therapies for acne, whenever possible, may offer significant advantages.
Keywords: antimicrobial resistance, antibiotic, acne, epidemiology, microbiome, Cutibacterium acnes, Propionibacterium acnes, infections
Introduction
Antimicrobial resistance is an increasing public health problem worldwide. The Centers for Disease Control (CDC) estimate that antimicrobial resistance infections cause one death every 15 minutes in the United States and antimicrobial resistance overall is a top three public health concern of the 21st century [1]. The interest of the focus on antimicrobial resistance in acne lies on the facts that acne vulgaris (acne) is the most common skin disease worldwide, that the bacterium Cutibacterium acnes (C. acnes) plays a key role in the pathogenesis of acne, while at the same time being part of a complex skin microbiome, and that antibiotics are commonly recommended for acne treatment.
Acne affects 80% of teenagers (of both sexes), while up to 50% of individuals (mostly female) may have acne in their adult life [2]. It may result in scarring and impact on the psychology and quality of life of patients [3]. Acne is a chronic inflammatory disease of the pilosebaceous units of the skin, located on sebaceous gland-rich body areas, such as the face and trunk (Figure 1) [4]. The major factors implicated in acne pathophysiology include disturbed sebaceous gland activity associated with hyperseborrhea and alterations in sebum fatty acid composition, follicular hyperkeratinization, changes in C. acnes (previously termed Propionibacterium acnes [5,6]) colonization, and dysfunction of the innate and adaptive immunity [4].
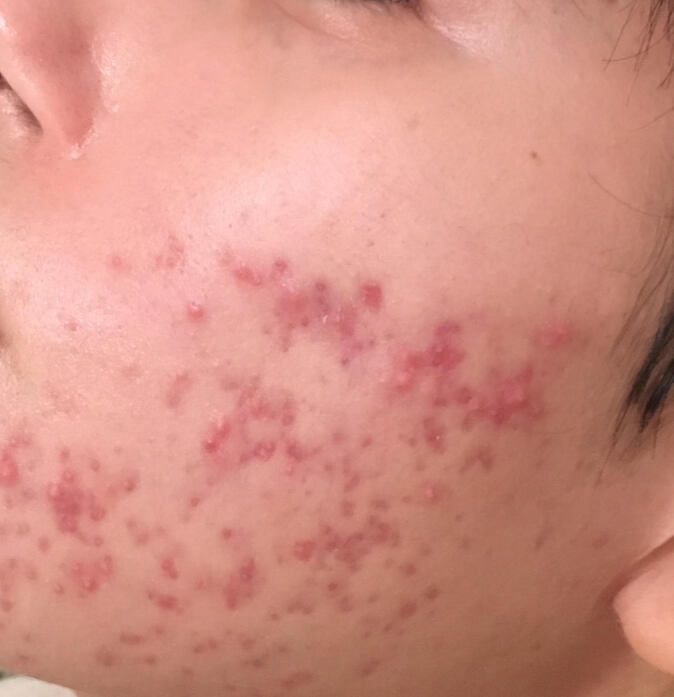
Figure 1.
A patient with acne vulgaris on the face, presenting with inflammatory papules and pustules and atrophic scarring. The patient had been previously using topical antibiotics without improvement.
Acne is a non-infectious disease and C. acnes that is implicated in its pathogenesis, is a commensal bacterium, ie, a member of the normal skin microbiota and an inhabitant of all humans’ skin [7]. Dysbiosis, defined as changes in the skin microbiota, has been implicated in various skin diseases, including acne, eczema, and chronic wounds [8]. In acne, similar relative abundance of C. acnes on the skin of patients and controls have been shown, however distinct “acnegenic” C. acnes phylotypes and a loss of C. acnes phylotype diversity are highly associated with acne [9-12]. In the complex pathways of acne pathophysiology, C. acnes acts on all the implicated key cell types, namely keratinocytes, immune cells, and sebocytes. “Acnegenic” C. acnes strains may modulate the differentiation of keratinocytes and increase local inflammation, supporting its key role in the development of inflammatory acne lesions as well as the formation of the microcomedo in the early stages of acne, while it has also been implicated in lipogenesis and sebum production from sebocytes (Figure 2) [7,13-15]. In addition, the effect of C. acnes on dermal fibroblasts was recently demonstrated in acne. Reactive adipogenesis – a process in which skin fibroblasts can undergo localized proliferation and differentiation into a preadipocyte lineage in response to bacteria stimuli – was shown in human acne lesions, while C. acnes induced reactive adipogenesis via TLR2 in mice. Adipocytes in turn mount an innate immune defense response that may contribute to acne pathophysiology [16].
Figure 2.
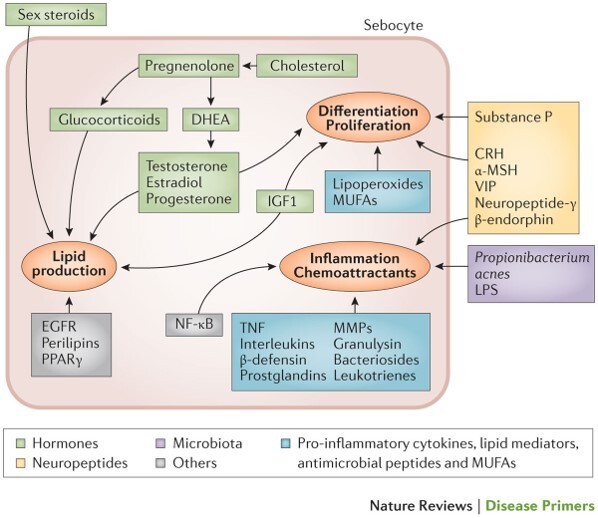
Pathophysiological processes involved in acne vulgaris. The pathogenesis of acne involves several processes including sebum production, and sebocytes differentiation, proliferation, and inflammation. These processes are regulated by circulating sex hormone levels as well as locally synthesized hormones, neuropeptides, the microbiota and pro-inflammatory cytokines, lipid mediators, antimicrobial peptides, and monounsaturated fatty acids (MUFAs). α-MSH, alpha-melanocyte-stimulating hormone; CRH, corticotropin-releasing hormone; DHEA, dehydroepiandrosterone; EGFR, epidermal growth factor receptors; IGF-1, insulin-like growth factor 1; LPS, lipopolysaccharide; MMP, matrix metalloproteinase; NF-κB, nuclear factor-κB; PPARγ, peroxisome proliferator-activated receptor-γ; TNF, tumor necrosis factor; VIP, vascular intestinal polypeptide. (Reproduced with permission from: Moradi Tuchayi S et al. [4])
Antibiotics have been traditionally used as treatments for acne, mainly due to their anti-inflammatory properties, resulting in clinical improvement of the disease. However, the overuse of topical and/or systemic antibiotics, the long treatment courses used for acne, and the availability of over-the-counter (OTC) antibiotic preparations in some countries, has led to the worldwide emergence of resistant strains [17,18]. In this review, we will discuss the epidemiological trends of antimicrobial resistance in acne, the need to avoid the perturbation of the skin microbiome caused by anti-acne antibiotics, and the clinical practice considerations related to the emergence of resistant strains in acne patients.
C. acnes Resistance in Acne: Why and How
The emergence of C. acnes resistance coincided with the introduction of topical antibiotic formulations in the 1970s [17]. The clinically relevant resistance of P. acnes to antibiotics, was first documented in 1983, in the US, in patients who were not responding well to oral antibiotic treatment [19]. In the past and over the years, various antibiotics have been extensively used in acne, including topical clindamycin and topical erythromycin, oral trimethoprim-sulfamethoxazole, oral macrolides, oral tetracyclines, amoxicillin, and cephalexin [20,21].
Since acne is treated at the outpatient setting, prescribing patterns are a critical target for antibiotic stewardship efforts. There are scarce data on the prescription practices concerning the duration of antibiotic treatment for acne. Although current acne guidelines recommend restricting the duration of oral antibiotics to up to 3 months [22], the reported length of antibiotic treatment in clinical practice is significantly longer [23]. In a study from the US MarketScan Commercial Claims and Encounters database (2008-2010) of 29,908 patients that were prescribed oral antibiotics for acne, the majority of courses were more than 3 months; 64% were treated for more than 3 months, including 17% treated for more than 6 months [23]. Another US retrospective study of 137 patients with acne (2005-2014), reported an average duration of 331 days for oral antibiotic use before isotretinoin. Only 15.3% of patients were prescribed antibiotics for 3 months or less, while the remaining majority received extended courses of oral antibiotics for 6 months or more. These results highlight the overuse and late recognition of antibiotic failure for these patients with severe inflammatory or nodulocystic acne [24].
Regarding prescribing patterns among physicians, a US study in data from the National Ambulatory Medical Care Survey (NAMCS) (1996-2005) reported that erythromycin was prescribed more frequently by pediatricians (in 7.2% of visits for acne) compared to dermatologists (2.8%) [25]. Another US study investigated prescribing patterns of systemic therapies among dermatologists and non-dermatologists of 572,630 patients treated for acne using the OptumInsight Clinformatics DataMart (2004-2013) [26]. Over the 10-year study period, there was no significant change in the mean duration of therapy among dermatologists and non-dermatologists, while one-third of both physician groups prescribed courses of oral antibiotics that exceeded 6 months in duration [26]. An internet questionnaire survey of 201 dermatologists and 147 family physicians in Turkey (2018) showed that there were concerns over antibiotic resistance from 88.5% of dermatologists and 79.5% of family physicians, highlighting a group of physicians that does not report concerns on this issue. Also, 23.9% of dermatologists and 31.9% of family physicians “had no idea” of the highest rate of resistance among antibiotics [27]. These findings underscore the need to raise awareness of the risk of antimicrobial resistance in health providers.
The molecular basis for resistance was first shown in the United Kingdom to be caused by point mutations in genes coding the 23S subunit in ribosomal RNA (rRNA) for erythromycin and 16S subunit in rRNA for tetracycline [28]. The same mutations were replicated in strains from the US, Australia, France, Japan, and Germany [29]. Cross resistance between erythromycin and clindamycin has been associated to point mutations in genes encoding the 23S subunit of rRNA, which give resistance to Macrolide-lincosamide (clindamycin)-streptogramin B (MLS) antibiotics [30]. Tetracycline resistance in C. acnes, in most cases, is associated with a mutation in the 16S rRNA of the small ribosomal subunit at E. coli equivalent base 1058 (G to C transition) [31]. MLS antibiotic resistance in C. acnes has been reported to be also mediated by an acquired Corynebacterium transposon Tn5432 carrying the erm(X) resistance gene [32]. The transposon-based erm(X) resistance determinant accounted for 8.9% of MLS-resistant isolates in six European countries in the study of Ross et al. [32].
The minimal inhibitory concentration (MIC) is the lowest concentration in vitro of an antibiotic that will prevent growth of a microorganism [18]. Recommendations for the interpretation of antibiotic susceptibility testing for anaerobes, have been provided by the Clinical and Laboratory Standard Institute (CLSI) [33]. Also, the European EUCAST guidelines for antibiotic susceptibility testing have provided relevant critical MIC breakpoints [34]. A MIC above the breakpoint value is defined as resistance.
Recommendations on the Use of Oral Antibiotics for Acne in Current Guidelines
The concern over antimicrobial resistance has shaped the current recommendations in international guidelines for the treatment of acne. Current European guidelines (2016) limit their recommendation for systemic antibiotics for acne, to doxycycline and lymecycline, to a treatment period of 3 months [22]. Also, considering the risk of antimicrobial resistance, topical antibiotics should preferably be avoided as monotherapy [22]. In the current US guidelines (2016), doxycycline and minocycline are more effective than oxytetracycline. In addition, although oral erythromycin and azithromycin can be effective in treating acne, their use should be limited to those who cannot use the tetracyclines (ie, pregnant women or children <8 years of age) [20]. Also, the use of systemic antibiotics, other than the tetracyclines and macrolides, is discouraged because there are limited data for their use in acne [20]. In current guidelines, the recommendations for oral antibiotics for acne, are based considering their effectiveness for acne, the risk of antimicrobial resistance, as well as their safety profile. In the US guidelines 2016, Canadian guidelines 2015, the UK NICE guidelines 2021, and the European guidelines 2016, doxycycline and lymecycline should be selected in preference to minocycline. The reasoning was the similar effectiveness across tetracyclines but the more frequent severe adverse events with minocycline [20,22,35,36].
More recently, sarecycline, a narrow-spectrum tetracycline derivative, was approved by the US FDA for moderate-to-severe acne vulgaris [37], after the guidelines were issued. The topical use of nadifloxacin for acne treatment has been questioned, considering the risk of antimicrobial resistance with the use of a broad-spectrum quinolone [38]. Along the same lines, a call was made by Sardana et al. to restrict the use of oral azithromycin for acne. The logic for this statement was based on the absence of regulatory approval of azithromycin for acne in US and Europe, the risk of resistance to azithromycin [39-42], the indications of azithromycin for other important diseases (including respiratory tract infections, tick-borne infections, donovanosis, cat-scratch disease, toxoplasmosis, urethritis, and cervicitis) and evidence showing that azithromycin is not superior to doxycycline or minocycline for acne [43-45].
Antimicrobial Resistance in Acne: Epidemiological Trends
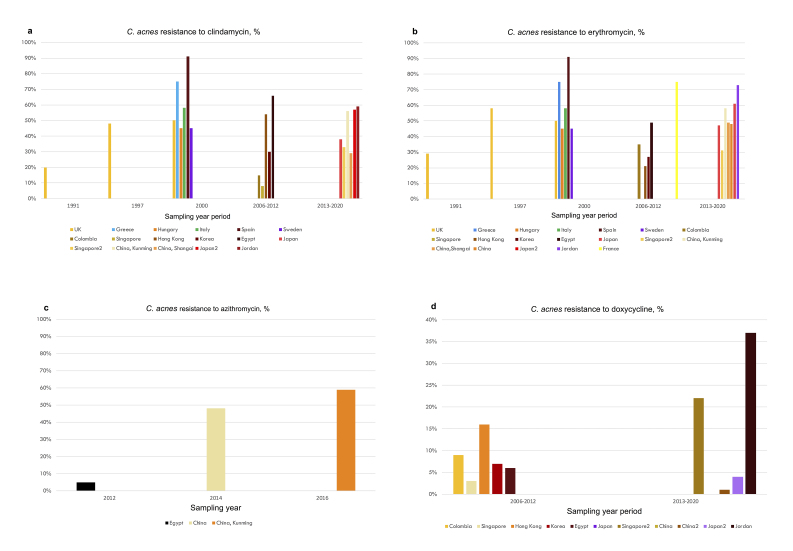
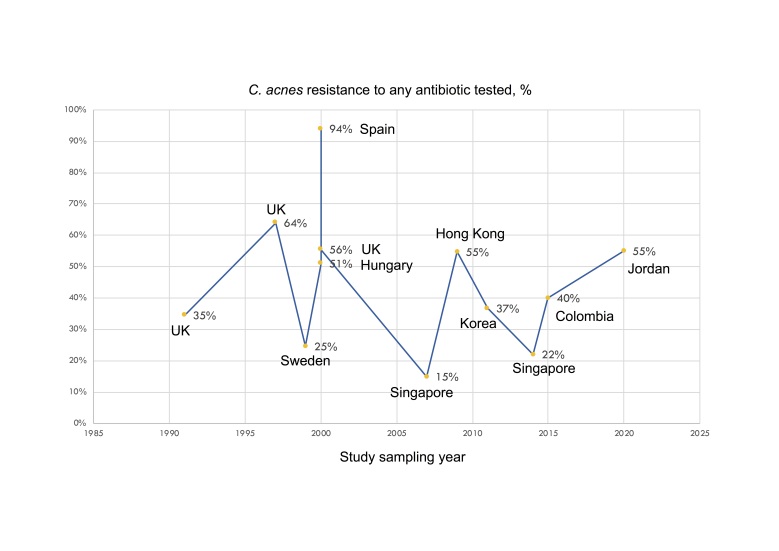
Worldwide efforts have struggled to describe resistant patterns in C. acnes and other bacteria isolated from acne patients. Epidemiological data on antimicrobial resistance in acne mainly refer to the emergence of C. acnes resistant strains due to antibiotic treatment for acne. The frequency of antimicrobial resistance in patients with acne in Europe and the UK, reported in published studies from 1990 to 2022, is summarized in Table 1 [17,46-49]. Of note, there are no national prevalence data for any country. The UK study of Coates et al. is the largest study on antibiotic-resistant propionibacteria, including 4,274 acne patients spanning 10 years (1991-2000). The frequency of resistant propionibacteria to any antibiotic rose from 34.5% in 1991 to 55.5% in 2000. Resistance to erythromycin was most prevalent and resistance to clindamycin was slightly lower, while resistance to tetracycline was less common [17]. The multicenter study by Ross et al. reported the frequency of resistance of C. acnes in Europe and the UK from 1999 to 2001 [46]. This study is important as it reports resistance patterns across various countries using a common, standard methodology and thus including a fairly homogeneous sample. It is also the largest study from Europe and the UK including 622 patients. A limitation is that it reported resistance for clindamycin and erythromycin and not for doxycycline (Table 1). The prevalence of resistant propionibacteria was lowest in Hungary (50.8%) and highest in Spain (93.6%). Resistance to tetracycline was lower compared to clindamycin or erythromycin in all the countries, and no isolates resistant to tetracycline were detected in Hungary or Italy [46]. The multicenter French study by Dumont et al. reported resistance of Propionibacteria to doxycycline in all the 26 patients that were also resistant to tetracycline [47] (Table 1). The frequency and patterns of antimicrobial resistance of C. acnes in acne patients in other countries, including Jordan, Japan, Israel, Egypt, Korea, Colombia, Hong Kong, Singapore, and China, are shown in Table 2 [41,42,50-59]. Overall, there are higher rates of clindamycin and erythromycin resistance and lower rates of doxycycline resistance of C. acnes. Azithromycin resistance has been scarcely studied; two studies in China reported similarly high rates of azithromycin- and erythromycin-resistance in their sampled patients [41,42]. On the other hand, a study in Egypt found low rates of azithromycin resistance despite high rates of resistance to erythromycin [53]. In addition, a study in 49 acne patients in northern Mexico, reported that 82% showed resistance to azithromycin [40], while a study in 52 C. acnes strains isolated from 80 patients in India reported 100% resistance to azithromycin [39]. The frequency of antimicrobial resistance of C. acnes to clindamycin, erythromycin, azithromycin, and doxycycline in various countries, is shown in Figure 3 (a-d). Figure 4 is a schematic diagram of the reported frequency of C. acnes resistance (to any antibiotic) over time. Reports from different studies are difficult to compare directly as results may vary depending on the chronological setting, prior acne treatments, sampling techniques, the body location sampled, and the methodology used to identify C. acnes strains [60] and to define resistance. However, resistance rates vary across countries even when the same methodology was used, reflecting – at least in some part – true differences due to varying antibiotic prescribing practices [46].
Table 1. Antimicrobial resistance of C. acnes in acne patients in Europe and UK, in the published literature (1990-2022). Studies including at least 100 patients are shown.
| Study, period | Country | Acne patients, n | Any antibiotic resistance, n (%) | Clin, n % | Ery, n % | Azi, n % | Doxy, n % | Mino, n % |
| Ross [46], 1999-2001 | Total | 622 | ||||||
| UK | 106 | NR | 50% | 50% | NS | NR | 0 | |
| Greece | 150 | NR | 75% | 75% | NS | NR | 0 | |
| Hungary | 68 | 51% | 45% | 45% | NS | NR | 0 | |
| Italy | 128 | NR | 58% | 58% | NS | NR | 0 | |
| Spain | 92 | 94% | 91% | 91% | NS | NR | 0 | |
| Sweden | 120 | NR | 45% | 45% | NS | NR | 0 | |
| Coates [17], 1991-2000 | UK | 4274 | 1991: 34.5% 1997: 64% 2000: 55.5% |
1991: 20% 1997: 48% 2000: 43% |
1991: 29% 1997: 57.6% 2000: 54% |
NR | NR | NR |
| Dumont-Wallon [47], 2010 | France | 273 | NR | NS | 75.1% | NS | 100% * (26 patients) | NS |
| Bettoli [48], 2000-2004 | Italy | 1206 patients (1146 Priopionibacteria isolates) | 56.5% | 40.9% | 49.8% | NS | NS | 0.6% |
| Oprica [49], 1999-2000 | Sweden | 130 patients (280 C. acnes isolates) | 24.6% | NR | NR | NS | NS | NS |
NR: not reported, NS: not studied, Clin: clindamycin resistance, Ery: erythromycin resistance, Azi: azithromycin resistance, Doxy: doxycycline resistance, Mino: minocycline resistance. * Only strains resistant to tetracycline were tested with doxycycline.
Table 2. Antimicrobial resistance of C. acnes in acne patients in other countries, in the published literature (1990-2022). Studies including at least 100 patients are shown.
| Study period | Country | Acne patients, n | Any antibiotic resistance, n (%) | Clin, n (%) | Ery, n (%) | Azi, n (%) | Doxy, n (%) | Mino, n (%) |
| Moon [54], 2011 | Korea | 100 (30 C. acnes isolates) | 11 (36.7) | 30% | 26.7% | NS | 2 (6.7) | 3 (10) |
| Mendoza [55], 2005, 2006 | Colombia | 100 | 40% | 15% | 35% | NS | 9% | 1% |
| Tan [57], 2007 | Singapore | 262 (174 C. acnes isolates) | 14.9% | 7.5% | 10.3% | NS | 3.4% | 1.7% |
| Yang [58], 2014 | Singapore | 149 (45 C. acnes isolates) | 15 (33%) | 15 (33%) | 14 (31%) | NS | 10 (22%) | NS |
| Luk [56], 2009 | Hong Kong | 111 (86 C. acnes) |
47 (54.7) | 53.5% | 18 (20.9) | NS | 14 (16.3) | 14 (16.3) |
| Abdel-Fattah [53], 2011-2012 | Egypt | 98 patients | NR | 66% | 49% | 5% | 6% | NS |
| Akhawaja [50], 2020 | Jordan | 100 | 55% | 59% | 73% | NS | 37% | 3% |
| Aoki [51], 2013-2018 | Japan | 212 patients (127 C. acnes isolates) | NR | 2013: 37.5% 2018: 56.5% |
2013: 46.9% 2018: 60.9% |
NS | 2013: 0 2018: 4.3% |
2013: 0 2018: 0 |
| Zhu [41], 2015-2017 | China, Kunming | 375 patients (227 C. acnes isolates) | NR | 55.5% | 57.7% | 58.6% | 1.3% | NS |
| Fan [42], 2014 | China, multicenter | 364 patients (312 C. acnes isolates) | NR | NR | 47.8% | 47.8% | 0.3% | 0 |
| Zhang [59], 2016-2017 | China, Shanghai | 100 (63 C. acnes isolates) | NR | 18 (28.6%) | 31 (49.2%) | NS | NS | 0 |
NR: not reported, NS: not studied, Clin: clindamycin resistance, Ery: erythromycin resistance, Azi: azithromycin resistance, Doxy: doxycycline resistance, Mino: minocycline resistance.
Figure 3.
The frequency of antimicrobial resistance of C. acnes in acne patients in various countries, for A. clindamycin, B. erythromycin, C. azithromycin, D. doxycycline. (Data is derived from Tables 1 and 2. For a study period lasting more than one year, the median year value is shown. Minocycline is not shown as its use is discouraged compared to doxycycline for acne in current guidelines).
Figure 4.
The frequency of antimicrobial resistance of C. acnes to any antibiotic in various countries, and the corresponding study period (data from studies included in Tables 1 and 2).
There are also reports of the antimicrobial resistance in other bacteria isolated from acne patients. In a study in Jordan, 35% of isolated S. aureus and 25% of S. epidermidis strains were resistant to an antibiotic tested [50]. In a study in Korean acne, patients reported high rates of resistance of S. epidermidis to tetracycline (31%), doxycycline (27%), clindamycin (33%), and erythromycin (58%) [54].
Another French study in 1,472 hospitalized (mostly orthopedics) patients reported that all Cutibacterium strains were susceptible to amoxicillin, ceftriaxone, vancomycin, and moxifloxacin. Fifteen percent of C. acnes strains were resistant to erythromycin and 4.1% were resistant to clindamycin, while 2.2% were resistant to tetracycline [61].
The Fine Equilibrium of the Skin Microbiome: Do Not Disturb!
The microbiota consists of an aggregate of microorganisms, including bacteria, archaea, protists, fungi, and viruses. The microbiome refers to the composition of all microbial genes in a community [8]. In the healthy skin microbiota, bacteria are the most abundant kingdom. The relative abundance of bacterial taxa depends on the body location, ie, sebaceous sites are dominated by lipophilic Propionibacterium species, while Staphylococcus and Corynebacterium species are abundant in moist areas, such as the bends of the elbows and the feet [8]. A diversity of the healthy human skin microbiota has been reported, with approximately equal inter-personal and intra-personal variation regarding the bacterial community membership and structure [62].
C. acnes is a commensal (resident) bacterium that forms a fine equilibrium with other microbial species of the skin microbiome. The possible interactions of C. acnes with other opportunistic pathogens, including Staphylococcus sp, are a field of continuing research. C. acnes has been identified in multi-species biofilm communities on the skin and within sebaceous glands [8]. A biofilm is defined by three essential components: the microbial cells, a surface where these cells adhere, and a self-produced extracellular polymeric matrix in which cells are embedded and form larger communities [18]. This biofilm protects bacteria from antibiotic therapy by limiting the penetration of effective antimicrobial concentrations [18]. Bacteria in biofilms may exist in a sessile state (called persister cells), they are able to communicate through “quorum sensing” and they are relatively metabolically inert and protected. In a planktonic state, they are free of the biofilm and capable to induce the expression of new proteins [63]. A recent study showed in vitro that the presence of C. acnes sterile supernatants reduced the biomass of S. aureus cultures and resulted in a defective biofilm maturation with greater susceptibility to antibiotic treatments. In contrast, C. acnes supernatant in planktonic S. aureus cultures increased antibiotic tolerance [64]. However, different results were shown by Gannesen et al. [65] and Tyner and Patel [66], reporting that C. acnes presence within a biofilm increased S. aureus biofilm biomass in anaerobic conditions, underscoring the difficulties of microbiological methodology and complex interactions. Furthermore, inter-species competition may exist; an endogenous extracellular nuclease, BmdE, secreted by the skin commensal Cutibacterium (Propionibacterium) granulosum was able to degrade C. acnes biofilm both in vivo and in vitro [67]. In addition, interactions between members of the microbiota prevent colonization by pathogenic bacteria in a process called “colonization resistance” [8]. These findings underscore the importance of avoiding the perturbation of the equilibrium of the skin microbiome caused by antibiotics.
Antimicrobial Resistance in Acne: Clinical Practice Considerations
Earlier reports considered the possible clinical therapeutic failure of oral or topical antibiotics in patients with acne and C. acnes strains resistant to the antibiotic used [68-70]. However, in these studies, patients were largely treated with antibiotic monotherapy and this concern has been currently mitigated given that monotherapy with antibiotics is contra-indicated for acne; instead the combination with topical benzoyl peroxide formulations is recommended [20,22]. Benzoyl peroxide shows anti-propionibacterial effects irrespectively of antibiotic susceptibility, may enhance the penetration and the concentration of topical antibiotics in the acne lesions, may act in synergy with topical antibiotics against some resistant strains [71], and may reverse the selectivity of topical clindamycin resistant C. acnes strains [72]. Also, oral tetracyclines exhibit anti-inflammatory properties that account, at least in part, for their effectiveness in acne, beyond their bacteriostatic effects [21]. It is recommended to be proactive and take measure to minimize the risk of antimicrobial resistance: limit the use of antibiotics for acne to less than 12 weeks, not use antibiotic monotherapy [22], and favor non-antibiotic pharmacological treatments further discussed below. Moreover, antibiotic failure in acne patients may be due to other causes, apart from antimicrobial resistance, such as low adherence to treatment regimen, high sebum excretion, inadequate duration of treatment or the development of gram-negative folliculitis [73].
Apart from the interactions with other members of the resident skin microbiome and the key roles in the pathophysiology of acne, C. acnes subspecies have been implicated in the pathophysiology of other diseases including sarcoidosis, prostate cancer, lumbar disc herniations, postoperative infection following shoulder arthroplasty and other open shoulder procedures, shoulder arthritis, and non-prosthetic spondylodiscitis [74-81]. Recently, changes in the skin microbiome, including C. acnes, correlated with cutaneous squamous cell carcinoma compared to actinic keratosis or healthy skin, although a causal relation in skin cancer progression has not been shown [82]. In this context, antimicrobial non-antibiotic pharmacological agents have demonstrated benefit to prevent shoulder infections. A randomized study in 60 patients, a benzoyl peroxide 5%/miconazole nitrate 2% cream was used on the skin of the shoulder for 7 days before surgery, with the aim to reduce the deep C. acnes tissue load before elective open shoulder surgery. This intervention significantly reduced the number of intraoperative deep subcutaneous and capsular shoulder samples that were positive for C. acnes compared with the control group [83]. Similarly, topical benzyl peroxide significantly decreased the C. acnes shoulder burden in two clinical trials [84,85] and in a systematic review [76].
In addition to the risk of developing C. acnes resistant strains, the overuse and misuse of antibiotics for acne may lead to emergence of other resistant bacterial pathogens, and thus compromise the effectiveness of antibiotics for other treatment indications [18]. A prospective randomized study investigated microbial alterations on the skin after administration of systemic doxycycline (20 mg or 100 mg), in healthy volunteers, with follow-up for up to 1 year. There was emergence of doxycycline-resistant staphylococci on the skin of all doxycycline-100mg subjects and in two of the four doxycycline-20mg subjects [86]. Another study in four patients with acne reported significant changes in the composition and diversity of skin microbiota after treatment with oral minocycline 100 mg twice daily for 4 weeks. There was a 1.4-fold reduction in the level of C. acnes, with recovery after treatment discontinuation. On the other hand, there was a transient 5.6-fold increase in the relative abundance of Pseudomonas species and a persistent 1.7-fold increase in the relative abundance of Streptococcus species. There was a 4.7-fold decrease in the relative abundance of Lactobacillus species, that persisted even 8 weeks after the antibiotic treatment discontinuation [87]. Furthermore, Coagulase-Negative Staphylococci, Staphylococcus aureus and Group A Streptococci can colonize the skin of many individuals as transient skin commensals, but they may also act as potential pathogens. The conversion of the cutaneous staphylococcal flora of acne patients from predominantly antibiotic sensitive to predominantly resistant during long term antibiotic therapy has been documented [88]. Also, the topical application of erythromycin was associated with an overgrowth of erythromycin-resistant coagulase-negative staphylococci. On the other hand, in a prospective study in 263 acne patients under acne treatment, there was significantly lower carriage rate of S. aureus in the anterior nares in patients treated with antibiotics compared to those that received non-antibiotic treatments. Resistant S. aureus isolates did not differ between the two groups [89].
Another concern is whether antibiotic resistant staphylococci colonizing the skin of antibiotic treated acne patients may transfer to and colonize untreated close contacts. A study in 41 family contacts of acne patients who had received sequential anti-acne antibiotics over a minimum period of 2 years, showed increased skin carriage of resistant coagulase-negative staphylococci compared to controls. High rates of resistance were shown independently for tetracycline, erythromycin, clindamycin, fusidic acid, chloramphenicol, trimethoprim, and kanamycin, even though the family contacts had not themselves received any antibiotic in the preceding 2 years [90]. Also, the multicenter study of Ross et al. reported that apart from patients and close contacts, 67% of participating dermatologists were also colonized on the face with resistant propionibacteria, including all those who specialized in treating acne. In contrast, none of the 27 physicians working in other outpatient departments carried resistant propionibacterial strains [46].
An important implication of the use of antibiotics for acne is the possible effect on the risk of infections beyond the skin. A UK retrospective cohort study by Margolis et al. assessed the effect of oral or topical antibiotic acne treatment (tetracyclines, erythromycin, or clindamycin) on upper respiratory or urinary tract infections. Among 118,496 individuals with acne, the risk of developing an upper respiratory tract infection developing within the first year, was two times greater in acne patients receiving antibiotic treatment compared to those not receiving antibiotic treatment [91]. Another UK study in 358 students with acne reported a significantly increased risk of pharyngitis in students taking an oral antibiotic compared to those that did not, however, there were only 36 patients that were treated with an oral antibiotic during the study period [92]. However, a systematic review on the association between treatment with antibiotics for acne and infection beyond the skin, reported the lack of high-quality evidence, with only two relevant studies [93]. In the study of Levy et al. there was a 3-fold increase in asymptomatic colonization of the oropharynx by Streptococcus pyogenes in acne patients treated with anti-acne antibiotics. Eighty-five percent of these strains were resistant to tetracyclines [94].
Last but not least, there are some preliminary reports indicating that antibiotics used for acne may have effects on the gut microbiome [95,96]. In in vitro models of the human colon, minocycline and doxycycline exposure resulted in a more significant and persistent impact on the gut microbiota composition and diversity, compared to sarecycline [96]. In turn, the gut microbiome has had increasingly recognized implications in human non-infectious diseases, including skin cancer and allergic and inflammatory skin diseases [97-99]. Nevertheless, the direct association of antibiotics used for acne with diseases via the alterations of the gut microbiome has not been studied.
The Spotlight on Non-antibiotic Pharmacological Treatments for Acne Patients
In light of the increasing risk of antimicrobial resistance, raising concerns over the use of antibiotics for acne, the selection of non-antibiotic therapies may offer significant advantages.
Regarding topical non-antibiotic treatments, approved topicals recommended for acne, include benzoyl peroxide (BPO), retinoids, azelaic acid, and clascoterone [22,100]. BPO is a topical treatment that clinically improves acne, does not induce bacterial resistance and shows a well-established antibacterial non-antibiotic action. In addition, BPO has been shown to reduce antibiotic-resistant C. acnes strains. BPO 5% gel treatment in acne patients, significantly reduced the surface and follicular C. acnes after 2 days of treatment, suggesting usefulness of short-course treatment to reduce the carriage of antibiotic-resistant C. acnes [101]. BPO had a bactericidal effect in vitro against both antibiotic-resistant and antibiotic-susceptible C. acnes. The minimum contact time needed in vitro was 60 min, 15 min, and 30 sec, with concentrations of 1.25%, 2.5%, and 5% respectively. The median MIC of BPO did not significantly differ between antibiotic-resistant and non-resistant C. acnes [102]. It has been recommended to consider a course of topical BPO, of at least 5 to 7 days, between antibiotic courses with the aim to reduce the emergence of cutaneous resistant strains [100]. Given that the combination of BPO with topical antibiotics may reduce antibiotic-resistant C. acnes strains [103,104], BPO has also been formulated in commercially available fixed-dose topical BPO-antibiotic combination treatments for acne that have largely replaced topical antibiotic monotherapy for acne [22]. Clascoterone was more recently FDA-approved in 2020, as a topical androgen receptor inhibitor for acne in patients 12 years and older [105]. A recent multicenter phase 2B randomized double-blind, vehicle-controlled trial showed the effectiveness and safety of a new class topical treatment with a selective peroxisome proliferator-activated receptor-γ (PPARγ) modulator (NAC-GED 5% gel) for moderate-to-severe facial acne vulgaris [106]. Topical probiotics with “healthy-skin” associated C. acnes phylotypes represent an ongoing area of research regarding their potential therapeutic benefit on the modulation and restoration of the cutaneous diversity of C. acnes phylotypes [107]. A pilot study assessed the topical application of C. acnes strains to the skin of 14 patients with acne for 5 weeks. A shift in the composition of the skin microbiome to the mixed strains present in the topical formulation was observed. Also, there was a significant reduction in non-inflamed lesions (comedones) and no change in the inflamed acne lesions [108].
On the other hand, there is a limited range of approved systemic non-antibiotic pharmacological treatments, consisting mainly of isotretinoin and combined oral contraceptives (COC) (varying approval status of COC formulations for acne across countries), highlighting the need of studies on new non-antibiotic treatments for moderate and severe acne [109-111]. Of note, COCs that are approved for the treatment of acne, are indicated for use only in women who also desire contraception [20]. COCs for acne, may be used in women with hyperandrogenism (eg, polycystic ovary syndrome, hirsutism) and in women without these findings [20]. A consideration of the risk-to-benefit profile and potential contra-indications is important [20,110].
Regarding oral probiotics (ie, live microorganisms with a potential to correct dysbiosis), there are a limited number of studies investigating their effects in patients with acne and their potential usefulness has not been established [107,112-114].
Conclusions and Outlook
Worldwide efforts have struggled to describe the resistant patterns in C. acnes and other bacteria isolated from acne patients. Epidemiological data on antimicrobial resistance in acne mainly refer to the increasing emergence of C. acnes resistant strains due to antibiotic treatment for acne. Overall, there are higher rates of clindamycin and erythromycin resistance and lower rates of doxycycline resistance of C. acnes, while there are more limited reports on resistance to azithromycin.
Apart from its key roles in the pathophysiology of acne, C. acnes is part of complex interactions with other members of the skin microbiome and C. acnes subspecies have been implicated in the pathophysiology of other diseases, beyond the skin. In addition to the risk of developing C. acnes resistant strains, the overuse of antibiotics for acne may lead to the emergence of other resistant bacterial pathogens, and thus compromise the effectiveness of antibiotics for other treatment indications. In addition, the possible effects of oral antibiotics used for acne on the gut microbiota and associated diseases, merit further investigation.
The call to reduce the risk of antimicrobial resistance and safeguard public health has fueled the initiative for antibiotic stewardship. Studies on the prescribing behavior of acne treatments among dermatologists and non-dermatologists have shown that a considerable part of courses with a systemic antibiotic were longer than 6 months, that there are physicians not expressing concerns for the risk of antimicrobial resistance, and that prescribing trends of systemic antibiotics in the US have not changed over a decade. These findings underscore the need to further raise awareness on the risk of antimicrobial resistance in health providers treating patients with acne.
The concern over antimicrobial resistance has shaped the current international guidelines for the treatment of acne, recommending limiting antibiotic use and select non-antibiotic treatments, when possible. In clinical practice, moving further away from antibiotic treatments in acne can be based on the future availability of additional non-antibiotic treatments with evidence-based efficacy and safety for patients with acne.
Glossary
- MIC
minimal inhibitory concentration
- BPO
benzoyl peroxide
- COC
combined oral contraceptives
Conflict of interest
Dr. Dessinioti has no conflict of interest to declare. Dr Katsambas has no conflict of interest to declare.
References
- George S, Muhaj FF, Nguyen CD, Tyring SK, Part I. Part I Antimicrobial resistance: bacterial pathogens of dermatologic significance and implications of rising resistance. J Am Acad Dermatol. 2022. Jun;86(6):1189–204. 10.1016/j.jaad.2021.11.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouboulis C, Dessinioti C, Antoniou C. Acne epidemiology and socioeconomic aspects. In: Zouboulis C, Katsambas A, Kligman AM, editors. Pathogenesis and treatment of acne and rosacea: Springer Berlin Heidelberg; 2014. p. 53-7.
- Chernyshov PV, Zouboulis CC, Tomas-Aragones L, Jemec GB, Manolache L, Tzellos T, et al. Quality of life measurement in acne. Position Paper of the European Academy of Dermatology and Venereology Task Forces on Quality of Life and Patient Oriented Outcomes and Acne, Rosacea and Hidradenitis Suppurativa. J Eur Acad Dermatol Venereol. 2018. Feb;32(2):194–208. 10.1111/jdv.14585 [DOI] [PubMed] [Google Scholar]
- Moradi Tuchayi S, Makrantonaki E, Ganceviciene R, Dessinioti C, Feldman SR, Zouboulis CC. Acne vulgaris. Nat Rev Dis Primers. 2015. Sep;1:15029. 10.1038/nrdp.2015.29 [DOI] [PubMed] [Google Scholar]
- Scholz CF, Kilian M. The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int J Syst Evol Microbiol. 2016. Nov;66(11):4422–32. 10.1099/ijsem.0.001367 [DOI] [PubMed] [Google Scholar]
- Dekio I, Asahina A, Shah HN. Unravelling the eco-specificity and pathophysiological properties of Cutibacterium species in the light of recent taxonomic changes. Anaerobe. 2021. Oct;71:102411. 10.1016/j.anaerobe.2021.102411 [DOI] [PubMed] [Google Scholar]
- Dessinioti C, Katsambas AD. The role of Propionibacterium acnes in acne pathogenesis: facts and controversies. Clin Dermatol. 2010. Jan-Feb;28(1):2–7. 10.1016/j.clindermatol.2009.03.012 [DOI] [PubMed] [Google Scholar]
- Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018. Mar;16(3):143–55. 10.1038/nrmicro.2017.157 [DOI] [PubMed] [Google Scholar]
- Fitz-Gibbon S, Tomida S, Chiu BH, Nguyen L, Du C, Liu M, et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol. 2013. Sep;133(9):2152–60. 10.1038/jid.2013.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Res. 2018;7. doi: 10.12688/f1000research.15659.1 [DOI] [PMC free article] [PubMed]
- McDowell A, Gao A, Barnard E, Fink C, Murray PI, Dowson CG, et al. A novel multilocus sequence typing scheme for the opportunistic pathogen Propionibacterium acnes and characterization of type I cell surface-associated antigens. Microbiology (Reading). 2011. Jul;157(Pt 7):1990–2003. 10.1099/mic.0.049676-0 [DOI] [PubMed] [Google Scholar]
- Dagnelie MA, Corvec S, Saint-Jean M, Bourdès V, Nguyen JM, Khammari A, et al. Decrease in Diversity of Propionibacterium acnes Phylotypes in Patients with Severe Acne on the Back. Acta Derm Venereol. 2018. Feb;98(2):262–7. 10.2340/00015555-2847 [DOI] [PubMed] [Google Scholar]
- Isard O, Knol AC, Ariès MF, Nguyen JM, Khammari A, Castex-Rizzi N, et al. Propionibacterium acnes activates the IGF-1/IGF-1R system in the epidermis and induces keratinocyte proliferation. J Invest Dermatol. 2011. Jan;131(1):59–66. 10.1038/jid.2010.281 [DOI] [PubMed] [Google Scholar]
- Kistowska M, Gehrke S, Jankovic D, Kerl K, Fettelschoss A, Feldmeyer L, et al. IL-1β drives inflammatory responses to propionibacterium acnes in vitro and in vivo. J Invest Dermatol. 2014. Mar;134(3):677–85. 10.1038/jid.2013.438 [DOI] [PubMed] [Google Scholar]
- Kistowska M, Meier B, Proust T, Feldmeyer L, Cozzio A, Kuendig T, et al. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J Invest Dermatol. 2015. Jan;135(1):110–8. 10.1038/jid.2014.290 [DOI] [PubMed] [Google Scholar]
- O’Neill AM, Liggins MC, Seidman JS, Do TH, Li F, Cavagnero KJ, et al. Antimicrobial production by perifollicular dermal preadipocytes is essential to the pathophysiology of acne. Sci Transl Med. 2022. Feb;14(632):eabh1478. 10.1126/scitranslmed.abh1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates P, Vyakrnam S, Eady EA, Jones CE, Cove JH, Cunliffe WJ. Prevalence of antibiotic-resistant propionibacteria on the skin of acne patients: 10-year surveillance data and snapshot distribution study. Br J Dermatol. 2002. May;146(5):840–8. 10.1046/j.1365-2133.2002.04690.x [DOI] [PubMed] [Google Scholar]
- Dessinioti C, Katsambas A. Propionibacterium acnes and antimicrobial resistance in acne. Clin Dermatol. 2017. Mar - Apr;35(2):163–7. 10.1016/j.clindermatol.2016.10.008 [DOI] [PubMed] [Google Scholar]
- Leyden JJ, McGinley KJ, Cavalieri S, Webster GF, Mills OH, Kligman AM. Propionibacterium acnes resistance to antibiotics in acne patients. J Am Acad Dermatol. 1983. Jan;8(1):41–5. 10.1016/s0190-9622(83)70005-8 [DOI] [PubMed] [Google Scholar]
- Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016. May;74(5):945–73.e33. 10.1016/j.jaad.2015.12.037 [DOI] [PubMed] [Google Scholar]
- Katsambas A, Dessinioti C. New and emerging treatments in dermatology: acne. Dermatol Ther. 2008. Mar-Apr;21(2):86–95. 10.1111/j.1529-8019.2008.00175.x [DOI] [PubMed] [Google Scholar]
- Nast A, Dréno B, Bettoli V, Bukvic Mokos Z, Degitz K, Dressler C, et al. European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016. Aug;30(8):1261–8. 10.1111/jdv.13776 [DOI] [PubMed] [Google Scholar]
- Lee YH, Liu G, Thiboutot DM, Leslie DL, Kirby JS. A retrospective analysis of the duration of oral antibiotic therapy for the treatment of acne among adolescents: investigating practice gaps and potential cost-savings. J Am Acad Dermatol. 2014. Jul;71(1):70–6. 10.1016/j.jaad.2014.02.031 [DOI] [PubMed] [Google Scholar]
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016. Feb;74(2):273–9. 10.1016/j.jaad.2015.09.046 [DOI] [PubMed] [Google Scholar]
- Yentzer BA, Irby CE, Fleischer AB Jr, Feldman SR. Differences in acne treatment prescribing patterns of pediatricians and dermatologists: an analysis of nationally representative data. Pediatr Dermatol. 2008. Nov-Dec;25(6):635–9. 10.1111/j.1525-1470.2008.00790.x [DOI] [PubMed] [Google Scholar]
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: A retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017. Sep;77(3):456–463.e4. 10.1016/j.jaad.2017.04.016 [DOI] [PubMed] [Google Scholar]
- Aslan Kayıran M, Karadağ AS, Mutlu HH, Goldust M, Sarıcaoğlu H. Comparison of dermatologists and family physicians in terms of prescribing antibiotics for the treatment of acne vulgaris. Dermatol Ther. 2020. Nov;33(6):e13973. 10.1111/dth.13973 [DOI] [PubMed] [Google Scholar]
- Eady EA, Ross JI, Cove JH. Multiple mechanisms of erythromycin resistance. J Antimicrob Chemother. 1990. Oct;26(4):461–5. 10.1093/jac/26.4.461 [DOI] [PubMed] [Google Scholar]
- Ross JI, Snelling AM, Eady EA, Cove JH, Cunliffe WJ, Leyden JJ, et al. Phenotypic and genotypic characterization of antibiotic-resistant Propionibacterium acnes isolated from acne patients attending dermatology clinics in Europe, the U.S.A., Japan and Australia. Br J Dermatol. 2001. Feb;144(2):339–46. 10.1046/j.1365-2133.2001.03956.x [DOI] [PubMed] [Google Scholar]
- Ross JI, Eady EA, Cove JH, Jones CE, Ratyal AH, Miller YW, et al. Clinical resistance to erythromycin and clindamycin in cutaneous propionibacteria isolated from acne patients is associated with mutations in 23S rRNA. Antimicrob Agents Chemother. 1997. May;41(5):1162–5. 10.1128/AAC.41.5.1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JI, Eady EA, Cove JH, Cunliffe WJ. 16S rRNA mutation associated with tetracycline resistance in a gram-positive bacterium. Antimicrob Agents Chemother. 1998. Jul;42(7):1702–5. 10.1128/AAC.42.7.1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JI, Eady EA, Carnegie E, Cove JH. Detection of transposon Tn5432-mediated macrolide-lincosamide-streptogramin B (MLSB) resistance in cutaneous propionibacteria from six European cities. J Antimicrob Chemother. 2002. Jan;49(1):165–8. 10.1093/jac/49.1.165 [DOI] [PubMed] [Google Scholar]
- Weinstein MP, Patel JB, Bobenchik AM, Campeau S, Cullen SK, Galas MF, et al. Performance Standards for Antimicrobial Susceptibility Testing. Available at: https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf. Access on August 6, 2022 USA: Clinical and Laboratory Standards Institute (CLSI); 2020. 30th edition:[
- EUCAST (European Committee On Antimicrobial Susceptibility Testing). Clinical breakpoints and dosing of antibiotics. V. 12.0. Available at: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf. Access on June 29, 2022 2022. [updated 1 Jan, 202229 June 2022]. Available from: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf
- Asai Y, Baibergenova A, Dutil M, Humphrey S, Hull P, Lynde C, et al. Management of acne: canadian clinical practice guideline. CMAJ. 2016. Feb;188(2):118–26. 10.1503/cmaj.140665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mavranezouli I, Kuznetsov L, Stephen Murphy M, Healy E, Guideline C, Guideline Committee. Management of acne vulgaris: summary of NICE guidance. BMJ. 2021. Sep;374:n1800. 10.1136/bmj.n1800 [DOI] [PubMed] [Google Scholar]
- Moore AY, Charles JE, Moore S. Sarecycline: a narrow spectrum tetracycline for the treatment of moderate-to-severe acne vulgaris. Future Microbiol. 2019. Sep;14:1235–42. 10.2217/fmb-2019-0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffier d’Epenoux L, Guillouzouic A, Bémer P, Dagnelie MA, Khammari A, Dréno B, et al. Should we consider broad-spectrum quinolone antibacterial agent as acne treatment in the antimicrobial resistance era? J Eur Acad Dermatol Venereol. 2022. Mar;36(3):e193–5. 10.1111/jdv.17727 [DOI] [PubMed] [Google Scholar]
- Sardana K, Gupta T, Kumar B, Gautam HK, Garg VK. Cross-sectional Pilot Study of Antibiotic Resistance in Propionibacterium Acnes Strains in Indian Acne Patients Using 16S-RNA Polymerase Chain Reaction: A Comparison Among Treatment Modalities Including Antibiotics, Benzoyl Peroxide, and Isotretinoin. Indian J Dermatol. 2016. Jan-Feb;61(1):45–52. 10.4103/0019-5154.174025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González R, Welsh O, Ocampo J, Hinojosa-Robles RM, Vera-Cabrera L, Delaney ML, et al. In vitro antimicrobial susceptibility of Propionibacterium acnes isolated from acne patients in northern Mexico. Int J Dermatol. 2010. Sep;49(9):1003–7. 10.1111/j.1365-4632.2010.04506.x [DOI] [PubMed] [Google Scholar]
- Zhu T, Zhu W, Wang Q, He L, Wu W, Liu J, et al. Antibiotic susceptibility of Propionibacterium acnes isolated from patients with acne in a public hospital in Southwest China: prospective cross-sectional study. BMJ Open. 2019. Feb;9(2):e022938. 10.1136/bmjopen-2018-022938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Hao F, Wang W, Lu Y, He L, Wang G, et al. Multicenter cross-sectional observational study of antibiotic resistance and the genotypes of Propionibacterium acnes isolated from Chinese patients with acne vulgaris. J Dermatol. 2016. Apr;43(4):406–13. 10.1111/1346-8138.13149 [DOI] [PubMed] [Google Scholar]
- Kim JE, Park AY, Lee SY, Park YL, Whang KU, Kim HJ. Comparison of the Efficacy of Azithromycin Versus Doxycycline in Acne Vulgaris: A Meta-Analysis of Randomized Controlled Trials. Ann Dermatol. 2018. Aug;30(4):417–26. 10.5021/ad.2018.30.4.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardana K, Gupta T, Garg VK, Ghunawat S. Antibiotic resistance to Propionobacterium acnes: worldwide scenario, diagnosis and management. Expert Rev Anti Infect Ther. 2015. Jul;13(7):883–96. 10.1586/14787210.2015.1040765 [DOI] [PubMed] [Google Scholar]
- Sardana K, Mathachan SR, Gupta T. Antibiotic resistance in acne an emergent need to recognize resistance to azithromycin and restrict its unapproved use in acne vulgaris. J Eur Acad Dermatol Venereol. 2021. May;35(5):e347–8. 10.1111/jdv.17099 [DOI] [PubMed] [Google Scholar]
- Ross JI, Snelling AM, Carnegie E, Coates P, Cunliffe WJ, Bettoli V, et al. Antibiotic-resistant acne: lessons from Europe. Br J Dermatol. 2003. Mar;148(3):467–78. 10.1046/j.1365-2133.2003.05067.x [DOI] [PubMed] [Google Scholar]
- Dumont-Wallon G, Moyse D, Blouin E, Dréno B. Bacterial resistance in French acne patients. Int J Dermatol. 2010. Mar;49(3):283–8. 10.1111/j.1365-4632.2009.04270.x [DOI] [PubMed] [Google Scholar]
- Bettoli V, Borghi A, Rossi R, Ferroni M, Rigolin F, Virgili A. Antibiotic resistance of propionibacteria. Four years’ experience of a large number of cases in Italy. Dermatology. 2006;212(2):206–7. 10.1159/000090665 [DOI] [PubMed] [Google Scholar]
- Oprica C, Emtestam L, Lapins J, Borglund E, Nyberg F, Stenlund K, et al. Antibiotic-resistant Propionibacterium acnes on the skin of patients with moderate to severe acne in Stockholm. Anaerobe. 2004. Jun;10(3):155–64. 10.1016/j.anaerobe.2004.02.002 [DOI] [PubMed] [Google Scholar]
- Alkhawaja E, Hammadi S, Abdelmalek M, Mahasneh N, Alkhawaja B, Abdelmalek SM. Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: a cross sectional study. BMC Dermatol. 2020. Nov;20(1):17. 10.1186/s12895-020-00108-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki S, Nakase K, Hayashi N, Nakaminami H, Noguchi N. Increased prevalence of doxycycline low-susceptible Cutibacterium acnes isolated from acne patients in Japan caused by antimicrobial use and diversification of tetracycline resistance factors. J Dermatol. 2021. Sep;48(9):1365–71. 10.1111/1346-8138.15940 [DOI] [PubMed] [Google Scholar]
- Sheffer-Levi S, Rimon A, Lerer V, Shlomov T, Coppenhagen-Glazer S, Rakov C, et al. Antibiotic Susceptibility of Cutibacterium acnes Strains Isolated from Israeli Acne Patients. Acta Derm Venereol. 2020. Oct;100(17):adv00295. 10.2340/00015555-3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel Fattah NS, Darwish YW. In vitro antibiotic susceptibility patterns of Propionibacterium acnes isolated from acne patients: an Egyptian university hospital-based study. J Eur Acad Dermatol Venereol. 2013. Dec;27(12):1546–51. 10.1111/jdv.12057 [DOI] [PubMed] [Google Scholar]
- Moon SH, Roh HS, Kim YH, Kim JE, Ko JY, Ro YS. Antibiotic resistance of microbial strains isolated from Korean acne patients. J Dermatol. 2012. Oct;39(10):833–7. 10.1111/j.1346-8138.2012.01626.x [DOI] [PubMed] [Google Scholar]
- Mendoza N, Hernandez PO, Tyring SK, Haitz KA, Motta A. Antimicrobial susceptibility of Propionibacterium acnes isolates from acne patients in Colombia. Int J Dermatol. 2013. Jun;52(6):688–92. 10.1111/j.1365-4632.2011.05403.x [DOI] [PubMed] [Google Scholar]
- Luk NM, Hui M, Lee HC, Fu LH, Liu ZH, Lam LY, et al. Antibiotic-resistant Propionibacterium acnes among acne patients in a regional skin centre in Hong Kong. J Eur Acad Dermatol Venereol. 2013. Jan;27(1):31–6. 10.1111/j.1468-3083.2011.04351.x [DOI] [PubMed] [Google Scholar]
- Tan HH, Tan AW, Barkham T, Yan XY, Zhu M. Community-based study of acne vulgaris in adolescents in Singapore. Br J Dermatol. 2007. Sep;157(3):547–51. 10.1111/j.1365-2133.2007.08087.x [DOI] [PubMed] [Google Scholar]
- Yang SS, Long V, Liau MM, Lee SH, Toh M, Teo J, et al. A profile of Propionibacterium acnes resistance and sensitivity at a tertiary dermatological centre in Singapore. Br J Dermatol. 2018. Jul;179(1):200–1. 10.1111/bjd.16380 [DOI] [PubMed] [Google Scholar]
- Zhang N, Yuan R, Xin KZ, Lu Z, Ma Y. Antimicrobial Susceptibility, Biotypes and Phylotypes of Clinical Cutibacterium (Formerly Propionibacterium) acnes Strains Isolated from Acne Patients: An Observational Study. Dermatol Ther (Heidelb). 2019. Dec;9(4):735–46. 10.1007/s13555-019-00320-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer H, McDowell A, Alexeyev OA. Understanding the role of Propionibacterium acnes in acne vulgaris: the critical importance of skin sampling methodologies. Clin Dermatol. 2017. Mar - Apr;35(2):118–29. 10.1016/j.clindermatol.2016.10.003 [DOI] [PubMed] [Google Scholar]
- Broly M, Ruffier d’Epenoux L, Guillouzouic A, Le Gargasson G, Juvin ME, Leroy AG, et al. Propionibacterium/Cutibacterium species-related positive samples, identification, clinical and resistance features: a 10-year survey in a French hospital. Eur J Clin Microbiol Infect Dis. 2020. Jul;39(7):1357–64. 10.1007/s10096-020-03852-5 [DOI] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Renaud G, Young AC, Bouffard GG, Blakesley RW, et al. NISC Comparative Sequencing Program. A diversity profile of the human skin microbiota. Genome Res. 2008. Jul;18(7):1043–50. 10.1101/gr.075549.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaper D, Assadian O, Edmiston CE. Approach to chronic wound infections. Br J Dermatol. 2015. Aug;173(2):351–8. 10.1111/bjd.13677 [DOI] [PubMed] [Google Scholar]
- Abbott C, Grout E, Morris T, Brown HL. Cutibacterium acnes biofilm forming clinical isolates modify the formation and structure of Staphylococcus aureus biofilms, increasing their susceptibility to antibiotics. Anaerobe. 2022. Aug;76:102580. 10.1016/j.anaerobe.2022.102580 [DOI] [PubMed] [Google Scholar]
- Gannesen AV, Lesouhaitier O, Racine PJ, Barreau M, Netrusov AI, Plakunov VK, et al. Regulation of Monospecies and Mixed Biofilms Formation of Skin Staphylococcus aureus and Cutibacterium acnes by Human Natriuretic Peptides. Front Microbiol. 2018. Dec;9:2912. 10.3389/fmicb.2018.02912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyner H, Patel R. Propionibacterium acnes biofilm - A sanctuary for Staphylococcus aureus? Anaerobe. 2016. Aug;40:63–7. 10.1016/j.anaerobe.2016.05.014 [DOI] [PubMed] [Google Scholar]
- Bronnec V, Eilers H, Jahns AC, Omer H, Alexeyev OA. Propionibacterium (Cutibacterium) granulosum Extracellular DNase BmdE Targeting Propionibacterium (Cutibacterium) acnes Biofilm Matrix, a Novel Inter-Species Competition Mechanism. Front Cell Infect Microbiol. 2022. Jan;11:809792. 10.3389/fcimb.2021.809792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady EA, Cove JH, Holland KT, Cunliffe WJ. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J Dermatol. 1989. Jul;121(1):51–7. 10.1111/j.1365-2133.1989.tb01399.x [DOI] [PubMed] [Google Scholar]
- Mills O Jr, Thornsberry C, Cardin CW, Smiles KA, Leyden JJ. Bacterial resistance and therapeutic outcome following three months of topical acne therapy with 2% erythromycin gel versus its vehicle. Acta Derm Venereol. 2002;82(4):260–5. 10.1080/000155502320323216 [DOI] [PubMed] [Google Scholar]
- Sadhasivam S, Sinha M, Saini S, Kaur SP, Gupta T, Sengupta S, et al. Heterogeneity and antibiotic resistance in Propionibacterium acnes isolates and its therapeutic implications: blurring the lines between commensal and pathogenic phylotypes. Dermatol Ther. 2016. Nov;29(6):451–4. 10.1111/dth.12391 [DOI] [PubMed] [Google Scholar]
- Eady EA, Farmery MR, Ross JI, Cove JH, Cunliffe WJ. Effects of benzoyl peroxide and erythromycin alone and in combination against antibiotic-sensitive and -resistant skin bacteria from acne patients. Br J Dermatol. 1994. Sep;131(3):331–6. 10.1111/j.1365-2133.1994.tb08519.x [DOI] [PubMed] [Google Scholar]
- Cunliffe WJ, Holland KT, Bojar R, Levy SF. A randomized, double-blind comparison of a clindamycin phosphate/benzoyl peroxide gel formulation and a matching clindamycin gel with respect to microbiologic activity and clinical efficacy in the topical treatment of acne vulgaris. Clin Ther. 2002. Jul;24(7):1117–33. 10.1016/s0149-2918(02)80023-6 [DOI] [PubMed] [Google Scholar]
- Dessinioti C, Katsambas A. Difficult and rare forms of acne. Clin Dermatol. 2017. Mar - Apr;35(2):138–46. 10.1016/j.clindermatol.2016.10.005 [DOI] [PubMed] [Google Scholar]
- Schupp JC, Tchaptchet S, Lützen N, Engelhard P, Müller-Quernheim J, Freudenberg MA, et al. Immune response to Propionibacterium acnes in patients with sarcoidosis—in vivo and in vitro. BMC Pulm Med. 2015. Jul;15:75. 10.1186/s12890-015-0070-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollason J, McDowell A, Albert HB, Barnard E, Worthington T, Hilton AC, et al. Genotypic and antimicrobial characterisation of Propionibacterium acnes isolates from surgically excised lumbar disc herniations. BioMed Res Int. 2013;2013:530382. 10.1155/2013/530382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belk JW, Kraeutler MJ, Smith JR, Littlefield CP, Bravman JT, Houck DA, et al. Prevention of Cutibacterium acnes infection in arthroscopic shoulder surgery: a systematic review. J Shoulder Elbow Surg. 2020. May;29(5):867–73. 10.1016/j.jse.2019.12.032 [DOI] [PubMed] [Google Scholar]
- Levy PY, Fenollar F, Stein A, Borrione F, Cohen E, Lebail B, et al. Propionibacterium acnes postoperative shoulder arthritis: an emerging clinical entity. Clin Infect Dis. 2008. Jun;46(12):1884–6. 10.1086/588477 [DOI] [PubMed] [Google Scholar]
- Daguzé J, Frénard C, Saint-Jean M, Dumont R, Touchais S, Corvec S, et al. Two cases of non-prosthetic bone and joint infection due to Propionibacterium acnes. J Eur Acad Dermatol Venereol. 2016. Nov;30(11):e136–7. 10.1111/jdv.13446 [DOI] [PubMed] [Google Scholar]
- Nakase K, Koizumi J, Midorikawa R, Yamasaki K, Tsutsui M, Aoki S, et al. Cutibacterium acnes phylogenetic type IC and II isolated from patients with non-acne diseases exhibit high-level biofilm formation. Int J Med Microbiol. 2021. Oct;311(7):151538. 10.1016/j.ijmm.2021.151538 [DOI] [PubMed] [Google Scholar]
- Brüggemann H, Al-Zeer MA. Bacterial signatures and their inflammatory potentials associated with prostate cancer. APMIS. 2020. Feb;128(2):80–91. 10.1111/apm.13021 [DOI] [PubMed] [Google Scholar]
- Brüggemann H, Salar-Vidal L, Gollnick HP, Lood R. A Janus-Faced Bacterium: Host-Beneficial and -Detrimental Roles of Cutibacterium acnes. Front Microbiol. 2021. May;12:673845. 10.3389/fmicb.2021.673845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt AY, Emiola A, Johnson JS, Fleming ES, Nguyen H, Zhou W, et al. Skin Microbiome Variation with Cancer Progression in Human Cutaneous Squamous Cell Carcinoma. J Invest Dermatol. 2022. Oct;142(10):2773–2782.e16. 10.1016/j.jid.2022.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterfrauner I, Wieser K, Catanzaro S, Uçkay I, Bouaicha S. Acne cream reduces the deep Cutibacterium acnes tissue load before elective open shoulder surgery: a randomized controlled pilot trial. J Shoulder Elbow Surg. 2022. May;31(5):897–905. 10.1016/j.jse.2022.01.115 [DOI] [PubMed] [Google Scholar]
- Scheer VM, Bergman Jungeström M, Lerm M, Serrander L, Kalén A. Topical benzoyl peroxide application on the shoulder reduces Propionibacterium acnes: a randomized study. J Shoulder Elbow Surg. 2018. Jun;27(6):957–61. 10.1016/j.jse.2018.02.038 [DOI] [PubMed] [Google Scholar]
- Kolakowski L, Lai JK, Duvall GT, Jauregui JJ, Dubina AG, Jones DL, et al. Neer Award 2018: Benzoyl peroxide effectively decreases preoperative Cutibacterium acnes shoulder burden: a prospective randomized controlled trial. J Shoulder Elbow Surg. 2018. Sep;27(9):1539–44. 10.1016/j.jse.2018.06.012 [DOI] [PubMed] [Google Scholar]
- Jo JH, Harkins CP, Schwardt NH, Portillo JA, Zimmerman MD, Carter CL, et al. NISC Comparative Sequencing Program. Alterations of human skin microbiome and expansion of antimicrobial resistance after systemic antibiotics. Sci Transl Med. 2021. Dec;13(625):eabd8077. 10.1126/scitranslmed.abd8077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien AL, Tsai J, Leung S, Mongodin EF, Nelson AM, Kang S, et al. Association of Systemic Antibiotic Treatment of Acne With Skin Microbiota Characteristics. JAMA Dermatol. 2019. Apr;155(4):425–34. 10.1001/jamadermatol.2018.5221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady EA, Cove JH, Holland KT, Cunliffe WJ. Superior antibacterial action and reduced incidence of bacterial resistance in minocycline compared to tetracycline-treated acne patients. Br J Dermatol. 1990. Feb;122(2):233–44. 10.1111/j.1365-2133.1990.tb08270.x [DOI] [PubMed] [Google Scholar]
- Delost GR, Delost ME, Armile J, Lloyd J. Staphylococcus aureus carriage rates and antibiotic resistance patterns in patients with acne vulgaris. J Am Acad Dermatol. 2016. Apr;74(4):673–8. 10.1016/j.jaad.2015.11.025 [DOI] [PubMed] [Google Scholar]
- Miller YW, Eady EA, Lacey RW, Cove JH, Joanes DN, Cunliffe WJ. Sequential antibiotic therapy for acne promotes the carriage of resistant staphylococci on the skin of contacts. J Antimicrob Chemother. 1996. Nov;38(5):829–37. 10.1093/jac/38.5.829 [DOI] [PubMed] [Google Scholar]
- Margolis DJ, Bowe WP, Hoffstad O, Berlin JA. Antibiotic treatment of acne may be associated with upper respiratory tract infections. Arch Dermatol. 2005. Sep;141(9):1132–6. 10.1001/archderm.141.9.1132 [DOI] [PubMed] [Google Scholar]
- Margolis DJ, Fanelli M, Kupperman E, Papadopoulos M, Metlay JP, Xie SX, et al. Association of pharyngitis with oral antibiotic use for the treatment of acne: a cross-sectional and prospective cohort study. Arch Dermatol. 2012. Mar;148(3):326–32. 10.1001/archdermatol.2011.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhate K, Lin LY, Barbieri JS, Leyrat C, Hopkins S, Stabler R, et al. Is there an association between long-term antibiotics for acne and subsequent infection sequelae and antimicrobial resistance? A systematic review. BJGP Open. 2021. Jun;5(3):BJGPO.2020.0181. 10.3399/BJGPO.2020.0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy RM, Huang EY, Roling D, Leyden JJ, Margolis DJ. Effect of antibiotics on the oropharyngeal flora in patients with acne. Arch Dermatol. 2003. Apr;139(4):467–71. 10.1001/archderm.139.4.467 [DOI] [PubMed] [Google Scholar]
- Thompson KG, Rainer BM, Antonescu C, Florea L, Mongodin EF, Kang S, et al. Minocycline and Its Impact on Microbial Dysbiosis in the Skin and Gastrointestinal Tract of Acne Patients. Ann Dermatol. 2020. Feb;32(1):21–30. 10.5021/ad.2020.32.1.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura IB, Grada A, Spittal W, Clark E, Ewin D, Altringham J, et al. Profiling the Effects of Systemic Antibiotics for Acne, Including the Narrow-Spectrum Antibiotic Sarecycline, on the Human Gut Microbiota. Front Microbiol. 2022. May;13:901911. 10.3389/fmicb.2022.901911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitali F, Colucci R, Di Paola M, Pindo M, De Filippo C, Moretti S, et al. Early melanoma invasivity correlates with gut fungal and bacterial profiles. Br J Dermatol. 2022. Jan;186(1):106–16. 10.1111/bjd.20626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polkowska-Pruszyńska B, Gerkowicz A, Krasowska D. The gut microbiome alterations in allergic and inflammatory skin diseases - an update. J Eur Acad Dermatol Venereol. 2020. Mar;34(3):455–64. 10.1111/jdv.15951 [DOI] [PubMed] [Google Scholar]
- Woo YR, Cho SH, Lee JD, Kim HS. The Human Microbiota and Skin Cancer. Int J Mol Sci. 2022. Feb;23(3):1813. 10.3390/ijms23031813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick H, Cunliffe W, Berson D, Dreno B, Finlay A, Leyden JJ, et al. Global Alliance to Improve Outcomes in Acne. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003. Jul;49(1 Suppl):S1–37. 10.1067/mjd.2003.618 [DOI] [PubMed] [Google Scholar]
- Bojar RA, Cunliffe WJ, Holland KT. The short-term treatment of acne vulgaris with benzoyl peroxide: effects on the surface and follicular cutaneous microflora. Br J Dermatol. 1995. Feb;132(2):204–8. 10.1111/j.1365-2133.1995.tb05014.x [DOI] [PubMed] [Google Scholar]
- Boonchaya P, Rojhirunsakool S, Kamanamool N, Khunkhet S, Yooyongsatit S, Udompataikul M, et al. Minimum Contact Time of 1.25%, 2.5%, 5%, and 10% Benzoyl Peroxide for a Bactericidal Effect Against Cutibacterium acnes. Clin Cosmet Investig Dermatol. 2022. Mar;15:403–9. 10.2147/CCID.S359055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady EA, Bojar RA, Jones CE, Cove JH, Holland KT, Cunliffe WJ. The effects of acne treatment with a combination of benzoyl peroxide and erythromycin on skin carriage of erythromycin-resistant propionibacteria. Br J Dermatol. 1996. Jan;134(1):107–13. [PubMed] [Google Scholar]
- Jackson JM, Fu JJ, Almekinder JL. A randomized, investigator-blinded trial to assess the antimicrobial efficacy of a benzoyl peroxide 5%/ clindamycin phosphate 1% gel compared with a clindamycin phosphate 1.2%/tretinoin 0.025% gel in the topical treatment of acne vulgaris. J Drugs Dermatol. 2010. Feb;9(2):131–6. [PubMed] [Google Scholar]
- Hebert A, Thiboutot D, Stein Gold L, Cartwright M, Gerloni M, Fragasso E, et al. Efficacy and Safety of Topical Clascoterone Cream, 1%, for Treatment in Patients With Facial Acne: Two Phase 3 Randomized Clinical Trials. JAMA Dermatol. 2020. Jun;156(6):621–30. 10.1001/jamadermatol.2020.0465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardo M, Cardinali C, La Placa M, Lewartowska-Białek A, Lora V, Micali G, et al. GEDACNE Study Group. Efficacy and safety of N-acetyl-GED-0507-34-LEVO gel in patients with moderate-to severe facial acne vulgaris: a phase IIb randomized double-blind, vehicle-controlled trial. Br J Dermatol. 2022. Oct;187(4):507–14. 10.1111/bjd.21663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessinioti C, Dreno B. Acne treatments: future trajectories. Clin Exp Dermatol. 2020. Dec;45(8):955–61. 10.1111/ced.14239 [DOI] [PubMed] [Google Scholar]
- Karoglan A, Paetzold B, Pereira de Lima J, Brüggemann H, Tüting T, Schanze D, et al. Safety and Efficacy of Topically Applied Selected Cutibacterium acnes Strains over Five Weeks in Patients with Acne Vulgaris: An Open-label, Pilot Study. Acta Derm Venereol. 2019. Dec;99(13):1253–7. 10.2340/00015555-3323 [DOI] [PubMed] [Google Scholar]
- Dessinioti C, Zouboulis CC, Bettoli V, Rigopoulos D. Comparison of guidelines and consensus articles on the management of patients with acne with oral isotretinoin. J Eur Acad Dermatol Venereol. 2020. Oct;34(10):2229–40. 10.1111/jdv.16430 [DOI] [PubMed] [Google Scholar]
- Katsambas AD, Dessinioti C. Hormonal therapy for acne: why not as first line therapy? facts and controversies. Clin Dermatol. 2010. Jan-Feb;28(1):17–23. 10.1016/j.clindermatol.2009.03.006 [DOI] [PubMed] [Google Scholar]
- Thiboutot D. Acne: hormonal concepts and therapy. Clin Dermatol. 2004. Sep-Oct;22(5):419–28. 10.1016/j.clindermatol.2004.03.010 [DOI] [PubMed] [Google Scholar]
- Jung GW, Tse JE, Guiha I, Rao J. Prospective, randomized, open-label trial comparing the safety, efficacy, and tolerability of an acne treatment regimen with and without a probiotic supplement and minocycline in subjects with mild to moderate acne. J Cutan Med Surg. 2013. Mar-Apr;17(2):114–22. 10.2310/7750.2012.12026 [DOI] [PubMed] [Google Scholar]
- Kim J, Ko Y, Park YK, Kim NI, Ha WK, Cho Y. Dietary effect of lactoferrin-enriched fermented milk on skin surface lipid and clinical improvement of acne vulgaris. Nutrition. 2010. Sep;26(9):902–9. 10.1016/j.nut.2010.05.011 [DOI] [PubMed] [Google Scholar]
- Fabbrocini G, Bertona M, Picazo Ó, Pareja-Galeano H, Monfrecola G, Emanuele E. Supplementation with Lactobacillus rhamnosus SP1 normalises skin expression of genes implicated in insulin signalling and improves adult acne. Benef Microbes. 2016. Nov;7(5):625–30. 10.3920/BM2016.0089 [DOI] [PubMed] [Google Scholar]