Abstract
Background
The WHO VISION 2020 global initiative against blindness, launched in 2000, prioritised childhood visual disability by aiming to end avoidable childhood blindness by 2020. However, progress has been hampered by the global paucity of epidemiological data concerning childhood visual disability. The British Childhood Visual Impairment and Blindness Study 2 (BCVIS2) was done to address this evidence gap.
Methods
BCVIS2 was a prospective UK-wide, cross-sectional, observational study to establish an inception cohort of children newly diagnosed with visual impairment. Ophthalmologists and paediatricians reported cases from 89 hospitals and community centres across the UK. We included children aged 18 years or younger who were newly diagnosed with any condition causing impaired visual acuity to a level of 0·5 logMAR or worse (worse than 6/18 Snellen) in each eye, or equivalent vision as assessed by standard qualitative measures, between Oct 1, 2015, and Nov 1, 2016. Eligible children were notified simultaneously but independently by their managing ophthalmologists and paediatricians via the two national active surveillance schemes, the British Ophthalmological Surveillance Unit and the British Paediatric Surveillance Unit. Standardised detailed demographic, socioeconomic, and clinical data about detection, management, and treatment were collected at diagnosis and 1 year later. We calculated incidence estimates and relative rates by key sociodemographic factors. We did descriptive analyses of underlying ophthalmic disorders and non-ophthalmic comorbidities.
Findings
61 (7%) of 845 eligible children initially notified were ineligible at follow-up because of improved vision after treatment. Thus, the study sample comprised 784 children with permanent newly-diagnosed all-cause visual impairment, severe visual impairment, or blindness. 559 (72%) of 778 children had clinically significant non-ophthalmic impairments or conditions. 28 (4%) of 784 children died within a year after diagnosis of visual disability (all had underlying systemic disorders). Incidence of visual disability in the first year of life was 5·19 per 10 000 children (95% CI 4·71–5·72), almost ten times higher than among 1-to-4-year-olds and between 20 times and 100 times higher than in the older age groups. The overall cumulative incidence (or lifetime risk) of visual impairment, severe visual impairment, or blindness was 10·03 per 10 000 children (9·35–10·76). Incidence rates were higher for those from any ethnic minority group, the lowest quintile of socioeconomic status, and those born preterm or with low birthweight. 345 (44%) of 784 children had a single affected anatomical site. Disorders of the brain and visual pathways affected 378 (48%) of 784 children.
Interpretation
BCVIS2 provides a contemporary snapshot of the heterogeneity, multi-morbidity, and vulnerability associated with childhood visual disability in a high-income country. These findings could facilitate developing and delivering health care and planning of interventional research. Our findings highlight the importance of including childhood visual disability as a sentinel event and metric in global child health initiatives.
Funding
Fight for Sight, National Institute for Health Research, and Ulverscroft Foundation.
Introduction
Most people intuitively recognise the profound impact of losing one's eyesight in adulthood.1, 2 However, perhaps fewer people will have given thought to those who are born with or grow up with impaired vision. An expanding literature is revealing the vital importance of vision to all aspects of child development3 at a time when optimising early childhood development, particularly as the foundation of adult health and wellbeing, is a global priority.4 There is also growing recognition of the diverse and deep impact of impaired vision on physical and mental health, quality of life, and social outcomes of the affected child and the adult they become.3, 5, 6
Childhood-onset visual disability arguably confers a greater burden than adult-onset visual impairment (mainly occurring in later adult life), in terms of years of sighted life lost and the associated financial and opportunity costs of care and loss of potential productivity.7 Childhood visual disability was prioritised in VISION 2020,8 the WHO global initiative to eliminate avoidable blindness by 2020. However, as recognised in the WHO Universal Eye Health Global Action Plan,9 progress has been hampered by a global paucity of robust epidemiological intelligence about childhood visual disability to inform primary, secondary, or tertiary preventive health care, policies, and strategies. The British Childhood Visual Impairment and Blindness Study (BCVIS)10 was done in 2000, as VISION 2020 launched, to address this evidence gap in the UK specifically, and provide an example of a high-income country setting. The study used national active surveillance methods for the first time in this arena to understand the epidemiology of childhood blindness, the so-called ‘tip of the iceberg’ of the full spectrum of impaired vision. In response to the continuing shortage of alternative data sources to inform planning and provision of services and policies, we built the methods and national collaborative research network from this previous study for the BCVIS2, a national epidemiological study of incident childhood full-spectrum visual disability (ie, spanning visual impairment to blindness), characterising this population and identifying their specific needs within the broader context of child health.
Research in context.
Evidence before this study
The WHO Universal Eye Health Global Action Plan articulates the global paucity of epidemiological data on childhood visual disability, which has resulted in children being subsumed within the subgroup of people younger than 50 years in the WHO global vision database. Therefore, data are lacking for planning primary, secondary, and tertiary preventive strategies for children with visual disability. We searched PubMed and Embase for papers published from inception to Dec 31, 2020, in any language with the search terms child*, vis* impairment, and blind*. Our search did not identify any national population-based epidemiological studies of incident full-spectrum childhood visual disability. The British Childhood Visual Impairment and Blindness Study, undertaken in 2000, investigated solely the epidemiology of childhood blindness, the subgroup of children at the most severe end of the full spectrum of visual disability.
Added value of this study
This study provides annual age-specific and cumulative incidence of all-cause full-spectrum childhood visual disability in a high-income country and shows variations in incidence by key sociodemographic metrics of disadvantage and early life adversity. We showed the predominance of aetiological factors operating prenatally or perinatally. We described the underlying ophthalmic conditions, of which there were two or more in most children, and reported the complex multi-morbidity, comprising diverse non-ophthalmic impairments or disorders, in this vulnerable population, including reduced life expectancy.
Implications of all the available evidence
Our findings should aid planning, implementation, and evaluation of clinical and public health services and health policies in the UK and similar countries. Progress in reducing the burden of childhood visual disability globally will require better integration of visual disability into child health strategies and policies. This strategy would be facilitated by considering visual disability a sentinel child health event and key metric in child health monitoring systems.
The aim of this study was to determine, for the first time to our knowledge, the incidence, causes, and short-term health outcomes for children with all-cause full spectrum visual disability (comprising visual impairment, severe visual impairment, and blindness) in the UK.
Methods
Study design and case definition
BCVIS2 was a prospective UK-wide, cross-sectional, observational study to establish an inception cohort of children newly diagnosed with visual impairment. Clinicians reported cases from 89 hospitals and community health centres. We included children aged 18 years or younger who were newly diagnosed with any condition causing impaired visual acuity to a level of 0·5 logMAR or worse (worse than 6/18 Snellen) in each eye, or equivalent vision as assessed by standard qualitative measures.10, 11 Thus, children with unilateral visual impairment or who had visual perceptual disorders but with acuity better than 0·5 LogMAR were ineligible.
Within the International Classification of Diseases, Tenth Revision (ICD-10), visual impairment comprises acuity between 0·5 and 1·0 logMAR (6/19 to 6/60 Snellen) and severe visual impairment and blindness comprise a narrower range of acuity of 1·01 logMAR or worse, including no perception of light. As a benchmark, in the UK the minimum threshold for a standard driving licence is 0·3 logMAR (6/12 Snellen), and 0·5 logMAR is a conventional threshold for anticipating additional educational support such as low vision aids or large print. In this study, we considered whether children had blindness isolated or plus (ie, children with an additional major non-ophthalmic disorder or impairment).
The UK Health Research Authority (ref 14/LO/1809) approved this study, with section 251 exemption from individual consent for use of data from the UK Confidentiality Advisory Group on the grounds of public interest.
Case ascertainment
In the UK, multidisciplinary assessment of children newly diagnosed as visually impaired or blind is recommended,12 and a proportion of children will first present to a paediatrician.10 Therefore, to maximise ascertainment of eligible cases and completeness of data collection, eligible children were identified simultaneously but independently through the two long-standing national active surveillance schemes in the UK for research on rare conditions in ophthalmology and paediatrics, the British Ophthalmological Surveillance Unit and the British Paediatric Surveillance Unit, respectively. In both schemes, which comprise all UK consultant or attending ophthalmologists (ie, general and specialist paediatric) and paediatricians, respectively, clinicians use a monthly reporting card to either notify any new cases or confirm they have no cases to report. Despite national guidance12 recommending all children with visual impairment are assessed by a multidisciplinary team including paediatricians, in practice children with the most severe visual impairment or blindness usually see a paediatrician around the time of diagnosis, but those with less severe visual impairment might not. Thus, ophthalmologists reported all eligible children (visual impairment, severe visual impairment, and blindness) and paediatricians reported those with severe visual impairment and blindness. Cases were ascertained over a 12-month period from Oct 1, 2015, to Nov 1, 2016, with 1 year follow-up data collection completed between Nov 1, 2016, and Oct 1, 2017.
Data collection
Data were collected at diagnosis and one year later using standardised proformas developed with our multidisciplinary clinical research network, the British Childhood Visual Impairment and Blindness Study Group (BCVISG). Data collected at diagnosis comprised sociodemographic characteristics (eg, age, sex, ethnicity, and family postcode) alongside detailed ophthalmic and systemic clinical information using ICD-10 definitions, and information about early management, including diagnostic tests and treatments. The disorders or condition(s) causing visual impairment, severe visual impairment, and blindness were categorised using the modified WHO dual taxonomy we used previously10 (ie, by both anatomical site[s] affected and aetiological factors [by timing of action]). Identifiers were used to match cases, exclude duplicate reports, and merge data obtained through both sources. Follow-up data were used to review or confirm eligibility, including confirmation that the visual disability was permanent, and collect additional information about management and outcomes. This additional information included status with respect to certification of sight impairment, the process by which individuals with visual impairment are offered inclusion in their local social care register to assist in accessing support, and governmental financial assistance.13
All incoming data returned by the managing consultant (attending) clinician were reviewed for completeness by a senior ophthalmologist (ALS). Reporting clinicians were contacted about missing data or for clarification, as required.
Statistical analysis
Children were grouped by age at diagnosis of visual impairment, severe visual impairment, or blindness (<1 year, 1–4 years, 5–9 years, 10–15 years, and 16–18 years), and also by absence or presence of other clinically significant non-ophthalmic impairments or conditions, referred to as visual impairment, severe visual impairment, or blindness isolated or plus, respectively, hereafter. Socioeconomic status was categorised using the Index of Multiple Deprivation (IMD), the standard UK measure derived from postcode,14 with the lowest quintile comprising the most deprived group. Child population at risk denominators were obtained from the UK Office for National Statistics.15 Descriptive analyses are presented as frequencies and percentages. Cumulative incidence (risk) and annual age group-specific incidence (rate) of permanent visual impairment, severe visual impairment, or blindness (ie, confirmed at follow-up), with 95% CIs, were calculated using person-time analysis.16 The denominator for the youngest age group (<1 year) was the total number of livebirths.17
Data were analysed using STATA (version 14·2). p≤0·05 was considered to indicate a statistically significant difference.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
61 (7%) of 845 eligible children initially notified were ineligible at follow-up because of improved vision after treatment. Thus, the study sample comprised 784 children with permanent newly-diagnosed all-cause visual impairment, severe visual impairment, or blindness.
Despite the surveillance schemes being independent, some ophthalmologists and paediatricians in the study collaborated, which improved data completeness and quality but precluded use of capture–recapture analysis to estimate completeness of ascertainment of the subset of children with severe visual impairment and blindness. No alternative data source existed for capture–recapture analysis of children with visual impairment. Denominators are reported individually because of missing data for some sociodemographic variables.
427 (55%) of 783 children were boys, 437 (63%) of 689 were White, and 264 (34%) of 772 were from the most deprived quintile for IMD score (table 1 ). 52 (7%) of 702 children were twins and two (<1%) of 702 children were from triplet births, proportions that were 4·7 times and 12 times higher than the proportion of twin and triplet births in the UK,17 respectively, in the study year.
Table 1.
Relative incidence rates of visual impairment, severe visual impairment, or blindness by sociodemographic characteristics
| All cases (n=784) | Total UK population (1000s) | Annual incidence (95% CI)* | Relative rate (95% CI) | ||
|---|---|---|---|---|---|
| Ethnic group† | |||||
| White | 437 (63%) | 12289·3 | 0·4 (0·3–0·4) | 1 (ref) | |
| South Asian‡ | 162 (24%) | 999·3 | 1·6 (1·4–1·9) | 4·6 (3·8–5·5) | |
| Pakistani | 86 (12%) | 462·6 | 1·9 (1·5–2·3) | 5·2 (4·2–6·6) | |
| Indian or Bangladeshi | 50 (7%) | 536·7 | 0·9 (0·7–1·2) | 2·6 (2·0–3·5) | |
| Black | 32 (5%) | 636·6 | 0·5 (0·4–0·7) | 1·4 (1·0–2·0) | |
| Mixed | 36 (5%) | 668·6 | 0·5 (0·4–0·7) | 1·5 (1·0–2·1) | |
| Other | 22 (3%) | 171·2 | 1·3 (0·8–2·0) | 3·6 (2·4–5·6) | |
| Sex§ | |||||
| Female | 356 (45%) | 7143·7 | 0·5 (0·5–0·6) | 1 (ref) | |
| Male | 427 (55%) | 7508·5 | 0·6 (0·5–0·6) | 1·1 (1·0–1·3) | |
| Deprivation (IMD) quintile¶ | |||||
| Quintile 1 (least deprived) | 112 (15%) | 2930·4 | 0·4 (0·3–0·5) | 1 (ref) | |
| Quintile 2 | 110 (14%) | 2930·4 | 0·4 (0·3–0·5) | 1·0 (0·8–1·3) | |
| Quintile 3 | 112 (15%) | 2930·4 | 0·4 (0·3–0·5) | 1·0 (0·8–1·2) | |
| Quintile 4 | 174 (23%) | 2930·4 | 0·6 (0·5–0·7) | 1·6 (1·3–1·9) | |
| Quintile 5 (most deprived) | 264 (34%) | 2930·4 | 0·9 (0·8–1·0) | 2·4 (2·0–2·8) | |
| Country of residence‖ | |||||
| England** | 712 (91%) | 12434·2 | 0·6 (0·53–0·62) | .. | |
| Scotland | 33 (4%) | 665·2 | 0·3 (0·21–0·42) | .. | |
| Wales | 31 (4%) | 1092·7 | 0·5 (0·33–0·66) | .. | |
| Northern Ireland | 8 (1%) | 460·1 | 0·2 (0·09–0·35) | .. | |
| Birthweight, g††‡‡ | |||||
| ≥2500 (normal) | 267 (69%) | 686·3 | 3·9 (3·4–4·4) | 1 (ref) | |
| 1500–2499 (low birthweight) | 71 (18%) | 44·61 | 15·9 (12·6–21·1) | 4·1 (3·1-5·4) | |
| <1500 (very low birthweight) | 49 (13%) | 7·52 | 65·2 (49·2–86·2) | 16·8 (12·4–22·8) | |
| Gestational age at birth††§§ | |||||
| Normal (≥37 weeks) | 383 (72%) | 688·65 | 5·3 (5·0–6·1) | 1 (ref) | |
| Moderate to late preterm (32–36 weeks) | 88 (17%) | 48·29 | 18·2 (14·8–22·5) | 3·3 (2·6–4·1) | |
| Very preterm (28–31 weeks) | 33 (6%) | 5·92 | 55·7 (39·6–78·4) | 10·0 (7·0–14·3) | |
| Extreme preterm (<28 weeks) | 27 (5%) | 3·33 | 81·1 (55·6–118·2) | 14·6 (9·9–21·6) | |
Data are n (%) unless otherwise indicated. IMD=Index of Multiple Deprivation.
Values are yearly incidence per 10 000 children aged 0–18 years, except for birthweight and preterm, which is yearly incidence per 10 000 livebirths.
n=689.
Includes 15 south Asian children of Asian other ethnicity.
n=783.
n=772.
n=784.
Including one child from Guernsey and one child from the Isle of Man.
Birthweight and preterm birth excludes cases from Northern Ireland as the denominator is unknown; values are yearly incidence per 10 000 children <1 year old.
n=387.
n=531.
559 (72%) of 778 children had clinically significant non-ophthalmic impairments or conditions (ie, childhood visual disability plus). 28 (4%) of 784 children died within a year after diagnosis of visual disability (all had underlying systemic disorders). A quarter of those who died were infants (under the age of 1 year); there was an infant mortality rate for children with visual impairment, severe visual impairment, or blindness of 17·4 per 1000 infants (95% CI 8·3–36·5), compared with an overall national infant mortality rate of 3·8 per 1000 infants.18
402 (51%) of 784 children were diagnosed with visual impairment, severe visual impairment, or blindness in the first year of life, and 182 (23%) of 784 were diagnosed after the age of five years (table 2 ). Incidence of visual disability in the first year of life was 5·19 per 10 000 children (95% CI 4·71–5·72), almost ten times higher than among 1-to-4-year-olds, and between 20 times and 100 times higher than in the older age groups. Variation in incidence by age group was similar for the two subpopulations with isolated and plus visual impairment, severe visual impairment, and blindness. The overall cumulative incidence (or lifetime risk) of visual impairment, severe visual impairment, or blindness was 10·03 per 10 000 children (95% CI 9·35–10·76). The cumulative incidence of visual disability plus was considerably higher (7·15 per 10 000 children, 95% CI 6·58–7·77) than for visual disability isolated (2·80 per 10 000 chlidren, 2·46–3·10).
Table 2.
Annual age group-specific incidence and cumulative incidence of visual impairment, severe visual impairment, or blindness per 10 000 children
|
Visual impairment, severe visual impairment, and blindness plus*(n=559) |
Visual impairment, severe visual impairment, and blindness isolated†(n=219) |
All (n=784)‡ |
Total UK population§(1000s) | |||||
|---|---|---|---|---|---|---|---|---|
| n (%) or n | Incidence (95%CI) | n (%) or n | Incidence (95%CI) | n (%) or n | Incidence (95%CI) | |||
| Age-specific incidence | ||||||||
| Age, years | ||||||||
| <1 | 299 (53%) | 3·86 (3·45–4·32) | 99 (45%) | 1·28 (1·05–1·56) | 402¶ (51%) | 5·19 (4·71–5·72) | 774·5 | |
| 1–4 | 151 (27%) | 0·47 (0·40–0·55) | 48 (22%) | 0·15 (0·11–0·20) | 200‖ (26%) | 0·62 (0·54–0·71) | 3231·8 | |
| 5–9 | 57 (10%) | 0·14 (0·11–0·18) | 42 (19%) | 0·10 (0·08–0·14) | 99 (13%) | 0·25 (0·20–0·30) | 4037·4 | |
| 10–15 | 43 (8%) | 0·10 (0·07–0·13) | 25 (11%) | 0·06 (0·04–0·09) | 69‖ (9%) | 0·16 (0·13–0·20) | 4338·3 | |
| 16–18 | 9 (2%) | 0·04 (0·02–0·08) | 5 (2%) | 0·02 (0·01–0·05) | 14 (2%) | 0·06 (0·04–0·10) | 2262·1 | |
| 0–18 | 559 (100%) | 0·38 (0·35–0·41) | 219 (100%) | 0·15 (0·13–0·17) | 784 (100%) | 0·54 (0·50–0·57) | 14644·2 | |
| Cumulative incidence | ||||||||
| Age, years | ||||||||
| 1 | 299 | 3·86 (3·45–4·32) | 99 | 1·28 (1·05–1·56) | 402¶ | 5·19 (4·71–5·72) | .. | |
| 5 | 450 | 5·73 (5·22–6·28) | 147 | 1·87 (1·59–2·20) | 602‖ | 7·67 (7·08–8·30) | .. | |
| 10 | 507 | 6·44 (5·90–7·02) | 189 | 2·39 (2·07–2·76) | 701 | 8·89 (8·26–9·58) | .. | |
| 16 | 550 | 7·03 (6·47–7·64) | 214 | 2·74 (2·40–3·13) | 770‖ | 9·85 (9·17–10·57) | .. | |
| 18 | 559 | 7·15 (6·58–7·77) | 219 | 2·80 (2·46–3·10) | 784 | 10·03 (9·35–10·76) | .. | |
Values are incidence per 10 000 children (95% CI), unless otherwise indicated.
Children with an additional major non-ophthalmic disorder or impairment.
Children with isolated visual loss (no major non-ophthalmic disorder or impairment).
Includes six children with unknown visual impairment plus or isolated status.
Using mid-year 2016 UK population estimates (Office for National Statistics15) by single year of age.
Includes four children with unknown visual impairment plus or isolated status.
Includes one child with unknown visual impairment plus or isolated status.
A year after diagnosis, 644 (82%) of 784 children had been certified as sight impaired or severely sight impaired. Certification had been deferred by health professionals or parents in most of the remaining children.
Incidence rates varied significantly by key sociodemographic factors potentially related to early life adversity (table 1). Children from any ethnic minority group, most notably south Asian, had significantly higher incidence of visual impairment, severe visual impairment, or blindness compared with White children. Incidence increased with decreasing socioeconomic status. There were gradients of increasing incidence with decreasing gestational age and with lower birthweight.
345 (44%) of 784 children had a single anatomical site affected, 288 (37%) had two anatomical sites affected, and 151 (19%) had three or more anatomical sites affected. The specific disorders causing visual impairment, severe visual impairment, or blindness are shown in table 3 .
Table 3.
Disorders causing visual impairment, severe visual impairment, or blindness grouped by anatomical site or sites affected
| Children with site affected*(n=784) | |||
|---|---|---|---|
| Cerebral or visual impairment | 378 (48%) | ||
| Hypoxic or ischaemic encephalopathy | 118 (15%) | ||
| Structural abnormalities | 113 (14%) | ||
| Non-accidental injury | 9 (1%) | ||
| Neurodegenerative disorders | 24 (3%) | ||
| Tumour | 23 (3%) | ||
| Metabolic disorder | 16 (2%) | ||
| Infection | 21 (3%) | ||
| Unknown disorder but evidence of cerebral or visual pathway involvement | 60 (8%) | ||
| Whole globe and anterior segment | 95 (12%) | ||
| Microphthalmia or anophthalmia | 40 (5%) | ||
| Anterior segment dysgenesis | 24 (3%) | ||
| Multiple site coloboma | 14 (2%) | ||
| Disorganised globe | 7 (1%) | ||
| Buphthalmos | 4 (1%) | ||
| Phthisis | 6 (1%) | ||
| Glaucoma | 42 (5%) | ||
| Primary congenital | 10 (1%) | ||
| Secondary | 32 (4%) | ||
| Cornea | 50 (6%) | ||
| Opacity | 29 (4%) | ||
| Dystrophy | 2 (<1%) | ||
| Other | 19 (2%) | ||
| Uvea | 30 (4%) | ||
| Aniridia | 17 (2%) | ||
| Coloboma (single site) | 4 (1%) | ||
| Uveitis | 4 (1%) | ||
| Other | 5 (1%) | ||
| Lens | 67 (9%) | ||
| Cataract or aphakia | 58 (7%) | ||
| Other | 9 (1%) | ||
| Retina | 286 (36%) | ||
| Retinopathy of prematurity | 31 (4%) | ||
| Retinal and macular dystrophies | 125 (16%) | ||
| Cone | 28 (4%) | ||
| Cone-rod | 34 (4%) | ||
| Leber's amaurosis | 5 (1%) | ||
| Stargardt's disease | 11 (1%) | ||
| Storage disorder (neuronal ceroid lipofuscinosis) | 4 (1%) | ||
| Congenital stationary night blindness | 8 (1%) | ||
| Retinitis pigmentosa | 13 (2%) | ||
| Unspecified macular dystrophy | 14 (2%) | ||
| Unspecified retinal dystrophy | 6 (1%) | ||
| Retinoschisis | 2 (<1%) | ||
| Oculocutaneous albinism | 60 (8%) | ||
| Retinitis | 4 (1%) | ||
| Retinal detachment | 36 (5%) | ||
| Retinoblastoma | 3 (<1%) | ||
| Other | 17 (2%) | ||
| Myelination of retina | 1 (<1%) | ||
| Other retinopathy | 1 (<1%) | ||
| Single site coloboma | 2 (<1%) | ||
| Vitreoretinal dysplasia | 4 (1%) | ||
| Foveal hypoplasia | 9 (1%) | ||
| Optic nerve | 222 (28%) | ||
| Hypoplasia | 116 (15%) | ||
| Septo-optic dysplasia | 32 (4%) | ||
| Isolated | 84 (11%) | ||
| Atrophy | 89 (11%) | ||
| Primary | 32 (4%) | ||
| Secondary | 57 (7%) | ||
| Neuritis or neuropathy | 17 (2%) | ||
| Other | 8 (1%) | ||
| Demyelinated optic nerve | 1 (<1%) | ||
| Morning glory anomaly | 2 (<1%) | ||
| Dysplasia | 2 (<1%) | ||
| Aplasia | 1 (<1%) | ||
| Optic nerve astrocytoma | 1 (<1%) | ||
| Coloboma single site | 1 (<1%) | ||
| Other | 14 (2%) | ||
| Isolated nystagmus | 9 (1%) | ||
| Isolated high refractive error† | 4 (1%) | ||
| Stickler syndrome | 1 (<1%) | ||
| Blepharophimosis syndrome | 1 (<1%) | ||
Data are n (%) unless otherwise indicated.
Subtotals represent the number of children with each ophthalmic site affected; this will be less than the sum of individual disorders as some children had multiple disorders per site so were counted more than once.
High refractive error was considered as ≥5·5 dioptres in the better eye.
Disorders of the brain and visual pathways (a heterogeneous group of conditions grouped under the umbrella term of cerebral visual impairment) affected 378 (48%) of 784 children (table 3). Disorders of the retina, mainly hereditary retinal dystrophies and albinism affected 286 (36%) of 784 children, including 31 (4%) children with retinopathy of prematurity, of whom 16 (52%) also had cerebral or visual impairment. Disorders of the optic nerve affected 222 (28%) of 784 children, predominantly optic nerve hypoplasia and optic atrophy.
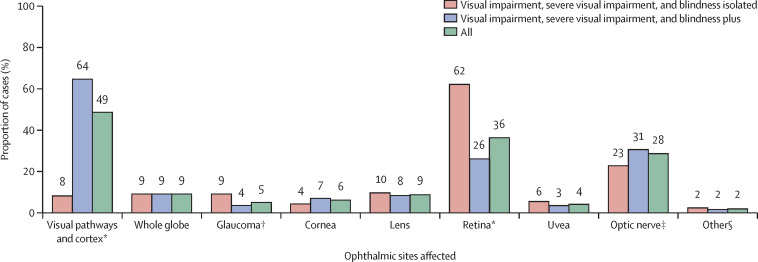
We observed marked differences in the relative importance of different anatomical sites between the two subpopulations of children with plus visual disability and isolated visual disability, for example, visual pathways and cortex accounting for 360 (64%) of 559 children and 18 (8%) of 219 children, respectively (figure 1 ).
Figure 1.
Disorders by anatomical sites
n=778. Totals exceed 100% as some children had multiple sites. *p<0·0001 for the difference in proportions test between visual impairment isolated and visual impairment plus. †p=0·0016. ‡p=0·031. §Idiopathic (isolated) nystagmus or high refractive error (not isolated but primary reason for loss of vision).
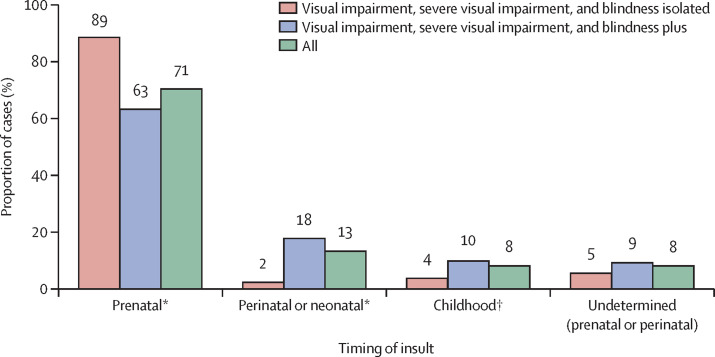
Underlying aetiological factors (where known) are shown in table 4 . Factors that acted prenatally accounted for 553 (71%) of 784 children (figure 2 ). Specifically, known hereditary conditions affected 482 (61%) of 784 children. The relative importance of hereditary factors varied somewhat by ethnicity or race, affecting 258 (59%) of 437 White children versus 109 (67%) of 162 south Asian children (Pakistani, Bangladeshi, or Indian; difference in proportions 8%, 95% CI −0·03 to 16·8; p=0·067), 20 (63%) of 32 Black children (difference in proportions 3%, −14·0 to 20·9; p=0·70), 20 (56%) of 36 mixed ethnicity children (difference in proportions 3%, −20·3 to 13·4; p=0·68), and 16 (73%) of 22 children in other ethnic groups (difference in proportions 14%, −5·5 to 32·9; p=0·20).
Table 4.
Aetiological factors causing visual impairment, severe visual impairment, or blindness (grouped by timing of effect)
| Children (n=784) | ||
|---|---|---|
| Prenatal*† | ||
| Hereditary | 482 (61%) | |
| Autosomal recessive | 162 (21%) | |
| Autosomal dominant | 46 (6%) | |
| X-linked | 18 (2%) | |
| Chromosomal | 29 (4%) | |
| Maternal inheritance | 10 (1%) | |
| Sporadic or uncertain | 217 (28%) | |
| Hypoxia ischaemia | 14 (2%) | |
| Infection in pregnancy | 19 (2%) | |
| Cytomegalovirus | 3 (<1%) | |
| Rubella | 2 (<1%) | |
| Toxoplasmosis | 2 (<1%) | |
| Herpes simplex | 1 (<1%) | |
| Hepatitis C | 1 (<1%) | |
| Group B Streptococcus | 7 (1%) | |
| HIV | 1 (<1%) | |
| Unknown or not specified | 2 (<1%) | |
| Maternal drug use | 9 (1%) | |
| Other | 2 (<1%) | |
| Twin–twin transfusion syndrome | 1 (<1%) | |
| Neonatal immune thrombocytopenia | 1 (<1%) | |
| Unknown (congenital, no further information) | 53 (7%) | |
| Perinatal or neonatal†‡ | ||
| Hypoxia ischaemia | 69 (9%) | |
| Infection | 19 (2%) | |
| Group B Streptococcus | 8 (1%) | |
| Herpes simplex | 1 (<1%) | |
| Pneumococcal | 1 (<1%) | |
| Other | 9 (1%) | |
| Unspecified meningitis | 5 (1%) | |
| Non-accidental injury | 2 (<1%) | |
| Other | 13 (2%) | |
| Hydrocephalus | 8 (1%) | |
| Epileptic encephalopathy | 2 (<1%) | |
| Neonatal hyperglycaemia or hypoglycaemia | 3 (<1%) | |
| Unknown | 18 (2%) | |
| Childhood (post-neonatal)§ | ||
| Tumour | 21 (3%) | |
| Astrocytoma | 2 (<1%) | |
| Glioma | 6 (1%) | |
| Medulloblastoma | 1 (<1%) | |
| Neuroblastoma | 2 (<1%) | |
| Craniopharyngioma | 3 (<1%) | |
| Tectal plate glioma | 1 (<1%) | |
| Rhabdomyosarcoma | 2 (<1%) | |
| Ependymoma | 2 (<1%) | |
| Prolactinoma | 1 (<1%) | |
| Unspecified brain tumour | 1 (<1%) | |
| Non-accidental injury | 9 (1%) | |
| Systemic disorders | 5 (1%) | |
| Homocystinuria | 1 (<1%) | |
| Acute lympoblastic lymphoma | 1 (<1%) | |
| Graft vs host disease | 1 (<1%) | |
| Erythema multiforme | 1 (<1%) | |
| Sickle cell disease | 1 (<1%) | |
| Hypoxia ischaemia | 9 (1%) | |
| Hydrocephalus or raised intracranial pressure | 3 (<1%) | |
| Infection | 3 (<1%) | |
| Epstein Barr virus | 1 (<1%) | |
| Group B Streptococcus | 1 (<1%) | |
| Unknown | 1 (<1%) | |
| Accidental injury | 5 (1%) | |
| Near drowning | 2 (<1%) | |
| Accidental physical trauma | 2 (<1%) | |
| Laser eye injury | 1 (<1%) | |
| Nutritional (vitamin A) deficiency | 1 (<1%) | |
| Unknown | 12 (2%) | |
Data are n (%). Total of some subcategories for each aetiological factor exceeds 100% as some children had multiple factors.
n=553 (71%).
63 (8%) children had unconfirmed timing (either prenatal, or perinatal or neonatal).
n=105 (13%).
n=63 (8%).
Figure 2.
Timing of insult leading to visual impairment, severe visual impairment, or blindness
Percentage totals exceed 100% due to multiple causes for some children. Children in the undetermined category had insults arising from either the prenatal or perinatal period, but the timing could not be reliably ascribed to a single cause with the information provided. *p<0·0001. †p=0·0044 for the difference in two proportions test.
Diverse clinically significant impairments and major non-ophthalmic conditions affected 559 (72%) of 778 children in the study (table 5 ). 105 (13%) of 784 children had hearing impairments and 167 (21%) of 784 children had speech and language impairments.
Table 5.
Non-ophthalmic impairments and conditions for children with visual impairment, severe visual impairment, or blindness
| Children (n=784) | |
|---|---|
| Impairments—key categories | |
| Hearing | 105 (13%) |
| Learning | 176 (23%) |
| Speech and language | 167 (21%) |
| Mobility | 204 (26%) |
| Main non-ophthalmic conditions | |
| Seizures or epilepsy | 177 (23%) |
| Developmental delay (including global delay) | 245 (31%) |
| Feeding problems | 89 (11%) |
| Cerebral palsy | 74 (9%) |
| Microcephaly | 62 (8%) |
| Hydrocephalus | 36 (5%) |
| Other neurological | 67 (9%) |
| Respiratory | 47 (6%) |
| Sleep related | 31 (4%) |
| Cardiac | 29 (4%) |
| Behavioural | 18 (2%) |
| Autism spectrum | 18 (2%) |
Data are n (%).
Discussion
To our knowledge, we report the results of the first national population-based epidemiological study of incident full-spectrum all-cause childhood visual disability. Although the underlying disorders are uncommon, the cumulative incidence (lifetime risk) of all-cause childhood visual disability is at least 10 per 10 000 children by age 18 years. Half of all children are affected from birth or during infancy. Incidence is markedly higher among those from socioeconomically disadvantaged backgrounds, any ethnic minority group, and those born preterm or with low birthweight. Almost three quarters of children had clinically significant additional impairments or disorders and the distributions of underlying disorders and aetiological factors in this group differed from those with isolated childhood visual disability. Overall, disorders of the brain and visual pathways (collectively known as cerebral visual impairment) account for almost half of all childhood visual disability. Among known causes of childhood visual disability, genetic or environmental influences acting prenatally or in the perinatal or neonatal periods predominate. The striking complexity and heterogeneity of visual disability illustrates a constellation of complex needs, underlined by the high proportion of children who die within the year after diagnosis.
We used the well-established national active surveillance schemes in ophthalmology and paediatrics in the UK to identify a representative study sample. Ascertainment was maximised by implementing the study through the BCVISG, established initially in 2000 and now comprising over 150 paediatric ophthalmologists and paediatricians. Given extant national guidance,12 it is unlikely that eligible children were managed by clinicians not in the BCVISG. In the absence of any alternative equivalent and independent data source, formal estimation of ascertainment using capture–recapture analysis was not possible. However, a larger number of children with incident severe visual impairment and blindness specifically were recorded than in BCVIS10 in 2000, supporting high ascertainment. Moreover, the cumulative incidence estimate of visual impairment, severe visual impairment, and blindness was considerably higher than the most recent estimate of sight impairment certification rates.13 Nevertheless, we report minimum estimates of the incidence of childhood visual disability in the UK. We observed low levels of missing data, with the exception of birthweight and gestation, but for both of these variables the gradient of relative rates is plausible and consistent with the type of disorders observed. Thus, our findings regarding the subgroups with highest rates, disorders causing impaired vision, and aetiological patterns are unlikely to be biased. This was a study of all-cause visual disability (ie, an outcome rather than a study of any individual disorder). Since this outcome reflects both the risk of disorder and the risk of worse outcome in both eyes, as children with the same conditions leading to unilateral disease or with mild visual impairment were not eligible for the study, and as there were no controls in this study, multivariable analysis to estimate the role and contribution of potential risk factors would not have been appropriate. We reported estimations of relative rates where population denominators are available.
To our knowledge, there are no other studies of full-spectrum (encompassing visual impairment, severe visual impairment, and blindness) all-cause incident childhood disability with which we can directly compare our findings. We previously undertook what remains, to our knowledge, the only national study of incident severe visual impairment and blindness in 200010 (with a subgroup of the population studied in the present study). Therefore, direct comparisons of incidence or causes are not appropriate. Furthermore, it is not possible to directly compare our findings about incident childhood visual disability with studies of prevalent visual disability,19, 20 given the populations studied for prevalence of visual disability reflect both survival and mortality and cohort effects in underlying risk factors. Our study is necessary because of this paucity of contemporary data required to characterise this population and provide a baseline for future monitoring, and to serve as the basis for developing and evaluating policies and services to meet health needs of children with visual disability. However counting—in the form of certification—of sight impairment has a long history in Britain13 and other high income countries. These certification systems were implemented primarily to address unmet social care and educational needs by flagging affected individuals to relevant services, and therefore sit outside and unconnected to generic health information systems. Even in settings with well-established universal health and social care provision and comprehensive health information systems, the impressive national-level linking of administrative, social-care, and health-care data excludes the registers of visual impairment.21 Given its purpose, certification in the UK is influenced by the perceived needs of the child, evidenced by an increasing certification of children with impaired visual processing rather than impaired visual function (acuity or visual fields), to facilitate appropriate educational support.13 Additionally, certification requires attribution to only one ophthalmic disorder and no additional information, for example, about non-ophthalmic conditions is collected. Our study shows this approach is inappropriate for capturing accurate information about visual impairment in children. Improvements in the British certification system relevant to children include adoption of the adapted WHO taxonomy for disorders used in the present study (developed for BCVIS10) and inclusion of an offer of certification to eligible individuals as part of quality standards for paediatric ophthalmologists.12 Unlike adults, childhood certification rates are not a Public Health England indicator.22 Some of these changes might account for the higher proportion of children certified within a year of diagnosis in BCVIS2 than in 2000.10 Nevertheless, counting childhood visual disability in isolation is not enough—our findings show the need for health intelligence that permits understanding in the context of child health.
The sociodemographic pattern, multimorbidity, long-term complex care needs, and truncated life expectancy observed in BCVIS2 shows that childhood visual disability epitomises the challenges to child health articulated in influential national and international child health initiatives and policies.4 Why then, rather than being an exemplar for developing models for ‘investing in children's health for lifelong intergenerational and economic benefits’,4 is consideration of visual disability lacking in key strategic documents? We suggest this is due to three factors. First, insufficient data necessary to understand the specific needs of this population, for example, children with visual disability are distributed throughout the analysis of mortality and each category of morbidity (communicable conditions, non-communicable conditions, and injuries) in children and adolescence in the Global Burden of Diseases Injuries and Risk Factors 2017 Study23 and are subsumed within the under 50 years group in the WHO's global vision database.24 Second, children with visual impairment are inadvertently sequestered away from the view of child health services and practitioners by virtue of their ophthalmic clinical management sitting within specialist ophthalmology or eye care services. Third, the potential impact of visual impairment is so self-evident that it is often overlooked in child health research.23 The findings of our study address some of these gaps. We suggest that our findings also show the value of inclusion of visual disability as a sentinel child health event, and a target condition in national and international child health research and strategies and policies.
The WHO-UNICEF-Lancet Commission4 rightly articulates the vital importance of optimising early childhood in a life course perspective of human development.4 Since Nobel prize-winning research on vision was instrumental to our understanding of brain plasticity and neurogenesis,25 it is regrettable that vision impairment has only recently been acknowledged to be a developmental emergency.3 This lack of prioritisation ill serves children with visual disability, of whom half, according to our study, are affected from birth or during the first year of life. Although multidisciplinary assessment of children newly diagnosed with visual impairment is advocated,12 practices and provision of vision-specific developmental support vary substantially, possibly reflecting structural boundaries between clinical specialties and primary, secondary, and tertiary health care. The UK National Health Service Long Term Plan26 makes ambitious pledges for child health but the sole commitment relating to vision is to eyesight services (comprising specialist optometric or optician assessment) for children with learning disabilities. Although welcome, our study shows this is relevant to around a fifth of all children with visual disability and does not address the substantial wider multimorbidity evidenced by BCVIS2.
The associations between all-cause childhood visual disability and socioeconomic disadvantage and ethnic minority status observed in our study reflect differences in the risk of specific conditions, access to health services, and outcomes of treatment. Nevertheless, these variations amplify the growing awareness of inequalities in childhood visual health, which are important in their own right and as the basis for inequalities in adult visual health,6 and closely mirror inequalities in other domains of child health. Since these disparities exist in the UK despite the universal, publicly-funded, free at the point of use health-care system, they can be reasonably assumed to exist elsewhere. As such, widening of visual health inequalities can be anticipated as part of the aftermath of the COVID-19 pandemic.27 Globally, among the most important indicators of child health-care impact are under-5 childhood mortality and stunted growth rates. Given our current findings and previous observations that the prevalence of childhood vision impairment aligns with under-5 childhood mortality, we suggest that childhood visual disability could be used as a sensitive and meaningful metric of the effectiveness of policies and programmes to reduce child health inequalities, particularly for neurodevelopmental outcomes.4, 9
The observed relative importance of different disorders in BCVIS2 reflects an evolution over time. A decline in preventable conditions, such as corneal scarring due to ophthalmia neonatorum and preventable prenatal infections such as rubella, occurred in tandem with improved outcomes via screening and treatment for key disorders such as retinopathy of prematurity and congenital cataract.28, 29
The predominance of disorders affecting the brain and visual pathways broadly echoes reports from other sources in similar settings.19, 20 Some of this predominance is attributable to neonatal encephalopathy due to birth trauma or hypoxia, which is recognised to be a growing issue,23 underlining the value of including vision outcomes in interventional research in this area. Equally, the significantly increased rate of childhood visual disability among those born preterm in the present study shows the importance of visual disability as a key metric in the substantial global efforts to prevent poor outcomes for the more than 1 in 10 children who are born too soon globally.30 Finally, the observed contribution of congenital ocular anomalies echoes their importance in child health. These findings show that effective interventions to reduce the current burden of childhood visual disability in the UK and similar populations are most likely to emerge by better interfacing of ophthalmology and paediatric services.
To better identify priorities and develop and implement integrated national eye health policies, plans, and programmes, there is a need to think more radically and consider new models of integrated live registers of childhood visual disability through clinician–patient and family partnerships. The ideal model would comprise a register able to pull through and push out the key high fidelity data from health, education, and social care. The promise of transformational changes in health care through implementation of electronic medical records has yet to be fully realised, but certainly offers a means of ensuring health information is both complete and up to date, capturing key information from all clinical specialties. Importantly, such a new model could also capture the perspectives of children and young people and their families, including through the use of patient-reported vision outcome measures as these become integrated into routine clinical practice,31 to enhance their value in health economics analyses.
In conclusion, the BCVIS2 provides a contemporary snapshot of childhood visual disability in a high-income country that is useful for developing and delivering health care and health policies and for planning interventional research. The longitudinal investigation of clinical, social, and educational outcomes in this unique inception cohort will afford further novel insights. This study has already shown that childhood visual disability is a marker of vulnerability and should be considered a sentinel child health event. This approach will require a shift from the current model of exceptionalism for visual disability created by health service structures and clinical boundaries. Without this change, childhood visual disability will remain simultaneously self-evidently important but invisible in national and international monitoring processes, and thus absent in our global ambitions for the future of child health.4
This online publication has been corrected. The corrected version first appeared at thelancet.com/child-adolescent on April 14, 2021
Data sharing
Individual patient data were collected and processed with section 251 support from the Confidentiality Advisory Group in England. We do not have permission to share these identifiable data.
Acknowledgments
Acknowledgments
This work was funded directly by a Fight for Sight grant (1525/26) and was supported by funding from the Ulverscroft Foundation for the Ulverscroft Vision Research Group. ALS received support from the National Institute for Health Research Biomedical Research Centre (NIHR BRC) based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology and an NIHR Lectureship, and is supported by an NIHR Clinician Scientist award (CS-2018-18-ST2-005). JSR is supported in part by the NIHR BRC based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology and an NIHR Senior Investigator award. This work was undertaken at UCL Institute of Child Health/Great Ormond Street Hospital for children, which received a proportion of funding from the Department of Health's NIHR BRC funding scheme. The funding organisations had no role in the design or conduct of this research. This paper presents independent research. The views expressed are those of the authors and not necessarily those of the UK National Health Service (NHS), the NIHR, or the UK Department of Health and Social Care.
Contributors
The study was conceived by JSR and designed by ALS and JSR. The data were collected by LJT and ALS, with oversight by JSR. All authors were involved in data analysis. The manuscript was drafted by all authors. All authors approved the final version of the manuscript for publication. ALS and JSR have verified the underlying data.
Declaration of interests
We declare no competing interests.
Contributor Information
British Childhood Visual Impairment and Blindness Study Interest Group:
Joe Abbott, Wajda Abdullah, Gill Adams, Louise Allen, Christopher Anderson, Karen Ansell, Samira Anwar, Isabel Ash, Jane Ashworth, Sher Aslam, Majunath Astagi, Colin Ball, Rajesh Balu, Victoria Barrett, Zahabiyah Bassi, Adam Bates, Dushyant Batra, Sarah Bell, Linda Belmour, James Benzimra, Ginny Birrell, Susmito Biswas, Andrew Blaikie, Michael Blundell, Kate Bolton, Ewoud Bos, Pamela Bowen, Richard Bowman, Natalie Boyle, John Bradbury, Maria Bredow, Marsel Bregu, Nicholas Brennan, Rosie Brennan, Paul Brittain, Charles Buchanan, Catey Bunce, Howard Bunting, Priscilla Burgess, Cathie Burke, Alexandra Kate Bush, Jeremy Butcher, Lucilla Butler, Clare Cane, Cathryn Chadwick, Ruth Charlton, Anne-Marie Childs, Jessy Choi, Vivi Choleva, Amanda Churchill, Michael Clarke, Peter Clayton, Luke Clifford, Alan Connor, Rachel Cox, Lyn Cresswell, Annegret Dahlmann-Noor, Angela D'Amore, Mehul Dattani, Fiona Dean, Anita Devlin, Luna Dhir, Cora Doherty, Suzanne Dorey, Fiona Drimmie, Tina Duke, Gordon Dutton, Fiona Eaton, Megan Eaton, Danielle Eckersley, Clive Edelsten, Rachel Elderkin, Julia Ennis, Julia Escardo-Paton, Ziad Estephen, Onajite Etuwewe, Anthony Evans, Adjoa Ezekwe, Jenny Fairfield, Kevin Falzon, Allison Ferguson, Brian Fleck, Mary Gainsborough, Alexandra Galloway, Naomi Gerson-Sofer, Caspar Gibbon, Patricia Gibson, Kevin Goss, Katherine Graham-Evans, Judith Gray, Anna Gregory, Arun Gulati, Deniz Gurtin-Zorkun, Emma Guy, Diab Haddad, Helen Haggerty, Paul Haigh, Julia Hale, Samer Hamada, Joanne Hancox, Kerry Hanna, Sian Harris, Christine Harrison, Phillip Harvey, Sophie Headland, Dominic Heath, Paul Heaton, Robert Henderson, Melanie Hingorani, Zoe Hirst, Claire Hogg, Wolfgang Hogler, Roger Holden, Janice Hoole, Karen Horridge, Delyth Howard, Rachel Howells, Vanessa Irvine, Clare Irving, Nicola Johnson, Ian Johnston, Alice Jollands, David Jones, Annie Joseph, Archana Joshi, Pugazhvendan Kandaswamy, Charles Kattakayam, Joseph Keenan, Anne Kelly, James Kersey, Awais Khan, Peng Khaw, Tina Kipioti, Sadia Kiran, Lesley Kneen, Ajay Kotagiri, Richa Kulshrestha, Rosemary Lambley, Tim Lavy, Joanna Lawson, Vicki Lee, Jane Leitch, Julie Lennon, Gabi Lipshen, Chris Lloyd, John Loftus, Tom Lomas, Vernon Long, Jane Mackinnon, Mary MacRae, Usman Mahmood, Anna Maino, Sarah Maling, David Mansfield, Elizabeth Marder, Richard Markham, Jane Marr, Catherine Marsh, Anna Maw, Eleanor McCartney, Helen McCullagh, Anna McDonald, Derek McPhee, Lawrence Miall, Shila Mistry, Benjamin Moate, Meyyammai Mohan, Helen Moore, Will Moore, Nicola Morgan, Claire Morton, Alan Mulvihill, Ranjit Nair, Bill Newman, Christiane Nitsch, Katy O'Connell, Ngozi Oluonye, Vittaldas Pai, Helen Palmer, Maria Papadopoulos, Shelagh Parkinson, Bina Parmar, Manoj Parulekar, Madhavi Parvathareddy, Dipesh Patel, Himanshu Patel, Kamal Patel, Philippa Pennefather, Flaudia Petrone, Marcus Pierrepoint, Rachel Pilling, Sally Pollard, Renata Puertas, Karen Pysden, Anthony Quinn, Philip Quinn, Diyaa Rachdan, Jyoti Raina, Saul Rajak, Laura Ramm, Catherine Rands, Tekki Rao, Mary Ray, Ashwin Reddy, Sheilla Reilly, Maralla Rekha, Greg Richardson, Andrew Riordan, Nerys Roberts, Helen Robertson, Gillian Robinson, Neil Rogers, Shakir Saeed, Caroline Salmon, Jenefer Sargent, Nagini Sarvananthan, Conrad Schmoll, James Self, P Sellar, Elaine Service, Ayad Shafiq, Shilpa Shah, Vinod Sharma, Jemima Sharp, Julia Shaw, Manjula Shenoy, Tamsin Sleep, Elisa Smit, Katherine Smyth, Lynne Speedwell, Katherine Spowart, P Standring, Paulo Stanga, Alison Stanley, Alan Stanton, David Steel, John Stephen, Catherine Stewart, Jessica Street, Sally Stucke, Shona Sutherland, Katya Tambe, Anamika Tandon, Alison Tappin, Kate Taylor, Robert Taylor, Katherine Teasdale, Maria Theodorou, Gareth Thomas, Megan Thomas, Paula Thomas, Dorothy Thompson, Stephen Thomson, Indrajit Thopte, Peter Tiffin, Angela Tillett, Heidi Traunecker, Maria Tsimpida, Vivienne Van Someren, Udupa Venkatesh, Zoe Vermaak, Michael Vincent, David Walker, Simon Walker, Deidre Walsh, Bronwyn Walters, Martin Ward Platt, Louise Watson, Patrick Watts, Siobhan West, Stephanie West, Cathy White, Joy White, Gabriel Whitlingum, Cathy Williams, Sophie Wilne, Janice Wilson, Chien Wong, Tamsin Woodbridge, Paul Wright, Martha Wyles, and Philip Wylie
References
- 1.Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 2.Scott AW, Bressler NM, Ffolkes S, Wittenborn JS, Jorkasky J. Public attitudes about eye and vision health. JAMA Ophthalmol. 2016;134:1111–1118. doi: 10.1001/jamaophthalmol.2016.2627. [DOI] [PubMed] [Google Scholar]
- 3.Sonksen PM, Dale N. Visual impairment in infancy: impact on neurodevelopmental and neurobiological processes. Dev Med Child Neurol. 2002;44:782–791. doi: 10.1017/s0012162201002936. [DOI] [PubMed] [Google Scholar]
- 4.Clark H, Coll-Seck AM, Banerjee A, et al. A future for the world's children? A WHO-UNICEF-Lancet Commission. Lancet. 2020;395:605–658. doi: 10.1016/S0140-6736(19)32540-1. [DOI] [PubMed] [Google Scholar]
- 5.WHO . World Health Organization; Geneva: 2007. Global initiative for the elimination of avoidable blindness: action plan 2006–2011. [Google Scholar]
- 6.Bountziouka V, Cumberland PM, Rahi JS. Trends in visual health inequalities in childhood through associations of visual function with sex and social position across 3 UK birth cohorts. JAMA Ophthalmol. 2017;135:954–961. doi: 10.1001/jamaophthalmol.2017.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deloitte Impact of low vision and blindness from paediatric eye disease. 2016. https://www2.deloitte.com/au/en/pages/economics/articles/socioeconomic-impact-low-vision-blindness-paediatric-eye-disease-australia.html
- 8.Pizzarello L, Abiose A, Ffytche T, et al. VISION 2020: the right to sight: a global initiative to eliminate avoidable blindness. Arch Ophthalmol. 2004;122:615–620. doi: 10.1001/archopht.122.4.615. [DOI] [PubMed] [Google Scholar]
- 9.WHO . World Health Organization; Geneva: 2013. Universal eye health: a global action plan 2014–2019. [Google Scholar]
- 10.Rahi JS, Cable N. Severe visual impairment and blindness in children in the UK. Lancet. 2003;362:1359–1365. doi: 10.1016/S0140-6736(03)14631-4. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . 2nd ed. World Health Organization; Geneva: 2004. ICD-10: international statistical classification of diseases and related health problems: tenth revision. [Google Scholar]
- 12.The Royal College of Ophthalmologists Ophthalmic Services for Children. 2012. https://www.rcophth.ac.uk/wp-content/uploads/2014/12/2012_PROF_182_Ophthalmic-Services-for-Children.pdf
- 13.NHS Digital Registered blind and partially sighted people, England 2016–17. Dec 7, 2017. https://digital.nhs.uk/data-and-information/publications/statistical/registered-blind-and-partially-sighted-people/registered-blind-and-partially-sighted-people-england-2016-17#summary
- 14.Ministry of Housing Communities & Local Government English indices of deprivation 2015. Sept 30, 2015. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2015
- 15.Office for National Statistics Population estimates for the UK, England and Wales, Scotland and Northern Ireland: mid-2016. June 22, 2017. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2016
- 16.Breslow NE, Day NE. Statistical methods in cancer research. Volume I—The analysis of case-control studies. IARC Sci Publ. 1980;32:5–338. [PubMed] [Google Scholar]
- 17.Office for National Statistics Birth characteristics. 2016. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/datasets/birthcharacteristicsinenglandandwales
- 18.Office for National Statistics Child and infant mortality in England and Wales. 2018. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/childhoodinfantandperinatalmortalityinenglandandwales/2018
- 19.Chong C, McGhee CNJ, Dai SH. Causes of childhood low vision and blindness in New Zealand. Clin Exp Ophthalmol. 2019;47:165–170. doi: 10.1111/ceo.13443. [DOI] [PubMed] [Google Scholar]
- 20.Royal Institute of Deaf and Blind Children Australian Childhood Vision Impairment Register (ACVIR) Newsletter April 2018. 2018. https://ridbc.org.au/renwick/acvir-newsletters
- 21.Bengtsson J, Dich N, Rieckmann A, Hulvej Rod N. Cohort profile: the DANish LIFE course (DANLIFE) cohort, a prospective register-based cohort of all children born in Denmark since 1980. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-027217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Public Health England Child and Maternal Health. Child Health Profiles. https://fingertips.phe.org.uk/profile/child-health-profiles/data#page/0/gid/1938133228/pat/6/par/E12000004/ati/202/are/E06000015/cid/4/page-options/cin-ci-4_ovw-do-0
- 23.Reiner RC, Jr, Olsen HE, Ikeda CT, et al. Diseases, injuries, and risk factors in child and adolescent health, 1990 to 2017: findings from the Global Burden of Diseases, Injuries, and Risk Factors 2017 study. JAMA Pediatr. 2019;173 doi: 10.1001/jamapediatrics.2019.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourne RRA, Flaxman SR, Braithwaite T, et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e888–e897. doi: 10.1016/S2214-109X(17)30293-0. [DOI] [PubMed] [Google Scholar]
- 25.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 26.National Health Service NHS Long Term Plan. Jan 7, 2019. https://www.longtermplan.nhs.uk/publication/nhs-long-term-plan/
- 27.Clark H, Coll-Seck AM, Banerjee A, et al. After COVID-19, a future for the world's children? Lancet. 2020;396:298–300. doi: 10.1016/S0140-6736(20)31481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solebo AL, Teoh L, Rahi J. Epidemiology of blindness in children. Arch Dis Child. 2017;102:853–857. doi: 10.1136/archdischild-2016-310532. [DOI] [PubMed] [Google Scholar]
- 29.Rahi J, Gilbert C. Taylor and Hoyt's Pediatric Ophthalmology and Strabismus. Elsevier; Amsterdam: 2017. Epidemiology and the worldwide impact of visual impairment in children. [Google Scholar]
- 30.Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7:e37–e46. doi: 10.1016/S2214-109X(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robertson AO, Tadić V, Cortina-Borja M, Rahi JS. A patient-reported outcome measure of functional vision for children and young people aged 8 to 18 years with visual impairment. Am J Ophthalmol. 2020;219:141–153. doi: 10.1016/j.ajo.2020.04.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual patient data were collected and processed with section 251 support from the Confidentiality Advisory Group in England. We do not have permission to share these identifiable data.