Abstract
Ni-catalyzed enantioselective hydrofunctionalizations of conjugated dienes are particularly demanding reactions to devise because they require not only addressing the inherent challenges associated with the development of an enantioselective transformation but also overcoming all other aspects of selective catalysis (chemoselectivity, regioselectivity, diastereoselectivity, etc.). However, the value-added nature of the chiral allylic and homoallylic derivatives obtained by these methods, the lack of efficient alternatives, and the use of an earth-abundant first-row transition metal have led to renewed interest over the past decade. In this Perspective, we give an overview of the developments in this field, from the original findings (often dating back to the last century) to the most recent contributions. Emphasis is placed on the nature of the hydrofunctionalization agent (C(sp), C(sp2), C(sp3), N, P, or O).
Keywords: nickel catalysis; selective catalysis; hydrofunctionalization; 1,3-dienes; reaction mechanism
1. Introduction
Transition-metal-catalyzed selective functionalizations of 1,3-dienes have gained important momentum over the last decades.1 These approaches are attractive because they convert simple hydrocarbon molecules into more complex polyfunctionalized entities. More specifically, selective hydrofunctionalization of one of the two carbon–carbon double bonds provides access to valuable, often chiral, allylic or homoallylic derivatives.2 Depending on the nature of the diene (cyclic vs acyclic) and its substitution pattern, the theoretical number of regio- and stereoisomers that can be generated varies but is typically >10 (Figure 1).
Figure 1.
Overview of the complexity inherent to transition metal-catalyzed selective hydrofunctionalizations of conjugated dienes. Only a subset of substitution patterns is displayed (FG: functional group).
While for a long time method development focused on naturally occurring dienes (i.e., 1,3-butadiene, isoprene, and myrcene) or on readily available linear dienes, recent advances in diene syntheses have greatly expanded the range of starting materials that can be explored for selective hydrofunctionalizations.3 Moreover, driven by economic and ecological imperatives, recent years have witnessed a renewed interest in the use of cheap, abundant, and environmentally friendly first-row transition metals.4 This Perspective aims to provide an overview of nickel-catalyzed hydrofunctionalizations of 1,3-dienes with an emphasis on enantioselective processes. Mechanistically, the majority of diene hydrofunctionalizations proceed via the formation of metal–allyl intermediates that can react with electrophilic or nucleophilic reagents. From a selectivity standpoint, diene hydrofunctionalization geared toward the isolation of a single product requires solving issues related to chemo-, regio-, diastereo-, and enantiocontrol while preventing hydrofunctionalization of the second carbon–carbon double bond or other kinetically competent and thermodynamically favorable side reactions (e.g., cycloadditions). This Perspective is structured around the nature of the hydrofunctionalization agent. It is modestly intended to complement two excellent Perspectives that have been published recently in this journal.1d,1e Hydrofunctionalizations of electronically biased dienes and difunctionalizations fall beyond the scope of this manuscript and will not be discussed herein.1b,1f
2. Nickel-Catalyzed Enantioselective Hydrocarbonation of 1,3-Dienes
C(sp) Reagents: Enantioselective Hydrocyanation
The transition-metal-catalyzed hydrocyanation of a carbon–carbon double bond is a fundamental chemical process that has unfailingly attracted academic and industrial attention. For instance, the Ni-catalyzed addition of 2 equiv of HCN to 1,3-butadiene is central to the industrial production of adiponitrile.5 Moreover, the enantioselective hydrocyanation of alkenes provides potential entry into the synthesis of chiral nitriles as well as amides, esters, and amines after functional group manipulation.6 Because of challenges associated with regioselectivity of addition, parasitic isomerizations, and catalyst deactivation, most research has focused on cyclic alkenes and vinylarenes. A handful of Ni-catalyzed enantioselective hydrocyanations of dienes have also been reported. In 2006, Vogt and co-workers showed that controlled addition of only 1 equiv of hydrogen cyanide to 1,3-cyclohexadiene afforded cyclohex-2-ene-1-carbonitrile (3a) with up to 86% ee using the C1-symmetric chiral bisphosphite ligand L1 (Figure 2).7 Based on deuterium-labeling experiments, the authors were able to assess the formal 1,2-/1,4-addition ratio dictated by the ligand and established that reductive elimination is the enantioselectivity-determining step of the reaction. Of note, these products cannot be distinguished when HCN is employed.
Figure 2.
Ni-catalyzed enantioselective hydrocyanation of cyclohexadiene.
The same year, Saha and RajanBabu disclosed the Ni-catalyzed hydrocyanation of diversely substituted cyclic and acyclic dienes using a sugar-based electron-deficient bisphosphite (L2).8 In the cases reported, a single regioisomer was obtained, but the regioselectivity of addition was dependent on the substitution pattern. For substrates containing an aromatic substituent, addition occurred at the less substituted C=C bond (3b–d). The products were isolated in moderate yields with enantioselectivity varying from 39% to 78% ee. For myrcene, an alkyl-substituted substrate, exclusive 1,4-regioselectivity was obtained, leading to the formation of a quaternary stereocenter, albeit with low ee (3e) (Figure 3).
Figure 3.
Ni-catalyzed enantioselective hydrocyanation of linear dienes.
More recently, using a cyanohydrin (4) as the HCN source, Fang and co-workers identified a C2-symmetric Taddol-based bisphosphite ligand (L3) for the enantioselective hydrocyanation of linear monosubstituted dienes as well as 1,1-, 1,2-, 1,4-disubstituted dienes and even trisubstituted dienes (Figure 4).9 Here again, the most accessible double bond underwent cyanide addition preferentially (>99 rr, with the exception of 3h). While excellent levels of enantioselectivity were obtained for aryl-substituted linear dienes (>90% ee), decreased enantiocontrol was observed for all other substitution patterns (typically 65–85% ee). Nonetheless, some of the most challenging nonsymmetric dienes underwent hydrocyanation with remarkable regioselectivity.
Figure 4.
Ni-catalyzed enantioselective hydrocyanation of linear and branched dienes using a cyanohydrin reagent.
C(sp) Reagents: Enantioselective Hydroalkynylation
To date, a single example of enantioselective hydroalkynylation of dienes has been reported (Figure 5). In 2010, Shirakura and Suginome10 showed that Taddol-based phosphoramidite ligands L4 were particularly effective at inducing high levels of regio- and stereocontrol for the hydroalkynylation of (E)-aryl-substituted linear dienes using terminal alkynes with a bulky siloxydialkyl substituent. The skipped enynes obtained, featuring a potentially stereolabile tertiary center, were isolated with high levels of enantiocontrol (6a–d; nine examples in total). Whereas reaction occurred preferentially at the terminal C=C bond, slow addition of the alkyne reagent was found to be crucial to minimize formation of the bisalkyne homocoupling product.
Figure 5.
Ni-catalyzed enantioselective hydroalkynylation of linear dienes.
Moreover, it should be noted that sterically demanding silanes that were found to be reactive in the non-asymmetric version of the reaction were not competent candidates for the enantioselective protocol.11 Finally, even though the structural constrains imposed on both reaction partners intrinsically limited the scope of the reaction, the authors demonstrated the utility of the chiral products obtained by conducting a highly enantiospecific desilylation/Rh-catalyzed conjugate addition sequence, affording 8a in 68% yield with 91% ee over two steps.
C(sp2) Reagents: Enantioselective Hydrovinylation
A seminal example of Ni-catalyzed enantioselective hydrovinylation of a cyclic diene was disclosed by Wilke and co-workers in 1972 (Figure 6A).12 Using menthol-derived trialkylphosphine L5, a Ni(II) precursor, and an aluminum cocatalyst (i.e., a strong Lewis acid activator) and working under a relatively high pressure of ethylene (20–30 bar), hydrovinylation of 1,3-cyclooctadiene was achieved, leading to 3-vinylcyclooctene. Based on combined derivatizations and degradation studies, the chiral product 10a was reported with an optical purity of 70%. Concomitant formation of 3-methylpentene (11a), resulting from ethylene trimerization, was also observed (optical purity: 64%).
Figure 6.
Ni-catalyzed enantioselective hydrovinylation of cyclic dienes.
Buono and co-workers showed in 1985 that polydentate aminophosphine/phosphinite (AMPP) ligand L6 derived from threonine induced excellent enantioselectivity in the Ni-catalyzed hydrovinylation of 1,3-cyclohexadiene using Et2AlCl as cocatalyst (10b: 93% optical purity; Figure 6B).13 No yields were disclosed, and no reports followed this promising initial work.
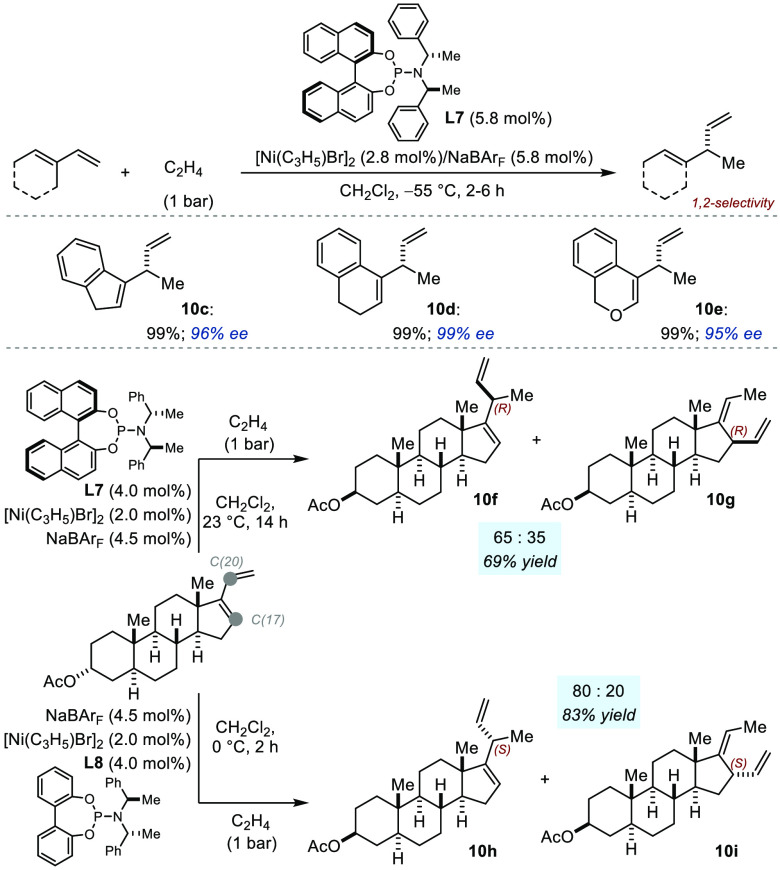
Breakthroughs came from the RajanBabu laboratory three decades later with the development of a method for the enantioselective hydrovinylation of 1-vinyl-substituted cycloalkenes (Figure 7).14 The modular phosphoramidite ligand L7 was found to provide excellent regiocontrol with preferred 1,2-addition to the less hindered olefin while inducing excellent levels of enantioselectivity (10c–e: 95–99% ee). Of note, linear 1,3-dienes were found to be unreactive using the optimized protocol. Shortly thereafter, the strategy was applied to install exocyclic stereocenters adjacent to the D ring of steroids, thus providing access to the so-called natural and unnatural C(20) configurations (even though this was achieved at the expense of the stereochemistry of C(17)).15,16 The authors found that the proportion of the 1,4-adduct could be reduced by conducting the reaction at low temperature. Finally, when L8 was used, hydrovinylation occurred preferentially at C(20), affording an 80:20 mixture of 10h and 10i in 83% yield. The product with the unnatural C(20) configuration was accessed using L7 but with reduced regioselectivity (10f:10g = 65:35; 69% yield).
Figure 7.
Ni-catalyzed enantioselective hydrocyanation of terminal dienes.
C(sp2) Reagents: Enantioselective Hydroarylation
In 2018, Zhou and co-workers disclosed a simple yet highly efficient Ni-catalyzed hydroarylation of styrenyl derivatives using arylboronic acids or arylboronic esters as C(sp2) sources.17 The process operates under mild reaction conditions and displayed broad functional group tolerance. The presence of a protic source/solvent was found to be crucial for reactivity. Subsequently, the reaction was extended to cyclic and acyclic 1,3-dienes. In the case of the latter, whereas aryl-substituted derivatives underwent hydroarylation with excellent 1,2-selectivity, alkyl-containing substrates led to mixtures of regioisomers. Preliminary mechanistic investigations indicated that the key active species is a [Ni(II)–H] generated by oxidative addition of MeOH to the Ni(0) precursor. Shortly thereafter, the enantioselective version of this reaction was reported by the same group (Figure 8A).18 Promising levels of enantioselectivity (77–84% ee) and exquisite 1,2-regioselectivity were obtained using aryl-substituted linear dienes and a chiral spiro amino–phosphine ligand (L9). In a following study, the Zhou laboratory established that nonprotected indoles were competent substrates for the selective hydro(hetero)arylation of 1,3-dienes (Figure 8B).19 With the use of a chiral bisphosphine ligand (L10 or L11) and diverse indole derivatives, excellent regio- and enantioselectivity were obtained for both aryl- and alkyl-substituted linear dienes over a range of ca. 40 combinations. On the basis of experimental and computational mechanistic investigations, the authors proposed that the reaction is initiated by a concerted ligand-to-ligand hydrogen transfer (LLHT) of a [Ni(alcohol)(diene)] complex.20
Figure 8.
Ni-catalyzed enantioselective hydroarylation of linear dienes using (A) arylboronic acids or (B) indoles.
In 2020, Meek and co-workers identified a sterically demanding axially chiral phosphoramidite ligand (L12) for the site-selective and enantioselective hydroarylation of terminal 1,3-dienes and unsymmetrically substituted internal 1,3-dienes (Figure 9).21 With the use of a protic solvent, the catalytic activity and regio- and enantioselectivity were systematically very high. In all cases, the hydroarylation product was generated as the E alkene isomer (E:Z > 93:7). Variations of both reaction partners showed that the method is compatible with aryl halides, various heterocycles, and sensitive Lewis basic functionalities (ethers, ketones, esters, and amides) (13c, 13e–h). Mechanistic experiments indicated that the reaction is stereoconvergent and funnels through rapidly interconverting [Ni–allyl] intermediates. The authors found that the identity of the boronate plays a critical role in the regioselectivity of insertion, with more sterically demanding boronates leading to increased site selectivity.
Figure 9.
Ni-catalyzed enantioselective hydroarylation of linear and internal dienes.
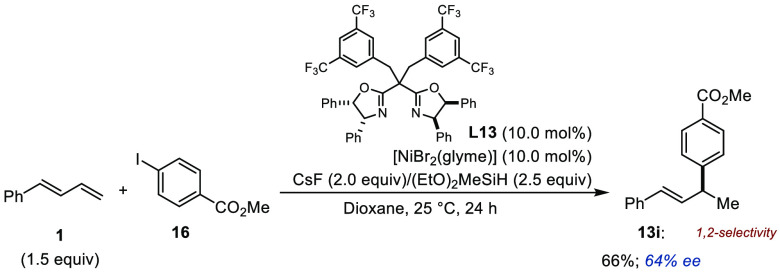
An isolated example of Ni-catalyzed enantioselective 1,2-hydroarylation of (E)-1,3-butadienylbenzene using an electron-deficient aryl iodide and the chiral bisoxazoline ligand L13 was reported by Wang, Ding, and co-workers (Figure 10).22 A single regioisomeric product (13i) was isolated in 66% yield with 64% ee. In contrast to the studies from the Zhou and Meek groups, the authors proposed that a catalytically competent [Ni(I)–H] is generated from a Ni(II) precatalyst using a combination of CsF and diethoxymethylsilane (DEMS), with the catalytic cycle proceeding via Ni(I)/Ni(III) intermediates.
Figure 10.
Ni-catalyzed enantioselective hydroarylation of (E)-1,3-butadienylbenzene under reductive conditions.
In late 2022, Huang, Ye, and colleagues disclosed a method for the Ni-catalyzed enantioselective hydro(hetero)arylation of a variety of conjugated dienes using pyridine derivatives (Figure 11).23 The reaction necessitated a temperature above the boiling point of the solvent (toluene, 135 °C) and the use of an aluminum cocatalyst to direct activation of one ortho C–H bond of the pyridine ring. An efficient chiral C1-symmetric diamine-derived phosphine oxide ligand (L14) was identified after a systematic screening campaign using (E)-buta-1,3-dienylbenzene as a model substrate. The product (18a) was obtained with exclusive Markovnikov 1,2-regioselectivity in 81% yield with 94% ee. A variety of functionalized pyridine derivatives but also quinolone and quinoxaline (18c) were found to be compatible with the optimized protocol. Practical yields and high levels of regio- and enantioselectivity were obtained systematically. Variation of the diene substitution pattern showed that while aryl-substituted dienes afforded the addition product (18f) with performances similar to that of the model substrate, an alkyl-substituted diene led to a reversal in regioselectivity (18g: 88:12 rr). A combined experimental and theoretical mechanistic study revealed that C–Hortho activation proceeds by reversible LLHT. It further confirmed the ability of the ligand to bind both the Al and Ni centers via the O and P atoms, respectively. The product-forming reductive elimination step was computed to be both rate- and enantiodetermining. Steric factors were proposed to account for the high degree of enantiodiscrimination imparted by the system.
Figure 11.
Ni-catalyzed enantioselective hydroarylation of conjugated dienes.
C(sp3) Reagents: Enantioselective Hydroalkylation
The earliest example of intramolecular enantioselective hydroalkylation of dienes was reported by Mori and co-workers in 2000 (Figure 12).24 Following their investigations on related non-enantioselective cyclizations, the authors designed a set of 1,3-dienes tethered to an aldehyde to access five- and six-membered carbocycles with two contiguous tertiary stereocenters. The optimized system required an excess of a silane reagent and operated in a polar nonprotic solvent (DMF or CH3CN) at low temperature. A chiral monophosphine ligand (L15) induced the highest levels of enantiocontrol. In several cases, the reaction led to a mixture of two cyclic products flanked with a propenyl (19a) or allyl chain (19b). Average yields and moderate levels of enantioselectivity were obtained across the restricted range of substrates investigated (17–86% ee). The origin of the product distribution is unclear. It could originate from different site selectivities of insertion of the putative [Ni–H], leading to intermediates equilibrating via [Ni–allyl] species. Concurrent isomerizations could parallel this process or simply be responsible for postcatalytic C=C bond migration between 19a and 19b.
Figure 12.
Intramolecular Ni-catalyzed enantioselective hydroalkylation of dienes.
An intermolecular variant of this reaction was published in 2007 by the Zhou group (Figure 13A).25 Using a catalytic combination of [Ni(acac)2] and a chiral spiro-phosphoramidite ligand (L16), ZnEt2 as reducing agent, and an aprotic solvent (i.e., toluene), the authors achieved the coupling of a variety of benzaldehyde derivatives with 1,4-diphenylbuta-1,3-diene, a symmetrically disubstituted diene. The new C–C bond was formed between one of the two benzylic positions of the diene and the carbon atom of the carbonyl function. All of the products were isolated in high yield with perfect anti/syn selectivity and high levels of enantioselectivity (85–96% ee). No variation of the diene was reported.
Figure 13.
Intermolecular Ni-catalyzed enantioselective hydroalkylation of terminal dienes using (A) aldehydes or (B) ketones.
A decade later, the same group reported the Ni-catalyzed enantioselective hydroalkylation of linear dienes using acetophenone derivatives or aliphatic ketones, providing access to enantioenriched γ,δ-unsaturated ketones featuring a potentially stereolabile tertiary α-stereocenter (Figure 13B).26 A novel C2-symmetric axially chiral bisphosphine ligand (L17) was found to exert high levels of regio- and enantiocontrol and delivered the 1,2-addition products in moderate to high yields. The reactions were conducted in refluxing ethanol in the presence of a catalytic amount of a strong base to activate the nonstabilized nucleophiles. The products derived from aryl-substituted dienes were obtained in higher yield and enantioselectivity than those derived from the corresponding alkyl-substituted substrates (compare 23a and 23e for instance). When non symmetric aliphatic methyl ketones were employed, the reaction occurred preferentially at the α-methylene unit rather than the α-methyl group, creating an opportunity for the development of a diastereoselective transformation. The resulting γ,δ-unsaturated ketones with two contiguous tertiary stereocenters were isolated in high yield and enantioselectivity but with low diastereoselectivity (23d). The deuterium-labeling experiment supported the formation of a catalytically competent [Ni–H] intermediate, the formation of which could occur either by an LLHT mechanism or by oxidative addition of the alcoholic solvent to the Ni(0) precursor.
In 2020, Shao et al. disclosed two regiodivergent methods for the stereoselective hydroalkylation of acyclic branched dienes with unstabilized C(sp3) nucleophiles.27 In addition to a Ni(0) source, the first catalytic system used an achiral C1-symmetric (P,N) ligand (L18), secondary or tertiary amides, and MeOK as the base for in situ generation of the corresponding enolates. The 1,4-addition products (25) were the only regioisomers generated. They were isolated in high yield with excellent stereocontrol of the internal olefin. Variations in nucleophilic and electrophilic components delineated a wide range of substrate combinations and showed that sensitive functionalities and heteroaromatic precursors were compatible with the reaction conditions (Figure 14A). Using the chiral phosphinoxazoline ligand L19, imides as carbon nucleophiles, and Barton’s base (BTMG), a complementary 3,4-addition hydroalkylation was developed that allowed the construction of two contiguous tertiary stereocenters in a single step (Figure 14B). The products (27) were isolated systematically with excellent enantioselectivity. The diastereoselectivity varied from moderate to high depending on the combination of substrates. A wide range of functional groups were shown to be compatible with the mild conditions employed, and several postcatalytic derivatizations were conducted to underscore the synthetic potential of the method.
Figure 14.
Stereodivergent Ni-catalyzed enantioselective hydroalkylation of terminal dienes: (A) 1,4-selective; (B) 3,4-selective.
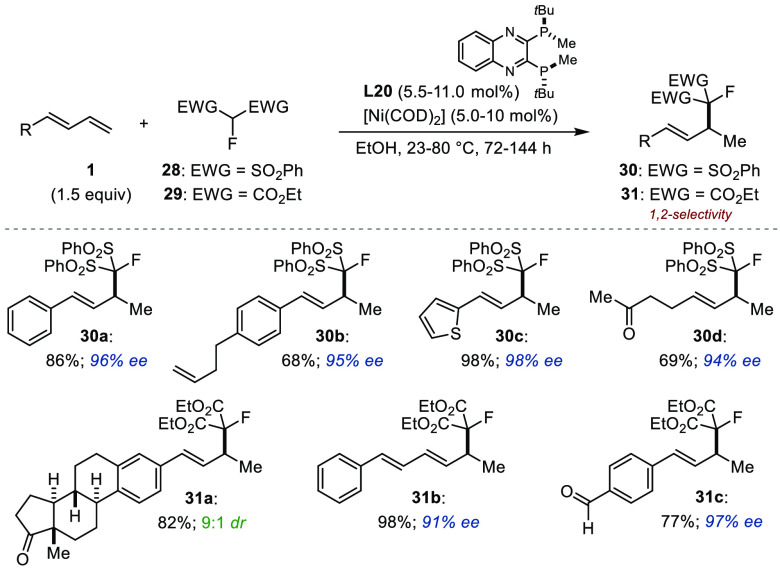
In late 2022, Yu and co-workers reported the hydroalkylation of linear dienes using activated monofluorinated alkylating agents 28 and 29 (Figure 15).28 With QuinoxP* (L20), the 1,2-addition products were obtained with perfect Markovnikov selectivity in high yield with excellent enantioselectivity (30a–d and 31a–c). Under the optimized reaction conditions, a wide number of substrates were found to be compatible, and several Lewis basic functionalities were tolerated. The usefulness of the products obtained was demonstrated through several derivatizations.29
Figure 15.
Ni-catalyzed enantioselective hydroalkylation of terminal dienes.
3. Nickel-Catalyzed Enantioselective Hydroamination of 1,3-Dienes
Ni-catalyzed homogeneous hydroamination of 1,3-dienes can be traced back to the early 1970s. Baker and co-workers showed that butadiene and a primary or secondary amine generated complex mixtures, with the major component resulting from the amination of telomerized butadiene (34b) (Figure 16A).30 The Hartwig group showed that alkylamines are competent nucleophiles for the catalytic hydroamination of cyclic dienes using an in situ-generated nickel complex (with L22) and trifluoroacetic acid (TFA). Importantly, the identification of exchange processes between the allylic amines produced and free amines emphasized the difficulty of developing an enantioselective variant of the process (Figure 16B).31 Nearly 20 years later, Tran et al. established a method for the Ni-catalyzed intermolecular enantioselective hydroamination of branched 1,3-dienes in the presence of trifluoroethanol using a chiral C2-symmetric bisphosphine ligand (L23) (Figure 16C).32 The method was found to be highly regio-, chemo-, and enantioselective and afforded value-added chiral allylic amines using 4 equiv of linear or α-branched aliphatic primary amines or secondary amines. Supporting organometallic syntheses, reaction monitoring, deuterium-labeling experiments, and kinetic analyses provided valuable mechanistic information. A Ni−π-allyl complex was identified as the resting state, and an outer-sphere nucleophilic attack of H-bonded amine aggregates was proposed to be the rate- and enantiodetermining step of the reaction. These findings guided the reoptimization of the initial experimental protocol and led to the identification of an improved catalytic system that required only 2 equiv of the amine reagent.
Figure 16.
(A) Ni-catalyzed hydroamination of butadiene. (B) Ni-catalyzed hydroamination of cyclic dienes. (C) Ni-catalyzed enantioselective hydroamination of branched dienes.
A few months later, Yin and co-workers published a related protocol for the Ni-catalyzed 1,2-enantioselective hydroamination of linear aryl- and alkyl-substituted 1,3-dienes using ligand L24 and a cocatalytic amount of phthalic acid (Figure 17).33 Remarkably, the reactivity, regioselectivity, and enantioselectivity were consistently very high. Moreover, the method was found to be compatible with primary and secondary alkylamines as well as with aniline derivatives. A variety of functional groups were also tolerated under the optimized reaction conditions. Consistent with some of Hartwig’s observations, hydroamination was found to be (slowly) reversible for secondary amines but not for primary amines.
Figure 17.
Ni-catalyzed enantioselective hydroamination of terminal dienes.
4. Nickel-Catalyzed Enantioselective Hydrophosphinylation of 1,3-Dienes
Regardless of the nature of the transition metal employed, enantioselective hydrophosphinylations remain scarce.34 Prior to 2022, no report had mentioned the use of nickel to achieve such a challenging transformation. Following their work on the seemingly related hydroamination, Yin and co-workers developed a [Ni/Brønsted acid]-catalyzed regio- and enantioselective hydrophosphinylation of 1,3-dienes using diarylphosphine oxides (Figure 18).35 Internal aryl,aryl- and aryl,alkyl-substituted dienes were found to be competent substrates and could be reacted with a variety of phosphine oxides. With Binap (L25) as the ligand and a catalytic amount of p-toluenesulfonic acid, exclusive 1,2-regioselectivity was observed, and the chiral allylic phosphine oxides were isolated in moderate to high yields with typically >90% ee. When unsymmetrically substituted phosphine oxides were employed, the catalytic activity and the regio- and enantioselectivity remained excellent, but the level of diastereocontrol was only modest (six examples, see 36f). Isotopic labeling experiments and crossover experiments indicated that C–P bond formation is certainly irreversible.
Figure 18.
Ni-catalyzed enantioselective hydrophosphinylation of internal dienes.
5. Nickel-Catalyzed Enantioselective Hydroalkoxylation of 1,3-Dienes
The development of a general, highly regio- and enantioselective hydroalkoxylation of 1,3-dienes has not been reported to date. Nonetheless, two studies are worthy of note. In 2017, Mifleur et al. conducted a combined experimental and computational study of the mechanism of the regioselective hydrofunctionalization of butadiene using SegPhos (L26) in combination with a Ni(0) source (Figure 19).36 The formation of the Markovnikov adduct 38a is favored over the anti-Markovnikov adduct 38b (∼2:1 rr). The conclusions of their investigations point to reversible postcatalytic isomerization and racemization processes that ultimately lead to the expected thermodynamic ratio of the butenyl ethers. During these investigations, enantioenriched allyl ether 38a was prepared by the Ni-catalyzed hydroalkoxylation of butadiene using L26 in toluene with an excess of EtOH (10 equiv). Subsequently, racemization of 38a was established by subjecting it to similar reaction conditions using an achiral bisphosphine nickel complex.
Figure 19.
Ni-catalyzed enantioselective hydroalkoxylation of butadiene.
In 2019, Tran and Mazet reported a quite general regioselective 3,4-hydroalkoxylation of branched dienes that operated with a low catalyst loading of nickel under mild conditions (Figure 20).37 The method appeared to be tolerant toward sensitive functional groups and provided access to a diverse range of allylic ethers in high yields with moderate to excellent regioselectivity. Control experiments showed that the Ni-catalyzed hydroalkoxylation was reversible and that it involved [Ni−π-allyl] species reminiscent of those observed in the hydroamination of dienes developed by the same group. Nonetheless, the authors showed that the reaction was amenable to enantioselective catalysis, and promising results were obtained with a ferrocenyl-based chiral phosphinooxazoline ligand (L27). Perhaps not surprisingly, the enantioselectivity of 38c was found to decrease as the reaction progressed.
Figure 20.
Ni-catalyzed enantioselective hydroalkoxylation of a branched diene.
6. Conclusions and Outlook
The content of this Perspective underlines the increased interest in Ni-catalyzed enantioselective hydrofunctionalization of 1,3-dienes observed in recent years. Independent of the nature of the hydrofunctionalization agent, these transformations are attractive from both the selectivity and synthesis points of view. Indeed, achieving high levels of enantiocontrol necessarily implies addressing chemo-, regio-, and even diastereocontrol issues at the same time. Moreover, from a synthetic standpoint, the functionalized chiral allylic and homoallylic derivatives obtained by this approach expand the chemical space significantly. In most cases, these value-added compounds would be difficult to prepare by conventional methods. It should be pointed out that many of the methods reported to date display broad functional group tolerance, rendering them attractive for applications in various fields. Some future directions appear obvious with the development of complementary methods aimed at generating all of the possible chiral regioisomers that are theoretically accessible from a given diene substitution pattern with excellent regio- and enantiocontrol. Exploring the periodic table further to identify additional hydrofunctionalization agents will create opportunities to expand our chemical horizons and will certainly pose challenging mechanistic questions. In this context, the use of dual catalytic systems employing two different earth-abundant transition metals certainly constitutes an appealing strategy.29 Finally, the role exerted by the chiral ligand in selective catalysis remains essential. Therefore, continued efforts in catalyst design and ligand development will be required.38
Acknowledgments
This work was supported by the Swiss National Science Foundation (200021_188490) and the University of Geneva.
Author Contributions
‡ A.F. and C.Z. contributed equally.
The authors declare no competing financial interest.
References
- a Huang L.; Arndt M.; Gooßen K.; Heydt H.; Gooßen L. J. Late Transition Metal-Catalyzed Hydroamination and Hydroamidation. Chem. Rev. 2015, 115, 2596. 10.1021/cr300389u. [DOI] [PubMed] [Google Scholar]; b Xiong Y.; Sun Y.; Zhang G. Recent Advances on Catalytic Asymmetric Difunctionalization of 1,3-Dienes. Tetrahedron Lett. 2018, 59, 347. 10.1016/j.tetlet.2017.12.059. [DOI] [Google Scholar]; c Holmes M.; Schwartz L. A.; Krische M. J. Intermolecular Metal-Catalyzed Reductive Coupling of Dienes, Allenes, and Enynes with Carbonyl Compounds and Imines. Chem. Rev. 2018, 118, 6026. 10.1021/acs.chemrev.8b00213. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Adamson N. J.; Malcolmson S. J. Catalytic Enantio- and Regioselective Addition of Nucleophiles in the Intermolecular Hydrofunctionalization of 1,3-Dienes. ACS Catal. 2020, 10, 1060. 10.1021/acscatal.9b04712. [DOI] [Google Scholar]; e Perry G. J. P.; Jia T.; Procter D. J. Copper-Catalyzed Functionalization of 1,3-Dienes: Hydrofunctionalization, Borofunctionalization and Difunctionalization. ACS Catal. 2020, 10, 1485. 10.1021/acscatal.9b04767. [DOI] [Google Scholar]; f Li G.; Huo X.; Jiang X.; Zhang W. Asymmetric Synthesis of Allylic Compounds via Hydrofunctionalisation and Difunctionalisation of Dienes, Allenes, and Alkynes. Chem. Soc. Rev. 2020, 49, 2060. 10.1039/C9CS00400A. [DOI] [PubMed] [Google Scholar]
- a Wäspi U.; Schweizer P.; Dudler R. Syringolin Reprograms Wheat to Undergo Hypersensitive Cell Death in a Compatible Interaction with Powdery Mildew. Plant Cell 2001, 13, 153. 10.1105/tpc.13.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Guzmán E. A.; Xu Q.; Pitts T. P.; Mitsuhashi K. O.; Baker C.; Linley P. A.; Oestreicher J.; Tendyke K.; Winder P. L.; Suh E. M.; Wright A. E. Leiodermatolide, a Novel Marine Natural Product, has Potent Cytotoxic and Antimitotic Activity Against Cancer Cells, Appears to Affect Microtubule Dynamics, and Exhibits Antitumor Activity. Int. J. Cancer 2016, 139, 2116. 10.1002/ijc.30253. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Xie P.; Fu W.; Wu Y.; Cai X.; Sun Z.; Li S.; Gao C.; Yang X.; Loh T. P. Allylic Phosphorus Ylides Directly Generated from Alcohols with Water as the Only Byproduct. Org. Lett. 2019, 21, 4168. 10.1021/acs.orglett.9b01349. [DOI] [PubMed] [Google Scholar]
- a Diver S. T.; Giessert A. J. Enyne Metathesis (Enyne Bond Reorganization). Chem. Rev. 2004, 104, 1317. 10.1021/cr020009e. [DOI] [PubMed] [Google Scholar]; b Kong J. R.; Krische M. J. Catalytic Carbonyl Z-Dienylation via Multicomponent Reductive Coupling of Acetylene to Aldehydes and α-Ketoesters Mediated by Hydrogen: Carbonyl Insertion into Cationic Rhodacyclopentadienes. J. Am. Chem. Soc. 2006, 128, 16040. 10.1021/ja0664786. [DOI] [PubMed] [Google Scholar]; c Borg T.; Tuzina P.; Somfai P. Lewis Acid-Promoted Addition of 1,3-Bis(Silyl)Propenes to Aldehydes: A Route to 1,3-Dienes. J. Org. Chem. 2011, 76, 8070. 10.1021/jo2013466. [DOI] [PubMed] [Google Scholar]; d Besset T.; Kuhl N.; Patureau F. W.; Glorius F. RhIII-Catalyzed Oxidative Olefination of Vinylic C–H Bonds: Efficient and Selective Access to Di-unsaturated α-Amino Acid Derivatives and Other Linear 1,3-Butadienes. Chem. - Eur. J. 2011, 17, 7167. 10.1002/chem.201101340. [DOI] [PubMed] [Google Scholar]; e De Paolis M.; Chataigner I.; Maddaluno J. Recent Advances in Stereoselective Synthesis of 1,3-Dienes. Top. Curr. Chem. 2012, 327, 87. 10.1007/128_2012_320. [DOI] [PubMed] [Google Scholar]; f Zheng C.; Wang D.; Stahl S. S. Catalyst–Controlled Regioselectivity in the Synthesis of Branched Conjugated Dienes via Aerobic Oxidative Heck Reactions. J. Am. Chem. Soc. 2012, 134, 16496. 10.1021/ja307371w. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Fiorito D.; Folliet S.; Liu Y.; Mazet C. A General Nickel-Catalyzed Kumada Vinylation for the Preparation of 2-Substituted 1,3-Dienes. ACS Catal. 2018, 8, 1392. 10.1021/acscatal.7b04030. [DOI] [Google Scholar]; h Šiaučiulis M.; Ahlsten N.; Pulis A. P.; Procter D. J. Transition-Metal-Free Cross-Coupling of Benzothiophenes and Styrenes in a Stereoselective Synthesis of Substituted (E,Z)-1,3-Dienes. Angew. Chem., Int. Ed 2019, 58, 8779. 10.1002/anie.201902903. [DOI] [PubMed] [Google Scholar]; i Scaringi S.; Mazet C. Kinetically Controlled Stereoselective Access to Branched 1,3-Dienes by Ru-Catalyzed Remote Conjugative Isomerization. ACS Catal. 2021, 11, 7970. 10.1021/acscatal.1c02144. [DOI] [Google Scholar]; j Soengas G. R.; Rodríguez-Solla H. Modern Synthetic Methods for the Stereoselective Construction of 1,3-Dienes. Molecules 2021, 26, 249. 10.3390/molecules26020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Peng J. B.; Wu F. P.; Wu X. F. First-Row Transition-Metal-Catalyzed Carbonylative Transformations of Carbon Electrophiles. Chem. Rev. 2019, 119, 2090. 10.1021/acs.chemrev.8b00068. [DOI] [PubMed] [Google Scholar]; b Wei D.; Darcel C. Iron Catalysis in Reduction and Hydrometalation Reactions. Chem. Rev. 2019, 119, 2550. 10.1021/acs.chemrev.8b00372. [DOI] [PubMed] [Google Scholar]; c Dalle K. E.; Warnan J.; Leung J. J.; Reuillard B.; Karmel I. S.; Reisner E. Electro- and Solar-Driven Fuel Synthesis with First Row Transition Metal Complexes. Chem. Rev. 2019, 119, 2752. 10.1021/acs.chemrev.8b00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Arthur P. Jr.; England D. C.; Pratt B. C.; Whitman G. M. Addition of Hydrogen Cyanide to Unsaturated Compounds. J. Am. Chem. Soc. 1954, 76, 5364. 10.1021/ja01650a034. [DOI] [Google Scholar]; b Brown E. S.; Rick E. A. Catalytic Addition of Hydrogen Cyanide to Non-activated Olefins. J. Chem. Soc. D 1969, 112b. 10.1039/c2969000112b. [DOI] [Google Scholar]; c Drinkard W. C.Hydrocyanation of Olefins Using Selected Nickel Phosphite Catalysts. US 3,496,215, 1970.; d Zhang H.; Su X.; Dong K. Recent Progress in Transition-Metal-Catalyzed Hydrocyanation of Nonpolar Alkenes and Alkynes. Org. Biomol. Chem. 2020, 18, 391. 10.1039/C9OB02374G. [DOI] [PubMed] [Google Scholar]
- a Elmes P. S.; Jackson W. R. Asymmetric Addition of Hydrogen Cyanide to Alkenes Catalyzed by a Zerovalent Palladium Compound. J. Am. Chem. Soc. 1979, 101, 6128. 10.1021/ja00514a049. [DOI] [Google Scholar]; b Casalnuovo A. L.; RajanBabu T. V.; Ayers T. A.; Warren T. H. Ligand Electronic Effects in Asymmetric Catalysis: Enhanced Enantioselectivity in the Asymmetric Hydrocyanation of Vinylarenes. J. Am. Chem. Soc. 1994, 116, 9869. 10.1021/ja00101a007. [DOI] [Google Scholar]; c Yan M.; Xu Q. Y.; Chan A. S. C. Asymmetric Hydrocyanation of Olefins Catalyzed by Chiral Diphosphite–Nickel Complexes. Tetrahedron Asymmetry 2000, 11, 845. 10.1016/S0957-4166(00)00026-4. [DOI] [Google Scholar]; d Li X.; You C.; Yang J.; Li S.; Zhang D.; Lv H.; Zhang X. Asymmetric Hydrocyanation of Alkenes without HCN. Angew. Chem., Int. Ed. 2019, 58, 10928. 10.1002/anie.201906111. [DOI] [PubMed] [Google Scholar]
- a Wilting J.; Janssen M.; Müller C.; Vogt D. The Enantioselective Step in the Nickel-Catalyzed Hydrocyanation of 1,3-Cyclohexadiene. J. Am. Chem. Soc. 2006, 128, 11374. 10.1021/ja064378u. [DOI] [PubMed] [Google Scholar]; b Wilting J.; Janssen M.; Müller C.; Lutz M.; Spek A. L.; Vogt D. Binaphtol-Based Diphosphite Ligands in Asymmetric Nickel-Catalyzed Hydrocyanation of Styrene and 1,3-Cyclohexadiene: Influence of Steric Properties. Adv. Synth. Catal. 2007, 349, 350. 10.1002/adsc.200600315. [DOI] [Google Scholar]
- Saha B.; RajanBabu T. V. Nickel(0)-Catalyzed Asymmetric Hydrocyanation of 1,3-Dienes. Org. Lett. 2006, 8, 4657. 10.1021/ol062002f. [DOI] [PubMed] [Google Scholar]
- Yu R.; Xing Y.; Fang X. Regio-, Chemo-, and Enantioselective Ni-Catalyzed Hydrocyanation of 1,3-Dienes. Org. Lett. 2021, 23, 930. 10.1021/acs.orglett.0c04133. [DOI] [PubMed] [Google Scholar]
- Shirakura M.; Suginome M. Nickel-Catalyzed Asymmetric Addition of Alkyne C-H Bonds across 1,3-Dienes Using Taddol-Based Chiral Phosphoramidite Ligands. Angew. Chem. Int. Ed 2010, 49, 3827. 10.1002/anie.201001188. [DOI] [PubMed] [Google Scholar]
- Shirakura M.; Suginome M. Nickel-Catalyzed Addition of C-H Bonds of Terminal Alkynes to 1,3-Dienes and Styrenes. J. Am. Chem. Soc. 2008, 130, 5410. 10.1021/ja800997j. [DOI] [PubMed] [Google Scholar]
- Bogdanovic B.; Henc B.; Meister B.; Pauling H.; Wilke G. A Catalyzed Asymmetric Synthesis. Angew. Chem. Int. Ed 1972, 11, 1023. 10.1002/anie.197210231. [DOI] [Google Scholar]
- Siv C.; Peiffer G.; Triantaphylides C.; Denis P.; Mortreux A.; Petit F.; Buono G. Threophos: A New Chiral Aminophosphine Phosphinite (AMPP) Ligand Highly Efficient in Asymmetric Hydrovinylation of Cyclohexa-1,3-diene Catalyzed by Nickel Complexes. J. Org. Chem. 1985, 50, 1781. 10.1021/jo00210a051. [DOI] [Google Scholar]
- Zhang A.; RajanBabu T. V. Hydrovinylation of 1,3-Dienes: A New Protocol, an Asymmetric Variation, and a Potential Solution to the Exocyclic Side Chain Stereochemistry Problem. J. Am. Chem. Soc. 2006, 128, 54. 10.1021/ja0561338. [DOI] [PubMed] [Google Scholar]
- Saha B.; Smith C. R.; RajanBabu T. V. Ligand Tuning in Asymmetric Hydrovinylation of 1,3-Dienes: A Stereoselective Route to Either Steroid-C20 (S) or -C20 (R) Derivatives. J. Am. Chem. Soc. 2008, 130, 9000. 10.1021/ja711475f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingate B. B.; Hazra B. G. A Concise Account of Various Approaches for Stereoselective Construction of the C-20(H) Stereogenic Center in Steroid Side Chain. Chem. Rev. 2014, 114, 6349. 10.1021/cr4004083. [DOI] [PubMed] [Google Scholar]
- Xiao L. J.; Cheng L.; Feng W. M.; Li M. L.; Xie J. H.; Zhou Q. L. Nickel(0)-Catalyzed Hydroarylation of Styrenes and 1,3-Dienes with Organoboron Compounds. Angew. Chem., Int. Ed. 2018, 57, 461. 10.1002/anie.201710735. [DOI] [PubMed] [Google Scholar]
- Lv X. Y.; Fan C.; Xiao L. J.; Xie J. H.; Zhou Q. L. Ligand-Enabled Ni-Catalyzed Enantioselective Hydroarylation of Styrenes and 1,3-Dienes with Arylboronic Acids. CCS Chem. 2019, 1, 328. 10.31635/ccschem.019.20190026. [DOI] [Google Scholar]
- Cheng L.; Li M. M.; Li M. L.; Xiao L. J.; Xie J. H.; Zhou Q. L. Nickel-Catalyzed Regio- and Enantioselective Hydroarylation of 1,3-Dienes with Indoles. CCS Chem. 2022, 4, 2612. 10.31635/ccschem.021.202101472. [DOI] [Google Scholar]
- a Guihaumé J.; Halbert S.; Eisenstein O.; Perutz R. N. Hydrofluoroarylation of Alkynes with Ni Catalysts. C–H Activation via Ligand-to-Ligand Hydrogen Transfer, an Alternative to Oxidative Addition. Organometallics 2012, 31, 1300. 10.1021/om2005673. [DOI] [Google Scholar]; b Cheng Q.; Dang Y. Mechanistic Studies of Nickel-Catalyzed Hydroarylation of Styrenes. Org. Lett. 2020, 22, 8998. 10.1021/acs.orglett.0c03395. [DOI] [PubMed] [Google Scholar]; c Gao H.; Hu L.; Hu Y.; Lv X.; Wu Y. B.; Lu G. Origins of Regioselectivity in Ni-catalyzed Hydrofunctionalization of Alkenes via Ligand-to-Ligand Hydrogen Transfer Mechanism. Chem. Commun. 2022, 58, 8650. 10.1039/D2CC02691K. [DOI] [PubMed] [Google Scholar]
- Marcum J. S.; Taylor T. R.; Meek S. J. Enantioselective Synthesis of Functionalized Arenes by Nickel-Catalyzed Site-Selective Hydroarylation of 1,3-Dienes with Aryl Boronates. Angew. Chem. Int. Ed 2020, 59, 14070. 10.1002/anie.202004982. [DOI] [PubMed] [Google Scholar]
- Wang C.; Guo Y.; Wang X.; Wang Z.; Ding K. Ni-Catalyzed Regioselective Hydroarylation of 1-Aryl-1,3-butadienes with Aryl Halides. Chem. - Eur. J. 2021, 27, 15903. 10.1002/chem.202102847. [DOI] [PubMed] [Google Scholar]
- Li J. F.; Pan D.; Wang H. R.; Zhang T.; Li Y.; Huang G.; Ye M. Enantioselective C2–H Alkylation of Pyridines with 1,3-Dienes via Ni–Al Bimetallic Catalysis. J. Am. Chem. Soc. 2022, 144, 18810. 10.1021/jacs.2c09306. [DOI] [PubMed] [Google Scholar]
- a Sato Y.; Saito N.; Mori M. A Novel Asymmetric Cyclization of ω-Formyl-1,3-dienes Catalyzed by a Zerovalent Nickel Complex in the Presence of Silanes. J. Am. Chem. Soc. 2000, 122, 2371. 10.1021/ja994059l. [DOI] [PubMed] [Google Scholar]; b Sato Y.; Saito N.; Mori M. A Asymmetric Cyclization of ω-Formyl-1,3-dienes Catalyzed by a Zerovalent Nickel Complex in the Presence of Silanes. J. Org. Chem. 2002, 67, 9310. 10.1021/jo020438c. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Zhu S. F.; Duan H. F.; Zhou C. Y.; Wang L. X.; Zhou Q. L. Asymmetric Reductive Coupling of Dienes and Aldehydes Catalyzed by Nickel Complexes of Spiro Phosphoramidites: Highly Enantioselective Synthesis of Chiral Bishomoallylic Alcohols. J. Am. Chem. Soc. 2007, 129, 2248. 10.1021/ja0693183. [DOI] [PubMed] [Google Scholar]
- Cheng L.; Li M. M.; Xiao L. J.; Xie J. H.; Zhou Q. L. Nickel(0)-Catalyzed Hydroalkylation of 1,3-Dienes with SimpleKetones. J. Am. Chem. Soc. 2018, 140, 11627. 10.1021/jacs.8b09346. [DOI] [PubMed] [Google Scholar]
- Shao W.; Besnard C.; Guénée L.; Mazet C. Ni-Catalyzed Regiodivergent and Stereoselective Hydroalkylation of Acyclic Branched Dienes with Unstabilized C(sp3) Nucleophiles. J. Am. Chem. Soc. 2020, 142, 16486. 10.1021/jacs.0c08319. [DOI] [PubMed] [Google Scholar]
- Liao L.; Zhang Y.; Wu Z.-W.; Ye Z.-T.; Zhang X.-X.; Chen G.; Yu J. S. Nickel-catalyzed regio- and enantio-selective Markovnikov hydromonofluoroalkylation of 1,3-dienes. Chem. Sci. 2022, 13, 12519. 10.1039/D2SC03958C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang and Mashima recently demonstrated that dual catalysis is a viable approach to enantioselective hydrofunctionalization of dienes. See:Xia J.; Hirai T.; Katayama S.; Nagae H.; Zhang W.; Mashima K. Mechanistic Study of Ni and Cu Dual Catalyst for Asymmetric C–C Bond Formation; Asymmetric Coupling of 1,3-Dienes with C-Nucleophiles to Construct Vicinal Stereocenters. ACS Catal. 2021, 11, 6643. 10.1021/acscatal.1c01626. [DOI] [Google Scholar]
- a Baker R.; Halliday D. E.; Smith T. N. Nickel Complex-Catalysed Reactions of Butadiene with Amines. J. Chem. Soc. D 1971, 23, 1583. 10.1039/c29710001583. [DOI] [Google Scholar]; b Baker R.; Onions A.; Popplestone R. J.; Smith T. N. Reactions of Amines and Active Methylene Compounds with Buta-1,3-diene and Isoprene: Catalysis by Nickel, Cobalt, Rhodium, and Iridium Complexes. J. Chem. Soc., Perkin Trans. 2 1975, 1133. 10.1039/p29750001133. [DOI] [Google Scholar]
- Pawlas J.; Nakao Y.; Kawatsura M.; Hartwig J. F. A General Nickel-Catalyzed Hydroamination of 1,3-Dienes by Alkylamines: Catalyst Selection, Scope, and Mechanism. J. Am. Chem. Soc. 2002, 124, 3669. 10.1021/ja017575w. [DOI] [PubMed] [Google Scholar]
- Tran G.; Shao W.; Mazet C. Ni-Catalyzed Enantioselective Intermolecular Hydroamination of Branched 1,3-Dienes Using Primary Aliphatic Amines. J. Am. Chem. Soc. 2019, 141, 14814. 10.1021/jacs.9b07253. [DOI] [PubMed] [Google Scholar]
- Long J.; Wang P.; Wang W.; Li Y.; Yin G. Nickel/Brønsted Acid-Catalyzed Chemo- and Enantioselective Intermolecular Hydroamination of Conjugated Dienes. iScience 2019, 22, 369. 10.1016/j.isci.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Lu J.; Ye J.; Duan W. L. Palladium-Catalyzed Asymmetric 1,6-Addition of Diarylphosphines to α,β,γ,δ-Unsaturated Sulfonic Esters: Controlling Regioselectivity by Rational Selection of Electron-Withdrawing Groups. Chem. Commun. 2014, 50, 698. 10.1039/C3CC46290K. [DOI] [PubMed] [Google Scholar]; b Yang X. Y.; Tay W. S.; Li Y.; Pullarkat S. A.; Leung P. H. Asymmetric 1,4-Conjugate Addition of Diarylphosphines to α,β,γ,δ-Unsaturated Ketones Catalyzed by Transition-Metal Pincer Complexes. Organometallics 2015, 34, 5196. 10.1021/acs.organomet.5b00787. [DOI] [Google Scholar]; c Yang X. Y.; Gan J. H.; Li Y.; Pullarkat S. A.; Leung P. H. Palladium Catalyzed Asymmetric Hydrophosphination of α,β- and α,β,γ,δ-Unsaturated Malonate Esters–Efficient Control of Reactivity, Stereo- and Regio-Selectivity. Dalton Trans. 2015, 44, 1258. 10.1039/C4DT02673J. [DOI] [PubMed] [Google Scholar]; d Nie S. Z.; Davison R. T.; Dong V. M. Enantioselective Coupling of Dienes and Phosphine Oxides. J. Am. Chem. Soc. 2018, 140, 16450. 10.1021/jacs.8b11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J.; Li Y.; Zhao W.; Yin G. Nickel/Brønsted Acid Dual-Catalyzed Regio- and Enantioselective Hydrophosphinylation of 1,3-Dienes: Access to Chiral Allylic Phosphine Oxides. Chem. Sci. 2022, 13, 1390. 10.1039/D1SC05651D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifleur A.; Merel D. S.; Mortreux A.; Suisse I.; Capet F.; Trivelli X.; Sauthier M.; Macgregor S. A. Deciphering the Mechanism of the Nickel-Catalyzed Hydroalkoxylation Reaction: A Combined Experimental and Computational Study. ACS Catal. 2017, 7, 6915. 10.1021/acscatal.7b00616. [DOI] [Google Scholar]
- Tran G.; Mazet C. Ni-Catalyzed Regioselective Hydroalkoxylation of Branched 1,3-Dienes. Org. Lett. 2019, 21, 9124. 10.1021/acs.orglett.9b03511. [DOI] [PubMed] [Google Scholar]
- a Wu K.; Doyle A. Parameterization of phosphine ligands demonstrates enhancement of nickel catalysis via remote steric effects. Nat. Chem. 2017, 9, 779. 10.1038/nchem.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Reid J. P.; Sigman M. S. Comparing quantitative prediction methods for the discovery of small-molecule chiral catalysts. Nat. Rev. Chem. 2018, 2, 290. 10.1038/s41570-018-0040-8. [DOI] [Google Scholar]; c Durand D. J.; Fey N. Computational Ligand Descriptors for Catalyst Design. Chem. Rev. 2019, 119, 6561. 10.1021/acs.chemrev.8b00588. [DOI] [PubMed] [Google Scholar]; d Falivene L.; Cao Z.; Petta A.; Serra L.; Poater A.; Oliva R.; Scarano V.; Cavallo L. Towards the online computer-aided design of catalytic pockets. Nat. Chem. 2019, 11, 872. 10.1038/s41557-019-0319-5. [DOI] [PubMed] [Google Scholar]