Abstract
In this study, the hsp60 and hsp70 heat shock protein antigens of Mycobacterium tuberculosis were tested as potential vaccine candidates, using purified recombinant protein antigens or antigens encoded in the form of a DNA plasmid vaccine. Guinea pigs vaccinated with a mixture of the two proteins showed no evidence of resistance to low-dose aerosol challenge infection and quickly developed severe lung damage characterized by necrotizing bronchointerstitial pneumonia and bronchiolitis. As a result, we turned instead to a DNA vaccination approach using a plasmid encoding the hsp60 antigen of M. tuberculosis. Although immunogenic in mice, vaccination with plasmid DNA encoding hsp60 was not protective in that model or in the guinea pig model and again gave rise to similar severe lung damage. This study seriously questions the safety of vaccines against tuberculosis that target highly conserved heat shock proteins.
When cultures of Mycobacterium tuberculosis are exposed to stress, notably an increase in temperature, they begin to synthesize an extensive family of chaperonin-like proteins which are collectively referred to as the heat shock proteins of the organism (12, 18, 19, 25–27). Under normal physiological conditions, however, only a few of these proteins can be detected; these include DnaK protein (hsp70) and the GroES protein (hsp10), which are well represented, and the GroEL 65-kDa protein (hsp60), which is found in only trace amounts (21).
Both the hsp70 and hsp60 molecules have recently been shown to have significant promise as vaccines against tuberculosis. Culture filtrates dominated by hsp70 (16) or purified hsp70 (6), when delivered in a powerful adjuvant, induce high levels of protection in the sensitive guinea pig challenge model. The hsp60 molecule, when delivered encoded in the form of a DNA vaccine, induces immunity that not only protects against subsequent challenge (13, 23) but also is capable of inducing sterilizing immunity when given as a postexposure vaccine (14).
We have recently reported (1, 15) that culture filtrate proteins of M. tuberculosis delivered in a relatively mild adjuvant and supplemented with the cytokine interleukin-2 (IL-2) do not have a direct effect on the lung bacterial load following a challenge infection in the guinea pig model but do appear to induce a lymphocytic response in the lungs that prevents the caseous necrosis that would otherwise develop. Given this information, we hypothesized that a mixture of hsp60 and hsp70 proteins should have a similar effect, perhaps with an element of direct protection given the previous results of others, in our model. However, no protection was observed, and the animals immunized with these proteins rapidly developed a necrotizing bronchointerstitial pneumonia and bronchiolitis after aerosol challenge.
Because of this negative result, we turned to the tactic of DNA vaccination, using hsp60 as an example, given previous reports of the very high effectiveness of this approach (13, 14). Here again, no protection was observed in either the mouse or guinea pig model despite direct evidence that the DNA was highly immunogenic. Histological analysis revealed a similar spectrum of moderate to severe, multifocal to disseminated necrotizing bronchointerstitial pneumonia with bronchiolitis.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free 6- to 8-week-old female C57BL/6 mice and female outbred Hartley guinea pigs were purchased from Charles River Laboratories (North Wilmington, Mass.) and held under barrier conditions in a level III biohazard laboratory. (C57BL/6 × BALB/c)F1 female mice were bred in the animal facilities at the Pasteur Institute, Brussels, Belgium. Guinea pigs weighed approximately 500 to 600 g at the beginning of the experiment and were housed two to a cage. All animals had free access to water and standard mouse or guinea pig chow.
Bacterial infections.
M. tuberculosis Erdman and H37Rv and Mycobacterium bovis BCG Pasteur and Copenhagen were grown to early mid-log phase in Proskauer Beck medium containing 0.02% Tween 80. Cultures were aliquoted into 1-ml tubes and stored at −70°C until used. Thawed aliquots were diluted in double-distilled sterile water to the desired inoculum concentrations. An aerosol generation device (Glas-Col, Terre Haute, Ind.) was used to expose the animals to an aerosol of M. tuberculosis and was calibrated to deliver approximately 20 bacilli into each guinea pig lung. Mice were infected in a similar manner with approximately 50 to 100 bacilli. In some experiments, mice were challenged intranasally with 2 × 104 CFU of M. tuberculosis H37Rv. The numbers of viable bacteria in target organs were determined at various time points by plating serial dilutions of whole organ homogenates on nutrient Middlebrook 7H11 agar and counting bacterial colonies after 20 days of incubation at 37°C. Data were expressed as the log10 of the mean number of bacteria recovered per organ.
Protein vaccine.
Recombinant M. bovis hsp60 and M. tuberculosis hsp70 were kindly provided from the World Health Organization repository by M. Singh (Braunschweig, Germany). Guinea pigs were immunized subcutaneously with a mixture of the two heat shock proteins (20 μg each) emulsified into an adjuvant vehicle (100 μg of monophosphoryl lipid A [MPL] [Ribi ImmunoChem Research, Hamilton, Mont.] solubilized in triethanolamine by sonication; stock solutions contained 0.02% triethanolamine and 0.4% dextrose) and supplemented with 20 μg of Proleukin-polyethylene glycol IL-2 (Chiron, Emeryville, Calif.). Animals were given the vaccine twice 3 weeks apart and then challenged 4 weeks later by aerosol exposure. As a positive control, animals were injected intradermally with BCG Copenhagen (103 bacilli/guinea pig) a single time, corresponding to the second set of injections.
DNA vaccines.
DNA vaccines encoding the hsp60 and Ag85A protein antigens of M. tuberculosis were constructed as previously described (7, 9) using the plasmid vector V1Jns-tPA. To verify immunogenicity, mice were injected three times at 3-week intervals with 100 μg of plasmid DNA encoding Ag85A or hsp60. Three weeks after the last immunization, spleens from five mice were analyzed individually for IL-2 and gamma interferon (IFN-γ) secretion in response to the specific antigens purified from M. bovis BCG culture filtrate (4, 5, 8) or to the polyclonal mitogen pokeweed mitogen (PWM).
Two months after the last DNA vaccination, mice were challenged by intranasal inoculation with M. tuberculosis H37Rv, and the bacterial load in the lungs was determined 1 month later as described above.
Guinea pigs were vaccinated using a biojector device (Bioject, Portland, Oreg.) three times at 3-week intervals. Each guinea pig was given 200 μg of plasmid DNA in saline into each hind quadriceps muscle (400 μg total per immunization). Positive control animals received BCG as described above. One month later all animals were given a low-dose aerosol challenge as described above. Lungs from one set of animals were harvested 30 days later to determine lung bacterial counts, while a second group were left to determine survival times. These animals were weighed regularly, and a 10% loss of body weight was used as a criterion for euthanasia.
Cytokine production.
DNA-vaccinated mice were sacrificed 3 weeks after the third DNA vaccination, and spleens were removed aseptically. Spleens from five mice were analyzed individually in each group. Spleen cells were adjusted to a concentration of 4 × 106 cells/ml and grown in round-bottomed microwell plates (Nunc) in RPMI 1640 medium (Gibco-BRL) supplemented with glutamine, HEPES, 50 mM 2-mercaptoethanol, antibiotics, and 10% heat-inactived fetal calf serum (Gibco-BRL). A volume of 180 μl of cell suspension was added to 20 μl of purified Ag85A or hsp60 (final concentration, 5 μg/ml) or PWM (dilution of 1:50 from a stock solution). Cells were incubated at 37°C in a humidified CO2 incubator, and supernatants were harvested after 24 h (IL-2) and 72 h (IFN-γ). Supernatants from three separate wells were pooled and stored frozen at −20°C until assay.
IL-2 activity was measured using a bioassay. Briefly, a volume of 100 μl of 24-h culture supernatant was added to 100 μl of CTLL-2 cells (105/ml) and incubated for 48 h. [3H]thymidine (Amersham) (8.3 Ci/ml) was added (0.4 μCi/well) during the last 6 h of culture. Cells were harvested on a Titertek cell harvester, and the radioactivity recovered on the fiber mats was counted in a Betaplate scintillation counter. Each sample was tested in duplicate. IL-2 levels are expressed as mean counts per minute, and the standard deviation was below 10%. In this assay, 10.000 cpm corresponded to 3.12 IU/ml or about 600 pg/ml, and the detection limit was around 10 pg/ml.
IFN-γ production was quantified in duplicate using a cytopathic effect reduction assay on mouse L929 fibroblastoid cells with vesicular stomatitis virus as the challenge virus on 72-h culture supernatants. Titers are expressed as mean log2 values obtained for five individual mice. The value of log2 = 1 corresponds to 110 pg/ml as measured in the Genzyme mouse IFN-γ DuoSet (catalog no. 80-3931-00). The detection limit of the bioassay is about 75 pg/ml.
Histological analysis.
Tissues were fixed in 10% neutral buffered formalin for routine microscopic processing. All tissues were stained with hematoxylin and eosin. In each case, the left caudal lung lobe was sagittally sectioned through the middle of the lobe. The tissues were coded and evaluated by a veterinary pathologist without prior knowledge of time or treatment group. Some lesion variability within vaccination groups was noted, presumably due to the use of outbred animals; however, this variability was much less pronounced than lesion variability between vaccine groups.
RESULTS
Vaccination of guinea pigs with the heat shock protein mixture.
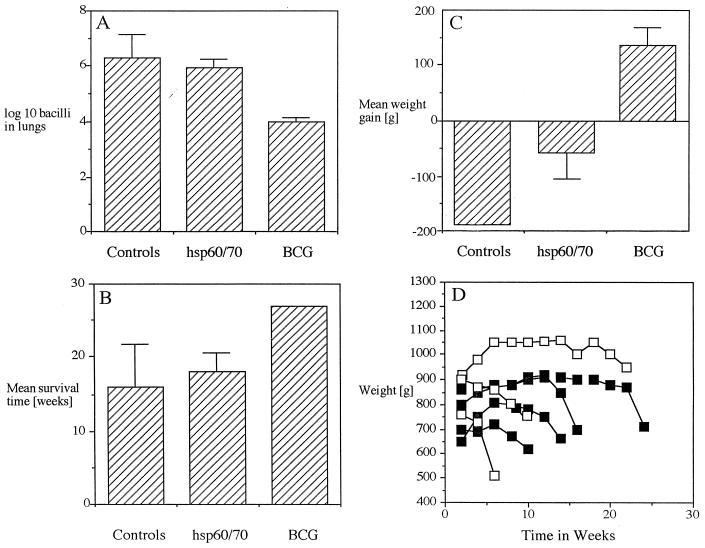
Guinea pigs were vaccinated with a mixture of hsp60 and hsp70 in MPL adjuvant containing a long-lived form of IL-2, using a vaccination protocol previously shown (1) to be efficacious for other mycobacterial proteins. Thirty days after aerosol challenge, animals were euthanized and bacterial loads were determined. Guinea pigs given the hsp60-hsp70 mixture had lung bacterial numbers similar to those in animals receiving the adjuvant–IL-2 negative control inoculum (Fig. 1A). In contrast, guinea pigs receiving BCG had a lung load reduction of 2 log10 units. This was also reflected in similar survival times for guinea pigs given the hsp60-hsp70 vaccine and the adjuvant control group (Fig. 1B), as well as weight gain or loss (Fig. 1C) and the kinetics of this loss (Fig. 1D).
FIG. 1.
Vaccination of guinea pigs with an hsp60-hsp70 protein vaccine mixture. (A) Bacterial load in lungs 30 days after aerosol challenge. (B) Mean survival. Note that none of the BCG-vaccinated animals had died when the experiment was stopped at 27 weeks. (C) Mean weight gain. For panels A to C, n = 4 and error bars indicate standard errors of the means. (D) Weight loss of individual animals. Open squares, adjuvant controls; closed squares, hsp60-hsp70-vaccinated animals.
Evidence for severe lung damage in guinea pigs vaccinated with the protein vaccine.
In our previous experience with other vaccine candidates, prevention or delay of lung necrosis could be achieved using proteins delivered in the MPL–IL-2 vehicle (1). In the present case, however, the reverse result was obtained, with animals immunized with hsp60-hsp70 showing evidence of severe lung damage. As shown in Fig. 2, vaccinated guinea pigs developed severe pneumonia with airway damage characterized by multifocal erosions and/or complete loss of bronchiolar epithelium and exposure of the underlying basement membrane. A direct physical connection between peribronchiolar inflammation and the airway lumen was often observed. Such lumens were filled with necrotic cellular debris, polymorphonuclear leukocytes (neutrophils), mucus, and exfoliated epithelial cells. Epithelial hyperplasia (repair) was often observed at the margins of these denuded areas of epithelium.
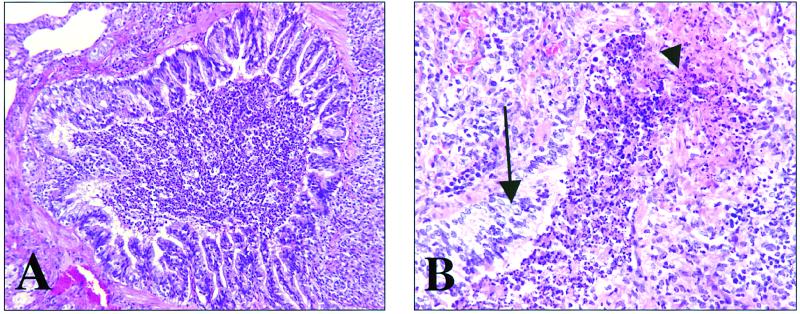
FIG. 2.
Representative photomicrographs of a lung from a guinea pig vaccinated with the hsp60-hsp70 protein mixture. (A) Terminal bronchiole surrounded by granulomatous-lymphocytic inflammation. The lumen is filled with mucus, sloughed epithelium, and necrosuppurative cellular debris. Magnification, ×160. (B) Terminal bronchiole with focal epithelial ulceration (arrowhead) and focal epithelial hyperplasia (arrow). The lumen is again filled with mucus, sloughed epithelium, and necrosuppurative cellular debris. Magnification, ×320. Hematoxylin and eosin staining was used.
DNA vaccine constructs were highly immunogenic.
Given the failure of the protein vaccine approach, we then used the tactic of generating a DNA vaccine against the hsp60 molecule, given recent success with this approach (1, 9, 13, 14). To establish the immunogenicity of the plasmid DNA encoding hsp60, mice were immunized with this material and tested for antigen-specific spleen cell IL-2 and IFN-γ secretion in vitro. A construct made in an identical manner but encoding the Ag85A molecule of M. tuberculosis was used for comparison. It was found (Table 1) that vaccination with both plasmids induced significant levels of IL-2 and IFN-γ from immune spleen cells.
TABLE 1.
Cytokine secretion by T cells from mice given DNA vaccinesa
| Stimulating antigen | IL-2 (cpm)
|
IFN (log2)
|
||
|---|---|---|---|---|
| Ag85A plasmid | hsp60 plasmid | Ag85A plasmid | hsp60 plasmid | |
| Control | 310 ± 94 | 288 ± 88 | 0.92 ± 0.62 | 0.85 ± 0.48 |
| Ag85A | 10,222 ± 3,550 | NDb | 5.87 ± 1.14 | ND |
| hsp60 | ND | 5,235 ± 3,602 | ND | 4.48 ± 0.78 |
| PWM | 20,630 ± 2,482 | 23,512 ± 4,471 | 7.57 ± 0.65 | 7.14 ± 0.44 |
n = 5 mice per assay.
ND, not determined.
DNA vaccination in the mouse model.
By a protocol that has previously shown that DNA vaccination of mice with a plasmid encoding the Ag85 antigen is protective against aerosol challenge, mice were immunized with either the hsp60 DNA vaccine or Ag85. Protective activity 30 days after intranasal challenge was observed in mice receiving the Ag85 DNA vaccine, but no reduction in the lung bacterial load was observed in mice immunized with the hsp60 DNA vaccine (Table 2).
TABLE 2.
Protection of mice after vaccination
| Vaccine | Lung bacterial load (log10)a | Protection (log10) |
|---|---|---|
| Control DNA | 4.87 ± 0.37 | |
| BCG | 3.96 ± 0.38 | 0.91 |
| Ag85A DNA | 4.34 ± 0.18 | 0.53 |
| hsp60 DNA | 5.17 ± 0.24 | None |
Measured 30 days after intranasal challenge with M. tuberculosis H37Rv.
The DNA vaccine encoding hsp60 was not protective in guinea pigs.
After vaccination with the hsp60 DNA plasmid, guinea pigs were challenged by aerosol, and the bacterial load in the lungs was determined 1 month later. No protection was seen in these animals compared to controls (Table 3).
TABLE 3.
Protection of guinea pigs after DNA vaccination
| Vaccine | Bacterial load (log10)a in:
|
|
|---|---|---|
| Lungs | Spleen | |
| Control DNA | 5.01 ± 0.21 | 5.19 ± 0.31 |
| BCG | 4.10 ± 0.31 | 1.78 ± 1.75 |
| hsp60 DNA | 5.11 ± 0.22 | 5.93 ± 0.35 |
Thirty days after low-dose aerosol exposure to M. tuberculosis H37Rv.
Evidence for severe pathology in vaccinated guinea pigs.
In guinea pigs vaccinated with BCG, a mild diffuse interstitial pneumonia with alveolar walls variably thickened by lymphocytes was observed in the lungs of these animals 30 days after aerosol challenge. For animals vaccinated with hsp60 DNA, about half of the animals exhibited a moderate to severe, multifocal to coalescing, granulomatous interstitial pneumonia with scattered aggregates of lymphocytes, whereas the other animals presented with a necrotizing granulomatous bronchointerstitial pneumonia (Fig. 3). All negative control animals given the empty plasmid showed the latter type of pathology. Additionally, within the hsp60 DNA-vaccinated group, bronchiolar epithelial erosion and/or complete ulceration similar to that seen in the guinea pigs vaccinated with the hsp60-hsp70 protein mixture was also observed. The bronchiolar lumens of the DNA-vaccinated animals were packed with polymorphonuclear leukocytes, mucus, sloughed epithelium, and necrotic cellular debris.
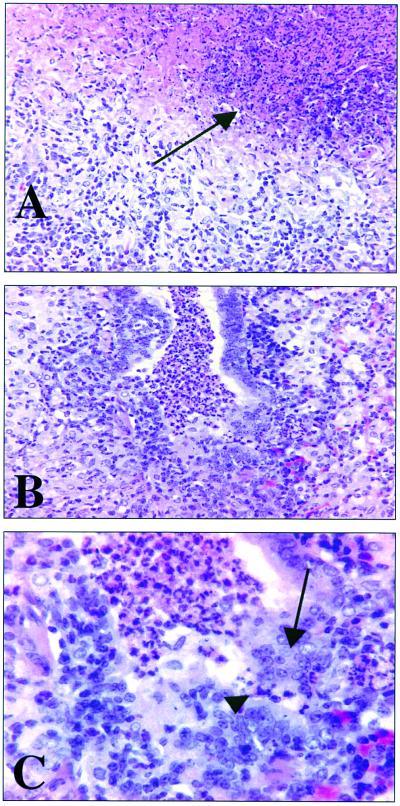
FIG. 3.
Lung pathology after DNA vaccination and aerosol challenge. (A) Plasmid vector control. Severe extensive granulomatous interstitial pneumonia with centralized area of necrosis (arrow) is seen. Magnification, ×160. (B) Guinea pig vaccinated with hsp60 plasmid DNA. Severe extensive granulomatous bronchointerstitial pneumonia with polymorphonuclear leukocytes, mucus, sloughed epithelium, and necrotic cellular debris filling the airway lumen is shown. Magnification, ×160. (C) High magnification of airway in panel B. Note transepithelial migration of polymorphonuclear cells and epithelial erosion (arrowhead) and epithelial hyperplasia (arrow). Magnification, ×310. Hematoxylin and eosin staining was used.
DISCUSSION
In this study, we examined the efficacy of two potential heat shock protein-based tuberculosis vaccine candidates. Neither the protein- nor the DNA-based vaccines had any protective effect, measured as a reduction in bacterial loads after M. tuberculosis challenge infections. In addition, the lung pathology in guinea pigs vaccinated with either of these candidates ranged from a moderate to severe necrotizing granulomatous bronchointerstitial pneumonia with bronchiolitis.
The finding of severe airway damage and complete lack of protection in guinea pigs vaccinated with a preparation containing hsp70 is in complete contrast to earlier work with this protein, in which the animals were significantly protected by this material delivered in a potent adjuvant vehicle. In our study, however, a much milder adjuvant formulation was used, which consistently has minimal influence on bacterial load in the guinea pig model but does tend to prevent adverse lung pathology and give rise to substantial long-term survival. Hence, the severe pathology seen in guinea pigs immunized with hsp70 in the MPL adjuvant is paradoxical and seems to imply that the protein induces immunity that is effective only just directly after the challenge infection is given, and then only if a much stronger adjuvant is used.
Because of these findings, we turned to the DNA vaccination approach, and further experiments were performed using a plasmid DNA vaccine encoding hsp60 of M. tuberculosis. Initial experiments with mice showed that the DNA was highly immunogenic, but subsequent experiments in which these animals were then challenged with M. tuberculosis did not indicate any protective effect for the hsp60-encoding plasmid. In contrast, vaccination with plasmid DNA encoding Ag85A significantly reduced the lung bacterial load compared to that in mice vaccinated with empty plasmid vector, confirming previous results using either aerosol or intravenous M. tuberculosis challenge (1, 9, 10). A similar outcome was seen in guinea pigs vaccinated using a highly effective biojector protocol, in which no reduction in lung bacterial load was observed and there was no evidence of any survival beyond that for negative control animals. Moreover, histological analysis of the lungs of these guinea pigs revealed a similar spectrum of necrotizing bronchointerstitial granulomatous pneumonia and bronchiolitis.
A number of DNA vaccine candidates have been shown to have activity in small animal models of tuberculosis (1, 7, 9, 10, 13–15, 22, 23, 28). For example, a DNA vaccine containing the M. tuberculosis gene encoding Ag85A (mycolyl transferase) had a protective effect in mice challenged by aerosol infection (1, 9). In guinea pigs challenged similarly, no significant effect on the lung bacterial load was initially observed, but these animals exhibited excellent long-term survival with the development of lymphocytic granulomas and no evidence of lung tissue necrosis (1). Other targets include ESAT-6, PstS-1, and PstS-3 (7, 10, 22, 28).
A very promising candidate, a DNA vaccine made from the hsp60 gene of Mycobacterium leprae, has been shown to dramatically reduce bacterial loads in mice infected intravenously when given both prior to (2, 13, 23) and after (14) the M. tuberculosis challenge. Moreover, in a Cornell-type model, therapeutic administration of the vaccine resulted in sterilizing immunity in some animals (14), although we should note here that the bacterial loads recovered in that study from steroid-treated infected control mice after prolonged isoniazid and pyrazinamide therapy seemed to us to be extraordinarily high given our own experience (3) and that of others (17). Moreover, in contrast to those results, we have completely failed to see any postexposure effects using two vaccines (a culture filtrate-based vaccine and the Ag85 DNA vaccine) shown previously to be effective if given prior to challenge (24).
We cannot offer an explanation here as to why our hsp60 DNA vaccine had no effect. It was engineered from the M. tuberculosis hsp60 gene (rather than M. leprae), stimulated substantial cytokine responses from T cells from immunized mice, and was inoculated into guinea pigs using a highly efficient biojector protocol. Perhaps much more troubling than the lack of protection, however, was the observation of severe pulmonary pathology in these vaccination approaches, which questions the safety of the hsp60 vaccine candidates. We do not know the etiology of the pathological process in these guinea pigs, but an obvious starting point would be the potential induction of autoimmunity given the highly conserved nature of this molecule in mycobacteria and mammals (11, 20, 26). In addition, moreover, there were certain similarities in the pathology in these animals to the broad clinical syndrome referred to as asthma, allergic bronchitis, or allergic pneumonia, which has been recognized in many mammalian species. In this syndrome, a prominent infiltration of eosinophils into an edematous, hyperemic bronchial lamina propria is classically seen, and airway lumens fill with a mix of mucus, sloughed epithelial cells and many eosinophils. Some of these features were observed within the guinea pigs tested in the present study, thus suggesting a form of an allergic phenomenon, although whether this is indeed the basis remains unproven.
ACKNOWLEDGMENTS
We thank K. Palfliet, F. Jurion, N. De Smet, and A. Vanonckelen for excellent technical assistance. We are very grateful to Donna Montgomery for providing the biojector device and to Marty Giedlin for IL-2.
A.T. holds a grant from the Damiaanaktie Belgium. This work was supported by grant G.0355.97 from the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen and by NIH grant AI-40488.
REFERENCES
- 1.Baldwin S L, D'Souza C, Roberts A D, Kelly B P, Frank A A, Lui M A, Ulmer J B, Huygen K, McMurray D M, Orme I M. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect Immun. 1998;66:2951–2959. doi: 10.1128/iai.66.6.2951-2959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonato V L, Lima V M, Tascon R E, Lowrie D B, Silva C L. Identification and characterization of protective T cells in hsp65 DNA-vaccinated and Mycobacterium tuberculosis-infected mice. Infect Immun. 1998;66:169–175. doi: 10.1128/iai.66.1.169-175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks J V, Furney S K, Orme I M. Metronidazole therapy in mice infected with tuberculosis. Antimicrob Agents Chemother. 1999;43:1285–1288. doi: 10.1128/aac.43.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Bruyn J, Bosmans R, Turneer M, Weckx M, Nyabenda J, Van Vooren J P, Falmagne P, Wiker H G, Harboe M. Purification, partial characterization, and identification of a skin-reactive protein antigen of Mycobacterium bovis BCG. Infect Immun. 1987;55:245–252. doi: 10.1128/iai.55.1.245-252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Bruyn J, Huygen K, Bosmans R, Fauville M, Lippens R, Van Vooren J P, Falmagne P, Weckx M, Wiker H G, Harboe M, et al. Purification, characterization and identification of a 32 kDa protein antigen of Mycobacterium bovis BCG. Microb Pathog. 1987;2:351–366. doi: 10.1016/0882-4010(87)90077-5. [DOI] [PubMed] [Google Scholar]
- 6.Horwitz M A, Lee B W, Dillon B J, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huygen K. DNA vaccines: application to tuberculosis. Int J Tuberc Lung Dis. 1998;2:971–978. [PubMed] [Google Scholar]
- 8.Huygen K, Abramowicz D, Vandenbussche P, Jacobs F, De Bruyn J, Kentos A, Drowart A, Van Vooren J P, Goldman M. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect Immun. 1992;60:2880–2886. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D'Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 10.Kamath A T, Feng C G, Macdonald M, Briscoe H, Britton W J. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect Immun. 1999;67:1702–1707. doi: 10.1128/iai.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamb J R, Bal V, Mendez-Samperio P, Mehlert A, So A, Rothbard J, Jindal S, Young R A, Young D B. Stress proteins may provide a link between the immune response to infection and autoimmunity. Int Immunol. 1989;1:191–196. doi: 10.1093/intimm/1.2.191. [DOI] [PubMed] [Google Scholar]
- 12.Lamb J R, Bal V, Rothbard J B, Mehlert A, Mendez-Samperio P, Young D B. The mycobacterial GroEL stress protein: a common target of T-cell recognition in infection and autoimmunity. J Autoimmun. 1989;2(Suppl):93–100. doi: 10.1016/0896-8411(89)90120-0. [DOI] [PubMed] [Google Scholar]
- 13.Lowrie D B, Silva C L, Colston M J, Ragno S, Tascon R E. Protection against tuberculosis by a plasmid DNA vaccine. Vaccine. 1997;15:834–838. doi: 10.1016/s0264-410x(97)00073-x. [DOI] [PubMed] [Google Scholar]
- 14.Lowrie D B, Tascon R E, Bonato V L, Lima V M, Faccioli L H, Stavropoulos E, Colston M J, Hewinson R G, Moelling K, Silva C L. Therapy of tuberculosis in mice by DNA vaccination. Nature. 1999;400:269–271. doi: 10.1038/22326. [DOI] [PubMed] [Google Scholar]
- 15.Orme I M. New vaccines against tuberculosis. The status of current research. Infect Dis Clin N Am. 1999;13:169–185. doi: 10.1016/s0891-5520(05)70049-0. , vii-viii. [DOI] [PubMed] [Google Scholar]
- 16.Pal P G, Horwitz M A. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect Immun. 1992;60:4781–4792. doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scanga C A, Mohan V P, Joseph H, Yu K, Chan J, Flynn J L. Reactivation of latent tuberculosis: variations on the Cornell murine model. Infect Immun. 1999;67:4531–4538. doi: 10.1128/iai.67.9.4531-4538.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinnick T M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987;169:1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shinnick T M, Sweetser D, Thole J, van Embden J, Young R A. The etiologic agents of leprosy and tuberculosis share an immunoreactive protein antigen with the vaccine strain Mycobacterium bovis BCG. Infect Immun. 1987;55:1932–1935. doi: 10.1128/iai.55.8.1932-1935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinnick T M, Vodkin M H, Williams J C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988;56:446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonnenberg M G, Belisle J T. Definition of Mycobacterium tuberculosis culture filtrate proteins by two-dimensional polyacrylamide gel electrophoresis, N-terminal amino acid sequencing, and electrospray mass spectrometry. Infect Immun. 1997;65:4515–4524. doi: 10.1128/iai.65.11.4515-4524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanghe A, Lefevre P, Denis O, D'Souza S, Braibant M, Lozes E, Singh M, Montgomery D, Content J, Huygen K. Immunogenicity and protective efficacy of tuberculosis DNA vaccines encoding putative phosphate transport receptors. J Immunol. 1999;162:1113–1119. [PubMed] [Google Scholar]
- 23.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 24.Turner J, Rhoades E R, Keen M, Belisle J T, Frank A A, Orme I M. Effective preexposure tuberculosis vaccines fail to protect when they are given in an immunotherapeutic mode. Infect Immun. 2000;68:1706–1709. doi: 10.1128/iai.68.3.1706-1709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young D B. Chaperonins and the immune response. Semin Cell Biol. 1990;1:27–35. [PubMed] [Google Scholar]
- 26.Young D B. The immune response to mycobacterial heat shock proteins. Autoimmunity. 1990;7:237–244. doi: 10.3109/08916939009087583. [DOI] [PubMed] [Google Scholar]
- 27.Young D B. Stress proteins and the immune response. Antonie Leeuwenhoek. 1990;58:203–208. doi: 10.1007/BF00548934. [DOI] [PubMed] [Google Scholar]
- 28.Zhu X, Venkataprasad N, Thangaraj H S, Hill M, Singh M, Ivanyi J, Vordermeier H M. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J Immunol. 1997;158:5921–5926. [PubMed] [Google Scholar]