ABSTRACT
Mycobacteria use specialized type VII secretion systems (T7SSs) to secrete proteins across their diderm cell envelope. One of the T7SS subtypes, named ESX-1, is a major virulence determinant in pathogenic species such as Mycobacterium tuberculosis and the fish pathogen Mycobacterium marinum. ESX-1 secretes a variety of substrates, called Esx, PE, PPE, and Esp proteins, at least some of which are folded heterodimers. Investigation into the functions of these substrates is problematic, because of the intricate network of codependent secretion between several ESX-1 substrates. Here, we describe the ESX-1 substrate PPE68 as essential for secretion of the highly immunogenic substrates EsxA and EspE via the ESX-1 system in M. marinum. While secreted PPE68 is processed on the cell surface, the majority of cell-associated PPE68 of M. marinum and M. tuberculosis is present in a cytosolic complex with its PE partner and the EspG1 chaperone. Interfering with the binding of EspG1 to PPE68 blocked its export and the secretion of EsxA and EspE. In contrast, esxA was not required for the secretion of PPE68, revealing a hierarchy in codependent secretion. Remarkably, the final 10 residues of PPE68, a negatively charged domain, seem essential for EspE secretion, but not for the secretion of EsxA and of PPE68 itself. This indicates that distinctive domains of PPE68 are involved in secretion of the different ESX-1 substrates. Based on these findings, we propose a mechanistic model for the central role of PPE68 in ESX-1-mediated secretion and substrate codependence.
KEYWORDS: ESX-1, EsxA, Mycobacterium, PPE, chaperones, protein transport, protein-protein interactions, tuberculosis, type VII secretion
INTRODUCTION
The genus Mycobacterium belongs to the phylum Actinobacteria and includes the major human pathogens Mycobacterium tuberculosis and Mycobacterium leprae. Mycobacteria have a distinctive cell envelope that is shared with other bacteria within the order Corynebacteriales. The unique feature of their cell envelope is the long-chained fatty acids, called mycolic acids, that assemble into a second hydrophobic bilayer, also known as the mycobacterial outer membrane (1, 2). Mycobacteria have acquired specialized secretion systems, called type VII secretion systems (T7SSs), to export proteins across this multilayered cell envelope. There are five paralogous T7SSs present in M. tuberculosis, called ESX-1 to ESX-5. The ESX-1 system is considered a major virulence factor of M. tuberculosis and the fish pathogen Mycobacterium marinum through its essential role in phagosomal rupture and subsequent translocation of the pathogen to the host cytosol (3–6). As a consequence, the absence of the esx-1 region results in severe attenuation of pathogenic mycobacteria, which is further illustrated by the observation that a large deletion within the same region (RD1) is the prime cause of the attenuation of the live vaccine strain Mycobacterium bovis BCG (7–9).
The ESX-1 system secretes over a dozen different proteins, which all belong to the EsxAB clan (Pfam CL0352) and can be categorized into four distinctive protein families, i.e., Esx/WxG100, PE, PPE, and Esp. The best-studied and highly immunogenic ESX-1 substrates EsxA and EsxB belong to the Esx/WxG100 protein family, which are characterized by a WxG protein motif and generally are 100 amino acids long. PE35 and PPE68 are encoded within the same operon as esxA and esxB and belong to PE and PPE protein families that share homologous N-terminal domains of approximately 110 amino acids and 180 amino acids, respectively, with conserved proline-glutamic acid (PE) and proline-proline-glutamic acid (PPE) motifs (10). While each T7SS secretes its own Esx proteins and often also PE and PPE substrates, the Esp proteins seem to be exclusively secreted by the ESX-1 system.
An intriguing feature of the mycobacterial T7SS substrates is that these proteins are secreted as folded heterodimers that are formed in the cytosol (11–14). While these heterodimers are formed by either Esx substrates or a PE protein with a specific PPE protein, available crystal structures reveal highly conserved features. Each complex forms a four-helix bundle, composed of two helix-turn-helix structures in an antiparallel orientation, with a conserved secretion motif (YxxxD/E) extending from the double-helix of one partner protein (15). In addition to the helix-turn-helix, the N-terminal domain of PPE proteins contains an extra region that is relatively hydrophobic and extends from the PE-PPE interface (16). This so-called helical tip is recognized by a cytosolic chaperone, called EspG, in an ESX system-specific manner, recognition of which is required for secretion of the PE-PPE pair (12, 13, 17). In addition to the conserved dimeric structure formed by the N-terminal PE and PPE domains, which is thought to be involved in substrate recognition and secretion of the heterodimers, many PE and PPE proteins have highly variable C-terminal domains that could potentially execute specific functions (18–20). Only limited structural information is available for the Esp substrates, although structure predictions indicate that most of these substrates form similar heterodimers as PE and PPE proteins (21–23).
EsxA has been indicated to play a central role in ESX-1-mediated virulence, including the perturbation of phagosomal membranes (3, 4, 6, 24–26). However, while in vitro biochemical analyses suggest that EsxA has membranolytic activity (25, 27–31), later reports have ascribed at least some of these observations to detergent contaminations during the purification of EsxA (30, 32). Another complicating factor is that mutations in esxA abolish the secretion of other ESX-1 substrates, such as EspA, EspF, EspJ, EspK, and EspB in M. tuberculosis and/or M. marinum (33–35). In numerous cases, the codependence for secretion is mutual, as the deletion of specific esp genes also results in abolished EsxA secretion (34, 36–39). This complicates the analysis and interpretation of the role of individual ESX-1 substrates in virulence.
Codependent secretion has also been observed for substrates of other ESX systems (40–42). The underlying reason for this phenomenon, however, remains unclear. Previously, we provided the first mechanistic insight into this process by the successful redirection of PE35_1/PPE68_1, the M. marinum paralogs of the esx-1 locus-encoded PE35/PPE68, from the ESX-1 to the ESX-5 system (43, 44). Surprisingly, the redirection of these two ESX-1 substrates also resulted in redirection of EsxA_1. This finding suggested a central role of the PE-PPE-EspG complex in the secretion of Esx substrates. Interestingly, deletion of espG1 results in a general secretion defect not only of respective PE/PPE and Esx substrates in M. marinum but also of Esp proteins (24, 33, 35, 39, 45, 46), indicating a central role for PE/PPE substrates in the secretion of all known ESX substrate classes. Here, we investigated the role of PPE68 in ESX-1-mediated secretion of EsxA and EspE in M. marinum, and we report that this PPE protein has a central function in the secretion of these two ESX-1 substrates.
RESULTS
The pe35/ppe68 pair is necessary for secretion of ESX-1 substrates EsxA and EspE and for ESX-1-mediated hemolysis.
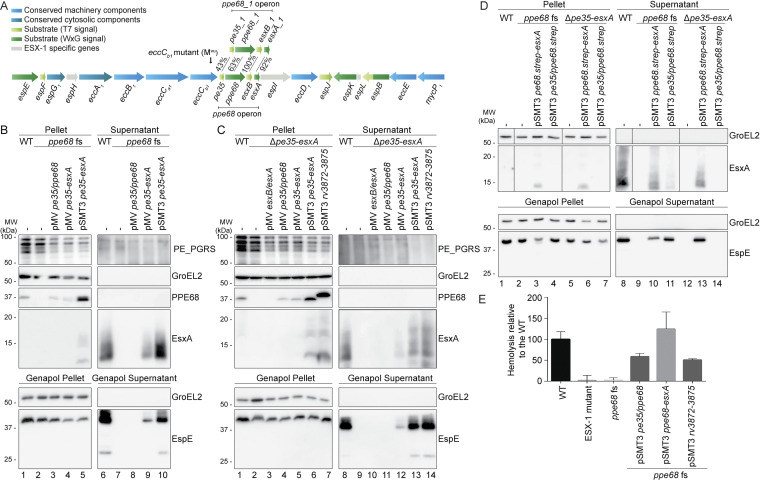
To study the effect of PPE68 on protein secretion, we created a frameshift mutation in M. marinum M to minimize polar effects, using the efficient genome editing tool CRISPR1-Cas9 (47). The generated ppe68 frameshift (fs) mutant that we selected contained a 1-bp insertion at position 181, which resulted in an early stop codon after amino acid 202 (see Fig. S1A in the supplemental material). As expected, PPE68 could not be detected in the pellet or supernatant fraction of this mutant (Fig. 1B, lanes 2 and 7). In the wild-type (WT) strain, we could identify PPE68 in the pellet fraction but not in the supernatant (Fig. 1B, lanes 1 and 6), which is in line with the observations in M. tuberculosis (48). The ppe68 mutant showed a loss of EsxA in the supernatant fraction, while no accumulation of EsxA in the pellet fraction was observed (Fig. 1B, lanes 2 and 7). Similarly, no EsxA accumulation was observed in the cell pellet of an eccCb1 core complex mutant (Fig. S1C, lane 2), an observation that has previously been linked to downregulated expression of esxA in the presence of an inactive ESX-1 secretion system by a WhiB6-controlled negative feedback mechanism (49, 50). In agreement, we observed that mRNA levels of esxA in the ppe68 fs mutant was reduced to a comparable level as in the eccCb1 mutant (Fig. S1D). Next, we analyzed the secretion of ESX-1 substrate EspE, which is an abundant cell surface protein of M. marinum and can be extracted by the mild detergent Genapol X-080 (51, 52). The ppe68 fs mutation prevented the secretion of EspE to the cell surface, while the intracellular levels of EspE in the mutant and the WT were comparable (Fig. 1B, lanes 2 and 7). Complementation of the ppe68 fs mutant with the pe35/ppe68 pair, expressed from an integrative pMV vector, failed to restore detectable EspE and EsxA secretion (Fig. 1B, lane 8), while the introduction of the complete ppe68 operon, encompassing pe35, ppe68, esxB, and esxA, using either the pMV or multicopy pSMT3 vector, restored EspE and EsxA secretion (Fig. 1B, lanes 9 and 10). Importantly, expression of pe35/ppe68 from the pSMT3 vector, with a Strep tag at the C terminus of PPE68 for detection purposes, also fully restored EspE secretion, while EsxA was secreted at lower levels than in the WT (Fig. 1D, lane 11). Notably, a similarly reduced amount of esxA transcripts was observed in this latter complementation strain as in the ppe68 fs mutant (Fig. S1D), showing that these mRNA levels were sufficient for EsxA secretion and therefore that downregulated transcription was not the main cause for the absence of EsxA in the culture supernatant of the mutant.
FIG 1.
pe35/ppe68 is involved in the secretion of ESX-1 substrates EsxA and EspE, and is required for ESX-1 mediated hemolysis. A) Schematic overview of the esx-1 gene cluster and its paralogous region. Genes are color-coded to their subcellular localization (key color). Notably, the pe gene upstream of ppe68_1 was originally named pe35, while the paralogous gene upstream of ppe68 in the esx-1 locus was named mmar_5447. In this study, we renamed these two genes pe35_1 and pe35, respectively. B, C, D) SDS-PAGE and immunostaining of the cell pellet, culture supernatant, Genapol pellet and Genapol supernatant fractions of M. marinum M wild type (WT) and the ppe68 fs mutant without and with various complementation constructs (B, D) or WT and the ppe68 operon mutant (Δpe35-esxA) with and without various complementation constructs (C, D). Genes were expressed from an integrative pMV vector or a multicopy pSMT3 vector under control of the hsp60 promotor, and for D) with a Strep-tag on the C-terminus of PPE68. In B and D) the WT, ppe68 fs mutant and the ppe68 fs mutant containing a pSMT3 vector also contained a pMV TdTomato plasmid. Proteins were visualized with anti-EsxA, anti-EspE, and anti-PPE68 antibodies (ESX-1 substrates). A processed band of 25 kDa was detected by the anti-EspE antibody in the Genapol supernatant fraction of the WT, which has been reported before (46). As a loading and lysis control, blots were incubated with antibodies directed against the cytosolic GroEL2 protein and secreted PE_PGRS proteins (ESX-5 substrates). In all blots, equivalent OD units were loaded: 0.2 OD for pellet and Genapol pellet and 0.4 OD for supernatant and Genapol supernatant fractions (B, C) or 0.2 OD for supernatant fractions and 2 OD for Genapol supernatant fractions (D). Data shown are representative of three independent experiments. E) Hemoglobin release after incubation of sheep red blood cells with M. marinum WT, an ESX-1 mutant (eccCb1 mutant; MVU), the ppe68 fs mutant, and the ppe68 fs mutant complemented with either the entire ppe68 operon of M. marinum, with only pe35/ppe68, or with the ppe68 operon of M. tuberculosis (rv3872-rv3875). Hemoglobin release was quantified by determining the OD450 of the medium after incubation. Data shown are from two independent experiments including 3 technical replicates each.
Generation and confirmation of M. marinum frameshift mutants, including phenotypic analysis of the ppe68 fs mutant. (A, B) Each panel shows the sgRNA binding region within the gene, i.e. in ppe68 (A) and mmar_2894 (B), with a panel underneath showing the sequencing results that were obtained after PCR. (C) SDS-PAGE and immunostaining of the cell pellet fraction of M. marinum WT, the eccCb1 mutant (MVU – ESX-1 mutant), the ppe68 fs mutant and the mmar_2894 fs mutant. The ppe68 operon mutant (Δpe35-esxA) with and without the ppe68 operon expressed from a multicopy pSMT3 vector under control of the hsp60 promotor served as the positive and negative control for EsxA production, respectively. The ppe68 fs mutant and mmar_2894 fs mutant also contained a pMV TdTomato plasmid. Proteins were visualized with an anti-EsxA antibody, and as a loading control, blots were incubated with antibodies directed against the cytosolic GroEL2 protein. An amount of 0.2 OD units was loaded for the pellet fraction. Data are representative of two independent experiments. (D) Relative esxA mRNA levels of an eccCb1 mutant (MVU, ESX-1 mutant), the ppe68 fs mutant, and the ppe68 fs mutant complemented with pe35/ppe68 expressed from the pMST3 vector, compared to the WT. Cycle threshold (CT) values were normalized for CT values of the household gene sigA and compared to the other CT values. Data shown are from two independent experiments including 3 technical replicates each. Download FIG S1, TIF file, 1.5 MB (1.6MB, tif) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To investigate the individual roles of pe35/ppe68 and esxB/esxA in ESX-1-mediated secretion, we created an M. marinum knockout strain that contained a deletion of the complete pe35-ppe68-esxA-esxB operon (Fig. 1A). Again, no EspE was detected on the cell surface of the ppe68 operon mutant, while the intracellular EspE levels were comparable to those in the WT (Fig. 1C, lanes 2 and 9). Introduction of either esxB/esxA or pe35/ppe68 on the integrative pMV vector, or for pe35/ppe68, also on the pSMT3 plasmid, failed to restore EspE and EsxA secretion (Fig. 1C, lanes 10 and 11, and Fig. 1D, lane 14). EspE and EsxA secretion was only restored when the entire ppe68 operon was present on the introduced pMV or pSMT3 vector (Fig. 1C, lanes 12 and 13). Expression of the entire ppe68 operon from the pSMT3 vector resulted in secretion levels similar to those in the WT. In addition, the ppe68 operon of M. tuberculosis was also able to restore EspE and EsxA secretion by the ppe68 operon mutant, showing that the function of the ppe68 operon in ESX-1 secretion was conserved (Fig. 1C, lane 14).
Next, we investigated the impact of the ppe68 fs mutation on ESX-1-mediated membrane lysis as an important readout for ESX-1-mediated virulence mechanisms (24). As expected, while WT M. marinum lysed sheep red bloods cells efficiently, the eccCb1 mutant showed no effect above background levels (Fig. 1E). Also, the ppe68 fs mutant completely lost its hemolytic capabilities, which could be fully complemented by the introduction of the ppe68 operon. Introduction of the pe35/ppe68 pair on a pSMT3 plasmid and of the ppe68 operon of M. tuberculosis partially restored hemolysis (Fig. 1E). In conclusion, ppe68 is involved in the hemolytic activity of M. marinum and the secretion of EsxA and EspE, a role that is conserved between the M. marinum and M. tuberculosis protein.
The EspG1-binding domain of PPE68 is important for its central role in ESX-1 secretion.
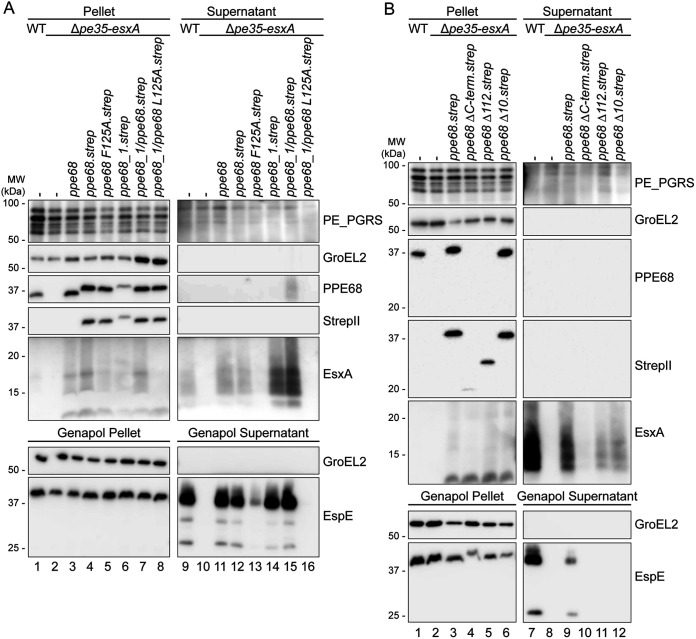
Previously, we reported that the EspG1-binding domain of PPE68_1 (MMAR_0186) is essential for secretion via the ESX-1 system (44). Specifically, a single amino acid substitution at position 125, located within this domain, abrogated PPE68_1 secretion to the culture supernatant without affecting its production. Similarly, the conserved amino acid in an ESX-5-dependent PPE protein (L125) was essential for EspG5 binding and its secretion (13). To investigate the role of EspG1 binding to PPE68, an equivalent F to A amino acid change at position 125 (F125A) was introduced in the ppe68 operon on the pSMT3 vector, again with a Strep tag to the C terminus of PPE68 for detection purposes. Immunoblot analysis showed that PPE68.strep was properly produced and, as expected, ran slightly higher on an SDS-PAGE gel (Fig. 2A, lane 4). In addition, the level of EsxA and EspE secretion by the complemented ppe68 operon mutant was not affected by the Strep tag (Fig. 2A, lane 12). While the F125A substitution did not affect the stability of this PPE68 variant (Fig. 2A, lane 5), it could not restore WT levels of EsxA secretion and showed severely reduced EspE secretion in the ppe68 operon mutant (Fig. 2A, lane 13). Notably, unlike for the ppe68 fs mutant, EsxA could be detected in the bacterial pellet, showing that this protein is stably produced from the PPE68 F125A operon plasmid. This result indicated that recognition of PPE68 by EspG1 is indeed important for its role in ESX-1 secretion.
FIG 2.
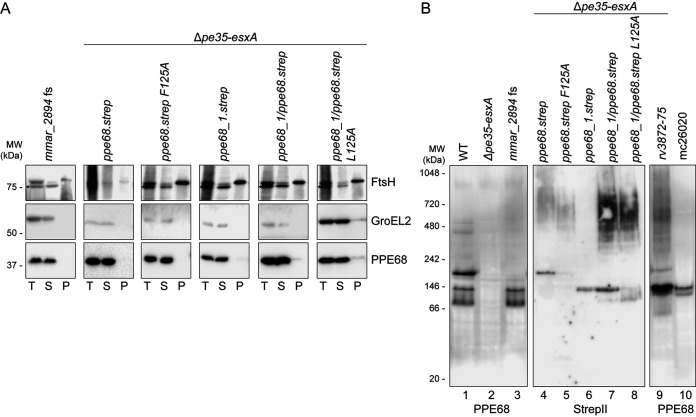
Conserved features of PPE68 are important for its central role in ESX-1 secretion. (A) Role of the EspG1-binding domain of PPE68. SDS-PAGE and immunostaining of the cell pellet, culture supernatant, Genapol pellet, and Genapol supernatant fractions of M. marinum WT, the ppe68 operon mutant (Δpe35-esxA), and the ppe68 operon mutant complemented with either the ppe68 operon, the ppe68_1 operon, or a hybrid operon containing pe35_1, the N-terminal domain of ppe68_1, or the C-terminal domain of ppe68, esxB, and esxA. Single amino acid substitutions were made in the helical tip of PPE68 and the PPE68_1/PPE68 hybrid to investigate the role of the EspG1-binding domain. (B) The role of the C-terminal domain of PPE68. SDS-PAGE and immunostaining of the cell pellet, culture supernatant, Genapol pellet, and Genapol supernatant fractions of M. marinum WT, the ppe68 operon mutant (Δpe35-esxA), and the ppe68 operon mutant complemented with either the WT or ppe68 operon variants, encoding PPE68 lacking either the entire (PPE68ΔC-term) or partial, i.e., the final 112 (PPE68Δ112) or 10 (PPE68Δ10) amino acids, of the C-terminal domain. In both panels A and B, the operons were expressed from a multicopy pSMT3 vector under control by the hsp60 promoter and with a Strep tag on the C terminus of PPE68 and its variants. Proteins were visualized with anti-EsxA, anti-EspE, anti-StrepII (PPE68), and anti-PPE68 antibodies (ESX-1 substrates). A processed band of 25 kDa was detected by the anti-EspE antibody in the Genapol supernatant fraction of the WT, which has been reported before (Phan et al., 2018 [46]). As a loading and lysis control, blots were incubated with antibodies directed against the cytosolic GroEL2 protein and secreted PE_PGRS proteins (ESX-5 substrates). In all blots, equivalent OD units were loaded: 0.2 OD for pellet and Genapol pellet and 0.4 OD for supernatant and Genapol supernatant fractions. Data shown in each panel are representative of three independent experiments.
As we were unable to directly assess the secretion of PPE68, but previously could observe secretion of PPE68_1 (43, 44), we further investigated the paralogous ppe68_1 operon, consisting of pe35_1, ppe68_1, esxB_1, and esxA_1 (Fig. 1A). While esxB_1 and esxA_1 were almost identical to their paralogs in the esx-1 gene cluster, pe35_1 and ppe68_1 showed less similarity, particularly for the C-terminal domains of the PPE proteins (Fig. 1A and Fig. S2). Notably, deletion of this four-gene paralogous region did not affect EspE secretion nor the hemolytic activity of M. marinum, showing that these endogenous genes are dispensable for these specific ESX-1-mediated processes (Fig. S3). To assess the importance of the C-terminal domain of the PPE68 paralogs in ESX-1 secretion, we constructed a pSMT3 plasmid containing the ppe68_1 operon (mmar_0185-88) and a ppe68/ppe68_1 hybrid operon construct, containing pe35_1, the N-terminal domain of ppe68_1 (PPE domain, residues 1 to 175) (Fig. S2), the C-terminal domain of ppe68 (encoding residues 176 to 367), esxA, and esxB. Again, a Strep tag was introduced to the C terminus of the PPE variants for detection purposes. Surprisingly, both the ppe68_1 operon and the hybrid operon reestablished EsxA and EspE secretion in the ppe68 operon mutant (Fig. 2A, lanes 14 and 15), while the ppe68_1 operon was also able to restore hemolytic activity in the ppe68 fs mutant (Fig. S3B). These results showed that the paralogous but divergent PPE68_1 is functionally similar to the PPE68 with regard to their role ESX-1 secretion. However, the fact that deleting the paralogous PPE68_1 region did not impact ESX-1 secretion indicated that this region was not expressed under the conditions used. Notably, while some small amounts of hemagglutinin (HA)- or FLAG-tagged PPE68_1 could previously be detected in culture supernatants of M. marinum M (43, 44), PPE68_1 with a Strep tag could not clearly be detected in this fraction (Fig. 2A, lane 14). In contrast, the PPE68_1/PPE68 hybrid could readily be observed in the culture supernatant using the anti-PPE68 antibody (Fig. 2A, lane 15). The anti-StrepII antibody failed to visualize this protein in this fraction, likely due to C-terminal processing of the PPE68_1/PPE68 hybrid upon secretion (see below). Most importantly, the introduction of an L125A mutation in the EspG1-binding domain of the PPE68_1/PPE68 hybrid completely blocked its ability to complement secretion of EspE and EsxA in the ppe68 operon mutant and also abrogated the secretion of the PPE68_1/PPE68 hybrid itself (Fig. 2A, lane 16). These combined results show that the amino acid in position 125, located within the helical tip of the EspG1-binding domain, is essential for the export of the PPE68/PPE68_1 hybrid, which in turn is necessary for the secretion EspE and EsxA.
Protein sequence alignment of PPE68 homologs of M. marinum and M. tuberculosis, including the secondary structure prediction of M. tuberculosis PPE68. The secondary structure prediction was produced by Alphafold2. The blue square indicates the residue at position 125 involved in EspG1 binding. Underlined sequences indicate the C-terminal truncations according to the color key. Rv3873, PPE68 of M. tuberculosis; MMAR_5448, PPE68 of M. marinum; MMAR_0186, PPE68_1 of M. marinum. Download FIG S2, TIF file, 1.6 MB (1.6MB, tif) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Deletion of the paralogous ppe68_1 operon does not affect EspE secretion or hemolytic activity of M. marinum. (A) SDS-PAGE and immunostaining were performed on the cell pellet, culture supernatant, Genapol pellet, and Genapol supernatant fractions of M. marinum WT, an ESX-1 mutant (eccCb1 mutant; MVU), the ppe68 operon mutant (Δpe35-esxA), and the ppe68_1 operon mutant (Δpe35_1-esxA_1). Proteins were visualized with anti-EspE and anti-PPE68 (ESX-1 substrates) antibodies. A processed band of 25 kDa was detected by the anti-EspE antibody in the Genapol supernatant fraction of the WT, which has been reported before (Phan et al., 2018 [46]). As a loading and lysis control, blots were incubated with antibodies directed against the cytosolic GroEL2 protein. In all blots, equivalent OD units were loaded: 0.2 OD for pellet and Genapol pellet and 0.4 OD for supernatant and Genapol supernatant fractions. Data shown are representative of two independent experiments. (B) Hemoglobin release after incubation of the same strains as in panel A and the ppe68 fs mutant expressing the ppe68_1 operon with sheep red blood cells. Hemoglobin release was quantified by determining the OD450 of the medium after incubation. Data shown are from two independent experiments including 3 technical replicates each. Download FIG S3, TIF file, 0.8 MB (885.4KB, tif) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The C-terminal domain of PPE68 is necessary for ESX-1-mediated secretion.
While the C-terminal domains of PPE68_1 and PPE68 are relatively dissimilar, they possess a well-conserved stretch of 15 amino acids consisting of an unusual high number of negatively charged residues at their C termini. To investigate the role of the C-terminal domain of PPE68 in more detail, several C-terminal truncations were made, namely, PPE68ΔC (residues 1 to 174), PPE68Δ112 (residues 1 to 256), and PPE68Δ10 (residues 1 to 357) (Fig. S2), including a C-terminal Strep tag and encoded by the pSMT3 vector together with pe35, esxB, and esxA. Immunoblot analysis of the pellet fractions using the anti-Strep antibody showed that the two more severe C-terminal truncations of PPE68 were detected in lower amounts, and only the PPE68Δ10 variant was produced at similar levels as the full-length protein (Fig. 2B, lanes 4 to 6). The anti-PPE68 antibody could only recognize the PPE68Δ10 variant, indicating that the antibody primarily recognized the C-terminal domain of this protein. Both PPE68Δ122 and PPE68Δ10 were able to restore EsxA secretion by the ppe68 operon mutant (Fig. 2B, lanes 11 and 12), whereas with the PPE68ΔC variant EsxA secretion could hardly be observed, most likely because this variant was not stably produced (Fig. 2B, lane 10). In contrast, while the intracellular protein levels of EspE were similar between all strains, none of the PPE68 variants showed detectable secretion of EspE (Fig. 2B, lanes 4 to 6 and 10 to 12). These results suggest that the C-terminal domain of PPE68 is not required for EsxA secretion, but it is necessary for the secretion of EspE.
PPE68 is secreted independently from EsxA and EsxB and processed on the cell surface.
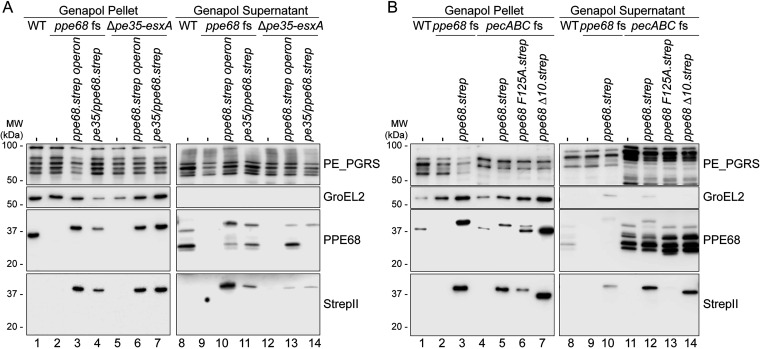
Although PPE68 contains all characteristics of an ESX substrate, we were not able to detect this protein in culture supernatants (Fig. 1 and 2). However, when we obtained a concentrated cell surface protein extract from larger cultures that were additionally grown without Tween 80, which has been shown to retain surface proteins (52), low levels of PPE68 were detected in the surface extract of WT M. marinum (Fig. 3A, lane 8). Here, PPE68 seemed to be processed, as multiple bands were recognized by the anti-PPE68 antibody. Only full-length PPE68 was observed by the anti-StrepII antibody, suggesting that the other bands detected by the anti-PPE68 antibody represented C-terminal truncates. C-terminal processing of the Strep tag was also observed for the secreted fraction of the PPE68_1/PPE68 hybrid (Fig. 2A). As we were now able to detect surface-localized PPE68, we analyzed whether the export of PPE68 was dependent on the coexpression of esxB and esxA. Importantly, a pSMT3 vector with only the pe35 and ppe68.strep genes fully restored PPE68 secretion to the cell surface by the two ppe68 mutants (Fig. 3A, lanes 11 and 14). As the ppe68 operon mutant did not contain a genomic copy of esxB or esxA, these results suggested that PPE68 can be secreted independently of EsxA (Fig. 3A, lane 14). Intriguingly, only full-length PPE68.strep was detected on the cell surface of the complemented ppe68 operon mutant, suggesting that surface processing of PPE68 might be dependent on the cosecretion of EsxA.
FIG 3.
PPE68 is secreted independently of esxB and esxA and processed on the cell surface of M. marinum. (A) SDS-PAGE and immunostaining on Genapol pellet and concentrated Genapol supernatant fractions of M. marinum WT, the ppe68 fs mutant, the ppe68 operon mutant, or both mutants complemented with pe35/ppe68 or the entire ppe68 operon. (B) Additional SDS-PAGE and immunostaining of Genapol pellet and concentrated Genapol supernatant fractions of the pecABC fs mutant overexpressing the WT ppe68 operon (ppe68.strep), the operon producing either the PPE68F125A with an amino acid substitution in the helical tip, PPE68Δ10 with a C-terminal truncation of the last 10 amino acids. Operons were expressed from a multicopy pSMT3 vector controlled by the hsp60 promoter and with a Strep tag on the C terminus of PPE68 and the variants. Proteins were visualized with anti-StrepII (PPE68) and anti-PPE68 antibodies (ESX-1 substrates). All ppe68 fs and pecABC fs mutants contained an additional pMV TdTomato plasmid. As a loading and lysis control, blots were incubated with antibodies directed against the cytosolic GroEL2 protein and the surface-localized PE_PGRS proteins. In all blots, equivalent OD units were loaded: 0.2 OD for Genapol pellet and 2 OD Genapol supernatant fractions. Data shown in each panel are representative of three independent experiments.
The various processed forms of PPE68 on the surface indicated that this protein is degraded upon secretion. Previously, the aspartic protease PecA was shown to cleave PE_PGRS proteins on the cell surface of M. marinum (18). We therefore hypothesized that this protein, together with its paralogs PecB and PecC, could be involved in cell surface processing of PPE68. Using a pecA-pecB-pecC triple-fs mutant, created using CRISPR1-Cas9 gene editing (unpublished data), we could confirm that, while PPE68 levels were similar in the Genapol pellet fractions (Fig. 3B, lanes 1 and 4), the amount of PPE68 protein that was detected in the upscaled cell surface extract was significantly higher in the pecABC fs mutant compared to that of the WT (Fig. 3B, lanes 8 and 11). This showed that the amount of exported PPE68 was in fact higher than previously assumed. As similar processing of surface-localized PPE68 was still observed in the pecABC fs mutant, another protease should be involved in this process. A prime candidate would be the serine protease MycP1, which is one of the conserved ESX-1 components. However, a point mutation in the active site of MycP1 (53) did not affect the surface processing of PPE68 (Fig. S4).
A point mutation in the active site of serine protease MycP1 does not affect the processing of PPE68 on the cell surface. SDS-PAGE and immunostaining of concentrated Genapol supernatant fractions of M. marinum WT and a mycP1 deletion mutant complemented with a mycP1 active site (S354A) variant, expressed from a pMV vector by the hsp60 promotor. Proteins were visualized with an anti-PPE68 antibody. As a loading and lysis control, blots were incubated with antibodies directed against the cytosolic GroEL2 protein and the surface-localized PE_PGRS proteins. In all blots, equivalent amounts, i.e. 2 OD units, of Genapol supernatant fractions were loaded. Data shown are representative of two independent experiments. Download FIG S4, TIF file, 0.6 MB (664.8KB, tif) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Next, we exploited the increased amount of cell surface-localized PPE68 in the pecABC fs mutant to further investigate the requirement for PPE68 secretion. When we disrupted the interaction with EspG1 by the F125A mutation, PPE68.strep was not detected in the surface fraction (Fig. 3B, lane 13), confirming the importance of the EspG1 interaction for secretion. Interestingly, deletion of the C-terminal 10 amino acids of PPE68, which did not majorly affect EsxA secretion but abrogated secretion of EspE, did not interfere with secretion of PPE68 itself (Fig. 3B, lane 14). To summarize, PPE68 is exported to the cell surface of M. marinum, where it is subsequently degraded. Secretion of PPE68 depends on its interaction with EspG1 but is independent of coexpression of esxB and esxA and its final 10 amino acids.
Intracellular PPE68 forms a complex with EspG1 and a PE protein.
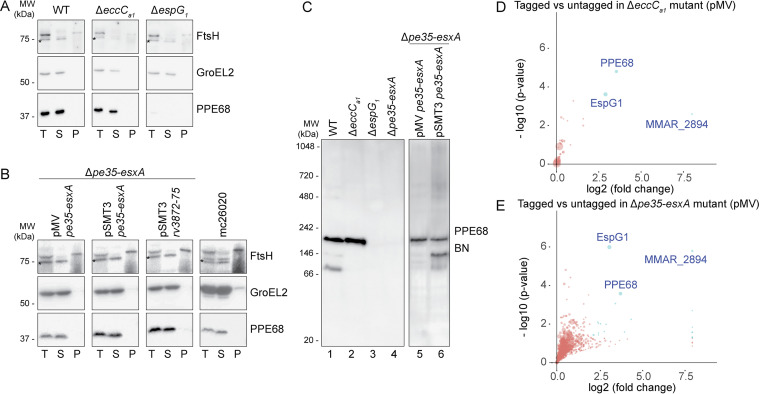
While small amounts of PPE68 could be extracted from the cell surface, a large portion of PPE68 remained cell associated upon detergent treatment (Fig. 3). In M. tuberculosis, PPE68 was shown to localize to the cell envelope fraction by subcellular fractionations (9, 48). However, when we fractionated M. marinum cells by high-pressure lysis followed by ultracentrifugation, we found the entire population of intracellular PPE68 to be present in the soluble fraction (Fig. 4A). In an eccCa1 deletion strain, production and solubility of PPE68 were similar to those in the WT, while deleting the espG1 chaperone gene drastically reduced the amount of PPE68. Exogenous expression of the entire ppe68 operon in the ppe68 operon mutant did not alter the solubility of PPE68 (Fig. 4B). To test whether the observed difference in solubility between PPE68 homologs was species specific or dependent on experimental procedures, we fractionated M. marinum expressing the ppe68 operon of M. tuberculosis and the auxotrophic M. tuberculosis strain mc26020 using the same procedure. In both cases, PPE68 of M. tuberculosis was again exclusively present in the soluble fraction (Fig. 4B); from this we conclude that the observed differences for PPE68 localization are a result of different protocols for cell lysis and subcellular fractionation.
FIG 4.
PPE68 of M. marinum and M. tuberculosis is soluble and forms complexes of different sizes. (A and B) SDS-PAGE and immunostaining of subcellular fractionation of M. marinum WT, an eccCa1 mutant, and an espG1 mutant (A) or the ppe68 operon mutant complemented with the ppe68 operon of M. marinum expressed from a pMV or pSMT3 vector, the ppe68 operon of M. tuberculosis H37Rv expressed from a pSMT3 vector, or auxotrophic M. tuberculosis strain mc26020 (B). Proteins were visualized with anti-PPE68, while antibodies against GroEL2 (cytosolic protein) and FtsH (membrane protein) were used to assess the purity of the fractions. Notably, the FtsH antiserum cross-reacted with a cytosolic protein (indicated by an asterisk). In all blots, equivalent amounts corresponding to 0.2 OD units were loaded. T, total lysate; S, supernatant or soluble proteins; P, pellet or cell envelope proteins. (C) Immunostaining of soluble fractions of the same strains as in panels A and B after blue native PAGE (BN-PAGE) gel electrophoresis. In all blots, an equivalent amount of 19 μg of total protein was loaded. Data shown in panels A, B, and C are representative of two independent experiments. (D and E) PPE68 interacts with its PE partner, MMAR_2894, and the EspG1 chaperone in the cytosol. Quantitative proteomics analysis comparing the PPE68 affinity purification from the soluble fraction of the eccCa1 mutant (D) and the ppe68 operon mutant (E), complemented with the ppe68 operon, produced PPE68 with or without a Strep tag on its C terminus from a pMV vector. Proteins that showed a log2 fold change of >2 and a −log10 P value of >1.3 between tagged and untagged PPE68 are indicated as blue dots. Sizes of the dots are correlated to the number of spectral counts. Data include two technical replicates per sample.
The current paradigm is that PPE proteins interact with a specific PE partner and a system-specific EspG chaperone in the cytosol prior to secretion (12, 13, 54). Analysis of PPE68 complex formation by blue native PAGE (BN-PAGE) revealed three major intracellular complexes ranging from ~70 to 180 kDa (Fig. 4C, lane 1). Both the ppe68 operon mutant and the ΔespG1 mutant did not show any PPE68 complex formation (Fig. 4C, lanes 3 and 4). In the soluble fraction of the ΔeccCa1 mutant, in which export of PPE68 is blocked, the largest (~180-kDa) and predominant complex was observed in similar amounts as in the WT fraction (Fig. 4C, lane 2), suggesting that a large portion of PPE68 in WT bacteria is retained in the cytosol. The additional two complexes were absent in the ΔeccCa1 mutant, indicating that these two assemblies are only formed in the presence of a functional ESX-1 membrane complex. Complementation of the ppe68 operon mutant with the ppe68 operon of M. marinum, either using the pMV or pSMT3 plasmid, restored formation of the ~180-kDa PPE68 complex, while the two smaller complexes were only clearly observed when complemented with the pSMT3 vector (Fig. 4C, lanes 5 and 6). This difference could be due to higher expression levels of the operon genes of the latter vector, compared to that of the integrative pMV vector.
To analyze the composition of the observed complexes, a Strep affinity purification was performed on the soluble fractions of the ΔeccCa1 and ppe68 operon mutants, producing Strep-tagged PPE68 from either the pMV or pSMT3 vector containing the full ppe68 operon. Both mutants containing the ppe68 operon without a Strep tag sequence were included as negative controls. BN-PAGE analysis of the purified samples showed a similar pattern of PPE68 complexes as seen for endogenous PPE68 (Fig. S5C). Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis showed that the PPE68.strep samples, purified from the ΔeccCa1 mutant, contained, as expected, PPE68 and the chaperone EspG1. The third protein that was present in high amounts was an M. marinum-specific PE protein, named MMAR_2894 (Fig. 4D and Table S1). Interestingly, this protein was already identified as an ESX-1 substrate (46). Notably, PE35 of M. marinum, unlike its ortholog in M. tuberculosis, lacks a general YxxxD/E secretion motif (Fig. S6) (15), which might explain why PPE68 binds an alternative partner in M. marinum. As PE35_1 was also not detected, MMAR_2894 seems to be the only PE partner of PPE68 in M. marinum. EspG1 and MMAR_2894 were also the predominant copurified proteins of PPE68 purified from the complemented ppe68 operon mutant, confirming that intracellular PPE68 localized to the cytosol also in the presence of a functional ESX-1 system (Fig. 4E and Table S1). PPE68.strep produced from the pSMT3 vector in both the ppe68 operon and ΔeccCa1 mutant also showed the specific copurification of EspG1 and MMAR_2894 (Fig. S5D and E).
Purification of PPE68 from the ppe68 operon mutant and the eccCa1 mutant containing a pMV or pSMT3 vector with the ppe68 operon of M. marinum. Genes were expressed from the hsp60 promotor with or without a Strep tag on the C terminus of PPE68. (A) Immunostaining of subcellular fractionations of the different strains. Proteins were visualized with anti-PPE68 and anti-StrepII antibodies, while anti-FtsH antibodies controlled for the loading and lysis. Notably, the FtsH antiserum cross-reacted with a cytosolic protein. In all blots, equivalent amounts corresponding to 0.2 OD units were loaded. T, total lysate; S, supernatant or soluble proteins; P, pellet or cell envelope proteins. (B) Separation of the PPE68 purification samples by SDS-PAGE and subsequent staining with Coomassie brilliant blue. (C) Separation of complexes in the PPE68 purification samples by BN-PAGE and subsequent staining with Coomassie brilliant blue. As a negative control, the purification sample of the ppe68 operon mutant containing a pSMT3 vector with the ppe68 operon, without C-terminal Strep tag on PPE68, was included. Data shown in panls A, B, and C are representative of two independent experiments. (D) Quantitative proteomics analysis comparing the PPE68 affinity purification from the soluble fraction of the ΔeccCa1 mutant and the ppe68 operon mutant (Δpe35-esxA) producing PPE68 with or without a Strep tag on the C terminus, from a pSMT3 vector. Data included two technical replicates per sample. Proteins that showed a log2 fold change of >2 and a −log10 P value of >1.3 between tagged and untagged PPE68 are indicated as blue dots. Size of the dots are correlated to the number of spectral counts. Download FIG S5, TIF file, 1.4 MB (1.4MB, tif) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Protein sequence alignment of PPE35 homologs of M. marinum and M. tuberculosis, including the secondary structure prediction of M. tuberculosis PE35. The secondary structure prediction was produced by Alphafold2. The blue square indicates the conserved YxxxD/E secretion motif. Note that PE35 of M. marinum lacks this conserved motif. Rv3872, PE35 of M. tuberculosis; PE35, MMAR_5447 of M. marinum; PE35_1, MMAR_0185 of M. marinum. Download FIG S6, TIF file, 1.3 MB (1.4MB, tif) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Significant hits of PPE68 interactors as determined by protein purification and mass spectrometry analysis (only proteins with an average normalized spectral count of >5 in the tagged samples, a log2 fold change of >2, and a −log10 P value of >1.3 between tagged and untagged samples are shown). Download Table S1, DOCX file, 0.01 MB (15.9KB, docx) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To confirm the presence of MMAR_2894 and EspG1 in the PPE68 complexes, we created an mmar_2894 fs mutant and analyzed the PPE68F125A variant, respectively. The mmar_2894 fs mutant, which contained a 13-bp deletion after 192 bp that resulted in an early stop codon after 68 amino acids (Fig. S1B), showed an ESX-1 secretion defect and loss of hemolytic capabilities similar to the ppe68 fs mutant (Fig. S7A to C). While PPE68 was detectable and soluble in the mmar_2894 fs mutant, BN-PAGE analysis showed that the major ~180-kDa complex was absent in this mutant. The smaller two complexes were still present, suggesting that only the predominant complex contained MMAR_2894 (Fig. 5B, lane 3). With the PPE68F125A variant, no complexes could be observed by BN-PAGE analysis (Fig. 5B, lane 5), while it was detectable and in the soluble fraction by SDS-PAGE analysis, suggesting that all three complexes contained EspG1.
FIG 5.
Verification of complex formation of PPE68 with MMAR_2894 and EspG1. (A) SDS-PAGE and immunostaining of subcellular fractions of the mmar_2894 fs mutant and the ppe68 operon mutant complemented with the WT ppe68 operon, the ppe68_1 operon, a hybrid operon containing pe35_1, the N-terminal domain of ppe68_1, the C-terminal domain of ppe68, and esxB and esxA or the ppe68 or hybrid operon expressing a PPE68 variant with a single amino acid substitution at position 125. Genes were expressed from a multicopy pSMT3 vector under the hsp60 promoter with a Strep tag on the C terminus of PPE68 or its variants. Proteins were visualized with anti-PPE68 antibodies, while antibodies against GroEL2 (cytosolic protein) and FtsH (membrane protein) were used to assess the purity of the fractions. Notably, the FtsH antiserum cross-reacted with a cytosolic protein (indicated by an asterisk). In all blots, equivalent amounts corresponding to 0.2 OD units were loaded. T, total lysate; S, supernatant or soluble proteins; P, pellet or cell envelope proteins. (B) BN-PAGE and immunostaining of soluble fractions of the same strains as in panel A, and also the ppe68 operon mutant expressing the ppe68 operon of M. tuberculosis from a pSMT3 vector and the auxotrophic M. tuberculosis strain mc26020. Proteins were visualized with anti-PPE68 and anti-StrepII antibodies. In all blots, an equivalent amount of 19 μg of total protein was loaded. Data shown in each panel are representative of two independent experiments.
Secretion and hemolytic analysis of the mmar_2894 fs mutant. (A) SDS-PAGE and immunostaining of the cell pellet, culture supernatant, Genapol pellet, and Genapol supernatant fractions of M. marinum, WT, an ESX-1 mutant (eccCb1 mutant; MVU), and the mmar_2894 fs mutant. Proteins were visualized with anti-EsxA, anti-EspE, and anti-PPE68 antibodies (ESX-1 substrates). A processed band of 25 kDa was detected by the anti-EspE antibody in the Genapol supernatant fraction of the WT, which has been reported before (Phan et al., 2018 [46]). As a loading and lysis control, blots were incubated with antibodies directed against the cytosolic GroEL2 protein. In all blots, equivalent OD units were loaded: 0.2 OD for pellet and Genapol pellet and 0.4 OD for supernatant and Genapol supernatant fractions. (B) SDS-PAGE and immunostaining of the Genapol pellet and upscaled Genapol supernatant fractions of M. marinum WT, the ppe68 fs mutant, and the mmar_2894 fs mutant. Proteins were visualized with an anti-PPE68 antibody (ESX-1 substrate). As a loading and lysis control, blots were incubated with antibodies directed against the cytosolic GroEL2 protein and the surface-localized PE_PGRS proteins. In all blots, equivalent OD units were loaded: 0.2 OD for Genapol pellet and 2 OD Genapol supernatant fractions. Data shown in panels A and B are representative of two independent experiments. (C) Hemoglobin release after incubation of the same strains shown in panels A and B with sheep red blood cells, quantified by determining the OD450 of the medium after incubation. Data shown are from two independent experiments that included 3 technical replicates each. Download FIG S7, TIF file, 1.4 MB (1.4MB, tif) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Finally, we also analyzed complex formation of PPE68 from M. tuberculosis using both heterologous expression of the ppe68 operon in M. marinum and the auxotrophic M. tuberculosis strain mc26020. The observed PPE68 complexes in both strains had the same size as PPE68_1.strep and the PPE68_1/PPE68.strep hybrid (Fig. 5B, lane 9 and 10). The smaller sizes of these complexes compared to the ~180-kDa PPE68mmar complex could be due to the sizes of their most likely PE partners, i.e., PE35_1 and PE35mtub, respectively, which are smaller (9 kDa) than MMAR_2894 (22 kDa). Structural analysis suggests that PE-PPE-EspG complexes are formed by a single copy of each protein (12, 13), resulting in a molecular weight for MMAR_2894-PPE68-EspG1 of M. marinum and both PE35_1-PPE68_1-EspG1 of M. marinum and PE35-PPE68-EspG1 of M. tuberculosis of 89 kDa and 74 kDa, respectively. The fact that the observed complexes had twice the molecular weight, i.e., ~180 kDa and ~150 kDa, respectively, indicated that the PE-PPE-EspG complexes form a dimer of trimers in the cytosol of mycobacteria. In summary, our fractionation and pulldown results suggest that cell-associated PPE68 of M. marinum and M. tuberculosis is mainly located in the cytosol, where it is in a complex together with EspG1 and its PE partner.
Role of PPE68 in ESX-1 secretion: a model.
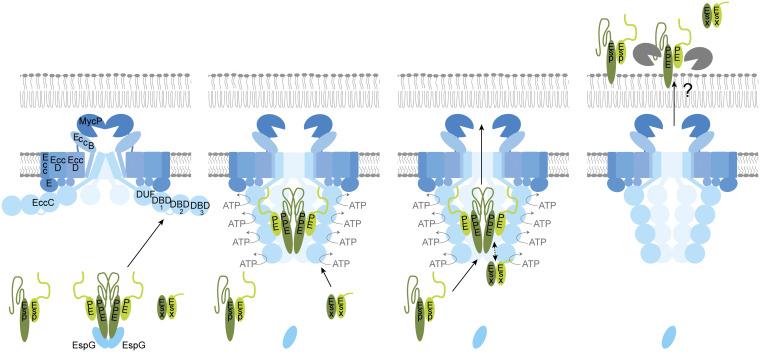
Based on our results, we propose a working model for PPE68 in the secretion of EsxA and EspE via the ESX-1 secretion system in M. marinum (Fig. 6). In the cytosol, PPE68 forms a stable complex together with its PE partner, i.e., MMAR_2894 in M. marinum, and chaperone EspG1. Upon targeting to the ESX-1 membrane complex, the PE/PPE pair binds to the central ATPase EccCab1, possibly to the linker 2 domain between nucleotide-binding domain 1 (NBD1) and NBD2, a previously proposed recognition site for PE/PPE substrates (55). Based on our observation that PPE68 does not require EsxA/EsxB for its secretion, this binding event is enough to trigger activation of the translocation channel. As a second step, EsxA/EsxB binds, based on in vitro binding studies and structural analysis, to NBD3 of EccCab1 (26, 56–58). The Esx pair probably also interacts with the PE/PPE pair at the membrane complex, as PE35_1/PPE68_1 has been shown to determine the system specificity of EsxB_1/EsxA_1 (43). As the third step, EspE is targeted with its predicted partner EspF and chaperone EspH (46) to the same ESX-1 complex. Secretion of the PE/PPE and Esx pairs probably does not depend on EspE, as mutations in EspH only affect secretion of EspE and EspF (46). Subsequently, the three substrate pairs are transported together through the channel at the cost of ATP hydrolysis by the EccC ATPases. EspE interacts with the C terminus of PPE68 during the translocation process, as these residues are important for the secretion of this Esp substrate specifically. The mechanism of translocation across the mycobacterial outer membrane remains unknown. While it is tempting to speculate that PPE68 plays a role in the process, the protein does not form a stable channel in this specific membrane. Instead, the main portion of PPE68 exists as a cytosolic pool, where it serves as a reservoir to facilitate secretion of the other ESX-1 substrates. Once PPE68 is exported to the cell surface, it is processed and/or degraded by PecABC and an unspecified protease.
FIG 6.
Working model for the role of PPE68 in ESX-1-mediated secretion. We propose that different ESX-1 substrates are secreted in a hierarchical fashion by a stepwise interaction with the secretion machinery. Here, the PE/PPE proteins activate the channel by interacting with the linker that connects NBD1 and NBD2 of the central EccC component. This interaction induces conformational changes in the ATPase and its full activation, after which the Esx substrates can bind NBD3, resulting in the cosecretion of the PE/PPE and Esx substrates across the cell envelope. The secretion of both the PE/PPE and Esx heterodimers is necessary for secretion of the final substrate family, the Esp proteins.
DISCUSSION
The ESX-1 substrate EsxA is an abundantly secreted and highly immunogenic protein of M. tuberculosis and M. marinum (59–61) and has therefore been a major focus to get mechanistic insights into the essential role of ESX-1 in phagosomal membrane rupture by these pathogens (3, 4, 6, 24–26). However, the investigation of EsxA, or other ESX-1 substrates, remains difficult due to the codependent secretion of these substrates (34, 36–39). Here, we report that ESX-1 substrate PPE68, which is encoded within the same operon as EsxA, plays a central role in the secretion of EsxA and EspE in M. marinum, and we provide mechanistic insights into the observed codependent secretion of these substrates.
First, we showed that PPE68 is exported in an ESX-1-dependent manner to the cell surface of M. marinum. While previous mass spectrometry analysis could also detect this protein in culture supernatants of M. marinum (33, 46, 62), we could not detect PPE68 in similar fractions by immunoblot analysis. Intriguingly, this protein is degraded upon secretion to the cell surface, as deletion of three Pec proteases (PecABC) increased the amount of PPE68 on the cell surface. This provided an explanation for the small amount of exported PPE68 we detected. The observation that the pecABC deletions did not affect the processing pattern of PPE68 protein suggested that an additional protease is involved in its processing and/or degradation.
A frameshift mutation in ppe68 abolished secretion of EsxA and EspE, and of hemolytic activity by M. marinum, which could be fully complemented by introduction of the pe35-ppe68-esxB-esxA operon. The role of PPE68 in the secretion process depended on the export of the PPE protein, as a point mutation in its EspG1-binding domain that blocked PPE export abolished secretion of the other ESX-1 substrates. Importantly, PPE68 export was independent of esxA/esxB expression, showing there is a hierarchy in codependent secretion. While the Esx pair has been considered to be important for secretion of all other ESX substrates, our results confirmed recently published data showing that MMAR_2894, the PE partner of PPE68 in M. marinum, is fully independent of esxB/esxA for its export (62).
Notably, we showed that PPE68 of M. tuberculosis can take over the central role of endogenous PPE68 of M. marinum in ESX-1-mediated secretion and hemolysis. Our observations and those made by Cronin et al. in M. marinum contrast results from previous studies in M. tuberculosis complex members that showed that interruption of the ppe68 gene did not affect secretion of EsxA in RD1-complemented Mycobacterium microti, M. bovis BCG, or M. tuberculosis H37Rv (48, 63). In the same studies, the ppe68 deletion in RD1-complemented M. microti and M. bovis BCG also did not decrease virulence in severe combined immunodeficient mice compared to the WT-complemented strains. However, in other studies, ppe68 transposon mutants did affect growth of M. tuberculosis H37Rv in a C57BL/6J mouse infection model (64) and also led to reduced cytotoxicity in THP-1 macrophages (65). Interestingly, host cell cytolysis, hemolytic activity, and in vivo virulence phenotypes of esx-1 mutants do not necessarily correlate, as reported in multiple studies (46, 62, 66). While the reason for the observed differences in requirements for EsxA secretion between M. marinum and M. tuberculosis remains unclear, these differences might be related to the different PE partner for PPE68 and/or the duplication of the pe35-esxA region in M. marinum. Alternatively, they might be caused by a variation in in vitro growth conditions, as previously observed for the importance of the conserved cytosolic component EccA1 in secretion (46). While analyzing a similar ppe68 fs mutant of M. tuberculosis would address the seemingly species-specific differences, our observations using the auxotrophic M. tuberculosis strain mc26020 and the M. marinum ppe68 fs mutant complemented with the ppe68 operon of M. tuberculosis showed that at least the subcellular location of PPE68 is conserved and that the M. tuberculosis protein can take over the essential role of its ortholog in ESX-1-mediated secretion in M. marinum.
A codependent relationship between substrates of the same ESX system could be explained by a more integral role of specific substrates in the translocation process itself. Recently, Cronin et al. proposed a model in which MMAR_2894/PPE68 forms the periplasmic part of the ESX-1 channel and EsxA/EsxB mediates translocation of ESX-1 substrates across the mycobacterial outer membrane (62). This model is supported by a previous observation that PPE68 of M. tuberculosis localizes to cell envelope fractions (9, 48). However, we observed that cell-associated PPE68 was soluble, both in M. marinum and the auxotrophic M. tuberculosis strain mc26020. These contrasting observations were probably caused by experimental differences. In addition, our observation that PPE68 still localized to the cell surface in the absence of esxB/esxA argues against a role of the Esx pair in outer membrane translocation. Although a function for PPE68 in outer membrane translocation is still possible, the fact that we found this protein predominantly in the cytoplasmic compartment points toward a role in substrate recognition and/or activation by the ESX-1 inner membrane complex. Our previous observation that PPE68_1 determines through which ESX system EsxA_1 is exported by M. marinum is in agreement with such a function (43). In addition, a domain in the EccC ATPase component, i.e., linker 2 between NBD1 and NBD2, has previously been shown to be involved in species-specific secretion of PE_PGRS proteins via the ESX-5 system (55). We therefore propose that binding of the PE/PPE pair to linker 2 on EccCab1 could trigger the activation of the ATPases and the opening of the ESX inner membrane complex.
Interestingly, we observed that the final 112 amino acids of PPE68 are dispensable for EsxA secretion. This suggested that the conserved N-terminal PPE domain of this protein, probably together with the PE domain of its partner, is involved in the secretion of other ESX-1 substrates. This involvement could be indirect, by activation of the ESX membrane channel, but it could also be mediated by a direct interaction of the two substrate pairs. In contrast, the C terminus of PPE68 seemed to be required for the secretion of EspE, i.e., deletion of only the C-terminal 10 amino acids already blocked EspE secretion. This charged C-terminal tail was conserved in the PPE68 homolog of M. tuberculosis and the PPE68_1 paralog in M. marinum, possibly explaining why these homologs are able to complement EspE secretion in the M. marinum ppe68 mutants. We propose that EspE interacts with the C-terminal domain of PPE68 during the secretion process.
The major portion of cytosolic PPE68 is in an ~180-kDa complex with the EspG1 chaperone and an unexpected PE protein, namely, MMAR_2894. MMAR_2894 has previously been identified as an ESX-1 substrate with a role in hemolysis (46, 66). Mutating mmar_2894 leads to the absence of the major cytosolic PPE68 complex and a secretion and hemolytic defect identical to that in the ppe68 fs mutant, which confirms that MMAR_2894 is the PE partner of PPE68 in M. marinum. This also means that PPE68 does not pair with the adjacently encoded PE35, which explains why PE35 lacks a T7SS signal (YxxxD/E). Apparently, during evolution PE35 was replaced by MMAR_2894 and lost its function, or vice versa. Analysis of multiple available M. marinum genomes (67) revealed a widespread lack of the T7SS signal in PE35, which extended to its close relatives Mycobacterium liflandii and Mycobacterium ulcerans, suggesting that this variation arose in a common ancestor. It seems that new associations between PE and PPE proteins can be formed, which also cautions the interpretation of adjacent PE and PPE genes as necessary secretion partners. Based on available structural data on PE:PPE:EspG complexes and observed molecular weights of the major cytosolic complex, we postulate that the MMAR_2894:PPE68:EspG1 complex forms a dimer of trimers. Dimers of EspG3 from M. tuberculosis and Mycobacterium smegmatis have been crystallized, suggesting that dimerization could occur via the chaperone (12, 68).
Overall, our study describes a central role for PPE68 in ESX-1-mediated secretion and function. We hypothesize that PPE68 is in complex together with its PE partner and EspG chaperone in the cytosol as a reservoir to aid in the secretion of other substrates across the inner and possibly also the outer membrane. Further studies should focus on the exact mechanism of codependence of substrates for secretion, potential interactions between secreted codependent heterodimers, and the role of the EccC ATPase in this process. The recent publications of the high-resolution structure of various ESX systems (69–72) will stimulate the field toward a mechanistic understanding of T7SSs.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
All strains included in this study are listed in Table S2 in the supplemental material. Mycobacterial strains were routinely grown on Middlebrook 7H10 agar with 10% oleic acid-albumin-dextrose-catalase (OADC; Difco) or in Middlebrook 7H9 liquid medium with 10% ADC (Difco) and 0.05% Tween 80. Additional supplement was necessary for culturing of M. tuberculosis mc26020 (ΔlysA ΔpanCD), for which 100 ng/mL l-lysine (Sigma) and 25 ng/mL d-pantothenic acid (Sigma) were included. Appropriate antibiotics were added when necessary, i.e., 50 μg/mL kanamycin (Sigma), 50 μg/mL hygromycin (Roche), or 30 μg/mL streptomycin (Sigma). M. marinum and M. tuberculosis cultures and plates were incubated at 30°C and 37°C, respectively. Escherichia coli DH5α was used for cloning and was grown on LB agar plates at 37°C. Antibiotics were added at the same concentrations as described above.
Strains and plasmids used in this study. Download Table S2, DOCX file, 0.03 MB (28.1KB, docx) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Plasmid construction.
Genes of interest were amplified from M. marinum M or M. tuberculosis H37Rv genomic DNA by PCR using primers that were synthesized by Sigma (Table S3). Hybrid combinations of genes or introduction of affinity tags was achieved with the use of nested primers. The Phusion polymerase (New England Biolabs [NEB]) was used throughout all cloning procedures. Generated constructs were digested with XmnI and HindIII or NheI and BamHI for ligation into pMV361 and pSMT3 vectors (73, 74), respectively. Restriction enzymes and ligation enzymes were provided by NEB. All plasmids were verified by Sanger sequencing (Macrogen) and are listed in Table S2.
Primers used in this study. Download Table S3, DOCX file, 0.02 MB (24.8KB, docx) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Generation of knockout strains and frameshift mutants.
Generation of the mmar_5447-50 and mmar_0185-88 knockout strains was performed in M. marinum M by allelic exchange using a specialized transducing mycobacteriophage as previously described (75). PCR was used to amplify the flanking regions of the genomic regions. Deletions were confirmed by PCR, after which the selection markers were removed with a temperature-sensitive phage encoding the γδ-resolvase (TnpR; a kind gift from Apoorva Bhatt, University of Birmingham, United Kingdom). The fs mutants were obtained by genome editing using Streptococcus thermophilus CRISPR1-Cas9 (47). Single-guide RNAs (sgRNAs) were produced by the annealing of oligonucleotides containing BsmBI overhangs (76). sgRNAs were ligated into BsmBI-digested pCRISPRx-Sth1Cas9-L5. The plasmids were sequenced and subsequently electroporated into competent M. marinum M cells. Bacteria were plated on 7H10 plates containing kanamycin and 100 ng/mL anhydrotetracycline (ATc; IBA Life sciences) for the induction of the S. thermophilus CRISPR1-Cas9 system. Single colonies were picked and screened for mutations in the gene of interest by PCR and sequencing. After verification, the CRISPR1-Cas9 integrative plasmid was removed by exchanging it with pTdTomato-L5, which integrates at the same site.
RNA isolation and quantitative RT-PCR.
RNA was isolated from bacterial cultures grown to an optical density at 600 nm (OD600) of 1. Bacteria were lysed by bead beating in the presence of buffer RA-1 (NucleoSpin RNA isolation kit; Mackery-Nagel) and β-mercaptoethanol (Sigma). RNA isolation and purification were continued according to the protocol supplied by the manufacturer (NucleoSpin RNA isolation kit; Mackery-Nagel). Samples were eluted in water with Ribolock (Thermoscientific), and an additional DNase treatment was performed with DNase I (Thermoscientific) according to the protocol supplied by the manufacturer. cDNA was synthesized from 400 ng RNA with the RevertAid first-strand cDNA synthesis kit (Thermoscientific). Prior to the PCRs, the cDNA was diluted 1:10 in RNase-free water. Reactions were set up using iTaq universal SYBR green supermix (Bio-Rad). Quantitative reverse transcription-PCR (RT-PCR) was performed with the QuantStudio 3 real-time PCR system (ThermoFisher). At the end of amplification, PCR product specificity was verified by melt curve analysis and agarose gel electrophoresis. The threshold cycle (CT) values were normalized with the CT of SigA.
Protein secretion.
M. marinum cultures were grown until mid-logarithmic phase (OD600 of 1 to 1.4), before they were washed with 7H9 liquid medium containing 0.2% dextrose and 0.05% Tween 80 to clear the cultures of bovine serum albumin (BSA). Cultures were set at an OD600 0.4 to 0.5 and grown for 16 h. Cells were pelleted (10 min at 3,000 × g), washed, and resuspended in phosphate-buffered saline (PBS) before lysing by bead beating, and a 1/4 volume of 5× SDS loading buffer (312.5 mM Tris-HCl [pH 6.8], 10% SDS, 30% glycerol, 500 mM dithiothreitol, and 0.2% bromophenol blue) was added, yielding the pellet fraction. Culture medium was passed through an 0.22-μm filter, and proteins were precipitated with trichloroacetic acid (TCA). TCA-precipitated pellets were washed with acetone and resuspended in 1× SDS loading buffer, yielding the supernatant fraction. Alternatively, a fraction of pelleted bacteria was incubated with 0.5% Genapol X-080 in PBS for 30 min with rotation at room temperature to extract cell surface-associated proteins. Genapol-treated cells were pelleted, washed, and resuspended in PBS before lysis by bead beating and addition of a 1/4 volume of 5× SDS buffer, yielding the Genapol pellet fraction. A 1/4 volume of 5× SDS buffer was also added to Genapol supernatants, yielding the Genapol supernatant fraction. All fractions were boiled for 10 min at 95°C before loading on SDS-PAGE gels.
Fractionation and Strep-tagged protein purification.
Mycobacterial cultures were grown to an OD600 of 1. Cells were harvested and washed with PBS before resuspension in PBS with 10% glycerol to a concentration of 50 to 100 OD/mL. Cells were lysed by passage through a One-Shot cell disrupter (Constant Systems) at 0.83 kbar. Unbroken cells were pelleted by centrifugation at 15,000 × g for 10 min at 4°C. The obtained supernatant was subjected to ultracentrifugation at 100,000 × g for 1 h at 4°C, yielding the cell envelope pellet and soluble fractions. Samples were boiled in SDS buffer for 10 min at 95°C before loading on SDS-PAGE gels, as explained above. Alternatively, soluble fractions were then separated under native conditions on a 4 to 16% NativePage Novex BisTris protein gel (Life Technologies) and transferred to a polyvinylidene difluoride (PVDF) membrane before immunostaining.
For the purification of intracellular Strep-tagged proteins, cells were harvested and lysed in buffer B (PBS with 10% glycerol [pH 8]). Soluble fractions were prepared and incubated with StrepTactin resin (IBA Lifesciences) for 30 min with rotation at 4°C. Beads were then washed with buffer B and proteins were eluted using buffer B supplemented with 10 mM desthiobiotin (IBA Lifesciences). Samples were taken at each step of the purification and loaded on SDS-PAGE gels to check the efficiency of affinity purification. Elution fractions were also separated under native conditions to ascertain the stability of the complexes after affinity purification.
Protein detection and sera.
SDS-PAGE and NativePage gels were stained with Brilliant Blue G-250 or R-250 (CBB; Bio-Rad), respectively. Alternatively, proteins were transferred to nitrocellulose (SDS-PAGE) or PVDF (BN-PAGE) membranes. Proteins were visualized using standard immunodetection techniques. Primary antibodies used in this study were anti-GroEL2 (CS44; Colorado State University), anti-PE_PGRS antibody (7C4.1F7) (77), anti-EsxA (Hyb76-8) (78), polyclonal anti-FtsH (79), polyclonal anti-PPE68 (9), polyclonal anti-EspE (51, 52), and polyclonal anti-Strep (Novusbio).
Mass spectrometry.
To identify the interaction partners of PPE68, elution fractions of the Strep-tagged protein purifications were analyzed by LC-MS/MS, essentially as described before (80). In short, SDS loading buffer was added to elution samples of Strep-tagged protein purification and boiled for 10 min at 95°C before loading on SDS-PAGE gels. SDS-PAGE gels were stained by CBB, and total protein lanes were excised, washed, and processed for in-gel digestion. Peptides were eluted from the gel pieces and analyzed by the Ultimate 3000 nanoLC-MS/MS system (Dionex LC-Packings, Amsterdam, The Netherlands). Proteins were identified from the resulting LC-MS/MS spectra by searching against the Uniprot M. marinum complete proteome (ATCC BAA-535M).
Hemolysis assay.
M. marinum strains were grown in 7H9 medium supplemented with ADC and 0.05% Tween 80 until mid-logarithmic phase. All strains were washed with PBS and diluted in Dulbecco's modified Eagle medium (DMEM) without phenol red (Gibco, Life Technologies). Numbers of bacteria were quantified by absorbance measurement at OD600 with an estimation of 2.5 × 108 bacteria in 1 mL of a 1.0 OD600 suspension. Defibrinated sheep erythrocytes (Oxoid-Thermo Fisher, The Netherlands) were washed and diluted in DMEM. A total of 4.15 × 107 erythrocytes and 1.04 × 108 bacteria (1:2.5 ratio) were added for one reaction mixture of 100 μL in a round-bottom 96-well plate, gently centrifuged for 5 min, and incubated at 32°C with 5% CO2. After 3 h of incubation, cells were resuspended and centrifuged. Supernatants were transferred to a flat-bottom 96-well plate and measured at an absorbance of 405 nm to quantify hemoglobin release.
ACKNOWLEDGMENTS
We thank Roy Ummels, Dagmar van Mourik, Daphne Fluitman, and Jobana Ananthasabesan for their contributions to plasmid design and the generation of mutants. We are grateful to Eric Brown (Genentech) and Roland Brosch (Institut Pasteur) for offering anti-EspE and anti-PPE68 antibodies, respectively. This work was funded by The Netherlands Organization of Scientific Research through an ALW open grant (ALWOP.319, to M.P.M.D.) and a VIDI grant (864.12.006, to E.N.G.H.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
M.P.M.D., A.S.M., E.M.K., and S.R.P. performed experiments; S.R.P., C.R.J., and C.P.K. helped in analyzing the results, M.P.M.D.; W.B., and E.N.G.H. designed the experiments; and M.P.M.D., W.B., and E.N.G.H. wrote the manuscript.
We declare that we have no conflict of interest.
Contributor Information
Edith N. G. Houben, Email: e.n.g.houben@vu.nl.
Sabine Ehrt, Weill Cornell Medical College.
REFERENCES
- 1.Hoffmann C, Leis A, Niederweis M, Plitzko JM, Engelhardt H. 2008. Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc Natl Acad Sci USA 105:3963–3967. doi: 10.1073/pnas.0709530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuber B, Chami M, Houssin C, Dubochet J, Griffiths G, Daffe M. 2008. Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J Bacteriol 190:5672–5680. doi: 10.1128/JB.01919-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houben D, Demangel C, van Ingen J, Perez J, Baldeon L, Abdallah AM, Caleechurn L, Bottai D, van Zon M, de Punder K, van der Laan T, Kant A, Bossers-de Vries R, Willemsen P, Bitter W, van Soolingen D, Brosch R, van der Wel N, Peters PJ. 2012. ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell Microbiol 14:1287–1298. doi: 10.1111/j.1462-5822.2012.01799.x. [DOI] [PubMed] [Google Scholar]
- 4.Simeone R, Bobard A, Lippmann J, Bitter W, Majlessi L, Brosch R, Enninga J. 2012. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog 8:e1002507. doi: 10.1371/journal.ppat.1002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stamm LM, Morisaki JH, Gao LY, Jeng RL, McDonald KL, Roth R, Takeshita S, Heuser J, Welch MD, Brown EJ. 2003. Mycobacterium marinum escapes from phagosomes and is propelled by actin-based motility. J Exp Med 198:1361–1368. doi: 10.1084/jem.20031072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, Pierson J, Brenner M, Peters PJ. 2007. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 129:1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 7.Lewis KN, Liao R, Guinn KM, Hickey MJ, Smith S, Behr MA, Sherman DR. 2003. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guerin attenuation. J Infect Dis 187:117–123. doi: 10.1086/345862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majlessi L, Brodin P, Brosch R, Rojas MJ, Khun H, Huerre M, Cole ST, Leclerc C. 2005. Influence of ESAT-6 secretion system 1 (RD1) of Mycobacterium tuberculosis on the interaction between mycobacteria and the host immune system. J Immunol 174:3570–3579. doi: 10.4049/jimmunol.174.6.3570. [DOI] [PubMed] [Google Scholar]
- 9.Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. 2002. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol 46:709–717. doi: 10.1046/j.1365-2958.2002.03237.x. [DOI] [PubMed] [Google Scholar]
- 10.Gey van Pittius NC, Sampson SL, Lee H, Kim Y, van Helden PD, Warren RM. 2006. Evolution and expansion of the Mycobacterium tuberculosis PE and PPE multigene families and their association with the duplication of the ESAT-6 (esx) gene cluster regions. BMC Evol Biol 6:95. doi: 10.1186/1471-2148-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jonge MI, Pehau-Arnaudet G, Fretz MM, Romain F, Bottai D, Brodin P, Honore N, Marchal G, Jiskoot W, England P, Cole ST, Brosch R. 2007. ESAT-6 from Mycobacterium tuberculosis dissociates from its putative chaperone CFP-10 under acidic conditions and exhibits membrane-lysing activity. J Bacteriol 189:6028–6034. doi: 10.1128/JB.00469-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekiert DC, Cox JS. 2014. Structure of a PE-PPE-EspG complex from Mycobacterium tuberculosis reveals molecular specificity of ESX protein secretion. Proc Natl Acad Sci USA 111:14758–14763. doi: 10.1073/pnas.1409345111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korotkova N, Freire D, Phan TH, Ummels R, Creekmore CC, Evans TJ, Wilmanns M, Bitter W, Parret AH, Houben EN, Korotkov KV. 2014. Structure of the Mycobacterium tuberculosis type VII secretion system chaperone EspG5 in complex with PE25-PPE41 dimer. Mol Microbiol 94:367–382. doi: 10.1111/mmi.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sysoeva TA, Zepeda-Rivera MA, Huppert LA, Burton BM. 2014. Dimer recognition and secretion by the ESX secretion system in Bacillus subtilis. Proc Natl Acad Sci USA 111:7653–7658. doi: 10.1073/pnas.1322200111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daleke MH, Ummels R, Bawono P, Heringa J, Vandenbroucke-Grauls CM, Luirink J, Bitter W. 2012. General secretion signal for the mycobacterial type VII secretion pathway. Proc Natl Acad Sci USA 109:11342–11347. doi: 10.1073/pnas.1119453109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strong M, Sawaya MR, Wang S, Phillips M, Cascio D, Eisenberg D. 2006. Toward the structural genomics of complexes: crystal structure of a PE/PPE protein complex from Mycobacterium tuberculosis. Proc Natl Acad Sci USA 103:8060–8065. doi: 10.1073/pnas.0602606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daleke MH, van der Woude AD, Parret AH, Ummels R, de Groot AM, Watson D, Piersma SR, Jimenez CR, Luirink J, Bitter W, Houben EN. 2012. Specific chaperones for the type VII protein secretion pathway. J Biol Chem 287:31939–31947. doi: 10.1074/jbc.M112.397596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burggraaf MJ, Speer A, Meijers AS, Ummels R, van der Sar AM, Korotkov KV, Bitter W, Kuijl C. 2019. Type VII secretion substrates of pathogenic mycobacteria are processed by a surface protease. mBio 10. doi: 10.1128/mBio.01951-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daleke MH, Cascioferro A, de Punder K, Ummels R, Abdallah AM, van der Wel N, Peters PJ, Luirink J, Manganelli R, Bitter W. 2011. Conserved Pro-Glu (PE) and Pro-Pro-Glu (PPE) protein domains target LipY lipases of pathogenic mycobacteria to the cell surface via the ESX-5 pathway. J Biol Chem 286:19024–19034. doi: 10.1074/jbc.M110.204966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sultana R, Vemula MH, Banerjee S, Guruprasad L. 2013. The PE16 (Rv1430) of Mycobacterium tuberculosis is an esterase belonging to serine hydrolase superfamily of proteins. PLoS One 8:e55320. doi: 10.1371/journal.pone.0055320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bunduc CM, Bitter W, Houben ENG. 2020. Structure and function of the mycobacterial type VII secretion systems. Annu Rev Microbiol 74:315–335. doi: 10.1146/annurev-micro-012420-081657. [DOI] [PubMed] [Google Scholar]
- 22.Korotkova N, Piton J, Wagner JM, Boy-Rottger S, Japaridze A, Evans TJ, Cole ST, Pojer F, Korotkov KV. 2015. Structure of EspB, a secreted substrate of the ESX-1 secretion system of Mycobacterium tuberculosis. J Struct Biol 191:236–244. doi: 10.1016/j.jsb.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solomonson M, Setiaputra D, Makepeace KAT, Lameignere E, Petrotchenko EV, Conrady DG, Bergeron JR, Vuckovic M, DiMaio F, Borchers CH, Yip CK, Strynadka NCJ. 2015. Structure of EspB from the ESX-1 type VII secretion system and insights into its export mechanism. Structure 23:571–583. doi: 10.1016/j.str.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Gao LY, Guo S, McLaughlin B, Morisaki H, Engel JN, Brown EJ. 2004. A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Mol Microbiol 53:1677–1693. doi: 10.1111/j.1365-2958.2004.04261.x. [DOI] [PubMed] [Google Scholar]
- 25.Hsu T, Hingley-Wilson SM, Chen B, Chen M, Dai AZ, Morin PM, Marks CB, Padiyar J, Goulding C, Gingery M, Eisenberg D, Russell RG, Derrick SC, Collins FM, Morris SL, King CH, Jacobs WR, Jr. 2003. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci USA 100:12420–12425. doi: 10.1073/pnas.1635213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanley SA, Raghavan S, Hwang WW, Cox JS. 2003. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc Natl Acad Sci USA 100:13001–13006. doi: 10.1073/pnas.2235593100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Leon J, Jiang G, Ma Y, Rubin E, Fortune S, Sun J. 2012. Mycobacterium tuberculosis ESAT-6 exhibits a unique membrane-interacting activity that is not found in its ortholog from non-pathogenic Mycobacterium smegmatis. J Biol Chem 287:44184–44191. doi: 10.1074/jbc.M112.420869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derrick SC, Morris SL. 2007. The ESAT6 protein of Mycobacterium tuberculosis induces apoptosis of macrophages by activating caspase expression. Cell Microbiol 9:1547–1555. doi: 10.1111/j.1462-5822.2007.00892.x. [DOI] [PubMed] [Google Scholar]
- 29.Peng X, Sun J. 2016. Mechanism of ESAT-6 membrane interaction and its roles in pathogenesis of Mycobacterium tuberculosis. Toxicon 116:29–34. doi: 10.1016/j.toxicon.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Refai A, Haoues M, Othman H, Barbouche MR, Moua P, Bondon A, Mouret L, Srairi-Abid N, Essafi M. 2015. Two distinct conformational states of Mycobacterium tuberculosis virulent factor early secreted antigenic target 6 kDa are behind the discrepancy around its biological functions. FEBS J 282:4114–4129. doi: 10.1111/febs.13408. [DOI] [PubMed] [Google Scholar]
- 31.Smith J, Manoranjan J, Pan M, Bohsali A, Xu J, Liu J, McDonald KL, Szyk A, LaRonde-LeBlanc N, Gao LY. 2008. Evidence for pore formation in host cell membranes by ESX-1-secreted ESAT-6 and its role in Mycobacterium marinum escape from the vacuole. Infect Immun 76:5478–5487. doi: 10.1128/IAI.00614-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conrad WH, Osman MM, Shanahan JK, Chu F, Takaki KK, Cameron J, Hopkinson-Woolley D, Brosch R, Ramakrishnan L. 2017. Mycobacterial ESX-1 secretion system mediates host cell lysis through bacterium contact-dependent gross membrane disruptions. Proc Natl Acad Sci USA 114:1371–1376. doi: 10.1073/pnas.1620133114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Champion MM, Williams EA, Pinapati RS, Champion PA. 2014. Correlation of phenotypic profiles using targeted proteomics identifies mycobacterial esx-1 substrates. J Proteome Res 13:5151–5164. doi: 10.1021/pr500484w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fortune SM, Jaeger A, Sarracino DA, Chase MR, Sassetti CM, Sherman DR, Bloom BR, Rubin EJ. 2005. Mutually dependent secretion of proteins required for mycobacterial virulence. Proc Natl Acad Sci USA 102:10676–10681. doi: 10.1073/pnas.0504922102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J, Laine O, Masciocchi M, Manoranjan J, Smith J, Du SJ, Edwards N, Zhu X, Fenselau C, Gao LY. 2007. A unique Mycobacterium ESX-1 protein co-secretes with CFP-10/ESAT-6 and is necessary for inhibiting phagosome maturation. Mol Microbiol 66:787–800. doi: 10.1111/j.1365-2958.2007.05959.x. [DOI] [PubMed] [Google Scholar]
- 36.Champion PA, Champion MM, Manzanillo P, Cox JS. 2009. ESX-1 secreted virulence factors are recognized by multiple cytosolic AAA ATPases in pathogenic mycobacteria. Mol Microbiol 73:950–962. doi: 10.1111/j.1365-2958.2009.06821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen JM, Zhang M, Rybniker J, Boy-Rottger S, Dhar N, Pojer F, Cole ST. 2013. Mycobacterium tuberculosis EspB binds phospholipids and mediates EsxA-independent virulence. Mol Microbiol 89:1154–1166. doi: 10.1111/mmi.12336. [DOI] [PubMed] [Google Scholar]
- 38.MacGurn JA, Raghavan S, Stanley SA, Cox JS. 2005. A non-RD1 gene cluster is required for Snm secretion in Mycobacterium tuberculosis. Mol Microbiol 57:1653–1663. doi: 10.1111/j.1365-2958.2005.04800.x. [DOI] [PubMed] [Google Scholar]
- 39.McLaughlin B, Chon JS, MacGurn JA, Carlsson F, Cheng TL, Cox JS, Brown EJ. 2007. A mycobacterium ESX-1-secreted virulence factor with unique requirements for export. PLoS Pathog 3:e105. doi: 10.1371/journal.ppat.0030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ates LS, Dippenaar A, Ummels R, Piersma SR, van der Woude AD, van der Kuij K, Le Chevalier F, Mata-Espinosa D, Barrios-Payan J, Marquina-Castillo B, Guapillo C, Jimenez CR, Pain A, Houben ENG, Warren RM, Brosch R, Hernandez-Pando R, Bitter W. 2018. Mutations in ppe38 block PE_PGRS secretion and increase virulence of Mycobacterium tuberculosis. Nat Microbiol 3:181–188. doi: 10.1038/s41564-017-0090-6. [DOI] [PubMed] [Google Scholar]
- 41.Shah S, Briken V. 2016. Modular Organization of the ESX-5 Secretion System in Mycobacterium tuberculosis. Front Cell Infect Microbiol 6:49. doi: 10.3389/fcimb.2016.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tufariello JM, Chapman JR, Kerantzas CA, Wong KW, Vilcheze C, Jones CM, Cole LE, Tinaztepe E, Thompson V, Fenyo D, Niederweis M, Ueberheide B, Philips JA, Jacobs WR, Jr. 2016. Separable roles for Mycobacterium tuberculosis ESX-3 effectors in iron acquisition and virulence. Proc Natl Acad Sci USA 113:E348–E357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damen MPM, Phan TH, Ummels R, Rubio-Canalejas A, Bitter W, Houben ENG. 2020. Modification of a PE/PPE substrate pair reroutes an Esx substrate pair from the mycobacterial ESX-1 type VII secretion system to the ESX-5 system. J Biol Chem 295:5960–5969. doi: 10.1074/jbc.RA119.011682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phan TH, Ummels R, Bitter W, Houben ENG. 2017. Identification of a substrate domain that determines system specificity in mycobacterial type VII secretion systems. Sci Rep 7:42704. doi: 10.1038/srep42704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lienard J, Nobs E, Lovins V, Movert E, Valfridsson C, Carlsson F. 2020. The Mycobacterium marinum ESX-1 system mediates phagosomal permeabilization and type I interferon production via separable mechanisms. Proc Natl Acad Sci USA 117:1160–1166. doi: 10.1073/pnas.1911646117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phan TH, van Leeuwen LM, Kuijl C, Ummels R, van Stempvoort G, Rubio-Canalejas A, Piersma SR, Jimenez CR, van der Sar AM, Houben ENG, Bitter W. 2018. EspH is a hypervirulence factor for Mycobacterium marinum and essential for the secretion of the ESX-1 substrates EspE and EspF. PLoS Pathog 14:e1007247. doi: 10.1371/journal.ppat.1007247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meijers AS, Troost R, Ummels R, Maaskant J, Speer A, Nejentsev S, Bitter W, Kuijl CP. 2020. Efficient genome editing in pathogenic mycobacteria using Streptococcus thermophilus CRISPR1-Cas9. Tuberculosis (Edinb) 124:101983. doi: 10.1016/j.tube.2020.101983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brodin P, Majlessi L, Marsollier L, de Jonge MI, Bottai D, Demangel C, Hinds J, Neyrolles O, Butcher PD, Leclerc C, Cole ST, Brosch R. 2006. Dissection of ESAT-6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. Infect Immun 74:88–98. doi: 10.1128/IAI.74.1.88-98.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdallah AM, Weerdenburg EM, Guan Q, Ummels R, Borggreve S, Adroub SA, Malas TB, Naeem R, Zhang H, Otto TD, Bitter W, Pain A. 2019. Integrated transcriptomic and proteomic analysis of pathogenic mycobacteria and their esx-1 mutants reveal secretion-dependent regulation of ESX-1 substrates and WhiB6 as a transcriptional regulator. PLoS One 14:e0211003. doi: 10.1371/journal.pone.0211003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bosserman RE, Nguyen TT, Sanchez KG, Chirakos AE, Ferrell MJ, Thompson CR, Champion MM, Abramovitch RB, Champion PA. 2017. WhiB6 regulation of ESX-1 gene expression is controlled by a negative feedback loop in Mycobacterium marinum. Proc Natl Acad Sci USA 114:E10772–E10781. doi: 10.1073/pnas.1710167114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carlsson F, Joshi SA, Rangell L, Brown EJ. 2009. Polar localization of virulence-related Esx-1 secretion in mycobacteria. PLoS Pathog 5:e1000285. doi: 10.1371/journal.ppat.1000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sani M, Houben ENG, Geurtsen J, Pierson J, de Punder K, van Zon M, Wever B, Piersma SR, Jimenez CR, Daffe M, Appelmelk BJ, Bitter W, van der Wel N, Peters PJ. 2010. Direct visualization by cryo-EM of the mycobacterial capsular layer: a labile structure containing ESX-1-secreted proteins. PLoS Pathog 6:e1000794. doi: 10.1371/journal.ppat.1000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Winden VJ, Ummels R, Piersma SR, Jimenez CR, Korotkov KV, Bitter W, Houben ENG. 2016. Mycosins are required for the stabilization of the ESX-1 and ESX-5 type VII secretion membrane complexes. mBio 7. doi: 10.1128/mBio.01471-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williamson ZA, Chaton CT, Ciocca WA, Korotkova N, Korotkov KV. 2020. PE5-PPE4-EspG3 heterotrimer structure from mycobacterial ESX-3 secretion system gives insight into cognate substrate recognition by ESX systems. J Biol Chem 295:12706–12715. doi: 10.1074/jbc.RA120.012698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bunduc CM, Ummels R, Bitter W, Houben ENG. 2020. Species-specific secretion of ESX-5 type VII substrates is determined by the linker 2 of EccC5. Mol Microbiol 114:66–76. doi: 10.1111/mmi.14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Champion PA, Stanley SA, Champion MM, Brown EJ, Cox JS. 2006. C-terminal signal sequence promotes virulence factor secretion in Mycobacterium tuberculosis. Science 313:1632–1636. doi: 10.1126/science.1131167. [DOI] [PubMed] [Google Scholar]
- 57.Rosenberg OS, Dovala D, Li X, Connolly L, Bendebury A, Finer-Moore J, Holton J, Cheng Y, Stroud RM, Cox JS. 2015. Substrates control multimerization and activation of the multi-domain ATPase motor of type VII secretion. Cell 161:501–512. doi: 10.1016/j.cell.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang S, Zhou K, Yang X, Zhang B, Zhao Y, Xiao Y, Yang X, Yang H, Guddat LW, Li J, Rao Z. 2020. Structural insights into substrate recognition by the type VII secretion system. Protein Cell 11:124–137. doi: 10.1007/s13238-019-00671-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andersen P, Andersen AB, Sorensen AL, Nagai S. 1995. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol 154:3359–3372. [PubMed] [Google Scholar]
- 60.Sorensen AL, Nagai S, Houen G, Andersen P, Andersen AB. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun 63:1710–1717. doi: 10.1128/iai.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ulrichs T, Munk ME, Mollenkopf H, Behr-Perst S, Colangeli R, Gennaro ML, Kaufmann SH. 1998. Differential T cell responses to Mycobacterium tuberculosis ESAT6 in tuberculosis patients and healthy donors. Eur J Immunol 28:3949–3958. doi:. [DOI] [PubMed] [Google Scholar]
- 62.Cronin RM, Ferrell MJ, Cahir CW, Champion MM, Champion PA. 2022. Proteo-genetic analysis reveals clear hierarchy of ESX-1 secretion in Mycobacterium marinum. Proc Natl Acad Sci USA 119:e2123100119. doi: 10.1073/pnas.2123100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Demangel C, Brodin P, Cockle PJ, Brosch R, Majlessi L, Leclerc C, Cole ST. 2004. Cell envelope protein PPE68 contributes to Mycobacterium tuberculosis RD1 immunogenicity independently of a 10-kilodalton culture filtrate protein and ESAT-6. Infect Immun 72:2170–2176. doi: 10.1128/IAI.72.4.2170-2176.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sassetti CM, Rubin EJ. 2003. Genetic requirements for mycobacterial survival during infection. Proc Natl Acad Sci USA 100:12989–12994. doi: 10.1073/pnas.2134250100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Danelishvili L, Everman J, Bermudez LE. 2016. Mycobacterium tuberculosis PPE68 and Rv2626c genes contribute to the host cell necrosis and bacterial escape from macrophages. Virulence 7:23–32. doi: 10.1080/21505594.2015.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bosserman RE, Nicholson KR, Champion MM, Champion PA. 2019. A new ESX-1 substrate in Mycobacterium marinum that is required for hemolysis but not host cell lysis. J Bacteriol 201. doi: 10.1128/JB.00760-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Das S, Pettersson BMF, Behra PRK, Mallick A, Cheramie M, Ramesh M, Shirreff L, DuCote T, Dasgupta S, Ennis DG, Kirsebom LA. 2018. Extensive genomic diversity among Mycobacterium marinum strains revealed by whole genome sequencing. Sci Rep 8:12040. doi: 10.1038/s41598-018-30152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tuukkanen AT, Freire D, Chan S, Arbing MA, Reed RW, Evans TJ, Zenkeviciutė G, Kim J, Kahng S, Sawaya MR, Chaton CT, Wilmanns M, Eisenberg D, Parret AHA, Korotkov KV. 2019. Structural variability of EspG chaperones from mycobacterial ESX-1, ESX-3, and ESX-5 type VII secretion systems. J Mol Biol 431:289–307. doi: 10.1016/j.jmb.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beckham KS, Ciccarelli L, Bunduc CM, Mertens HD, Ummels R, Lugmayr W, Mayr J, Rettel M, Savitski MM, Svergun DI, Bitter W, Wilmanns M, Marlovits TC, Parret AH, Houben ENG. 2017. Structure of the mycobacterial ESX-5 type VII secretion system membrane complex by single-particle analysis. Nat Microbiol 2:17047. doi: 10.1038/nmicrobiol.2017.47. [DOI] [PubMed] [Google Scholar]
- 70.Bunduc CM, Fahrenkamp D, Wald J, Ummels R, Bitter W, Houben ENG, Marlovits TC. 2021. Structure and dynamics of a mycobacterial type VII secretion system. Nature 593:445–448. doi: 10.1038/s41586-021-03517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Famelis N, Rivera-Calzada A, Degliesposti G, Wingender M, Mietrach N, Skehel JM, Fernandez-Leiro R, Bottcher B, Schlosser A, Llorca O, Geibel S. 2019. Architecture of the mycobacterial type VII secretion system. Nature 576:321–325. doi: 10.1038/s41586-019-1633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poweleit N, Czudnochowski N, Nakagawa R, Trinidad D, Murphy KC, Sassetti CM, Rosenberg OS. 2019. The structure of the endogenous ESX-3 secretion system. Elife 8:e52983. doi: 10.7554/eLife.52983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hayward CM, O'Gaora P, Young DB, Griffin GE, Thole J, Hirst TR, Castello-Branco LR, Lewis DJ. 1999. Construction and murine immunogenicity of recombinant Bacille Calmette Guerin vaccines expressing the B subunit of Escherichia coli heat labile enterotoxin. Vaccine 17:1272–1281. doi: 10.1016/S0264-410X(98)00350-8. [DOI] [PubMed] [Google Scholar]
- 74.Stover CK, de la Cruz VF, Fuerst TR, Burlein JE, Benson LA, Bennett LT, Bansal GP, Young JF, Lee MH, Hatfull GF. 1991. New use of BCG for recombinant vaccines. Nature 351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- 75.Bardarov S, Bardarov S, Pavelka MS, Sambandamurthy V, Larsen M, Tufariello J, Chan J, Hatfull G, Jacobs WR. 2002. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology (Reading) 148:3007–3017. doi: 10.1099/00221287-148-10-3007. [DOI] [PubMed] [Google Scholar]
- 76.Rock JM, Hopkins FF, Chavez A, Diallo M, Chase MR, Gerrick ER, Pritchard JR, Church GM, Rubin EJ, Sassetti CM, Schnappinger D, Fortune SM. 2017. Programmable transcriptional repression in mycobacteria using an orthogonal CRISPR interference platform. Nat Microbiol 2:16274. doi: 10.1038/nmicrobiol.2016.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abdallah AM, Verboom T, Weerdenburg EM, Gey van Pittius NC, Mahasha PW, Jimenez C, Parra M, Cadieux N, Brennan MJ, Appelmelk BJ, Bitter W. 2009. PPE and PE_PGRS proteins of Mycobacterium marinum are transported via the type VII secretion system ESX-5. Mol Microbiol 73:329–340. doi: 10.1111/j.1365-2958.2009.06783.x. [DOI] [PubMed] [Google Scholar]
- 78.Harboe M, Malin AS, Dockrell HS, Wiker HG, Ulvund G, Holm A, Jorgensen MC, Andersen P. 1998. B-cell epitopes and quantification of the ESAT-6 protein of Mycobacterium tuberculosis. Infect Immun 66:717–723. doi: 10.1128/IAI.66.2.717-723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Bloois E, Dekker HL, Froderberg L, Houben EN, Urbanus ML, de Koster CG, de Gier JW, Luirink J. 2008. Detection of cross-links between FtsH, YidC, HflK/C suggests a linked role for these proteins in quality control upon insertion of bacterial inner membrane proteins. FEBS Lett 582:1419–1424. doi: 10.1016/j.febslet.2008.02.082. [DOI] [PubMed] [Google Scholar]
- 80.Ates LS, Ummels R, Commandeur S, van de Weerd R, van der Weerd R, Sparrius M, Weerdenburg E, Alber M, Kalscheuer R, Piersma SR, Abdallah AM, Abd El Ghany M, Abdel-Haleem AM, Pain A, Jiménez CR, Bitter W, Houben ENG. 2015. Essential role of the ESX-5 secretion system in outer membrane permeability of pathogenic mycobacteria. PLoS Genet 11:e1005190. doi: 10.1371/journal.pgen.1005190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Generation and confirmation of M. marinum frameshift mutants, including phenotypic analysis of the ppe68 fs mutant. (A, B) Each panel shows the sgRNA binding region within the gene, i.e. in ppe68 (A) and mmar_2894 (B), with a panel underneath showing the sequencing results that were obtained after PCR. (C) SDS-PAGE and immunostaining of the cell pellet fraction of M. marinum WT, the eccCb1 mutant (MVU – ESX-1 mutant), the ppe68 fs mutant and the mmar_2894 fs mutant. The ppe68 operon mutant (Δpe35-esxA) with and without the ppe68 operon expressed from a multicopy pSMT3 vector under control of the hsp60 promotor served as the positive and negative control for EsxA production, respectively. The ppe68 fs mutant and mmar_2894 fs mutant also contained a pMV TdTomato plasmid. Proteins were visualized with an anti-EsxA antibody, and as a loading control, blots were incubated with antibodies directed against the cytosolic GroEL2 protein. An amount of 0.2 OD units was loaded for the pellet fraction. Data are representative of two independent experiments. (D) Relative esxA mRNA levels of an eccCb1 mutant (MVU, ESX-1 mutant), the ppe68 fs mutant, and the ppe68 fs mutant complemented with pe35/ppe68 expressed from the pMST3 vector, compared to the WT. Cycle threshold (CT) values were normalized for CT values of the household gene sigA and compared to the other CT values. Data shown are from two independent experiments including 3 technical replicates each. Download FIG S1, TIF file, 1.5 MB (1.6MB, tif) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Protein sequence alignment of PPE68 homologs of M. marinum and M. tuberculosis, including the secondary structure prediction of M. tuberculosis PPE68. The secondary structure prediction was produced by Alphafold2. The blue square indicates the residue at position 125 involved in EspG1 binding. Underlined sequences indicate the C-terminal truncations according to the color key. Rv3873, PPE68 of M. tuberculosis; MMAR_5448, PPE68 of M. marinum; MMAR_0186, PPE68_1 of M. marinum. Download FIG S2, TIF file, 1.6 MB (1.6MB, tif) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Deletion of the paralogous ppe68_1 operon does not affect EspE secretion or hemolytic activity of M. marinum. (A) SDS-PAGE and immunostaining were performed on the cell pellet, culture supernatant, Genapol pellet, and Genapol supernatant fractions of M. marinum WT, an ESX-1 mutant (eccCb1 mutant; MVU), the ppe68 operon mutant (Δpe35-esxA), and the ppe68_1 operon mutant (Δpe35_1-esxA_1). Proteins were visualized with anti-EspE and anti-PPE68 (ESX-1 substrates) antibodies. A processed band of 25 kDa was detected by the anti-EspE antibody in the Genapol supernatant fraction of the WT, which has been reported before (Phan et al., 2018 [46]). As a loading and lysis control, blots were incubated with antibodies directed against the cytosolic GroEL2 protein. In all blots, equivalent OD units were loaded: 0.2 OD for pellet and Genapol pellet and 0.4 OD for supernatant and Genapol supernatant fractions. Data shown are representative of two independent experiments. (B) Hemoglobin release after incubation of the same strains as in panel A and the ppe68 fs mutant expressing the ppe68_1 operon with sheep red blood cells. Hemoglobin release was quantified by determining the OD450 of the medium after incubation. Data shown are from two independent experiments including 3 technical replicates each. Download FIG S3, TIF file, 0.8 MB (885.4KB, tif) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
A point mutation in the active site of serine protease MycP1 does not affect the processing of PPE68 on the cell surface. SDS-PAGE and immunostaining of concentrated Genapol supernatant fractions of M. marinum WT and a mycP1 deletion mutant complemented with a mycP1 active site (S354A) variant, expressed from a pMV vector by the hsp60 promotor. Proteins were visualized with an anti-PPE68 antibody. As a loading and lysis control, blots were incubated with antibodies directed against the cytosolic GroEL2 protein and the surface-localized PE_PGRS proteins. In all blots, equivalent amounts, i.e. 2 OD units, of Genapol supernatant fractions were loaded. Data shown are representative of two independent experiments. Download FIG S4, TIF file, 0.6 MB (664.8KB, tif) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Purification of PPE68 from the ppe68 operon mutant and the eccCa1 mutant containing a pMV or pSMT3 vector with the ppe68 operon of M. marinum. Genes were expressed from the hsp60 promotor with or without a Strep tag on the C terminus of PPE68. (A) Immunostaining of subcellular fractionations of the different strains. Proteins were visualized with anti-PPE68 and anti-StrepII antibodies, while anti-FtsH antibodies controlled for the loading and lysis. Notably, the FtsH antiserum cross-reacted with a cytosolic protein. In all blots, equivalent amounts corresponding to 0.2 OD units were loaded. T, total lysate; S, supernatant or soluble proteins; P, pellet or cell envelope proteins. (B) Separation of the PPE68 purification samples by SDS-PAGE and subsequent staining with Coomassie brilliant blue. (C) Separation of complexes in the PPE68 purification samples by BN-PAGE and subsequent staining with Coomassie brilliant blue. As a negative control, the purification sample of the ppe68 operon mutant containing a pSMT3 vector with the ppe68 operon, without C-terminal Strep tag on PPE68, was included. Data shown in panls A, B, and C are representative of two independent experiments. (D) Quantitative proteomics analysis comparing the PPE68 affinity purification from the soluble fraction of the ΔeccCa1 mutant and the ppe68 operon mutant (Δpe35-esxA) producing PPE68 with or without a Strep tag on the C terminus, from a pSMT3 vector. Data included two technical replicates per sample. Proteins that showed a log2 fold change of >2 and a −log10 P value of >1.3 between tagged and untagged PPE68 are indicated as blue dots. Size of the dots are correlated to the number of spectral counts. Download FIG S5, TIF file, 1.4 MB (1.4MB, tif) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Protein sequence alignment of PPE35 homologs of M. marinum and M. tuberculosis, including the secondary structure prediction of M. tuberculosis PE35. The secondary structure prediction was produced by Alphafold2. The blue square indicates the conserved YxxxD/E secretion motif. Note that PE35 of M. marinum lacks this conserved motif. Rv3872, PE35 of M. tuberculosis; PE35, MMAR_5447 of M. marinum; PE35_1, MMAR_0185 of M. marinum. Download FIG S6, TIF file, 1.3 MB (1.4MB, tif) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Significant hits of PPE68 interactors as determined by protein purification and mass spectrometry analysis (only proteins with an average normalized spectral count of >5 in the tagged samples, a log2 fold change of >2, and a −log10 P value of >1.3 between tagged and untagged samples are shown). Download Table S1, DOCX file, 0.01 MB (15.9KB, docx) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Secretion and hemolytic analysis of the mmar_2894 fs mutant. (A) SDS-PAGE and immunostaining of the cell pellet, culture supernatant, Genapol pellet, and Genapol supernatant fractions of M. marinum, WT, an ESX-1 mutant (eccCb1 mutant; MVU), and the mmar_2894 fs mutant. Proteins were visualized with anti-EsxA, anti-EspE, and anti-PPE68 antibodies (ESX-1 substrates). A processed band of 25 kDa was detected by the anti-EspE antibody in the Genapol supernatant fraction of the WT, which has been reported before (Phan et al., 2018 [46]). As a loading and lysis control, blots were incubated with antibodies directed against the cytosolic GroEL2 protein. In all blots, equivalent OD units were loaded: 0.2 OD for pellet and Genapol pellet and 0.4 OD for supernatant and Genapol supernatant fractions. (B) SDS-PAGE and immunostaining of the Genapol pellet and upscaled Genapol supernatant fractions of M. marinum WT, the ppe68 fs mutant, and the mmar_2894 fs mutant. Proteins were visualized with an anti-PPE68 antibody (ESX-1 substrate). As a loading and lysis control, blots were incubated with antibodies directed against the cytosolic GroEL2 protein and the surface-localized PE_PGRS proteins. In all blots, equivalent OD units were loaded: 0.2 OD for Genapol pellet and 2 OD Genapol supernatant fractions. Data shown in panels A and B are representative of two independent experiments. (C) Hemoglobin release after incubation of the same strains shown in panels A and B with sheep red blood cells, quantified by determining the OD450 of the medium after incubation. Data shown are from two independent experiments that included 3 technical replicates each. Download FIG S7, TIF file, 1.4 MB (1.4MB, tif) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Strains and plasmids used in this study. Download Table S2, DOCX file, 0.03 MB (28.1KB, docx) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Primers used in this study. Download Table S3, DOCX file, 0.02 MB (24.8KB, docx) .
Copyright © 2022 Damen et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.