Abstract
Thrombospondin-related anonymous protein (TRAP), a candidate malaria vaccine antigen, is required for Plasmodium sporozoite gliding motility and cell invasion. For the first time, the ability of antibodies against TRAP to inhibit sporozoite infectivity in vivo is evaluated in detail. TRAP contains an A-domain, a well-characterized adhesive motif found in integrins. We modeled here a three-dimensional structure of the TRAP A-domain of Plasmodium yoelii and located regions surrounding the MIDAS (metal ion-dependent adhesion site), the presumed business end of the domain. Mice were immunized with constructs containing these A-domain regions but were not protected from sporozoite challenge. Furthermore, monoclonal and rabbit polyclonal antibodies against the A-domain, the conserved N terminus, and the repeat region of TRAP had no effect on the gliding motility or sporozoite infectivity to mice. TRAP is located in micronemes, secretory organelles of apicomplexan parasites. Accordingly, the antibodies tested here stained cytoplasmic TRAP brightly by immunofluorescence. However, very little TRAP could be detected on the surface of sporozoites. In contrast, a dramatic relocalization of TRAP onto the parasite surface occurred when sporozoites were treated with calcium ionophore. This likely mimics the release of TRAP from micronemes when a sporozoite contacts its target cell in vivo. Contact with hepatoma cells in culture also appeared to induce the release of TRAP onto the surface of sporozoites. If large amounts of TRAP are released in close proximity to its cellular receptor(s), effective competitive inhibition by antibodies may be difficult to achieve.
Transmission of malaria occurs by the injection of Plasmodium sporozoites during the bloodmeal of an infected mosquito. Once in the bloodstream, sporozoites rapidly and efficiently invade the liver cells in which they will multiply. Two parasite proteins, CS (circumsporozoite protein) and TRAP (thrombospondin-related anonymous protein; also called Ssp2), are known to play roles in this process. CS uniformly coats the surface of the sporozoite and binds specifically to heparan sulfate proteoglycans on hepatocytes. This binding occurs via region II-plus, a motif within the thrombospondin type 1 repeat (TSR) of CS (10, 16, 27). CS also contains a repeat region, and antibodies against these repeats immobilize sporozoites (34) and neutralize their infectivity (28, 33). Though recombinant and peptide Plasmodium falciparum CS vaccines have had some success in human trials, sustaining protective anti-CS titers is difficult (35, 36). Since CS most likely functions to arrest sporozoites in the liver, antibodies against a parasite protein involved in the invasion process may synergize with anti-CS antibodies. Several lines of evidence suggest that TRAP plays such a role.
TRAP is required for sporozoite gliding motility and cell invasion (20, 37). It belongs to a family of proteins (which includes MIC2 of Toxoplasma gondii) that are found in the micronemes of invasive stages of apicomplexan parasites. Members of the TRAP family are type 1 transmembrane proteins that contain at least one copy of the TSR (containing a region II-plus motif), and most contain one or more A-domains. TRAP carries one of each of these two adhesive domains, followed by a stretch of repeats in its extracellular portion (Fig. 1A). Most A-domains, such as those found in several integrin α-subunits, contain a MIDAS (metal ion-dependent adhesion site) (14, 19, 23). Ligand binding sites typically lie near the MIDAS, on the A-domain's metal binding face (11, 18, 29, 40, 43). Point mutations of the amino acids in the MIDAS of the TRAP A-domain greatly impaired the ability of sporozoites to invade hepatocytes (23). Although its function is unknown, TRAP also contains a 10-amino-acid region at its N terminus (designated N-10) that is conserved in all species of mammalian malaria parasites examined so far (reference 38; Table 1).
FIG. 1.
The domains of TRAP. (A) Schematic diagram of TRAP: SS, signal sequence; N-10, conserved N-terminal 10 amino acids; A-domain; TSR, thrombospondin type 1 repeat; TM, transmembrane domain; CD, cytoplasmic domain. (B and C) The crystal structure of the CD11b A-domain (22) (B) was used as the template to model the P. yoelii TRAP A-domain (C). Shown in magenta are Mg2+ ions coordinated by the MIDAS. The B-cell epitopes of the TRAP A-domain are highlighted as follows: yellow, B1; red, B2; green, B3.
TABLE 1.
TRAP N-10 region of Plasmodium spp.
| Host | Species | N-10 amino acid sequence |
|---|---|---|
| Rodents | P. yoelii | DEIKYSEEVC |
| P. berghei | ---------- | |
| Monkeys | P. knowlesi | ---------- |
| Humans | P. vivax | ---------- |
| P. falciparuma | -----R---- | |
| Birds | P. gallinaceum | ---T-N-QI- |
P. falciparum isolates vary between the amino acids R and S in the sixth position.
Because of TRAP's central role in cell invasion, we decided to evaluate the ability of antibodies against TRAP to block P. yoelii sporozoite infectivity in vivo. Since it is difficult to raise antibodies against the region II-plus of native CS (5; E. Nardin, personal communication), we targeted the other extracellular regions of TRAP: N-10, the repeat region, and the A-domain.
MATERIALS AND METHODS
Modeling of the TRAP A-domain.
Modeling was performed with the Molsoft ICM program, which was developed for molecular modeling and structure predictions by global restrained energy optimization of arbitrarily constrained molecules (1, 2). The template structure used was the A-domain of CD-11b (22).
Vaccine constructs.
Synthetic peptides T (SFERFEIFPKE; a T-cell epitope from influenza virus hemagglutinin protein), B1 (IPNDLPRSTAVVHQLKRKH), B2 (RFILAHLQNNYSPNGNTN), B3 (VGAGVNNEYNRILVG), Ser-TGGB1 (SSFERFEIFPKEGGIPNDLPRSTAVVHQLKRKH), Ser-TGGB2 (SSFERFEIFPKEGGRFILAHLQNNYSPNGNTN), and Ser-TGGB3 (SSFERFEIFPKEGGVGAGVNNEYNRILVG) were made by solid-phase synthesis on a model 430A machine (Applied Biosystems) using the improved tert-butoxycarbonyl chemistry (32) and were purified by reversed-phase high-pressure liquid chromatography (HPLC) and analyzed by electrospray ionization mass spectrometry as previously described (31). The N-terminal Ser residues of the diepitopic peptides (peptides TGGB1- to -3) were oxidized with periodate and, for each one, a tetraoxime was formed between the resulting glyoxylyl peptides and a tetrabranched core, (NH2OCH2CO)4-Lys2-Lys-Ser-Ser-Lys(Pam3Cys)-(Lys)4-OH (26, 42). Tetraoximes were purified by HPLC and characterized by electrospray mass spectrometry. Purified peptides and tetraoximes eluted as single components on analytical HPLC, and their masses were in very close agreement with calculated values. Peptide N-10 (DEIKYSEEVC) was synthesized by AnaSpec, Inc. (San Jose, Calif.), whereas it was purified by HPLC and analyzed by mass spectrometry. Peptide N-10 was then conjugated to keyhole limpet antigen for the production of antisera. The B1 epitope was also expressed in Escherichia coli as a fusion protein with hepatitis B core antigen (B1-HBcAg) using methodology similar to that described previously (44).
Immunizations and production of antibody reagents.
Groups of eight female, 6- to 8-week-old BALB/c mice were given a first injection of 100 μg of TGGB1 polyoxime, either subcutaneously, in the case of the vaccine given with phosphate-buffered saline (PBS) alone or with TiterMax (CytRx Corporation, Norcross, Ga.), or intraperitoneally, when given with complete Freund's adjuvant. Two boosters were given consisting of 50 μg of TGGB1 tetraoxime in either PBS, TiterMax, or incomplete Freund's adjuvant at 3-week intervals after the primary injection. TGGB3 tetraoxime was given as described above without adjuvant. Twenty micrograms of B1-HbcAg was given intraperitoneally with complete Freund's adjuvant. Titers were assessed by enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence (IF). To produce monoclonal antibodies against A-domain region B1, spleens from immunized mice were removed 1 week after the third injection for fusion with MOPC-21 (17). Hybridoma supernatants were screened for reactivity with air-dried P. yoelii sporozoites by IF staining. Positive hybridomas were cloned twice by limiting dilution, and antibodies were purified using HiTRAP-protein A columns (Pharmacia). In the case of monoclonal antibody 291-17 (immunoglobulin M [IgM]), purification was performed using gel filtration chromatography with a Superose 6 10/30 column (Pharmacia). Tetraoximes TGGB1, -2, and -3, as well as peptide N-10/KLH, were used to generate rabbit polyclonal antisera. New Zealand White rabbits were injected with 100 μg of antigen with TiterMax adjuvant subcutaneously and boosted twice at 3-week intervals. Sera were screened by ELISA and IF staining. The monoclonal antibody NYS1 (4), which is against the repeat region of P. yoelii CS, was kindly provided by Y. Charoenvit (Naval Medical Research Institute, Bethesda, Md.), and the monoclonal antibody against the repeat region of TRAP, F3B5, was provided by E. Nardin (New York University School of Medicine, New York, N.Y.).
ELISA.
Wells of Falcon Pro-Bind assay plates (Becton Dickinson, Lincoln Park, N.J.) were coated with 10 μg of the relevant peptide (B1 or B3) antigen per ml for 2 h at 37°C and then blocked with 3% bovine serum albumin (BSA)-PBS for 1 h at 37°C. Dilutions of primary and secondary antibodies were in 1% BSA-PBS, and incubations for each were for 1 h at 37°C. The substrate and the secondary antibodies (goat anti-mouse or rabbit IgG conjugated to horseradish peroxidase; Kierkegaard and Perry Laboratories, Gaithersburg, Md.) were used according to the manufacturer's instructions. Duplicate wells were tested for each measurement.
Cultivation and infection of HepG2 cells.
HepG2 cells were cultured in Dulbecco modified Eagle medium containing 10% heat-inactivated fetal calf serum, 100 U of penicillin, and 100 μg of streptomycin per ml at 37°C in a humid atmosphere with 5% CO2. Two days before infection, cells were seeded onto coverslips (12 mm in diameter; 104 cells/cm3) in a 24-well plate. Then, 105 salivary gland sporozoites were added to subconfluent HepG2 cells. To facilitate the contact between sporozoites and cells, the 24-well plates were centrifuged at 400 × g and 4°C for 1 min. Specimens were incubated at 37°C for 10 min before fixing and staining.
Immunofluorescence stainings.
For the screening of antisera and hybridoma supernatants, 2 × 103 P. yoelii sporozoites were permeabilized by air drying on glass slides. To visualize TRAP, the parasites were incubated with primary antibody for 1 h, followed by incubation with anti-mouse or rabbit fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (Kierkegaard and Perry Laboratories) for 30 min. For the ionophore experiments, 105 sporozoites/sample were initially fixed in 2% paraformaldehyde for 15 min at room temperature and then quenched with 0.1 M glycine. Surface-exposed TRAP was stained by incubation with 50 μg of F3B5 per ml for 2 h, followed by treatment with FITC-conjugated anti-mouse secondary antibodies for 1 h. For staining sporozoites that had been in contact with HepG2 cells, the preparations were first fixed in 2% paraformaldehyde. Surface labeling of TRAP was performed with F3B5 and FITC-conjugated anti-mouse secondary antibodies. The specimens were then permeabilized with 0.5% Triton X-100, and the actin cytoskeleton of the HepG2 cells was labeled using rhodamine-conjugated phalloidin (Molecular Probes, Eugene, Oreg.) for 10 min at room temperature. The parasites were then labeled a second time with F3B5 followed by rhodamine-conjugated anti-mouse secondary antibodies. Phalloidin does not stain parasite actin in T. gondii (24) or in P. yoelii sporozoite actin (data not shown). Thus, the red signal from the sporozoite is exclusively due to TRAP. All incubations with antibodies were performed at 37°C, and the stained specimens were mounted in Vectashield anti-fading medium (Vector Laboratories, Burlingame, Calif.).
Sporozoite challenge of immunized mice and neutralization experiments.
Immunized mice were each injected via the tail vein with 100 μl of freshly dissected P. yoelii sporozoites at 105/ml in RPMI 1640 medium. For the neutralization experiments, sporozoites were incubated with the indicated concentration of monoclonal antibody or serum for 30 min at room temperature and then directly injected as described above. Forty hours after injection into mice, parasites in the liver were quantitated by competitive reverse transcription-PCR (RT-PCR) of parasite rRNA as previously described (6).
Gliding motility.
Gliding motility was assessed in a blinded fashion after incubating the sporozoites with antibodies as described above. Sporozoites were placed on glass slides, and 40 sporozoites from each treatment were observed for 1 min each.
Ionophore treatments.
Tubes containing 105 freshly dissected sporozoites on ice were transferred to 37°C for 2 min to warm them. Then, 1 μl of a 100× stock of A23187 (CalBiochem; diluted in dimethyl sulfoxide [DMSO]) or a DMSO control was added to each tube, and the tubes were returned to 37°C for 2 min. Sporozoites were immediately pelleted at 4°C for 5 min at 4,000 × g, fixed, and stained for surface-exposed TRAP by IF with F3B5 as described above. In some experiments, before warming and the addition of ionophore, sporozoites were incubated for 10 min on ice with 1 μl of BAPTA/AM (CalBiochem; diluted in DMSO) or a DMSO control. One hundred sporozoites from each treatment were examined in a blinded fashion by phase-contrast microscopy, counting the number that displayed a bright cap-like pattern of fluorescence.
Fluorescence confocal microscopy and image processing.
Fluorescence images were obtained with a confocal laser scanning microscope equipped with dual detectors and an argon (Ar-Kr) laser for simultaneous scanning of two fluorochromes (Sarastro 2000; Molecular Dynamics, Sunnyvale, Calif.). Images were processed using Adobe Photoshop software (Adobe, San Jose, Calif.).
RESULTS
TRAP A-domain modeling and epitope selection.
We constructed a three-dimensional model of the P. yoelii TRAP A-domain, using the A-domain of CD11b as the template (Fig. 1). Three regions, B1, -2, and -3, of the TRAP A-domain were chosen as B-cell epitopes (Fig. 1C) based on the following criteria: they were predicted to be near the MIDAS, they were surface exposed, and they were predicted to have a minimum of secondary structure. In addition, the corresponding regions have ligand binding capacities in the A-domains of several integrins (11, 18, 29, 40, 43). These TRAP A-domain regions have no known sequence homology to mammalian proteins. Tetravalent polyoxime vaccines (called TGGB1, -2, and -3) were produced, each containing four copies of one of the three A-domain regions. To generate T-cell help, we included an immunodominant epitope from the influenza hemagglutinin protein. The tetraoximes also contained a Pam3Cys lipid moiety, which acts as a built-in adjuvant (42). The B1 region, which was studied in more detail, was also expressed as a hepatitis B core antigen fusion protein (B1-HBcAg).
Immunizations with A-domain constructs.
Groups of mice were immunized with the tetraoxime (TGGB1 or -3) or B1-HBcAg constructs. Serum antibody titers against peptides representing the A-domain B-cell epitopes were high when measured by ELISA (Table 2). However, reactivity against intact parasites was much lower. The addition of exogenous adjuvants did not increase the immunogenicity of the tetraoximes. The mice were challenged with live sporozoites, and infection was quantitated by competitive RT-PCR of liver RNA using primers for parasite rRNA. None of the constructs tested conferred protection relative to the controls (Table 2).
TABLE 2.
TRAP A-domain vaccine constructs testeda
| Expt | Vaccine | Adjuvant | Parasite rRNA (group avg [±SD])c | Antibody titerd
|
|
|---|---|---|---|---|---|
| ELISA | IF | ||||
| 1 | TGGB1 | None | 3.4 (0.7) | 1:104–105 | 1:10–102 |
| Control | None | 2.7 (1.1) | |||
| 2 | TGGB1 | TiterMax | 5.1 (0.6) | 1:103–105 | 1:10–102 |
| Control | TiterMax | 5.5 (0.3) | |||
| 3 | TGGB1 | QS-21 | 3.4 (1.0) | 1:104–105 | 1:10–102 |
| Control | QS-21 | 3.4 (0.6) | |||
| 4 | TGGB1 | Freund'sb | 12.4 (2.4) | 1:104–105 | 1:10–102 |
| Control | Freund's | 11.4 (1.7) | |||
| HbcAg-B1 | Freund's | 11.7 (1.8) | 1:104–105 | 1:10–102 | |
| HbcAg (control) | Freund's | 11.9 (1.5 | |||
| 5 | TGGB3 | None | 4.6 (1.0) | 1:104–105 | |
| Control | None | 4.3 (1.3) | |||
Groups of eight mice were immunized and then challenged with 104 P. yoelii sporozoites/mouse.
The initial injections were performed with complete Freund's adjuvant, and the boosters were done with incomplete Freund's adjuvant.
Values indicate the group averages (± the standard deviations) of parasite rRNA.
Titers represent the range within each group. ELISAs were performed against the peptide, B1 or B3, that is present in the antigen used for immunization. IF labeling was performed on air-dried P. yoelii sporozoites.
Neutralization experiments.
The lack of protection in immunized mice might have been due to the low titers of antiparasite antibodies. In an attempt to overcome the problem, experiments were performed in which P. yoelii sporozoites were preincubated with high titers of anti-TRAP antibodies before being injected into naive mice. Several antibodies were tested: monoclonal antibodies against the A-domain B1 epitope and the repeat region and also rabbit polyclonal antisera against the B1 and B2 epitopes of the A-domain and the N-10 region (Table 3). All anti-TRAP antibodies reacted strongly with sporozoites, showing the typical cytoplasmic TRAP staining pattern by IF (Table 3; Fig. 2A). In addition, these antibodies reacted with a single 140-kDa band by Western blotting of crude P. yoelii sporozoite extract (data not shown). However, none of the antibodies inhibited sporozoite infectivity (Tables 4 and 5). As positive controls, groups of five mice were injected with sporozoites that had been preincubated with a monoclonal antibody against the P. yoelii CS repeats (NYS1). As expected, the infectivity of these sporozoites was greatly inhibited (Tables 4 and 5). To control for the linearity of the RT-PCR assay, 10-fold-fewer sporozoites were injected into one group of mice. This resulted in an ∼10-fold reduction in the amount of parasite rRNA in these mice (Table 4). Finally, there was no inhibition of gliding motility after incubation of sporozoites with any of the anti-TRAP antibodies compared to the controls (72.5 to 90% of sporozoites were observed to glide). In contrast, the NYS1 anti-CS antibody profoundly inhibited gliding at 50 μg/ml (0 to 2.5% gliding).
TABLE 3.
Antibodies used in neutralization experiments
| Antibody | Epitope | Type | IFa |
|---|---|---|---|
| 250-30 | B1 | Monoclonal IgG1, mouse | 0.1 μg/ml |
| 141-32 | B1 | Monoclonal IgG1, mouse | 1 μg/ml |
| 297-17 | B1 | Monoclonal IgM, mouse | 10 μg/ml |
| PAb-B1 | B1 | Polyclonal, rabbit | 1:1,000 |
| PAb-B2 | B2 | Polyclonal, rabbit | 1:500 |
| PAb-N10 | N10 | Polyclonal, rabbit | 1:2,000 |
| F3B5 | TRAP repeat | Monoclonal IgG1, mouse | 0.1 μg/ml |
| NYS1 | CS repeat | Monoclonal IgG3, mouse | 0.01 μg/ml |
Value shown for each antibody is the minimum concentration at which IF could be detected against air-dried P. yoelii sporozoites.
FIG. 2.
Confocal images of P. yoelii sporozoites stained for TRAP by IF. (A) Staining pattern seen in all permeabilized sporozoites. (B) Typical faint TRAP surface staining pattern. (C) Bright cap-like TRAP surface staining pattern rarely seen on sporozoites. All sporozoites were stained with monoclonal antibody F3B5. All images were scanned under identical conditions. Scale bar units are micrometers.
TABLE 4.
Neutralization experiments with anti-TRAP monoclonal antibodiesa
| Expt | Parasite rRNA (group avg [±SD])b with:
|
||||||
|---|---|---|---|---|---|---|---|
| Control | F3B5 (500 μg/ml) | 250-30 (500 μg/ml) | 141-32 (500 μg/ml) | 291-17 (500 μg/ml) | NYS1 (50 μg/ml) | NYS1 (250 μg/ml) | |
| 1 | 8.3 (1.3) | 9.4 (1.4) | 8.8 (1.4) | 9.5 (1.0) | ND | ND | ND |
| 2 | 9.1 (0.7) | 9.0 (0.6) | 8.6 (0.6) | 8.3 (1.0) | ND | 3.1 (1.0) | ND |
| 3 | 9.8 (1.3) | 10.6 (1.2) | 10.9 (1.1) | 10.0 (1.5) | ND | 2.5 (0.6) | 0.4 (0.2) |
| 4 | 9.3 (1.3) | ND | ND | ND | 8.2 (1.0) | ND | ND |
Groups of five mice were injected with 104 P. yoelii sporozoites preincubated for 30 min with the indicated concentrations of monoclonal antibodies or rabbit sera (at a 1:5 dilution). ND, not done.
Values indicate group averages (± the standard deviations) of parasite rRNA.
TABLE 5.
Neutralization experiments with anti-TRAP polyclonal antiseraa
| Expt | Controlsb
|
PAb-B1 | PAb-B2 | PAb-N-10 | |
|---|---|---|---|---|---|
| I | II | ||||
| 1 | 5.3 (0.8) | 0.5 (0.1) | 5.7 (0.5) | 5.1 (0.9) | ND |
| 2 | 4.1 (0.7) | ND | ND | ND | 4.5 (0.5) |
| 3 | 8.2 (1.0) | ND | 8.0 (0.4) | 8.6 (1.2) | 7.6 (1.1) |
Values indicate group averages (± the standard deviations) of parasite rRNA. ND, not done.
Mice in group I were injected with 104 P. yoelii sporozoites, while group II mice received 103 sporozoites.
Assessment and induction of TRAP surface exposure.
It could be argued that the inability of anti-TRAP antibodies to neutralize sporozoites was due to TRAP's sequestration in sporozoite micronemes (3, 30), and its release during host cell invasion. Based on a large number of observations, approximately 50 to 70% of sporozoites were found to have detectable surface-exposed TRAP by IF staining. This surface staining is most often in a very faint, punctate pattern (Fig. 2B). Only a small proportion (ca. 3%) of sporozoites display one or more bright patches of surface fluorescence. Bright surface staining was commonly in the form of a “cap” over one pole of the sporozoite (Fig. 2C; references 20 and 30). This is a typical staining pattern of MIC2 of Toxoplasma gondii and other apicomplexan micronemal proteins that are released onto the parasite surface upon target cell contact (9, 15, 39). Bright surface caps were distinct from the bipolar, nucleus-sparing staining of TRAP obtained when sporozoites are permeabilized (Fig. 2A; references 13 and 30).
Agents that increase intracellular calcium induce T. gondii microneme secretion, resulting in relocalization of MIC2 to the parasite surface, and a transient inhibition of invasiveness (7, 8). To verify if a similar relocalization of TRAP occurs in Plasmodium spp., sporozoites were treated with a calcium ionophore, A23187, and examined for surface TRAP staining by IF. Incubation with A23187 increased the number of sporozoites with bright caps of surface TRAP staining by ∼5-fold (Fig. 3). This effect was reversed by pretreating sporozoites with BAPTA/AM, a chelator of intracellular calcium (Fig. 3B). Microneme secretion in T. gondii tachyzoites exhibited a similar dose response to A23187 treatment and was also prevented by BAPTA/AM (8).
FIG. 3.
Calcium ionophore A23187 increases the proportion of sporozoites with caps of bright TRAP surface staining. (A) Sporozoites were incubated with the indicated concentrations of A23187 for 2 min before fixation and IF labeling of surface-exposed TRAP. (B) The effect was reversed by pretreatment with BAPTA/AM, a membrane-permeant calcium chelator. For each treatment, 100 sporozoites were counted in blinded fashion. Each bar represents the average of two independent experiments.
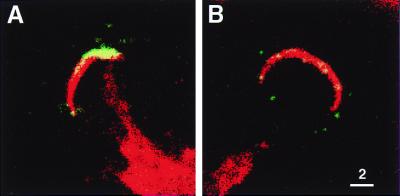
We next examined if TRAP release was also induced by cell contact. Sporozoites were incubated with HepG2 cells for 10 min at 37°C, followed by IF labeling of surface-exposed TRAP. To reliably determine whether sporozoites were in contact with cells, confocal microscopy was used. Those sporozoites that appeared to be in contact with HepG2 cells often displayed bright caps of TRAP surface labelling (Fig. 4A), while little or no TRAP staining could be detected on the surface of sporozoites not in contact with cells (Fig. 4B).
FIG. 4.
Confocal images of P. yoelii sporozoites incubated with HepG2 cells. Sporozoites were stained for surface-exposed TRAP (green) prior to permeabilization, followed by intracellular TRAP staining (red), using the monoclonal antibody F3B5. The actin of HepG2 cells, but not sporozoites, was stained with phalloidin (red). Thus, the red staining of parasites is due exclusively to TRAP. (A) Sporozoites in contact with cells often displayed bright cap patterns of surface TRAP labeling. (B) Only weak surface TRAP staining was typically detectable on sporozoites that were not in contact with cells. Images were scanned under identical conditions. Scale bar units are micrometers.
DISCUSSION
Recent findings highlight the essential role of TRAP in sporozoite motility and cell invasion (20, 37; K. Matuschewski, A. Nunes, V. Nussenzweig, and R. Ménard, Woods Hole Mol. Parasitol. Meet. X, abstr. 105, 1999). Our working hypothesis is that sporozoite infectivity depends on the attachment of the extracellular domains of TRAP (the A-domain and region II-plus) to target cell receptors. This is the first detailed evaluation of the in vivo activity of anti-TRAP antibodies and the first attempt to target antibodies to the TRAP MIDAS and N-10 region. Previously, two passive immunization experiments using high concentrations of antibodies against the TRAP repeats showed protection against sporozoite challenge in zero out of ten and one out of eight mice, respectively (13, 41). Another study showed no protection of mice despite high titers against undetermined epitopes of TRAP (21). There are, however, reports of modest in vitro inhibition of sporozoite cell invasion (12, 25, 30).
In this study, neither active immunizations with A-domain peptides nor passive immunization with monoclonal or polyclonal antibodies to the A-domain or other TRAP regions had any effect on the ability of sporozoites to infect mice. As a possible explanation for these results, it could be argued that the appropriate epitopes were not targeted. Alternatively, as discussed in detail below, antibodies may be ineffective because the majority of TRAP is sequestered within micronemes and only discharged upon target cell contact.
The first possibility does not seem likely because antibodies against multiple conserved extracellular regions of TRAP, with the exclusion of region II-plus, were tested. As mentioned, repeated attempts to generate antibodies to region II-plus that react with sporozoites have been unsuccessful, presumably because of the prevalence of the TSR in mammalian proteins (33).
Most of our efforts focused on peptides that surround the MIDAS of the TRAP A-domain. The selection of B-cell epitopes near the MIDAS was performed based on the structural conservation of A-domains. In several integrins, these epitopes correspond to ligand binding sites (11, 18, 29, 40, 43). Importantly, antibodies against these epitopes inhibit integrin function (11, 18, 43). Transfection studies performed with P. berghei provide a strong additional rationale for targeting the MIDAS of the TRAP A-domain. Point mutations introduced into the DxSxS-T-D residues that comprise the MIDAS of TRAP resulted in a dramatic impairment of sporozoite infectivity (Matuschewski et al., Woods Hole Mol. Parasitol. Meet. X, 1999).
Antibodies to two other TRAP regions, the repeats and N-10, were also not inhibitory. The TRAP repeat region was studied based on a report that anti-repeat antibodies partially inhibited P. falciparum sporozoite cell invasion in vitro (12). However, the monoclonal antibody against the TRAP repeats tested here (F3B5) did not inhibit in vivo infectivity of P. yoelii sporozoites, even when incubated at 500 μg/ml.
The rationale for studying the inhibitory properties of antibodies against the N-10 region of TRAP is its remarkably high degree of sequence conservation. Aside from a single amino acid that varies in some isolates of P. falciparum, this region is identical among Plasmodium species that infect mammals (Table 1; reference 38). Notably, in a Plasmodium species that infects birds, this region is significantly divergent (Table 1). Because sporozoites of mammalian and avian malaria parasites infect different host cells (hepatocytes versus macrophages and endothelial cells, respectively), we hypothesized that antibodies against N-10 might interfere with sporozoite infectivity. However, anti-N-10 polyclonal antisera, which reacted strongly with sporozoites by IF, did not reduce infectivity.
Another possible explanation for the lack of effect of antibodies is that TRAP is not readily accessible to antibody binding on the sporozoite surface. TRAP is abundant within the cytoplasm of all sporozoites. However, most of them (>90%) display very little TRAP on their surface that, when detectable by IF staining, appears as a very faint, punctate “dusting” of fluorescence. In agreement with a previous report (30), we found bright patches of TRAP staining on the surface of only a small proportion (<10%) of sporozoites. This bright TRAP surface staining was typically at one pole of the parasite, like a cap. This mirrors MIC2 localization in T. gondii tachyzoites. Little MIC2 is detectable on the surface of the tachyzoite until it comes in contact with its target cell, when it is released from micronemes, and caps the apical parasite surface (9). T. gondii microneme release is dependent on calcium signaling (7, 8). Likewise, here we show that treatment of sporozoites with a calcium ionophore induced caps of TRAP staining on the surface of sporozoites and that similar caps were often found on sporozoites that appeared to be in contact with hepatoma cells. These findings probably reflect what occurs upon sporozoite contact with its target cell in vivo. Therefore, the release of micronemal stores of TRAP may be a process that is spatially and temporally focused immediately on the target cell. Because the local concentration of TRAP at this interface may be high, it may be difficult for antibodies to compete with the binding of TRAP to its target cell receptor(s). In agreement with this model, anti-MIC2 antibodies are also unable to significantly affect invasion of T. gondii tachyzoites (L. D. Sibley, personal communication).
Because of its potential to elicit cell-mediated protection against infected hepatocytes in animal models (21, 41), there is continuing interest in TRAP as a malaria vaccine component. Based on in vitro data, however, Charoenvit et al. found little support for a vaccine designed to induce only anti-TRAP antibodies (12). Furthermore, protection of mice induced by melanoma cells expressing the majority of the TRAP sequence is abolished after CD8+ T-cell depletion, although the animals had high titers of anti-TRAP antibodies in their sera (21). Our in vivo results strengthen the conclusion that TRAP is a poor target for achieving antibody-mediated immunity to sporozoite infection. However, further study of the molecular mechanisms of sporozoite cell invasion may garner new antibody targets for pre-erythrocytic malaria vaccines.
ACKNOWLEDGMENTS
We thank R. Ménard, J. Raper, and P. Sinnis for critically reviewing the manuscript; L. Chacko, J. M. Li, and V. Mairov for technical assistance; R. Chappuis, I. Rossitto, and J. Vizzavona for help preparing the peptides and polyoximes; and Y. Charoenvit and E. Nardin for their generous gifts of antibodies.
This work was supported by a grant from the National Institutes of Health to V.N., a grant from the Swiss National Science Foundation to K.R., and by fellowships from the Wenner-Gren Foundation to C.P. and the New York University School of Medicine M.D./Ph.D. Program to S.G.
ADDENDUM IN PROOF
Wengelnik et al. (K. Wengelnik, R. Spaccapelo, S. Naitza, K. J. Robson, C. J. Janse, F. Bistoni, A. P. Waters, and A. Crisanti, EMBO J. 18:5195–5204, 1999) recently reported that antibodies against TRAP dramatically blocked parasite motility, in contradiction to the results reported here. Since this issue has implications for malaria vaccine development, we propose an exchange of antisera to TRAP between the two groups.
REFERENCES
- 1.Abagyan R, Totrov M. Biased probability Monte Carlo conformational searches and electrostatic calculations for peptides and proteins. J Mol Biol. 1994;235:983–1002. doi: 10.1006/jmbi.1994.1052. [DOI] [PubMed] [Google Scholar]
- 2.Abagyan R A, Totrov M M, Kuznetsov D A. ICM: a new method for structure modeling and design—applications to docking and structure prediction from the distorted native conformation. J Comp Chem. 1994;15:488–506. [Google Scholar]
- 3.Aikawa M, Atkinson C T, Beaudoin L M, Sedegah M, Charoenvit Y, Beaudoin R. Localization of CS and non-CS antigens in the sporogonic stages of Plasmodium yoelii. Bull W H O. 1990;68(Suppl.):165–171. [PMC free article] [PubMed] [Google Scholar]
- 4.Ak M, Bower J H, Hoffman S L, Sedegah M, Lees A, Carter M, Beaudoin R L, Charoenvit Y. Monoclonal antibodies of three different immunoglobulin G isotypes produced by immunization with a synthetic peptide or native protein protect mice against challenge with Plasmodium yoelii sporozoites. Infect Immun. 1993;61:2493–2497. doi: 10.1128/iai.61.6.2493-2497.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballou W R, Rothbard J, Wirtz R A, Gordon D M, Williams J S, Gore R W, Schneider I, Hollingdale M R, Beaudoin R L, Maloy W L, et al. Immunogenicity of synthetic peptides from circumsporozoite protein of Plasmodium falciparum. Science. 1985;228:996–999. doi: 10.1126/science.2988126. [DOI] [PubMed] [Google Scholar]
- 6.Briones M R, Tsuji M, Nussenzweig V. The large difference in infectivity for mice of Plasmodium berghei and Plasmodium yoelii sporozoites cannot be correlated with their ability to enter into hepatocytes. Mol Biochem Parasitol. 1996;77:7–17. doi: 10.1016/0166-6851(96)02574-1. [DOI] [PubMed] [Google Scholar]
- 7.Carruthers V B, Moreno S N, Sibley L D. Ethanol and acetaldehyde elevate intracellular [Ca2+] and stimulate microneme discharge in Toxoplasma gondii. Biochem J. 1999;342(Pt. 2):379–386. [PMC free article] [PubMed] [Google Scholar]
- 8.Carruthers V B, Sibley L D. Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol Microbiol. 1999;31:421–428. doi: 10.1046/j.1365-2958.1999.01174.x. [DOI] [PubMed] [Google Scholar]
- 9.Carruthers V B, Sibley L D. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- 10.Cerami C, Frevert U, Sinnis P, Takacs B, Clavijo P, Santos M J, Nussenzweig V. The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell. 1992;70:1021–1033. doi: 10.1016/0092-8674(92)90251-7. [DOI] [PubMed] [Google Scholar]
- 11.Champe M, McIntyre B W, Berman P W. Monoclonal antibodies that block the activity of leukocyte function-associated antigen 1 recognize three discrete epitopes in the inserted domain of CD11a. J Biol Chem. 1995;270:1388–1394. doi: 10.1074/jbc.270.3.1388. [DOI] [PubMed] [Google Scholar]
- 12.Charoenvit Y, Fallarme V, Rogers W O, Sacci J B, Jr, Kaur M, Aguiar J C, Yuan L F, Corradin G, Andersen E, Wizel B, Houghten R A, Oloo A, De la Vega P, Hoffman S L. Development of two monoclonal antibodies against Plasmodium falciparum sporozoite surface protein 2 and mapping of B-cell epitopes. Infect Immun. 1997;65:3430–3437. doi: 10.1128/iai.65.8.3430-3437.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charoenvit Y, Leef M F, Yuan L F, Sedegah M, Beaudoin R L. Characterization of Plasmodium yoelii monoclonal antibodies directed against stage-specific sporozoite antigens. Infect Immun. 1987;55:604–608. doi: 10.1128/iai.55.3.604-608.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickeson S K, Santoro S A. Ligand recognition by the I domain-containing integrins. Cell Mol Life Sci. 1998;54:556–566. doi: 10.1007/s000180050184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Entzeroth R, Kerckhoff H, Konig A. Microneme secretion in coccidia: confocal laser scanning and electron microscope study of Sarcocystis muris in cell culture. Eur J Cell Biol. 1992;59:405–413. [PubMed] [Google Scholar]
- 16.Frevert U, Sinnis P, Cerami C, Shreffler W, Takacs B, Nussenzweig V. Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. J Exp Med. 1993;177:1287–1298. doi: 10.1084/jem.177.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goding J W. Monoclonal antibodies: principles and practice. 3rd ed. London, England: Academic Press, Ltd.; 1996. [Google Scholar]
- 18.Gotwals P J, Chi-Rosso G, Ryan S T, Sizing I, Zafari M, Benjamin C, Singh J, Venyaminov S Y, Pepinsky R B, Koteliansky V. Divalent cations stabilize the alpha 1 beta 1 integrin I domain. Biochemistry. 1999;38:8280–8288. doi: 10.1021/bi982860m. [DOI] [PubMed] [Google Scholar]
- 19.Haas T A, Plow E F. Integrin-ligand interactions: a year in review. Curr Opin Cell Biol. 1994;6:656–662. doi: 10.1016/0955-0674(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 20.Kappe S, Bruderer T, Gantt S, Fujioka H, Nussenzweig V, Ménard R. Conservation of a gliding motility and cell invasion machinery in apicomplexan parasites. J Cell Biol. 1999;147:937–944. doi: 10.1083/jcb.147.5.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khusmith S, Charoenvit Y, Kumar S, Sedegah M, Beaudoin R L, Hoffman S L. Protection against malaria by vaccination with sporozoite surface protein 2 plus CS protein. Science. 1991;252:715–718. doi: 10.1126/science.1827210. [DOI] [PubMed] [Google Scholar]
- 22.Lee J O, Rieu P, Arnaout M A, Liddington R. Crystal structure of the A domain from the alpha subunit of integrin CR3 (CD11b/CD18) Cell. 1995;80:631–638. doi: 10.1016/0092-8674(95)90517-0. [DOI] [PubMed] [Google Scholar]
- 23.Michishita M, Videm V, Arnaout M A. A novel divalent cation-binding site in the A domain of the beta 2 integrin CR3 (CD11b/CD18) is essential for ligand binding. Cell. 1993;72:857–867. doi: 10.1016/0092-8674(93)90575-b. [DOI] [PubMed] [Google Scholar]
- 24.Morisaki J H, Heuser J E, Sibley L D. Invasion of Toxoplasma gondii occurs by active penetration of the host cell. J Cell Sci. 1995;108(Pt. 6):2457–2464. doi: 10.1242/jcs.108.6.2457. [DOI] [PubMed] [Google Scholar]
- 25.Müller H M, Reckmann I, Hollingdale M R, Bujard H, Robson K J, Crisanti A. Thrombospondin related anonymous protein (TRAP) of Plasmodium falciparum binds specifically to sulfated glycoconjugates and to HepG2 hepatoma cells suggesting a role for this molecule in sporozoite invasion of hepatocytes. EMBO J. 1993;12:2881–2889. doi: 10.1002/j.1460-2075.1993.tb05950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nardin E H, Calvo-Calle J M, Oliveira G A, Clavijo P, Nussenzweig R, Simon R, Zeng W, Rose K. Plasmodium falciparum polyoximes: highly immunogenic synthetic vaccines constructed by chemoselective ligation of repeat B-cell epitopes and a universal T-cell epitope of CS protein. Vaccine. 1998;16:590–600. doi: 10.1016/s0264-410x(97)00238-7. [DOI] [PubMed] [Google Scholar]
- 27.Pancake S J, Holt G D, Mellouk S, Hoffman S L. Malaria sporozoites and circumsporozoite proteins bind specifically to sulfated glycoconjugates. J Cell Biol. 1992;117:1351–1357. doi: 10.1083/jcb.117.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potocnjak P, Yoshida N, Nussenzweig R S, Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med. 1980;151:1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rieu P, Sugimori T, Griffith D L, Arnaout M A. Solvent-accessible residues on the metal ion-dependent adhesion site face of integrin CR3 mediate its binding to the neutrophil inhibitory factor. J Biol Chem. 1996;271:15858–15861. doi: 10.1074/jbc.271.27.15858. [DOI] [PubMed] [Google Scholar]
- 30.Rogers W O, Malik A, Mellouk S, Nakamura K, Rogers M D, Szarfman A, Gordon D M, Nussler A K, Aikawa M, Hoffman S L. Characterization of Plasmodium falciparum sporozoite surface protein 2. Proc Natl Acad Sci USA. 1992;89:9176–9180. doi: 10.1073/pnas.89.19.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose K. Facile synthesis of homogenous artificial proteins. J Am Chem Soc. 1994;116:30–33. [Google Scholar]
- 32.Schnolzer M, Alewood P, Jones A, Alewood D, Kent S B. In situ neutralization in Boc-chemistry solid phase peptide synthesis. Rapid, high yield assembly of difficult sequences. Int J Peptide Protein Res. 1992;40:180–193. doi: 10.1111/j.1399-3011.1992.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 33.Sinnis P, Nussenzweig V. Preventing sporozoite invasion of hepatocytes. In: Hoffman S L, editor. Malaria vaccine development. Washington, D.C.: American Society for Microbiology Press; 1996. pp. 15–33. [Google Scholar]
- 34.Stewart M J, Nawrot R J, Schulman S, Vanderberg J P. Plasmodium berghei sporozoite invasion is blocked in vitro by sporozoite-immobilizing antibodies. Infect Immun. 1986;51:859–864. doi: 10.1128/iai.51.3.859-864.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoute J A, Kester K E, Krzych U, Wellde B T, Hall T, White K, Glenn G, Ockenhouse C F, Garcon N, Schwenk R, Lanar D E, Sun P, Momin P, Wirtz R A, Golenda C, Slaoui M, Wortmann G, Holland C, Dowler M, Cohen J, Ballou W R. Long-term efficacy and immune responses following immunization with the RTS,S malaria vaccine. J Infect Dis. 1998;178:1139–1144. doi: 10.1086/515657. [DOI] [PubMed] [Google Scholar]
- 36.Stoute J A, Slaoui M, Heppner D G, Momin P, Kester K E, Desmons P, Wellde B T, Garcon N, Krzych U, Marchand M. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 37.Sultan A A, Thathy V, Frevert U, Robson K J, Crisanti A, Nussenzweig V, Nussenzweig R S, Ménard R. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell. 1997;90:511–522. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- 38.Templeton T J, Kaslow D C. Cloning and cross-species comparison of the thrombospondin-related anonymous protein (TRAP) gene from Plasmodium knowlesi, Plasmodium vivax and Plasmodium gallinaceum. Mol Biochem Parasitol. 1997;84:13–24. doi: 10.1016/s0166-6851(96)02775-2. [DOI] [PubMed] [Google Scholar]
- 39.Tomley F M, Bumstead J M, Billington K J, Dunn P P. Molecular cloning and characterization of a novel acidic microneme protein (Etmic-2) from the apicomplexan protozoan parasite, Eimeria tenella. Mol Biochem Parasitol. 1996;79:195–206. doi: 10.1016/0166-6851(96)02662-x. . (Erratum, 82:271.) [DOI] [PubMed] [Google Scholar]
- 40.Tuckwell D S, Xu Y, Newham P, Humphries M J, Volanakis J E. Surface loops adjacent to the cation-binding site of the complement factor B von Willebrand factor type A module determine C3b binding specificity. Biochemistry. 1997;36:6605–6613. doi: 10.1021/bi963155l. [DOI] [PubMed] [Google Scholar]
- 41.Wang R, Charoenvit Y, Corradin G, De La Vega P, Franke E D, Hoffman S L. Protection against malaria by Plasmodium yoelii sporozoite surface protein 2 linear peptide induction of CD4+ T cell- and IFN-gamma-dependent elimination of infected hepatocytes. J Immunol. 1996;157:4061–4067. [PubMed] [Google Scholar]
- 42.Zeng W, Jackson D C, Rose K. Synthesis of a new template with a built-in adjuvant and its use in constructing peptide vaccine candidates through polyoxime chemistry. J Peptide Sci. 1996;2:66–72. doi: 10.1002/psc.51. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Plow E F. Amino acid sequences within the alpha subunit of integrin alpha M beta 2 (Mac-1) critical for specific recognition of C3bi. Biochemistry. 1999;38:8064–8071. doi: 10.1021/bi990141h. [DOI] [PubMed] [Google Scholar]
- 44.Zheng J, Schodel F, Peterson D L. The structure of hepadnaviral core antigens. Identification of free thiols and determination of the disulfide bonding pattern. J Biol Chem. 1992;267:9422–9429. [PubMed] [Google Scholar]