Abstract
Background
The NIBIT-MESO-1 study demonstrated the efficacy and safety of tremelimumab combined with durvalumab in patient with unresectable mesothelioma followed up for a median of 52 months [IQR 49–53]. Here, we report 4-year survival and outcomes after retreatment, and the role of tumour mutational burden (TMB) in identifying patients who might have a better outcome in response to combined therapy.
Methods
NIBIT-MESO-1 was an open-label, non-randomised, phase 2 trial of patients with unresectable pleural or peritoneal mesothelioma who received intravenous tremelimumab (1 mg/kg bodyweight) and durvalumab (20 mg/kg bodyweight) every 4 weeks for four doses, followed by maintenance intravenous durvalumab at the same dose and schedule for nine doses. In this follow-up study, patients with disease progression following initial clinical benefit—ie, a partial repsonse or stable disease—were eligible for retreatment and with the same doses and schedules for tremelimumab and durvalumab as used in the NIBIT-MESO-1 trial. The primary endpoint, immune-related objective response rate, was evaluated per immune-related modified Response Evaluation Criteria in Solid Tumors (RECIST) or immune-related RECIST 1.1 criteria for patients with pleural or peritoneal malignant mesothelioma, respectively. Key secondary endpoints were overall survival and safety, and TMB was also evaluated post hoc in patients who had tumour tissue available before treatment. The intention-to-treat population was used for analysis of all efficacy endpoints. This study is registered with ClinicalTrials.gov, number NCT02588131.
Findings
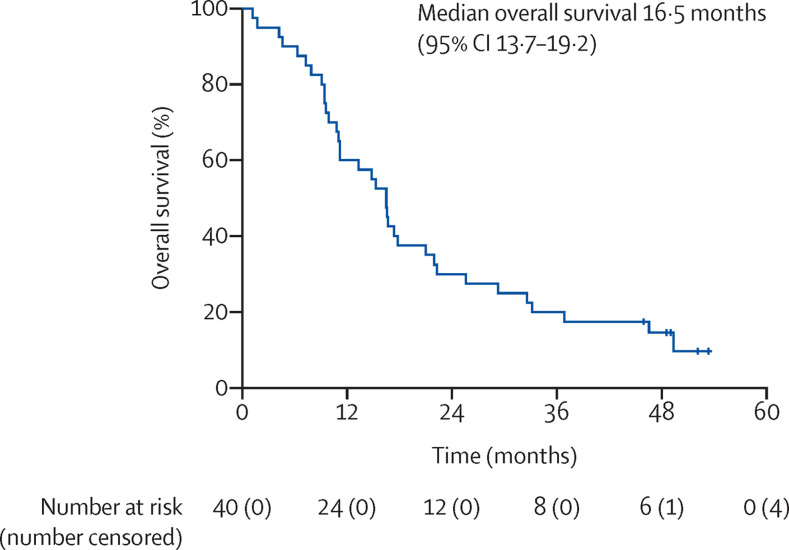
40 patients were enrolled in the NIBIT-MESO-1 study between Oct 30, 2015, and Oct 12, 2016. At data cut-off, April 30, 2020, five (13%) of 40 patients were alive, and 35 (88%) patients had died of progressive disease. At a median follow-up of 52 months (IQR 49–53), median overall survival was 16·5 months (95% CI 13·7–19·2). Survival was 20% (eight of 40 patients) at 36 months and 15% (six of 40 patients) and 48 months. 17 (43%) of 40 patients met the criteria for enrolment in the retreatment study and were retreated with at least one dose of tremelimumab and durvalumab. No immune-related objective responses were observed in the 17 retreated patients. Seven (41%) of 17 patients achieved immune-related stable disease. From the start of retreatment to a median follow-up of 24 months (22·0–25·0), median overall survival was 12·5 months (95% CI 0·0–25·8), and survival at 12 months was 52·9%, at 18 months was 35·3%, and at 24 months was 23·5%. There were no grade 3–4 immune-related adverse events in the retreatment cohort. In a post-hoc analysis of 28 patients for whom tumour tissue before treatment was available, patients with a TMB higher than the median value of 8·3 mutations per Mb had a higher median overall survival compared with patients with TMB below the median value, but this difference was non-significant. Moreover, when patients were additionally stratified for ICI retreatment (n=13), there was a significant difference in survival between those with a TMB higher than the median of 8·3 mutations per Mb and those with TMB lower than the median in the retreated cohort (41·3 months vs 17·4 months; p=0·02).
Interpretation
Tremelimumab combined with durvalumab was associated with long-term survival in patients with mesothelioma. Retreatment was safe and resulted in clinically meaningful outcomes, thus suggesting its potential application in the clinical practice of mesothalioma patients.
Funding
NIBIT Foundation, Fondazione AIRC, AstraZeneca.
Introduction
Malignant mesothelioma is an aggressive and lethal tumour, commonly associated with asbestos exposure.1 The prognosis for patients with mesothelioma remains poor, largely due to the absence of effective therapeutic options. Front-line systemic treatment for patients with unresectable disease has not changed substantially in the past two decades, and in most countries still comprises platinum combined with pemetrexed;2 however, the combination of platinum plus pemetrexed and bevacizumab is used in selected countries based on the results of the MAPS-trial.3 The therapeutic landscape for patients with mesothelioma who have been previously treated is even worse, with limited treatment options and no agents currently approved in this setting.4
Research in context.
Evidence before this study
We searched PubMed for reports published in English from Jan 1, 2010, to Dec 31, 2020, using the search terms “mesothelioma”, “anti-CTLA-4”, “anti-PD-1”, “anti-PD-L1”, “anti-CTLA-4 in combination with anti-PD-1/PD-L1”, “retreatment” and “immunotherapy”, and filtered for clinical trials. We did not find any data about a longer follow-up with immune checkpoint inhibitor (ICI) combinations in mesothelioma or data from retreatment with ICIs in patients with ICI-refractory mesothelioma. Treatment for patients with malignant mesothelioma remains unsatisfactory, with an urgent unmet need for new agents. Therapeutic blockade, with the use of ICIs, has shown antitumour activity in early phase studies of patients with mesothelioma. We have previously reported the clinical activity of combined treatment with tremelimumab and durvalumab in the first-line or second-line setting for patients with pleural or peritoneal mesothelioma (NIBIT-MESO-1 study). These results have since been corroborated by the phase 2 MAPS-2 and INITIATE studies in patients with previously-treated pleural mesothelioma, while the efficacy of first-line combination ICI treatment in pleural mesothelioma has been confirmed in the randomised, phase 3 CheckMate743 study.
Added value of this study
To our knowledge, this is the first extended follow-up study to look at the efficacy and safety of retreatment with a combination of two ICIs (tremelimumab and durvalumab) in patients with mesothelioma. We found that treatment with tremelimumab and durvalumab in patients with mesothelioma is associated with durable survival at 36 months and 48 months, 20% and 15% of patients with mesothelioma, respectively, suggesting a role of this combination in ICI rechallenge of ICI-refractory patients, who had progression of disease following disease control. We also identified tumour mutational burden (TMB) as a potential molecular marker to select patients with mesothelioma who might benefit most from retreatment with combined tremelimumab and durvalumab.
Implications of all the available evidence
For patients with mesothelioma, who have disease progression after an initial clinical benefit—ie, partial response or stable disease—with tremelimumab and durvalumab, retreatment with these same agents might be a useful option. TMB at baseline could be a potential molecular marker to identify patients who might have a better outcome in response to retreatment with this combined therapy.
Promising results have emerged on the use of targeted treatment with immune checkpoint inhibitors (ICIs),5, 6 particularly when given in combination regimens.7 NIBIT-MESO-1 was the first study to demonstrate encouraging signs of efficacy with tremelimumab (an anti-cytotoxic T lymphocyte antigen [CTLA]-4 monoclonal antibody [mAb]) plus durvalumab (an anti-programmed cell death ligand-1 [PD-L1] mAb) as first-line or second-line treatment in patients with malignant mesothelioma. 11 (28%) of 40 patients achieved an immune-related objective response and 26 (65%) of patients achieved immune-related disease control. Median overall survival was 16·6 months (95% CI 13·1–20·1).8 Similar results were subsequently reported in the MAPS-1 study9 and the INITIATE study,10 looking at the combination of ipilimumab and nivolumab in patients with pleural mesothelioma who had been previously treated. The results of the phase 3 CheckMate-743 study11 further support the efficacy of combined ICI therapy in mesothelioma; first-line nivolumab plus ipilimumab significantly improved survival of patients with pleural mesothelioma compared with platinum plus pemetrexed. Based on the results of these studies,9, 10, 11 the combination of nivolumab and ipilimumab has recently been approved by the US Food and Drug Administration as a first-line therapy for patients with pleural mesothelioma.
Despite these exciting results, primary and secondary resistance to treatment is emerging as a major limitation of ICI therapy.12 Several mechanisms of resistance have been identified to date, and strategies to overcome them represent an area of active investigation. In this context, very limited information is available about the therapeutic efficacy of retreatment in ICI-resistant patients and, to the best of our knowledge, no biomarkers are available to identify patients with mesothelioma who are appropriate candidates for ICI therapy and retreatment.
Here, we report the 36-month and 48-month survival analysis of patients treated with tremelimumab and durvalumab, as well as the efficacy and safety of ICI retreatment of patients who experienced disease progression following initial disease control.
Methods
Study design, patient population, procedures, and outcomes
We conducted a milestone, follow-up analysis of patients enrolled in the NIBIT-MESO-1 study.8 The study design, patient eligibility criteria, and treatment regimen for the NIBIT-MESO-1 study have already been described.
Briefly, the NIBIT-MESO-1 was an open-label, single-arm, phase 2 study. Patients were eligible for inclusion if they were aged 18 years or older, had histologically confirmed unresectable pleural or peritoneal mesothelioma, had refused or progressed during or after one platinum-based chemotherapy regimen, had measurable disease (assessed at baseline by CT or MRI) according to the modified Response Evaluation Criteria in Solid Tumors [RECIST] for pleural mesothelioma13 or RECIST 1.1 for peritoneal mesothelioma14), and had a life expectancy of 3 months or more (as judged by clinicians), adequate organ function, and an Eastern Cooperative Oncology Group (ECOG) performance status of 1 or less. Eligible patients received induction treatment comprising tremelimumab at a dose of 1 mg/kg bodyweight every 4 weeks (a cycle) for four doses in combination with durvalumab at a dose of 20mg/kg bodyweight every 4 weeks for four doses, followed by maintenance dosing every 4 weeks with only intravenous durvalumab 20mg/kg bodyweight for an additional nine doses, or until confirmed progressive disease, unacceptable toxicity, investigator's decision, or patient's withdrawal of consent.
For this follow-up study, following the NIBIT-MESO-1 protocol, patients were eligible for retreatment if they had completed 4 cycles of tremelimumab plus durvalumab and had obtained a partial response or stable disease, followed by progressive disease during maintenance with durvalumab for nine doses or during the follow-up phase. Additional eligibility criteria8 were ECOG performance status of 0 or 1; life expectancy of 3 months or more (as judged by clinicians); adequate organ function (liver, blood, kidney, and heart); and measurable disease, defined as at least one lesion that could be accurately assessed at baseline by CT scan or MRI that was suitable for repeated measurements according to the modified RECIST criteria for pleural mesothelioma,13 or RECIST 1.1 for peritoneal mesothelioma.14
Patients eligible for retreatment received the same schedule of treatment as used in the NIBIT-MESO-1 study.8 Briefly, intravenous tremelimumab (AstraZeneca–MedImmune, Gaithersburg, MD, USA) at 1 mg/kg bodyweight and intravenous durvalumab (AstraZeneca–MedImmune) at 20 mg/kg bodyweight were given every 4 weeks for four doses (re-induction phase), followed by durvalumab (20 mg/kg bodyweight) every 4 weeks for an additional nine doses (maintenance phase) for a total treatment duration of 48 weeks. Routine clinical and laboratory assessments were conducted at baseline and throughout the study. No further retreatment was allowed in case of disease progression. Radiological tumour assessments (brain, neck, chest, abdomen, and pelvis CT scan) were undertaken at re-screening and every 12 weeks during retreatment.
Endpoints were the same as for initial treatment in the NIBIT-MESO-1 study.8 The primary endpoint, immune-related objective response rate, was evaluated according to immune-related modified RECIST or immune-related RECIST 1.1 criteria for patients with pleural or peritoneal malignant mesothelioma, respectively. Secondary endpoints included immune-related disease control rate, immune-related progression-free survival, overall survival, and safety. In a post-hoc analysis we compared median survival of patients retreated with ICIs versus those not retreated.
Adverse events were recorded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0. No dose reduction was allowed. All treatment-related toxicity had to be resolved to grade 1 or lower before giving the next dose of the combination or maintenence durvalumab. Patients were permanently discontinued from the study if two consecutive doses were missed because of ongoing treatment-related toxicity.
Retreatment as a part of the NIBIT-MESO-1 study was already approved by the University Hospital of Siena's independent Ethics Committee, and was done in accordance with the ethical principles of the Declaration of Helsinki and the International Conference on Harmonization of Good Clinical Practice. All participants or their legal representatives provided written informed consent before enrolment in retreatment.
DNA extraction and next-generation sequencing
In order to assess tumour mutation burden (TMB), a post-hoc analysis, genomic DNA was isolated from formalin-fixed and paraffin-embedded (FFPE) tissue sections from 28 patients with mesothelioma enrolled in the NIBIT-MESO-1 study at baseline (of whom 13 patients entered entered the ICI retreatment phase) using the GeneRead DNA FFPE Kit (Qiagen, Hilden, Germany) and following the manufacturer's instructions. Analyses on the impact of TMB on outcomes were performed in patients stratified based on the median value of TMB at baseline and then according to their retreatment. DNA concentrations were measured with the Qubit 2.0 Fluorometer with Qubit dsDNA HS (High Sensitivity) Assay Kit (Life Technologies, Carlsbad, CA, USA). Next-generation sequencing analyses were performed using the Ion GeneStudio S5 System with the Comprehensive Cancer Panel that includes a total of 409 cancer-related genes arranged in four primer pools.15 Libraries were generated starting from 10 ng of DNA per primer pool for a total of 40 ng of input DNA, using the Ion AmpliSeq Library Kit Plus, barcoded with Ion Xpress Barcode Adapters (Life Technologies) and purified with Agencourt Ampure XP Beads (Beckman Coulter Life Sciences, Indianapolis, IN, USA). The obtained PCR amplicons were diluted to a final concentration of 70 pM, and pooled together; emulsion PCR and Chip (Ion 540) loading steps were performed with the Ion Chef Instrument. Sequencing of libraries was done with the Ion S5TM System (Thermofisher, Monza Italy). Sequencing data were processed with the Ion Torrent platform-specific pipeline software (Torrent Suite, V5.2.1). Ion Reporter Software V5.10 and Integrative Genome Viewer software) were used for variant annotation and reads visualisations, respectively. TMB values were directly calculated by the Ion ReporterSoftware using the specific panel analysis workflow.
Statistical analysis
The Kaplan–Meier method was utilised to estimate median follow-up (reverse method), median overall survival, and median progression-free survival, as well as survival rates, with two-sided 95% confidence intervals based on a normal approximation. Survival curves were compared using the log-rank test. The restricted mean survival time (RMST) was calculated as previously described.16 Two-sided 95% confidence intervals for immune-related objective response and immune-related disease control were estimated using an exact probability method. Statistical analyses for TMB and survival rates used GraphPad Prism version 8. All other statistical analyses used IBM-SPSS version 23·0. The study was registered with the European Union Clinical Trials Register, number 2015-001995-23, and ClinicalTrials.gov, number NCT02588131.
Role of the funding source
The study was sponsored by the NIBIT Foundation who designed, coordinated, collected, analysed, and interpreted the data, and decided to submit the manuscript for publication. All authors had full access to all the data in the study and accept responsibility to submit for publication.
Results
At data cut-off, April 30, 2020, five (13%) of 40 patients enrolled in the NIBIT-MESO-1 study were alive, and 35 (88%) patients had died of progressive disease. With a median follow-up of 52 months (IQR 49–53), the median overall survival was 16·5 months (95% CI 13·7–19·2; figure 1 ). Survival was 20% (eight of 40 patients) at 36 months and 15% (six of 40 patients) at 48 months.
Figure 1.
Kaplan–Meier curve of overall survival in patients with mesothelioma (n=40) treated with tremelimumab plus durvalumab
Vertical lines indicate censored observations.
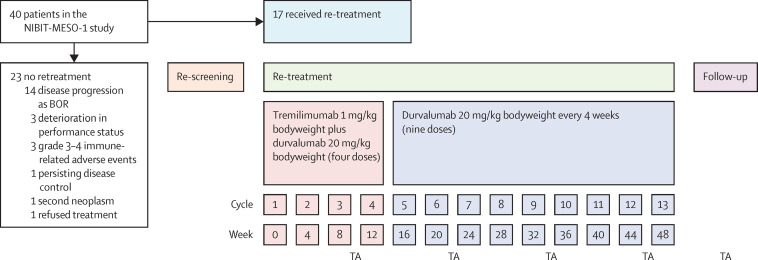
17 (43%) of 40 patients who had disease progression met the criteria for retreatment with tremelimumab and durvalumab (table 1 ; figure 2 ). Among these 17 patients, who had a median progression-free survival of 11·3 months (95% CI 9·0–13·5) at baseline in NIBIT-MESO-1 up to the point of starting retreatment, eight (47%) patients had disease progression during the initial maintenance phase in NIBIT-MESO-1 prior to retreatment, and nine (53%) had disease progression during the first follow up phase. Between Sept 6, 2016, and March 29, 2019, the 17 patients who received retreatment had a median of three doses (range 1–4) of tremelimumab and four doses (range 1–13) of durvalumab. Eight (47%) of 17 patients completed the re-induction phase with tremelimumab and durvalumab, of whom seven (41%) started the retreatment maintenance phase with durvalumab and one (5%) entered the follow-up phase.
Table 1.
Baseline characteristics of patients retreated with tremelimumab and durvalumab
| Study population (N=17) | ||
|---|---|---|
| Sex | ||
| Male | 11 (65%) | |
| Female | 6 (35%) | |
| Median age, years | 65 (49–69) | |
| ECOG performance status | ||
| 0 | 10 (59%) | |
| 1 | 7 (41%) | |
| Histology | ||
| Epithelioid | 14 (82%) | |
| Biphasic | 3 (18%) | |
| Site | ||
| Pleural | 16 (94%) | |
| Peritoneal | 1 (6%) | |
| Previous treatments | ||
| One* | 4 (23%) | |
| Two† | 13 (77%) | |
Data are n (%), or median (IQR). ECOG=Eastern Cooperative Oncology Group.
First-line treatment with tremelimumab and durvalumab in the NIBIT-MESO-1 study.8
First line treatment with platinum plus pemetrexed; second-line treatment with tremelimumab and durvalumab in the NIBIT-MESO-1 study.
Figure 2.
Patient disposition and schedule of retreatment with tremelimumab and durvalumab
BOR=best overall response. TA=tumour assessment.
Overall, seven (41%) of 17 patients included in the retreatment study achieved immune-related stable disease (disease control), which lasted over 11 months in three patients. No immune-related objective responses were observed. Four (24%) of 17 patients were treatment-naïve when they were first enrolled in the NIBIT-MESO-1 study (appendix p 1). Nine (53%) of 17 retreated patients had a partial response as best overall response (BOR) with the first course of therapy in the NIBIT-MESO-1 study; of these, six (67%) had stable disease and three (33%) had progressive disease as BOR with retreatment in the follow-up study. The remaining eight (47%) patients had stable disease as BOR with the first course of treatment, of whom one (13%) achieved stable disease as BOR at retreatment and seven (87%) had progressive disease as BOR at retreatment (appendix p 1).
From start of retreatment to a median follow-up of 24 months (IQR 22·0–25·0), the median immune-related progression-free survival of the 17 patients who were retreated was 3·5 months (95% CI 3·2–3·8), the median overall survival was 12·5 months (0·0–25·8). Survival at 12 months was 52·9% (nine of 17 patients), at 18 months was 35·3% (six of 17), and 24 months was 23·5% (four of 17). At data cut-off, April 30, 2020, all 17 patients who received retreatment had discontinued treatment because of disease progression; 13 (76%) patients received subsequent chemotherapy or immunotherapy.
23 (58%) of 40 patients enrolled in the NIBIT-MESO-1 study were not eligible for ICI retreatment due to: disease progression as BOR (14 [35%] of 40 patients); deterioration of clinical condition due to disease progression (three [8%]); grade 3–4 toxicity that required permanent treatment discontinuation (three [8%]); persisting disease control (one [3%]); occurrence of a second neoplasm (one [3%]); and refused ICI retreatment (one [3%]; figure 2). Of these 23 patients who did not receive ICI retreatment, 15 (38%) received further chemotherapy.
A post-hoc analysis was performed to compare the survival of 17 ICI retreated patients with survival of the 15 patients who did not qualify for ICI retreatment but received additional chemotherapy. At a median follow up of 48 months (IQR 46·0–49·0) from the start of NIBIT-MESO-1, the median overall survival of patients who recieved retreatment was significantly higher than for patients who recieved chemotherapy (25·6 months [95% CI 9·6–41·6] vs 11·0 months [9·4–12·6]; p=0·009; figure 3 ). Furthermore, RMST analysis demonstrated a mean survival time of 21·3 months (95% CI 16·3–26·2) for all 40 patients enrolled in the original study, 29·3 months (22·1–36·5) for the 17 retreated patients included in the follow-up study, and 16·7 months (9·8–23·5) for patients (n=23) who did not receive ICI retreatment.
Figure 3.
Kaplan–Meier curve of overall survival of patients retreated with tremelimumab and durvalumab (ICI retreated) versus non-ICI retreated patients who received additional chemotherapy (post-hoc analysis)
Vertical lines indicate censored observations. ICI=immune checkpoint inhibitor.
Overall, retreatment with tremelimumab and durvalumab was well-tolerated. Six (35%) of 17 patients experienced grade 1–2 immune-related adverse events, the most common of which were dermatological, and generally manageable and reversible per protocol guidelines (table 2 ). No grade 3–4 immune-related adverse events were observed.
Table 2.
Summary of immune-related adverse events
| Grade 1–2 | Grade 3–4 | |
|---|---|---|
| Any immune-related adverse events | 6 (35%) | 0 |
| Dermatological (rash, pruritus, erythema multiforme, psoriasis) | 4 (24%) | 0 |
| Gastrointestinal (diarrhoea, nausea, vomiting) | 2 (12%) | 0 |
| Pancreatic (amylases or lipase increase) | 1 (6%) | 0 |
| Pneumonitis | 2 (12%) | 0 |
| Articular and muscle pain | 1 (6%) | 0 |
| Decrease appetite | 1 (6%) | 0 |
Data are number of patients (%). Population included 17 patients.
In a post-hoc analysis, the impact of TMB on outcomes was analysed in 28 patients for whom tumour tissue before treatment was available, of whom 13 participated in the retreatment study. Patients with TMB higher than the median value of 8·3 mutations per Mb demonstrated an improved median overall survival than patients with TMB lower than the median value (30·9 months vs 14·9 months; p=0·06), although this difference did not reach statistical significance (figure 4A ). However, when patients were additionally stratified for ICI retreatment (n=13), there was a significant difference in survival between those with a TMB higher than the median of 8·3 mutations per Mb and those with TMB lower than the median in the retreated cohort (41·3 months vs 17·4 months; p=0·02; figure 4B).
Figure 4.
Kaplan–Meier cumulative survival analyses according to TMB (post-hoc analysis)
Kaplan–Meier overall survival for all patients in the NIBIT-MESO-1 study with samples available for TMB analysis. Vertical lines indicate censored observations. (A) Overall survival of patients stratified by median TMB of less than 8·3 and more than 8·3 mutations per Mb (n=28). (B) Overall survival of patients stratified by median TMB of less than 8·3 and more than 8·3 mutations per Mb in patients retreated with tremelimumab and durvalumab (n=13). TMB=tumour mutational burden.
Discussion
To our knowledge, the NIBIT-MESO-1 study has the longest follow-up of patients with mesothelioma treated by co-targeting the CTLA-4 and PD-L1 immune checkpoints. Our updated survival analysis shows that tremelimumab combined with durvalumab is associated with durable survival at 36 months and 48 months, in 20% and 15% of patients with mesothelioma, respectively, supporting the hypothesis that simultaneously targeting multiple immune checkpoints might be an effective strategy to induce long-term survival in this population, similar to that already demonstrated for other tumour types.17, 18, 19 Furthermore, first-line combination treatment with anti-PD-1 and anti-CTLA-4 mAbs has been shown to significantly improve overall survival compared with platinum-based therapy for patients with pleural mesothelioma,11 is likely to become the new standard of care in this setting worldwide.
Little evidence is available on the therapeutic efficacy of ICI retreatment in cancer patients with disease progression after initial clinical benefit (a partial response or stable disease) on these agents. To our knowledge, the NIBIT-MESO-1 study is the first study that prospectively investigated the efficacy and safety of ICI retreatment in ICI-refractory patients with mesothelioma. Although the primary endpoint of objective response was not observed, ICI retreatment resulted in disease stabilisation in 41% of patients, preferentially in those who had achieved an objective response during the first course of treatment of the NIBIT-MESO-1 study. Consistently, the median progression-free survival (11·3 months) of patients who had retreatment compared favourably with the median progression-free survival (8·0 months) observed for all patients in the first course of therapy in the NIBIT-MESO-1 study.8 Even more interesting was the impact on overall survival in post hoc analysis, which was significantly improved in patients who were retreated with tremelimumab and durvalumab compared with those who were not retreated and received additional chemotherapy (25·6 months vs 11·0 months). Supporting the efficacy of ICI-retreatment, RMST analysis identified a mean survival time of 29·3 months for ICI retreated patients versus 16·7 months for patients who did not receive ICI retreatment. Although this is a non-randomised study and the size of the retreated cohort was small, the results suggest that ICI retreatment might restore long-lasting tumour control in 24% of ICI-refractory patients with mesothelioma. This approach could be particularly relevant in previously-treated patients with mesothelioma for whom no effective therapies are currently available. However, it has to be acknowledged that in this follow-up study, patients who were eligible for retreatment were also those who benefitted (ie, partial response or stable disease) from the initial therapy with ICI and had no primary resistance to ICIs.
To date, we do not fully understand the efficacy of ICI retreatment, and current knowledge is primarily based on retrospective studies of small subsets of patients treated with either the anti-CTLA-4 mAb, ipilimumab, or with anti-PD-1/PD-L1 mAbs in larger trials.20, 21, 22, 23, 24, 25, 26 Indeed, in an ipilimumab expanded access programme, 55% of patients with metastatic melanoma who were retreated regained disease control, and 42% were alive 2 years after the first induction dose of ipilimumab.20 In the KEYNOTE-006 study,21 13 patients with metastatic melanoma, previously treated with pembrolizumab for 2 years, were retreated at disease progression with the same anti-PD-1 mAb. Seven (54%) of 13 retreated patients achieved an objective response. In other reports, rechallenge with anti-PD-1 or PD-L1 mAbs has been associated with objective response rates of 25–27% and stable disease rates of 18–75% in various tumour types, including melanoma, non-small-cell lung cancer, colorectal cancer, urothelial cancer, and breast cancer.22, 23, 24 Similar to our results, however, two further studies in patients with non-small-cell lung cancer reported primarily stable disease (with a single partial response among 50 retreated patients) as the best response to anti-PD-1 mAb rechallenge.25, 26 Taken together, the available data indicate that ICI retreatment is clinically active in some patients regardless of tumour histology, and merits further investigation in dedicated larger prospective studies.
Retreatment with tremelimumab and durvalumab was associated with a good tolerability profile; no grade-3–4 immune-related adverse events occurred in retreated patients. These findings are consistent with safety data for this combination in the NIBIT-MESO-1 study,8 as well as in other tumour types,27, 28 and support further exploration of retreatment with tremelimumab and durvalumab without safety concerns.
An additional ongoing challenge is the identification of biomarkers to enable selection of patients with mesothelioma who will derive most benefit from ICI therapy, and also from ICI retreatment. In this context, the role of PD-L1 expression by neoplastic cells remains controversial.29 Along this line, we found no correlation between PD-L1 expression and clinical outcome either in patients initially enrolled in the NIBIT-MESO-1 study,8 or in those who underwent ICI retreatment (data not shown). Similarly, PD-L1 expression did not correlate with the outcome of salvage chemotherapy (data not shown). Furthermore, the potential correlation between TMB at different cut-off values and the efficacy of ICI therapy has been evaluated in several clinical trials;27, 30, 31 however, no studies so far have validated pre-determined TMB cut-offs to select patients who are the best candidates to benefit the most from ICI therapy. In this evolving scenario, we found a correlation between baseline TMB higher than the median population value and improved survival in the whole patient population, although the results did not reach statistical significance. Furthermore, among patients who had ICI retreatment, the highest survival was observed in patients with a TMB higher than the median value. While these findings need to be interpreted with caution, due to being preliminary, and being derived from a post-hoc analysis of a small number of patients, a high mutation load at baseline seems to identify patients with mesothelioma who are most likely to benefit from ICI therapy and retreatment with these agents. This latter finding is intriguing and needs to be further explored, ideally comparing tumor biopsies at disease progression with archival samples, though its feasibility in mesothelioma is clearly limited by the very invasive procedures it would require. Nonetheless, the ability of TMB to identify patients with a favourable outcome to ICI, including those who are suitable candidates for rechallenge, warrants prospective investigation in a larger study.
Anti-tumour immunotherapy with immune checkpoint blockade in patients with mesothelioma has yet to reach its full potential. There is considerable scope to improve treatment outcomes and to overcome resistance to this class of immunotherapy. New ways of using these agents, including optimising the duration of ICI treatment or rechallenge, are under active investigation, and will hopefully improve the clinical efficacy of ICIs against this still fatal and difficult-to-treat disease. Despite the limitations of the study, including the small number of patients who received ICI retreatment and the non-randomised study design, which prevent drawing firm conclusions, the clinical results support the efficacy and safety of ICI retreatment in patients who become refractory to inital ICI treatment, and further investigation is warranted for potential application of retreatment in the clinical practice.
Data sharing
The Italian Network for Tumour Biotherapy (NIBIT) Foundation states that individual participant data from the NIBIT-MESO-1 trial will be available (including data dictionaries). The data that will be shared include individual participant data that underlie the results reported in this Article, after de-identification (text, table, figures, and appendices). The study protocol, statistical analysis plan, and analytic code will also be available. Data will be available between 9 months and 36 months after the Article has been published. Data will be made available to researchers who provide a methodologically sound proposal as reviewed by the Board of the NIBIT Foundation. The Board will ask the researchers for a complete protocol with the whole list of data needed for their research project and their analysis plan to achieve specific aims in the proposal. The protocol will then be reviewed by the Board of the NIBIT Foundation, which will make a recommendation on the scientific value of the project. Based on these recommendations, the Board will or will not accept sharing of the requested data, providing the researchers with the scientific reasons for their decision. In case of approval by the NIBIT Foundation, and to gain access, data requestors will need to sign a data access agreement specifying the policy for publication and the inclusion of the principal investigators and collaborators of the NIBIT-MESO-1 study in the list of authors of any scientific article or communication resulting from the research project. Proposals should be directed to segreteria@fondazionenibit.org.
Declaration of interests
LC has served as consultant or advisor to Bristol-Myers Squibb, Roche, and Merck Sharp & Dohme, and received compensated educational activities from Bristol Myers Squibb, AstraZeneca, and Sanofi. AMDG has served as a consultant or advisor to Incyte, Pierre Fabre, GlaxoSmith Kline, Bristol-Myers Squibb, Merck Sharp & Dohme, and Sanofi. MM has served as a consultant or advisor to Roche, Bristol-Myers Squibb, Merck Sharp & Dohme, Incyte, AstraZeneca, Amgen, Pierre Fabre, Eli Lilly, GlaxoSmith Kline, Sciclone, Sanofi, Alfasigma, and Merck Serono. MM and AC own shares in Epigen Therapeutics. GP currently has or has previously had an advisory role for Bristol Myers Squibb, Incyte, Merck Sharp & Dohme, Novartis, Pierre Fabre, and Roche–Genentech. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank our research nurses, our comprehensive laboratory staff, and data managers for their professional support in conducting this study. We thank the patients who participated in this study and their families. The study was sponsored by the NIBIT Foundation, the Fondazione AIRC under 5 per Mille 2018–ID 21073 program (principal investigator MM), and by AstraZeneca. AstraZeneca provided the study drugs and also financial support to the NIBIT Foundation for conducting the study. Editorial assistance was provided by Jean Scott and was funded by the NIBIT Foundation.
Contributors
LC and MM designed and supervised the study, had access to and verified all the data reported in the study. AM reviewed the radiological data. GR, CR, DG, MGD, and MC collected the data. LC, GR, AC, CF, OC, MA, AMDG, and GP analysed the data. LC and MM interpreted the data. All authors reviewed and provided comments on the initial version of the manuscript, and reviewed and approved the final draft for publication.
Supplementary Material
References
- 1.Delgermaa V, Takahashi K, Park EK, Le GV, Hara T, Sorahan T. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ. 2011;89:716–724. doi: 10.2471/BLT.11.086678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase 3 study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 3.Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387:1405–1414. doi: 10.1016/S0140-6736(15)01238-6. [DOI] [PubMed] [Google Scholar]
- 4.Buikhuisen WA, Hiddinga BI, Baas P, van Meerbeeck JP. Second line therapy in malignant pleural mesothelioma: a systematic review. Lung Cancer. 2015;89:223–231. doi: 10.1016/j.lungcan.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Calabrò L, Morra A, Fonsatti E, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2013;14:1104–1111. doi: 10.1016/S1470-2045(13)70381-4. [DOI] [PubMed] [Google Scholar]
- 6.Calabrò L, Morra A, Fonsatti E, et al. Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, single-arm, phase 2 study. Lancet Respir Med. 2015;3:301–309. doi: 10.1016/S2213-2600(15)00092-2. [DOI] [PubMed] [Google Scholar]
- 7.Calabrò L, Ceresoli GL, D'Incecco A, Scherpereel A, Aerts J, Maio M. Immune checkpoint therapy of mesothelioma: pre-clinical bases and clinical evidences. Cytokine Growth Factor Rev. 2017;36:25–31. doi: 10.1016/j.cytogfr.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Calabrò L, Morra A, Giannarelli D, et al. Tremelimumab combined with durvalumab in patients with mesothelioma (NIBIT-MESO-1): an open-label, non-randomised, phase 2 study. Lancet Respir Med. 2018;6:451–460. doi: 10.1016/S2213-2600(18)30151-6. [DOI] [PubMed] [Google Scholar]
- 9.Scherpereel A, Mazieres J, Greillier L, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019;20:239–253. doi: 10.1016/S1470-2045(18)30765-4. [DOI] [PubMed] [Google Scholar]
- 10.Disselhorst MJ, Quispel-Janssen J, Lalezari F, et al. Ipilimumab and nivolumab in the treatment of recurrent malignant pleural mesothelioma (INITIATE): results of a prospective, single-arm, phase 2 trial. Lancet Respir Med. 2019;7:260–270. doi: 10.1016/S2213-2600(18)30420-X. [DOI] [PubMed] [Google Scholar]
- 11.Baas P, Scherpereel A, Nowak A, et al. First-line nivolumab plus ipilimumab vs chemotherapy in unresectable malignant pleural mesothelioma: CheckMate 743. Presented at the IASLC WCLC 2020 Virtual Presidential Symposium. Aug 8, 2020. https://wclc2020.iaslc.org/wp-content/uploads/2020/08/Abstracts-Virtual-Presidential-Symposium.pdf
- 12.Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20:25–39. doi: 10.1038/s41577-019-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15:257–260. doi: 10.1093/annonc/mdh059. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumors: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Heydt C, Rehker J, Pappesch R, et al. Analysis of tumor mutational burden: correlation of five large gene panels with whole exome sequencing. Sci Rep. 2020;10 doi: 10.1038/s41598-020-68394-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harhay MO, Porcher R, Cantu E, et al. An alternative approach for the analysis of time-to-event and survival outcomes in pulmonary medicine. Am J Respir Crit Care Med. 2018;198:684–687. doi: 10.1164/rccm.201801-0189LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonia SJ, Borghaei H, Ramalingam SS, et al. 4-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol. 2019;20:1395–1408. doi: 10.1016/S1470-2045(19)30407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamid O, Robert C, Daud A, et al. A. 5-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30:582–588. doi: 10.1093/annonc/mdz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topalian SL, Hodi FS, Brahmer JR, et al. 5-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol. 2019;5:1411–1420. doi: 10.1001/jamaoncol.2019.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiarion-Sileni V, Pigozzo J, Ascierto PA, et al. Ipilimumab retreatment in patients with pretreated advanced melanoma: the expanded access programme in Italy. Br J Cancer. 2014;110:1721–1726. doi: 10.1038/bjc.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20:1239–1251. doi: 10.1016/S1470-2045(19)30388-2. [DOI] [PubMed] [Google Scholar]
- 22.Nomura M, Otsuka A, Kondo T, et al. Efficacy and safety of retreatment with nivolumab in metastatic melanoma patients previously treated with nivolumab. Cancer Chemother Pharmacol. 2017;80:999–1004. doi: 10.1007/s00280-017-3444-0. [DOI] [PubMed] [Google Scholar]
- 23.Bernard-Tessier A, Baldini C, Martin P, et al. Outcomes of long-term responders to anti-programmed death 1 and anti-programmed death ligand 1 when being rechallenged with the same anti-programmed death 1 and anti-programmed death ligand 1 at progression. Eur J Cancer. 2018;101:160–164. doi: 10.1016/j.ejca.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Niki M, Nakaya A, Kurata T, et al. Immune checkpoint inhibitor re-challenge in patient with advanced non-small cell lung cancer. Oncotarget. 2018;9:32298–32304. doi: 10.18632/oncotarget.25949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujita K, Yamamoto Y, Kanai O, et al. Retreatment with anti-PD-1 antibody in non-small cell lung cancer patients previously treated with anti-PD-L1 antibody. Thorac Cancer. 2020;11:15–18. doi: 10.1111/1759-7714.13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katayama Y, Shimamoto T, Yamada T, et al. Retrospective efficacy analysis of immune checkpoint inhibitor rechallenge in patients with non-small cell lung cancer. J Clin Med. 2019;9:102. doi: 10.3390/jcm9010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizvi NA, Cho BC, Reinmuth N, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non–small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 2020;6:661–674. doi: 10.1001/jamaoncol.2020.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferris RL, Haddad R, Even C, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase 3 study. Ann Oncol. 2020;31:942–950. doi: 10.1016/j.annonc.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 29.De Gooijer CJ, Borm FJ, Scherpereel A, Baas P. Immunotherapy in malignant pleural mesothelioma. Front Oncol. 2020;10:187. doi: 10.3389/fonc.2020.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hellmann MD, Ciuleanu T-E, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellmann MD, Callahan MK, Awad MM, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell. 2018;33:853–861. doi: 10.1016/j.ccell.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Italian Network for Tumour Biotherapy (NIBIT) Foundation states that individual participant data from the NIBIT-MESO-1 trial will be available (including data dictionaries). The data that will be shared include individual participant data that underlie the results reported in this Article, after de-identification (text, table, figures, and appendices). The study protocol, statistical analysis plan, and analytic code will also be available. Data will be available between 9 months and 36 months after the Article has been published. Data will be made available to researchers who provide a methodologically sound proposal as reviewed by the Board of the NIBIT Foundation. The Board will ask the researchers for a complete protocol with the whole list of data needed for their research project and their analysis plan to achieve specific aims in the proposal. The protocol will then be reviewed by the Board of the NIBIT Foundation, which will make a recommendation on the scientific value of the project. Based on these recommendations, the Board will or will not accept sharing of the requested data, providing the researchers with the scientific reasons for their decision. In case of approval by the NIBIT Foundation, and to gain access, data requestors will need to sign a data access agreement specifying the policy for publication and the inclusion of the principal investigators and collaborators of the NIBIT-MESO-1 study in the list of authors of any scientific article or communication resulting from the research project. Proposals should be directed to segreteria@fondazionenibit.org.