Abstract
In this study, we present an 18-month serological follow-up of 294 patients with COVID-19 pneumonia. The aim was to assess the dynamics of serological response and its correlation with clinical worsening, as well as to describe clinical worsening determinants. Results of the study showed an early immunoglobulin M response, which clearly diminished starting at 4 months, but nonetheless, a small group of patients remained positive. As for immunoglobulin G, levels were higher up to 6 months in patients who presented clinical worsening during hospitalization. High titers of the immunoglobulin were maintained in all patients during follow-up, which would indicate that humoral immunity due to infection is long-lasting. Male sex, presence of myalgias and extensive radiological affectation were significantly correlated with clinical worsening.
Keywords: COVID-19 pneumonia, SARS-COV-2, Serological response, Clinical worsening
Introduction
Millions of cases and deaths have been confirmed around the world due to the disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) more than 2 years after this coronavirus emerged in Wuhan (Hubei Province, China).
In the context of respiratory outbreaks, accurate and rapid diagnosis is crucial to isolate those people affected in time to stop epidemics [1]. Currently, there are three types of tests to assess SARS-CoV-2 infection: (1) nucleic acid amplification tests such as real-time polymerase chain reaction (RT-PCR) assay, which remains the standard diagnostic technique, (2) antigen tests and (3) serology tests. The first two types are used to diagnose acute infection whereas serology is mostly used for epidemiological surveillance [2].
However, in the first wave of the pandemic in our country, there were no antigen tests, and since RT-PCR tests were scarce, they were mostly performed on patients admitted to hospital. In addition, a test sensitivity as low as 60–70% [3, 4] was reported at that time, meaning that a considerable number of patients were not diagnosed.
Compared to RT-PCR assays, serological tests are less expensive, easier to use and accessible to untrained laboratory personnel. Moreover, antibody response might be prompted in the infection course and can be monitored across time, meaning that it can help define treatment and immunization strategies. For these reasons, serological techniques still play a role in viral infections’ assessment and can complement molecular techniques [5].
In viral infections, following an initial innate response, the adaptative immune system is activated with T cells destroying infected cells and B cells working in parallel to produce effective neutralizing antibodies against the virus [6]. After an initial immunoglobulin M (IgM) response, class-switched antibodies are generated, mostly immunoglobulin G (IgG). In the case of acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and Middle East Respiratory Syndrome-Coronavirus (MERS-CoV), both IgM and IgG became detectable in patients’ serum as early as 11 to 15 days post-illness onset. On the other hand, individuals who suffer from seasonal coronaviruses tend to maintain immunity for about 1 year, while antibody quantification from SARS-CoV-1 and MERS-CoV infection significantly decreases 2 to 3 years following the onset of symptoms. Furthermore, these pathogens tend to alter their structure to escape the immunity given by antibodies developed from previous infections and hence, individuals are susceptible to reinfection [7, 8].
Regarding SARS-CoV-2, immune response has been broadly studied since the beginning of the pandemic [9–11]. A similar scenario has been observed in patients with COVID-19 as IgM and IgG have been detected from 5 to 14 days after initial symptoms [12], with a slower response in less severe cases. In addition, a correlation between severe COVID-19 and higher levels of antibodies has been described [13, 14]. However, immunoglobulin titers seem to start declining between 4 and 6 months from the onset of infection, with patients with milder disease presenting an earlier reduction [15]. It has been observed that this decrease is related to the immunoglobulin peak response, the subtypes of antibodies implied and the varying contribution of short-lived and long-lived plasma cells to the circulating IgG levels [6]. Despite that, reinfection by the same variant is uncommon, given that antigen-specific memory B cells are rapidly activated and T cellular response also plays a major role [16]. Nonetheless, as seen with seasonal coronaviruses, new variants of SARS-CoV-2 have been arising from mutations in antibody epitopes in the S glycoprotein, compromising neutralization by antibodies produced from prior infection and vaccination [6, 17–20]. Currently, immunity induced by vaccination still offers considerable protection against severe forms of the disease caused by the new variants, but it is plausible that viral evolution will eventually overcome this protection [21].
In this study, we present a long 18 months serological follow-up of patients with COVID-19 pneumonia. The aim of the study is twofold: first, to assess serological response and its behavior over time, considering not only persistence of antibodies, but also their levels. It was expected that immune response, especially IgM, would develop very early, and that IgG would be maintained over time and with similar levels of antibodies throughout evolution. Moreover, we analyzed the predictors of immunoglobulin dynamics. Second, to assess the factors associated as well as the serological response to clinical worsening during hospitalization, considering that patients who presented progression of the infection would exhibit higher immunoglobulins titers.
Materials and methods
Design
This is an observational prospective Spanish cohort study of 294 hospitalized patients with COVID-19 pneumonia followed up during 18 months.
Patients and data collection
All patients admitted for hospital care with a clinicoradiological diagnosis of COVID-19 pneumonia between March 28th and June 15th 2020 were evaluated. Serum samples were extracted and IgM and IgG responses to COVID-19 pneumonia along this period were analyzed.
Patients’ selection
Inclusion criteria were:
Adults older than 18 years old
Grant verbal informed consent, which was recorded in medical history
Reliable diagnosis of SARS-CoV-2 infection confirmed by RT-PCR tests on nasopharyngeal and/or oropharyngeal swabs
Chest X-ray compatible with pneumonia
Hospital admission either due to the presence of pneumonia or to other severity criteria that justified admission.
Definitions
A case was defined as a patient with symptoms compatible with SARS-CoV-2 infection, pneumonia confirmed by chest X-ray or thoracic computed tomography (CT) scan and RT-PCR positive for SARS-CoV-2.
Severe cases were defined when at least one of the following criteria was met: (1) need for mechanical ventilation; (2) arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio (PF ratio, [PFR]) ≤ 100 mmHg (1 mmHg = 0.90 kPa); (3) shock; (4) multiorgan failure; or (5) chest X-ray images showing an evident progression of previous lesions in 24–48 h and a PFR < 200 mmHg.
Moderate cases were defined with at least one of the following criteria: (1) respiratory rate ≧ 30 breaths/min; (2) peripheral oxygen saturation (SpO2) ≤ 93% at rest; or (3) PFR ≤ 300 mmHg.
Finally, mild cases were defined when one of the following criteria was presented: (1) SpO2 > 93% at rest; (2) respiratory rate < 30 breaths/min; or (3) PFR > 300 mmHg.
Clinical worsening was defined as progression during hospitalization from mild to moderate disease or from moderate to severe disease.
Samples obtention
Antibody detection was performed from serum samples collected in appropriate separator tubes, using DiaSorin Liaison-XL SARS-CoV-2 immunoglobulin G (IgG) anti-spike S1/S2 assay (DiaSorin). The Liaison SARS-CoV-2 is an assay designed to detect IgG antibodies in the serum of subjects exposed to SARS-CoV-2. The assay consists of paramagnetic microparticles coated with S1 and S2 fragments of the viral surface spike protein. Recombinant fusion antigens were expressed in human cells to ensure proper folding, oligomer formation and glycosylation, providing capture moieties which are more similar to those of the viral spike proteins, as processed by natural cellular cleavage.
A precision study was performed to validate the analytical and clinical performance of this assay, with satisfactory results, as previous research has reported [22].
The Liaison SARS-CoV-2 uses the spike protein S1/S2 from an ancestral strain, which has shown to be highly immunogenic. S protein has already been proved before and it is widely recognized as the most specific with regard to production of protective and neutralizing antibodies. It has good sensitivity and specificity: sensitivity at 15 days 97.9% and specificity 95.7% (according to manufacturer’s specifications) [23].
In the last determination, we performed an assay designed to detect antibodies target to the nucleocapsid in the serum of patients that have suffered COVID-19 pneumonia and that have been vaccinated. The Elecsys Anti-SARS-CoV-2assay (Roche diagnostics), an Electro-chemiluminescence immunoassay (ECLIA), uses a recombinant protein representing the nucleocapsid (N) antigen for the determination of antibodies against SARS-CoV-2.
The diagnostic value of the ELISA-based IgM and IgG antibody test was based on sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and precision according to manufacturer’s recommendations.
Follow-up
Although there was a defined protocol, organization was very difficult at the beginning of the pandemic and it was not possible to collect the first samples for some patients.
Serological follow-up of the patients was carried out at different stages:
At the time of admission, a baseline sample was obtained, which corresponded to the first determination.
2 weeks after admission or, if that was not possible, on the day of hospital discharge. This deviation of protocol was accepted to avoid unnecessary displacement of patients during the pandemic period.
3 weeks after baseline determination.
6 weeks after the first one.
Thirteen weeks after the initial determination.
28 weeks from the initial one.
43 weeks after the baseline determination, but at that point, the majority of patients (especially those over 60 years) had already been vaccinated and, therefore, the sample was significantly smaller.
60 weeks after the initial one in a small group of patients that had not been vaccinated yet, just before vaccination.
2 months after patient vaccination, 18 months after the first determination, which corresponds to the final serological determination.
Statistical analysis
All statistical analyses were performed with Stata S/E vs 13.0. Quantitative variables were expressed as mean (standard deviation [SD]) or median (interquartile range [IQR] 25–75%) as appropriate. Qualitative variables were expressed as proportions and confidence intervals 95% (CI95). The log normal distribution was used to fit the temporal distribution of the IgM and IgG antibody seropositive rate. Furthermore, percentage and CI95 of the presence of immunology response IgG and IgM (as qualitative dependents binary variables yes/no) was calculated in the baseline and each time defined in the follow-up. Differences of IgG titles/logarithms and IgG/IgM seropositive rates between groups (worsening/no worsening) were assessed by U Mann–Whitney/Student’s t test or Pearson Chi2/Fisher’s exact test, as appropriate. In addition, relation between clinical worsening and other study variables was analyzed by those statistical tests. We performed exploratory linear regression models between the IgG quantitative dynamics, as dependent variables along study period, with other study variables (age, sex, streptococcal coinfection, length of hospital stay, immunosuppression, intensive care unit [ICU] admission as well as lymphocytic, C-reactive protein [CRP] and D-dimer counts at baseline, during hospitalization and at discharge). Those variables with statistical significance were included in multivariate linear regression model including the variable called “worsening”. A p value < 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
A total of 294 patients admitted to hospital during the study period with a diagnosis of COVID-19 pneumonia were analyzed. The mean age of the patients was 66 ± 26 years, with a predominance of men over women (176/118, 60%). The most prevalent comorbidities of the cohort were hypertension (38%), diabetes (17.3%), obesity (13.8%), chronic lung diseases (11.2%) and chronic kidney disease (9.9%), as shown in Table 1.
Table 1.
Patients’ demographic characteristics, total n = 294
| Characteristic | Value |
|---|---|
| Age, years; mean (standard deviation [SD]) | 66 (26) |
| Men, no. (%) | 176 (60) |
| Toxic habits, no. (%) | |
| Tobacco | Smokers 18 (6.4) |
| Former smokers 48 (17) | |
| Alcohol consumption | Active 12 (4.3) |
| Former 10 (3.6) | |
| Comorbidities, no. (%) | |
| Hypertension | 112 (38) |
| Diabetes | Non-complicated 44 (15) |
| Complicated 7 (2.4) | |
| Ischemic cardiopathy | 20 (6.8) |
| Heart failure | 23 (7.8) |
| Obesity | 39 (13.8) |
| Peripheric arteriopathy | 11 (3.7) |
| Stroke | 15 (5.1) |
| Dementia | 24 (8.1) |
| Chronic respiratory diseases | |
| COPD | 23 (7.9) |
| Asthma | 13 (4.4) |
| SAHS | 15 (5.1) |
| Hepatopathy | Mild 5 (1.7) |
| Severe 3 (1) | |
| Chronic renal failure | |
| GF 60–41 ml/min | 32 (10.85) |
| GF 40–21 ml/min | 15 (5.1) |
| GF 20 ml/min and below | 3 (1.1) |
| Hypothyroidism | 18 (6.1) |
| Malignancy | |
| Solid tumor | Localized 16 (5.4) |
| Distant 3 (1) | |
| Hematologic cancer | Leukemia 3 (1) |
| Lymphoma 3 (1) | |
| Immunosuppression | |
| Chronic immunosuppressive treatment | |
| Active chemotherapy | 7 (2.4) |
| AIDS | |
| Connective tissue disease | 3 (1) |
| 2 (0.7) | |
| 6 (2) | |
At the time of diagnosis, up to 83% of the patients did not identify any family member as close previous contact with confirmed COVID-19. Nonetheless, this proportion was only 30% at the end of the follow-up period. The mean duration of symptoms until admission was 7.7 ± 2 days. The most frequent symptoms were fever (79.9%), cough (62.2%), dyspnea (50%), myalgia (36.1%) and diarrhea (15.3%). The mean length of hospital ward stay was 30 ± 6 days. Most of the patients suffered from bilateral pneumonia (76.6%). Concomitant Streptococcus pneumoniae coinfection was documented in 66 patients (22.5%).
According to the severity criteria previously defined, 60.2% of the patients presented with mild disease, 34.6% had moderate disease and in 5.1% of cases it was severe. During admission, 119 patients (41.9%) presented clinical worsening, of which 62% were moderate and 31% were severe. 41 patients (14%) required ICU admission with a mean stay of 26 ± 12 days. Of these patients, 23 (56%) required invasive mechanical ventilation, 11 (26.8%) required non-invasive mechanical ventilation, 2 patients (4.9%) required high-flow nasal cannula and 5 (12%) were managed with high-flow oxygen therapy. Mortality rate among patients admitted to the ICU was 41.5%. Clinical characteristics are displayed in Table 2 in more detail.
Table 2.
Clinical characteristics of COVID-19
| Characteristic | Value |
|---|---|
| Symptoms, no. (%) | |
| Fever | 235 (79.9) |
| Cough | 183 (62.2) |
| Dyspnea | 147 (50) |
| Myalgia | 106 (36.1) |
| Diarrhea | 45 (15.3) |
| Headache | 35 (11.9) |
| Anosmia | 32 (10.9) |
| Dysgeusia | 11 (3.7) |
| Ageusia | 9 (3.1) |
| Asthenia | 9 (3.1) |
| Odynophagia | 6 (2) |
| Initial severity, no. (%) | |
| Mild | 177 (60.2) |
| Moderate | 102 (34.7) |
| Severe | 15 (5.1) |
| Clinical progression, no. (%) | |
| Moderate | 74 (62) |
| Severe | 37 (31) |
| Initial radiological findings, no. (%) | |
| Chest X-ray infiltrate | 256 (87.1) |
| Distribution | |
| Bilateral | 196 (76.6) |
| Extension | |
| Multilobular | 132 (51.6) |
| Diffuse | 77 (30.1) |
| Initial ventilatory support no. (%) | |
| Nasal cannula | 43 (14.6) |
| Venturi oxygen therapy | 119 (40.5) |
| High-flow oxygen therapy | 13 (4.4) |
| High-flow nasal cannula | 1 (0.4) |
| Non-invasive mechanical ventilation | 3 (1) |
| Invasive mechanical ventilation | 3 (1) |
| Initial analytic parameters; mean (SD) | |
| Lymphocytic count | 208 (514) |
| D-Dimer | 1881 (5827) |
| C-reactive protein | 86 (90) |
| Length of hospital stay, days; mean (SD) | 30 (6) |
| Symptoms duration until admission, days; mean (SD) | 7.7 (2) |
Regarding treatment, initially 95% of patients received hydroxychloroquine for a mean of 18.7 days. Lopinavir/ritonavir treatment was prescribed in 90% of cases and it was administered concomitant treatment with a beta-lactam antibiotic or azithromycin in 95% of patients. 164 patients (56%) received glucocorticoids, most of them dexamethasone at a dose of 6 mg every 8 h for a mean of 10.3 days. Finally, 56 patients (19%) were treated with tocilizumab, all with a single dose except for 3 patients who received an additional dose.
A total of 34 patients (11.6%) were diagnosed with pulmonary embolism concomitant or after COVID-19 pneumonia and received anticoagulant treatment during a mean of 6 ± 2 months until the end of follow-up.
In-hospital mortality rate was 7%. Patients were followed up for a mean of 275.35 days.
Humoral response
IgM and IgG prevalence across time
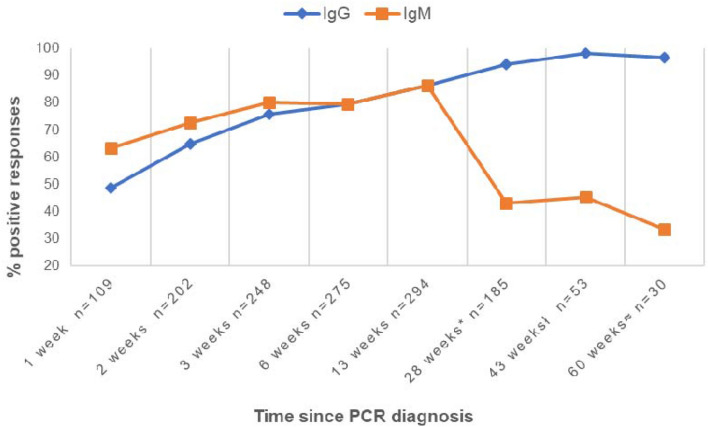
Results are shown in Fig. 1 regarding time since PCR diagnosis. Baseline determination was obtained in 109 patients with a mean of 6.2 days from admission (1 week), being IgM present in 63.3% of the cases and IgG in 48.6%. Regarding the second determination, it was performed in 202 patients with a mean of 16.4 days from admission (2 weeks), obtaining a prevalence of 72.7% for IgM and 64.85% for IgG. Third determination was performed in 248 patients with a mean of 21 days (3 weeks) showing 80.2% of IgM prevalence and 75.8% of IgG prevalence. Fourth determination was obtained in 275 patients with a mean of 45 days (6 weeks), revealing a prevalence of 79.3% for both IgM and IgG. As regards to the fifth determination, it was performed in 294 patients with a mean of 90 days (13 weeks), being IgM and IgG both present in 86.35% of the cases. The sixth determination was taken in 185 patients with a mean of 6.5 months (28 weeks) observing that IgM prevalence was 43.1% and IgG prevalence 94.1%. Among those who did not present seroconversion at 6 months (11 patients, 5.9%) there were 4 patients under immunosuppressive treatment and 1 patient with advanced neoplasm under no current treatment. Concerning seventh determination, it was obtained in 53 patients 10 months from the initial one (43 weeks) detecting IgM in 45.1% of the cases and IgG in 98.1%. At this point, the sample was significantly smaller because at the time some patients, especially those over 60, had already been vaccinated. As for the eighth determination, it was taken at 15 months (60 weeks) in a small group of 30 patients just before vaccination, evincing that IgM prevalence was 33.3%, whereas IgG prevalence was 96.7%. Finally, in a small group of 15 patients, a sample was obtained at 18 months, 2 months after vaccination. In this determination, IgM was still present in 28% of the group, while IgG was detectable in all the patients due to the vaccine. Moreover, the nucleocapsid antibody was also positive in 100% of the cases, meaning that humoral immunity given by infection was maintained.
Fig. 1.
IgM and IgG prevalence over time since PCR diagnosis in the study cohort. *Evaluated between 5.1 and 7.8 months (mean: 6.5 months ± 0.39); ł evaluated between 9 and 11 months (mean: 9.9 ± 0.27); ≈ evaluated between 13 and 15 months (mean 14.3 ± 0.47)
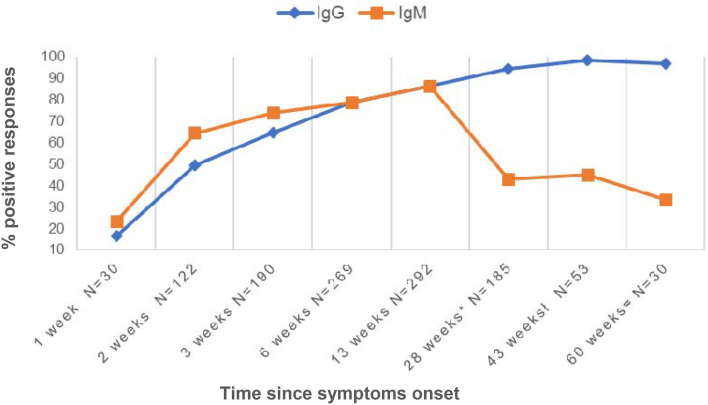
Humoral response regarding symptoms onset was also evaluated and it is represented in Fig. 2, finding a similar dynamic to that observed from PCR diagnosis.
Fig. 2.
IgM and IgG prevalence over time since symptoms onset in the study cohort. *Evaluated between 5.1 and 7.8 months (mean: 6.5 months ± 0.39); ł evaluated between 9 and 11 months (mean: 10.1 ± 0.33); ≈evaluated between 13 and 15 months (mean 14.6 ± 0.49)
Quantitative IgG dynamics
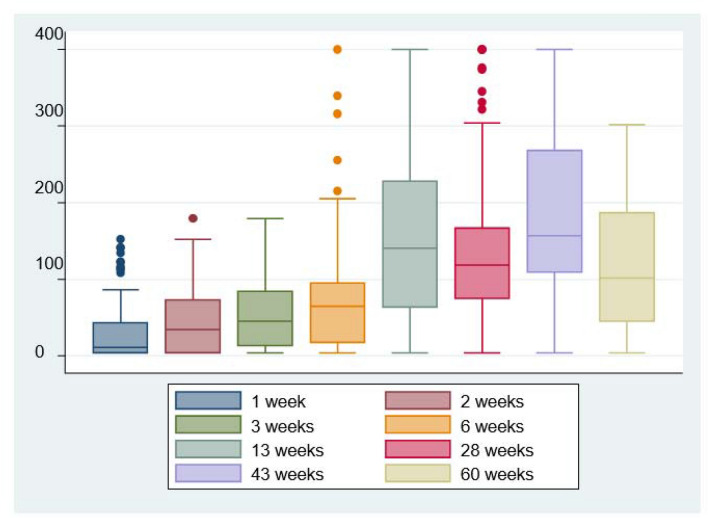
As for IgG quantification, it was demonstrated that patients maintained high immunoglobulin titers in all determinations throughout follow-up (Fig. 3). At first week, since PCR diagnosis, median (IQR 25–75%) IgG levels (UA/mL) were 11 (3.8–43.3), at second week 34.5 (3.8–72.6), at 13 weeks 140.5 (63–228), at 28 weeks (6 months) 118.5 (74.3–166.5), at 15 months just before vaccination patients still maintained mean quantitative levels of 158 (109–268) and, finally, at 18 months were 101.5 (45.5–185).
Fig. 3.
Quantitative IgG values expressed as UA/mL over time in the study cohort
Linear regression models with quantitative IgG along the study period only found statistical significance between IgG at 6 months regarding age (regression coefficient:0.92, CI 95:0.01–1.8; p = 0.05) and regarding length of hospital stay (regression coefficient: 1.5, CI 95: 0.4–2.6; p = 0.01). However, those associations did not longer persist when we performed multivariate analysis including worsening.
Clinical worsening determinants and antibody response
Determinants for clinical worsening in the cohort were analyzed. Results showed that male sex (p = 0.003), presence of myalgias (p = 0.005) and extensive initial radiological affectation including bilateral (p = 0.002), multilobar or diffuse infiltrates (p = 0.016) were significantly correlated with clinical worsening during hospitalization. In addition, when analyzing laboratory parameters, results showed a significant correlation between clinical worsening and lymphocytic count during hospitalization (p = 0.01), CRP at admission and during hospitalization (p < 0.001) and D-dimer peak value (p < 0.001. Table 3 includes all the variables analyzed that presented a p value ≤ 0.2 and mortality.
Table 3.
Determinants of clinical worsening
| Characteristic | Worsening N (%) | Not worsening N (%) | p value |
|---|---|---|---|
| Demographic characteristics | |||
| Agea | 62.6 (13.9) | 59.4 (17.9) | 0.09 |
| Male sex | 86 (69.9) | 90(52.6) | 0.003 |
| Former smokers | 26 (23) | 22 (13.2) | 0.10 |
| Comorbidities | |||
| Diabetes mellitus | 27 (22) | 24 (14.1) | 0.08 |
| Obesity | 21 (53.9) | 18 (46.2) | 0.12 |
| Symptoms | |||
| Dyspnea | 68 (46.6) | 78 (53.4) | 0.11 |
| Myalgias | 55 (45.1) | 49 (29) | 0.005 |
| Radiological findings | |||
| Bilateral infiltrates | 97 (85.8) | 98 (69.5) | 0.002 |
| Multilobar infiltrates | 61 (54) | 70 (49.7) | 0.02 |
| Diffuse infiltrates | 40 (35.4) | 37 (26.2) | 0.02 |
| Mortality | 10 (8.1) | 9 (5.3) | 0.32 |
| Analytic parametersb | |||
| Lymphocytic count (at admission) | (0.7–2) | 1.2 (0.8–2) | 0.13 |
| Lymphocytic count during hospitalization) | 1.0 (0.8–1.8) | 1.4 (0.9–2.2) | 0.01 |
| C-reactive protein [CRP (at admission) | 79.5 (37.5–155) | 39.8 (13–92) | < 0.001 |
| CRP (during hospitalization) | 94 (52–180) | 41.4 (18–99.6) | < 0.001 |
| D-Dimer (peak value) | 1576 (952–4724) | 1121.5 (546–1980) | < 0.001 |
aMean (SD)
bMedian (IQR 25–75%)
On the other hand, antibody response regarding clinical worsening during hospitalization was assessed in all determinations during follow-up.
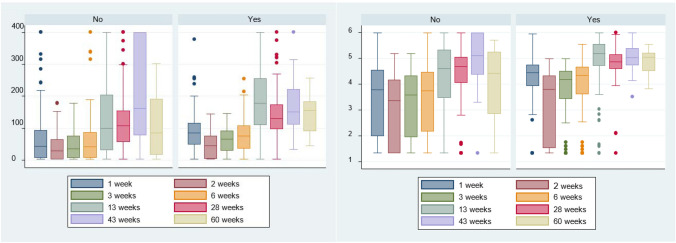
In relation to quantitative IgG dynamics, an upward trend up to 6 months was observed both in the group that had presented clinical worsening and the one that had not. Results for the first group at 6 months showed an absolute median of 130.5 (IQR 25–75%: 99.1–173) whereas second group presented an absolute median of 108 (IQR 25–75%: 157.7–154), with a p value of 0.001. As for logarithmic values, the group that had showed clinical progression presented a median of 4.8 (SD ± 0.67), while the second group presented a median of 4.4 (SD ± 1.1), with a p value of 0.0009. All groups maintained high IgG levels throughout follow-up although at 15 months a slight decrease in the immunoglobulin titer in the group that had not presented clinical progression was observed, without statistically significant differences. All absolute quantitative values and logarithmic values are shown in Fig. 4a, b.
Fig. 4.
a (left) Absolute IgG values according to clinical worsening (no/yes) since diagnosis of SARS-CoV-2 infection by RT-PCR. b (right) Logarithm of IgG response according to clinical worsening (no/yes) since diagnosis of SARS-CoV-2 infection by RT-PCR
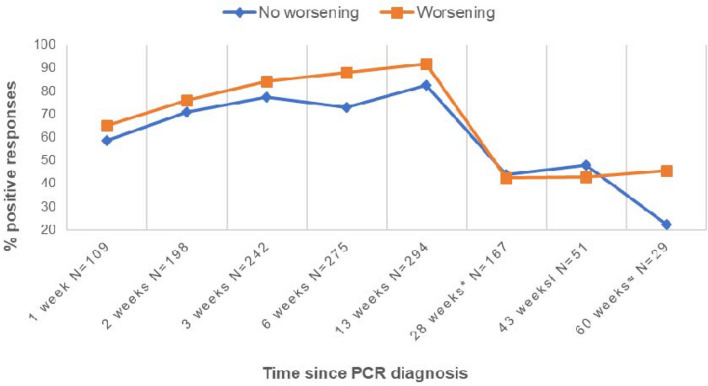
With respect to IgM prevalence related to clinical worsening, statistical analysis proved that at 45 days (clinical worsening group 87.9% vs not worsening group 73%; p value = 0.002) and 90 days (clinical worsening group 91.9% vs not worsening group 82.3%; p value = 0.02) patients who had presented clinical progression showed higher IgM prevalence, being the difference statistically significant. A correlation between IgM prevalence and clinical worsening was dismissed afterwards. Results are shown in Fig. 5.
Fig. 5.
IgM prevalence with regard to clinical worsening during hospitalization. *Evaluated between 5.1 and 7.8 months (mean: 6.5 months ± 0.39); ł evaluated between 9 and 11 months (mean: 9.9 ± 0.27); ≈ evaluated between 13 and 15 months (mean 14.3 ± 0.47)
With respect to IgM prevalence related to clinical worsening, univariate statistical analysis proved that at 45 days (clinical worsening group 87.9% vs not worsening group 73%; p value = 0.002) and 90 days (clinical worsening group 91.9% vs not worsening group 82.3%; p value = 0.02) patients who had presented clinical progression showed higher IgM prevalence, being the difference statistically significant. No association was found between IgM prevalence and clinical worsening for the rest of the follow-up. Results are shown in Fig. 5.
Discussion
Our study characterized the dynamics of serum IgM and IgG antibodies against SARS-CoV-2 and its behavior related to clinical progression by conducting a long follow-up in a large cohort of hospitalized patients due to COVID-19 pneumonia. Two important findings have emerged from the present study. First, IgG response to COVID-19 pneumonia is strong and long-lasting. Some studies have already shown that serological response is maintained up to 1 year after SARS-CoV-2 infection [24, 25]. In the present study that only includes patients with pneumonia, we detected high IgG titers in most patients during the 18-month follow-up. Second, patients who deteriorated during the course of infection seem to present a more robust antibody response. These patients showed higher IgG titers during the first 6 months compared to patients who did not show clinical worsening and who presented a slight decline in immunoglobulin levels at 15 months. According to regression analyses, at 6 months IgG titers were lower in elderly patients and those with a short in-hospital stay, but these differences did not persist when performing a multivariate analysis including clinical worsening. This highlights the fact that clinical worsening is one of the most important predictors of humoral response. Furthermore, in our study, we found that male sex, presence of myalgias and extensive radiological affectation as well as lymphocytic count and inflammation parameters (CRP and D-dimer) are significantly correlated with clinical worsening during hospitalization.
On the other hand, results exhibit that IgM and IgG antibodies against SARS-CoV-2 can be detected shortly after symptoms onset, with an IgM response clearly earlier than IgG response, as expected. The IgG assay’s sensitivity increases significantly as the immune response matures. Likewise, in other studies, less than 3% of patients (most of them immunocompromised) did not seroconvert during follow-up.
In most studies, IgM response appears to decrease steadily from 4 to 6 weeks after symptom onset [26]. In our study, clinical worsening correlated with higher IgM prevalence up to 90 days after diagnosis; afterwards, a small proportion of patients showed IgM positivity more than 1 year after infection, despite any clinical correlation. This could suggest an inability to class switch efficiently depending upon the individual patient or it could be related to inflammation levels as it has been previously described in other viral infections such as cytomegalovirus and Epstein–Barr [27, 28].
Regarding IgG response, it is remarkable that all 15 patients who were not vaccinated and could be tested at 18 months presented positivity for antibody nucleocapsid, which suggests that humoral immunity due to infection is long-lasting. In addition, this group showed a strong humoral immune response after vaccination, as it has been reported before [29].
Overall, cohort characteristics regarding age, gender and comorbidities are consistent with the literature [9, 13]. The pneumococcal coinfection rate, also noteworthy, has been reported broadly albeit with highly variable prevalence depending on patient’s characteristics as well as diagnostic technique [30].
Most of our data are for adult populations who are not immunocompromised. The time course of PCR positivity and seroconversion may vary in this population and other groups, such as children and asymptomatic individuals who go undiagnosed without active surveillance [26]. Therefore, our results must be interpreted with caution.
An important limitation of our work is the decrease in the sample size throughout the study due to loss to follow-up, but especially due to vaccination. In addition, the difficult organization of the first weeks of the pandemic meant that despite the existing protocol, some first samples were not obtained. However, we corroborated that there were no significant differences when performing the statistical analyses only with the patients that had a first sample and, hence, we finally decided to include all the patients. On the other hand, standards of laboratory techniques have been changing during the pandemics, but in our study, we used the same technique and cutoff points in all determinations. Although this has allowed us to easily compare results from different determinations, it makes the extrapolation of our data to other populations more complex.
In summary, humoral response due to COVID-19 pneumonia appears to be maintained and long-lasting, mostly in the case of IgG. In addition, clinical worsening seems to be correlated with sex, presence of myalgias and extensive radiological affectation, and IgG response seems to be more robust in patients who presented clinical worsening during the course of the disease. Nevertheless, the emergence of variants of concern has challenged protection from natural infection as well as from vaccination. Recent studies prove that the different vaccines developed provide an effective protection limited in time and only for some variants of concern [31]. Further studies are needed in order to fully understand the global immune response to this new pathogen and help design the best possible treatment and immunization strategy.
Acknowledgements
COMUTE Study Group Collaborators: Beatriz Dietl, Lucía Boix-Palop, Lucía Gómez, Mireia Cairó, Esther Calbo, Josep Trenado, Laura M. Gisbert, Komal Malik, Cristina Castrillo, Lluis Simón-Pascua, Maria José de la Asunción, Tere Moreno-López, Oriol Llargués, Aina Mateu, Franklyn Ferney Meza, Ginebra Libori-Roch, Siena Molina, David Clemente, and Ana Martínez-Urrea.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Declarations
Conflict of interest
The authors declare that there are no competing interests.
Ethical approval
The study was approved by the Ethics and Clinical trials Committee of our hospital and complies with data protection laws.
Informed consent
Verbal informed consent was obtained from all the participants and recorded in their medical history.
Human and animal rights
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Fundació Mútua Terrassa.
Footnotes
The members of "COMUTE Study Group (COVID-19 Mutua Terrassa Study Group)" are mentioned in acknowledgement section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gemma Grau Gómez, Email: gemma.grau27@gmail.com.
COMUTE Study Group (COVID-19 Mutua Terrassa Study Group):
Beatriz Dietl, Lucía Boix-Palop, Lucía Gómez, Mireia Cairó, Esther Calbo, Josep Trenado, Laura M. Gisbert, Komal Malik, Cristina Castrillo, Lluis Simón-Pascua, Maria José de la Asunción, Tere Moreno-López, Oriol Llargués, Aina Mateu, Franklyn Ferney Meza, Ginebra Libori-Roch, Siena Molina, David Clemente, and Ana Martínez-Urrea
References
- 1.Louie JK, Hacker JK, Mark J, et al. SARS and common viral infections. Emerging Infect Dis. 2004;10:1143–1146. doi: 10.3201/eid1006.030863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peeling RW, Heymann DL, Teo YY, et al. Diagnostics for COVID-19: moving from pandemic response to control. Lancet. 2022;399:757–768. doi: 10.1016/S0140-6736(21)02346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;296:115–117. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:32–40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burrell CJ, Howard CR, Murphy FA. Laboratory diagnosis of virus diseases. Fenner and White’s Med Virol. 2017;1:135–154. doi: 10.1016/B978-0-12-375156-0.00010-2. [DOI] [Google Scholar]
- 6.Castro X, Ols S, Karin L, et al. Immunity to SARS-CoV-2 induced by infection or vaccination. J Inter Med. 2022;291(1):32–50. doi: 10.1111/joim.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C. Pathogenesis of coronaviruses through human monocytes and tissue macrophages. Viral Immunol. 2021;34(9):597–606. doi: 10.1089/vim.2021.0038. [DOI] [PubMed] [Google Scholar]
- 8.Arabi YM, Hajeer AH, Balkhy H, et al. Kinetics of antibody response in critically ill patients with Middle East respiratory syndrome and association with mortality and viral clearance. Sci Rep. 2021;11(1):22548. doi: 10.1038/s41598-021-01083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Abellán J, Padilla S, Fernández-González M, et al. Antibody response to SARS-CoV-2 is associated with long-term clinical outcome in patients with COVID-19: a longitudinal study. J Clin Immunol. 2021;41(7):1490–1501. doi: 10.1007/s10875-021-01083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shang Y, Liu T, Li J, et al. Factors affecting antibody response to SARS-CoV-2 in patients with severe COVID-19. J Med Virol. 2021;93(2):612–614. doi: 10.1002/jmv.26379. [DOI] [PubMed] [Google Scholar]
- 11.Han Y, Liu P, Qiu Y. Effective virus-neutralizing activities in antisera from the first wave of survivors of severe COVID-19. JCI Insight. 2021;6(4):e146267. doi: 10.1172/jci.insight.146267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YL, Liao CH, Liu PY, et al. Dynamics of anti-SARS-Cov-2 IgM and IgG antibodies among COVID-19 patients. J Infect. 2020;81(2):e55–e58. doi: 10.1016/j.jinf.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legros V, Denolly S, Vogrig M. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021;18:318–327. doi: 10.1038/s41423-020-00588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeewandara C, Jayathilaka D, Gomes L, et al. SARS-CoV-2 neutralizing antibodies in patients with varying severity of acute COVID-19 illness. Sci Rep. 2021;11:2062. doi: 10.1038/s41598-021-81629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varona JF, Madurga R, Penalver F, et al. kinetics of anti-SARS-CoV-2 antibodies over time. Results of 10 month follow up in over 300 seropositive health care workers. Eur J Intern Med. 2021;89:97–103. doi: 10.1016/j.ejim.2021.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellerstein M. What are the roles of antibodies versus a durable, high quality T cell response in protective immunity against SARS-CoV-2? Vaccine X. 2020;6:100076. doi: 10.1016/j.jvacx.2020.100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lumley SF, Wei J, O'Donnell D, et al. The duration, dynamics and determinants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody responses in individual healthcare workers. Clin Infect Dis. 2021;73(3):e699–e709. doi: 10.1093/cid/ciab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anichini G, Gandolfo C, Terrosi C, et al. Antibody response to SARS-CoV-2 in infected patients with different clinical outcome. J Med Virol. 2021;93(4):2548–2552. doi: 10.1002/jmv.26789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagunas-Rangel FA, Chávez-Valencia V. What do we know about the antibody responses to SARS-CoV-2? Immunobiology. 2021;226(2):152054. doi: 10.1016/j.imbio.2021.152054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chvatal-Medina M, Mendez-Cortina Y, Patiño PJ, et al. Antibody responses in COVID-19: a review. Front Immunol. 2021;12:633184. doi: 10.3389/fimmu.2021.633184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tea F, Ospina Stella A, Aggarwal A, et al. SARS-CoV-2 neutralizing antibodies: Longevity, breadth, and evasion by emerging viral variants. PLoS Med. 2021;18(7):e1003656. doi: 10.1371/journal.pmed.1003656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plebani M, Padoan A, Negrini D, et al. Diagnostic performances and thresholds: the key to harmonization in serological SARS-CoV-2 assays? Clin Chim Acta. 2020;509:1–7. doi: 10.1016/j.cca.2020.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonelli F, Sarasini A, Zierold C, et al. Clinical and analytical performance of an automated serological test that identifies S1/S2-neutralizing IgG in COVID-19 patients semi quantitatively. J Clin Microbiol. 2020;58(9):e01224–e1320. doi: 10.1128/JCM.01224-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yun J, Yang JS, Young S, et al. Duration of humoral immunity and cross-neutralizing activity against the alpha, beta, and delta variants after wild-type severe acute respiratory syndrome coronavirus 2 infection: a prospective cohort study. J Infect Dis. 2022 doi: 10.1093/infdis/jiac050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi D, Weng T, Wu J, et al. Dynamic characteristic analysis of antibodies in patients with COVID-19: a 13-month study. Front Immunol. 2021;12:708184. doi: 10.3389/fimmu.2021.708184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundararaj SJ, Nandini S, Akihide R. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323(22):2249–2251. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 27.Evans AS, Niederman JC, Cenabre LC, et al. A prospective evaluation of heterophile and Epstein-Barr virus-specific IgM antibody tests in clinical and subclinical infectious mononucleosis: specificity and sensitivity of the tests and persistence of antibody. J Infect Dis. 1975;132:546–554. doi: 10.1093/infdis/132.5.546. [DOI] [PubMed] [Google Scholar]
- 28.Torii Y, Yoshida S, Yanase Y, et al. Serological screening of immunoglobulin M and immunoglobulin G during pregnancy for predicting congenital cytomegalovirus infection. BMC Pregnancy Childbirth. 2019;19:205. doi: 10.1186/s12884-019-2360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrari D, Di Resta C, Tomaiuolo R, et al. Long-term antibody persistence and exceptional vaccination response on previously SARS-CoV-2 infected subjects. Vaccine. 2021;39(31):4256–4260. doi: 10.1016/j.vaccine.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sreenath K, Batra P, Vinayaraj EV, et al. Coinfections with other respiratory pathogens among patients with COVID-19. ASM J. 2021;9(1):ee00163–ee221. doi: 10.1128/Spectrum.00163-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fei G, Meng W, Chen L, et al. Neutralizing antibodies to SARS-CoV-2 variants of concern including Delta and Omicron in subjects receiving mRNA-1273, BNT162b2, and Ad26.COV2.S vaccines. J Med Virol. 2022;94:5678–5690. doi: 10.1002/jmv.28032. [DOI] [PMC free article] [PubMed] [Google Scholar]