Abstract
Dendritic cells (DCs) are potent antigen-presenting cells and play a crucial role in initiation and modulation of specific immune responses. Various pathogens are able to persist inside DCs. However, internalization of the gram-positive bacterium Listeria monocytogenes into human DCs has not yet been shown. In the present study, we demonstrate that human monocyte-derived immature DCs can efficiently phagocytose L. monocytogenes. This uptake is independent of listerial adhesion factors internalin A and internalin B but requires cytoskeletal motion and factors present in human plasma. A major portion of internalized bacteria is found in membrane-bound phagosomes and is rarely free in the cytosol, as shown by transmission electron microscopy and by using an L. monocytogenes strain expressing green fluorescent protein when in the host cell cytosol. The infection caused maturation of the immature DCs into mature DCs displaying high levels of CD83, CD25, major histocompatibility complex class II, and the CD86 costimulator molecule. This effect appeared to be largely mediated by listerial lipoteichoic acid. Although L. monocytogenes infection is known to induce death in other cell types, infection of human DCs was found to induce necrotic but not apoptotic death in fewer than 20% of DCs. Therefore, the ability of DCs to act as effective antigen-presenting cells for listerial immunity is probably enhanced by their resistance to cell death, as well as their ability to rapidly differentiate into mature, immunostimulatory DCs upon encountering bacteria.
Dendritic cells (DCs) play a critical role in antigen presentation and are involved in the induction of primary T-cell responses (1, 30, 34). They are constitutively rich in major histocompatibility complex (MHC) class II molecules and can be induced to express costimulatory molecules such as B7.1 and B7.2, which are essential for the activation of naive T cells. DCs exist in two functional stages. Immature DCs develop from hematopoetic precursors and are scattered throughout the body in nonlymphoid organs, where they exert sentinel functions. DCs pick up and process antigens and subsequently migrate into lymphoid organs, a process which is paralleled by maturation. This maturation process is necessary to elicit an immune response and is induced in principle by inflammatory stimuli, such as cytokines, lipopolysaccharides, CpG-containing oligodeoxynucleotides, and cell-cell and cell-matrix contacts (1, 24, 32, 42, 45). In lymphoid organs mature DCs select and stimulate antigen-specific T cells.
DCs are the critical antigen-presenting cells (APCs) involved in the immune response against microbes. A variety of pathogens such as human immunodeficiency virus type 1, measles virus, bacteria, or protozoa use DCs as host cells (7, 23, 37, 38, 40). However, internalization of Listeria monocytogenes into primary human DCs has not yet been shown.
L. monocytogenes is a gram-positive human pathogenic bacterium, which is taken up via the orogastric route. After invading mucosal surfaces in the small intestine, they are confronted with the Peyer's patch-based immune system, including intestinal DCs. L. monocytogenes is capable of intracellular replication in a variety of mammalian cells, including both professional and nonprofessional phagocytic cells (9, 15). Therefore, it has been widely used as a model system of facultative intracellular bacteria (26, 41). Recently, many different murine and human cell types have been infected with L. monocytogenes (13, 18, 19, 20, 33) to study cell-mediated immunity and the cell biology of infection. L. monocytogenes is actively internalized by host cells. Once inside the host cells the bacteria lyse the phagosomal membrane and escape into the cytoplasm where the bacteria replicate and spread from cell to cell. A number of listerial virulence determinants involved in the intracellular life cycle of L. monocytogenes have been characterized. Internalin A and internalin B, which are members of the family of internalins discovered in L. monocytogenes, trigger the uptake by several normally nonphagocytic cell types (9, 11, 12, 14, 17, 35).
Listeriolysin, a pore-forming cytolysin, is required, along with two phospholipases, for lysis of the phagosome membrane. ActA, a listerial cell wall protein, promotes F-actin-driven intracellular movement. The expression of most of these virulence factors is controlled by the positive regulatory factor PrfA (reviewed in reference 26). Bacterial virulence factors are thought to interact with host cell components, resulting in specific transient or persistent activation of host cell signal transduction pathways (27, 28).
In the present study we demonstrate the efficient phagocytosis of L. monocytogenes by human blood-derived DCs employing several techniques. Our results suggest that effective DC-mediated immunity to L. monocytogenes infections may hinge critically on the ability of the DC to ingest, kill, and process bacteria for antigen presentation while avoiding cell death, which could contribute to further disease dissemination.
MATERIALS AND METHODS
Bacteria.
The L. monocytogenes EGD wild-type strain, the isogenic deletion mutants, and the other Listeria strains used in this study are described in Table 1. The bacteria were cultured aerobically in brain heart infusion (BHI) at 37°C until they reached the mid-log phase of growth.
TABLE 1.
Listeria strains used in this study
| Strain | Genotype | Vector | Resistance marker | Source or reference |
|---|---|---|---|---|
| L. monocytogenes | ||||
| Sv 1/2a EGD | Wild type | Institute strain collection | ||
| Sv 1/2a EGD | Wild type | PactA-gfp | Tetracycline | Dietrich et al. (10) |
| 10403S | Wild type | Jones and Portnoy (25) | ||
| DP-L2161 | Δhly | PactA-gfp | Tetracycline | Jones and Portnoy (25) |
| WL-112 | ΔinlAB | PactA-gfp | Tetracycline | Greiffenberg et al. (18) |
| L. innocua Sv6a | Wild type | PactA-gfp | Tetracycline | Institute strain collection |
Isolation of human DCs from peripheral blood.
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized leukocyte-enriched buffy coats of healthy adult donors by Lymphoprep (1.077 g/ml; Nycomed, Oslo, Norway) density gradient centrifugation applying 400 × g at room temperature. PBMCs were plated on tissue culture dishes (3003; Falcon Labware, Oxnard, Calif.) at a density of 5 × 106 cells/ml in RPMI 1640 medium (Gibco), supplemented with l-glutamine (2 mM), 1% autologous human plasma, and 100 U of granulocyte-macrophage colony-stimulating factor (GM-CSF) for 45 min at 37°C. Nonadherent cells were washed free with warm phosphate-buffered saline (PBS), and adherent cells were cultured for 7 days without antibiotics in RPMI 1640 medium, supplemented with 1% autologous human plasma, 2 mM l-glutamine, 1,000 U of recombinant human interleukin-4 (rhIL-4) (PBH, Hanover, Germany), and 800 U of rhGM-CSF (Leukomax; Sandoz, Basel, Switzerland) per ml. Cytokines were replenished every other day.
Cellular uptake assay.
On day seven nonadherent DCs were collected prior to infection by moderately vigorous aspiration and transferred to new 6-, 12-, or 24-well plates at a density of 5 × 105 cells/ml. DCs were infected with logarithmically growing bacteria. After two washes with PBS the bacteria were diluted in RPMI 1640 medium and added at the desired multiplicity of infection (MOI) to each well. The cultures were incubated in RPMI 1640 medium with 1% autologous human plasma at 37°C for 1 h to allow the bacteria to invade the cells. For selective removal of extracellular bacteria, 50 μg of gentamicin (Gibco) per ml was added to each well, and the cells were washed twice.
In order to detect intracellular bacteria in DCs, we took advantage of an L. monocytogenes strain carrying the green fluorescent protein gene (gfp) under transcriptional control of the PrfA-dependent actA promoter. This construct restricts expression of the gfp gene to L. monocytogenes organisms that have escaped the phagolysosome, resulting in detectable expression of gfp (5, 8).
Fluorescence microscopy.
Green fluorescent protein (GFP)-expressing L. monocytogenes was visualized by using a fluorescence-equipped inverted phase-contrast microscope and photographed with a digital-imaging system camera (Visitron). Images are computer generated with the help of MetaMorph Imaging software by overlaying black-and-white photographs and fluorescent images.
Determination of viable bacterial counts.
Infected DCs were lysed at different time points postinfection (p.i.) by the addition of ice-cold distilled water and incubating them for 10 min on ice, followed by sonication for 1 s with a Branson sonicator. Viable bacterial counts were determined by plating serial dilutions on BHI agar.
Transmission electron microscopy.
DCs were infected with L. monocytogenes and its mutants as described above. At three time points (1.5, 3, and 6 h) cells were washed, fixed in 2.5% glutaraldehyde, postfixed in 2% osmium tetroxide, stained with 0.5% uranyl acetate, dehydrated in graded alcohols, and finally embedded in Lowicryl K4M.
Giemsa staining.
DCs were infected with L. monocytogenes and its mutants as described above. At different time points (1.5, 3, 6, 16, and 36 h) cells were washed, centrifuged on coverslips, fixed with methanol for 5 min, stained with Giemsa at 1:20 (Merck, Darmstadt, Germany) for 20 min, and then examined using a Leitz Dialux 20 microscope (oil immersion objective).
Uptake inhibition studies.
The uptake assay was performed as described above, except that the medium contained 2 μg of the inhibitor cytochalasin D (Sigma, C 8273) per ml throughout the duration of the experiment. After 45 min of preincubation of the DCs with 2 μg of cytochalasin D per ml, the bacteria were added.
Isolation of LTA.
Lipoteichoic acid (LTA) from heat-inactivated and mechanically disintegrated L. monocytogenes cells were extracted with 40% (vol/vol) phenol at 65°C for 45 min, and the phases were separated by centrifugation. The aqueous phase containing LTA was dialyzed against 0.05 M sodium acetate (pH 4.0). LTA was then purified by separation on an octyl-Sepharose CL-4B column (Pharmacia) with a linear gradient (0 to 66% [vol/vol] propanol in 0.05 M sodium acetate; pH 4.0). Phosphate-containing fractions eluted within the propanol gradient were pooled, dialyzed against double-distilled water, and lyophilized (39).
Analysis of cell death process.
The cytoplasmic appearance of fragmented DNA was assessed by using Cell Death Detection ELISAPlus (Boehringer, Mannheim, Germany) according to the manufacturer's instructions. For visualizing the nuclear morphology, DCs were incubated with bisbenzimide dye (Hoechst 33342; 5 μg/ml) at 37°C for 15 min. In addition, the nuclear morphology was determined by transmission electron microscopy. To assess DNA fragmentation in infected cells, total DNA from 5 × 106 DCs was isolated by the DNAzol method (DNAzol Reagent [Gibco-BRL] 10503-027) and loaded onto a 1% agarose gel containing ethidium bromide. Percent cell death was determined by measuring propidium iodide (1 μg/ml)-positive DCs by flow cytometry.
Flow cytometry.
Flow cytometry was used to monitor the expression of surface markers of uninfected and infected DCs. Indirect immunofluorescence was performed according to standard techniques using murine monoclonal antibodies revealed by phycoerythrin-conjugated anti-mouse immunoglobulin (Dianova, Hamburg, Germany). The primary antibodies used were α-HLA class II DR (L243) and α-HLA class II DR/DQ (9.3F10) (American Type Culture Collection, Manassas, Va.), CD25 (clone MA251; Pharmingen, Hamburg, Germany), CD83 (HB15a; Immunotech, Hamburg, Germany), and CD86 (IT2.2; Pharmingen). The stained cells were analyzed on an EPICS XL-MCL (Coulter Immunotech Diagnotics, Krefeld, Germany).
Statistical analysis.
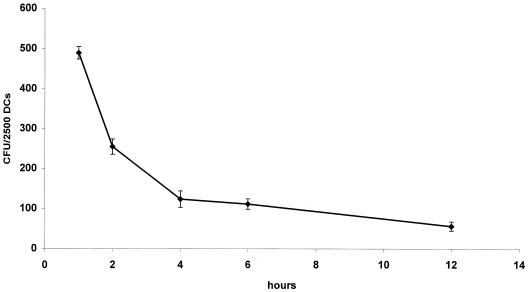
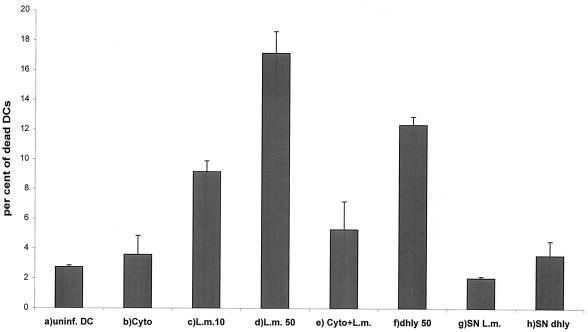
The data in Fig. 3 and 8 are presented as the means and standard deviations (error bars) of representative experiments run at least in triplicate.
FIG. 3.
Intracellular viability of L. monocytogenes EGD in human DCs. After 1 h of coincubation of L. monocytogenes with DCs (MOI = 50), cell cultures were washed and gentamicin was added. Thereafter, cells were lysed at different intervals, and the number of CFU recovered per well was determined. The results are presented as mean values and the standard deviations (error bars) of three independent experiments.
FIG. 8.
Determination of the percentage of dead DCs at 6 h p.i. by flow cytometry after propidium iodide staining. (a) Uninfected DCs (negative control). (b) Uninfected DCs after treatment with cytochalasin D (2 μg/ml). (c) L. monocytogenes wild type (10403S) infection at an MOI of 10. (d) L. monocytogenes wild type (10403S) infection at an MOI of 50. (e) L. monocytogenes wild type (10403S) infection at an MOI of 50 after pretreatment with cytochalasin D (2 μg/ml). (f) L. monocytogenes Δhly infection at an MOI of 50. (g) Addition of L. monocytogenes wild type supernatant (20%). (h) Addition of L. monocytogenes Δhly supernatant (20%). Results are presented as the mean values and the standard deviation (error bars) of three independent experiments.
RESULTS
L. monocytogenes is phagocytosed by human monocyte-derived immature DCs.
To determine whether and how human DCs internalize L. monocytogenes, we infected human DCs with this bacterium. Prior to infection we checked the quality and purity of the DCs. According to the forward scatter-side scatter of flow cytometry, 80 to 90% of the cells were CD1a+ and HLA class II+ DCs. Neither CD14 nor CD64 as markers for human monocytes/macrophages were detected. At this stage of DC maturation, the expression of the mature-DC-restricted marker CD83 was less than 30%. Therefore, the gated cell population exhibited the typical surface marker pattern of immature DCs (3).
Immature DCs were incubated with live bacterium-to-DC ratios of 10:1, 50:1, and 100:1. The uptake of bacteria into DCs was analyzed by transmission electron microscopy, by Giemsa staining, by listerial GFP expression in the host cell cytoplasma, and by determination of viable bacterial cell counts.
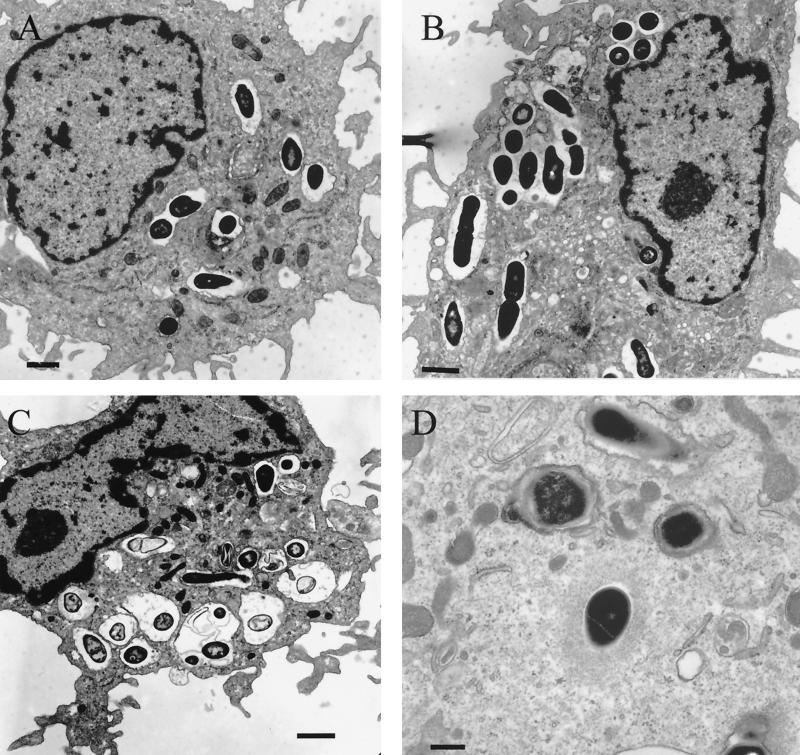
As quickly as 1.5 h after incubation in the presence of human plasma, L. monocytogenes was found in most DCs (>80%), mainly within membrane-bound phagosomes (Fig. 1A). At this time point, the majority of the phagosomes contained a single bacterium. Transmission electron microscopy revealed some bacteria (<5% of infected DCs) in the cytosol that had initiated actin polymerization (Fig. 1D). The number of phagocytosed bacteria varied from 1 to >50, a result clearly dependent on the chosen MOI. Using a bacterium-to-cell ratio of 50:1, the number of bacteria present in each cell was determined to be between 10 and 35.
FIG. 1.
(A) Human DCs phagocytose L. monocytogenes. At 1.5 h after incubation of DCs with L. monocytogenes EGD (MOI = 50), they are found in a great proportion (>80%) of DCs, mostly within membrane-bound phagosomal organelles (bar, 1.1 μm). (B and C) At 3 and 6 h p.i., L. monocytogenes cells are mostly located in the phagosome at different stages of degradation. Previous single phagosomes fuse to large vacuoles (bars, 1.1 μm). (D) Transmission electron microscopy reveals a cytosolic bacterium beginning actin polymerization at 1.5 h p.i. (bar, 0.4 μm).
After 3 and 6 h of infection, listeriae in DCs were still located in the phagosome in different stages of degradation (Fig. 1B and C). Occasionally, fusion of phagosomes occurred, producing large vacuoles.
Using L. monocytogenes PactA-gfp, we detected by phase-contrast microscopy and fluorescent microscopy, as early as 4 h p.i., intracellular fluorescent bacteria in up to 5% of DCs, whereas extracellular bacteria showed no fluorescence (Fig. 2A).
FIG. 2.
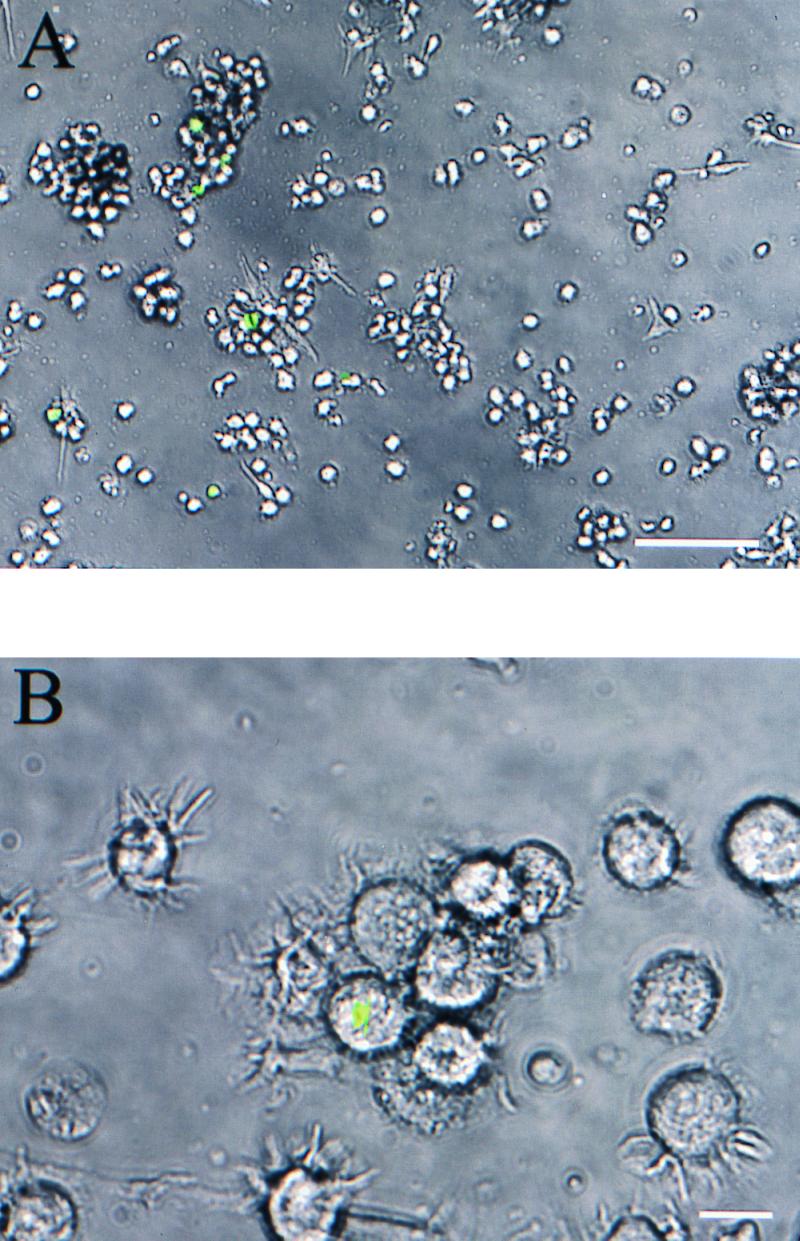
(A) Detection of living cytoplasmic L. monocytogenes in human DCs by an L. monocytogenes strain carrying the gfp gene under transcriptional control of the PrfA-dependent actA promoter (12 h p.i.; MOI = 50; bar, 100 μm). (B) Infected human DCs show morphological changes typical for mature DCs (24 h p.i.; MOI = 50; bar, 10 μm). There are still some fluorescent cytoplasmic listeriae. Green fluorescence was visualized by using a fluorescence-equipped inverted phase-contrast microscope and photographed with a digital-imaging system (combined phase-contrast and fluorescence images).
We confirmed by flow cytometry that cells containing cytosolic fluorescent bacteria expressed the typical surface markers of DCs. After pretreatment of DCs with cytochalasin D, which disturbs actin filament polymerization and therefore inhibits phagocytosis, no GFP-expressing bacteria were detected. Therefore, the L. monocytogenes construct expresses GFP only when the bacteria are inside the host cell cytosol.
The number of viable listeriae in DCs (MOI of 50, determined as CFU after lysing of the host cells) at 1 h p.i. was up to 20% of the DCs. This number of viable bacteria rapidly decreased within the next few hours. Thereafter, a further slow decrease of viable listeriae was observed over a 12-h period (Fig. 3).
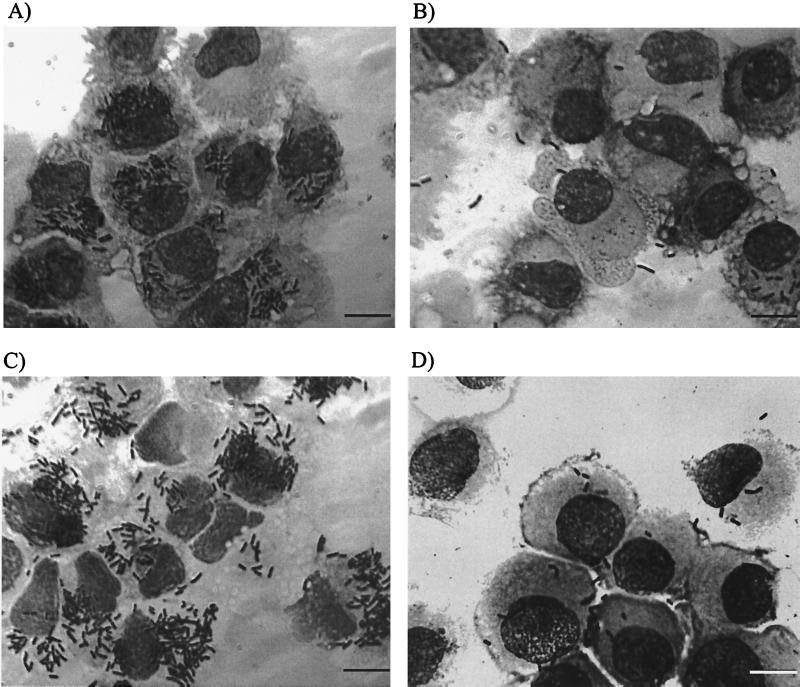
Interestingly, in contrast to immature DCs, incubation of CD83+ DCs matured by treatment with a cytokine cocktail (36) were not infected by coculture with L. monocytogenes. Only a few bacteria were observed upon cytospinning of DCs by Giemsa staining (Fig. 4D). We assume that these were externally bound, since no viable bacteria could be recovered after lysing of the mature DCs. Moreover, no fluorescent cytosolic bacteria were detected using the L. monocytogenes PactA-gfp strain.
FIG. 4.
Various numbers of phagocytosed listeriae in DCs by using different bacterial strains and different conditions during infection (conditions common to all: MOI = 50; Giemsa staining at 1.5 h p.i.). (A) Internalized L. monocytogenes EGD. (B) Internalized L. monocytogenes EGD carrying out the infection without the presence of human plasma (the uptake of L. monocytogenes was strongly decreased). (C) Internalized L. monocytogenes ΔinlAB with an infection rate comparable to that of the wild type. (D) Infection of mature DCs (few bacteria are phagocytosed by DCs). Bar, 10 μm.
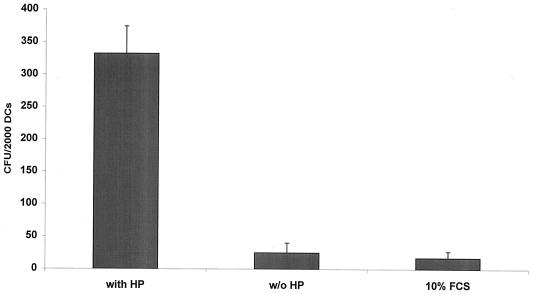
The use of a ΔinlAB (18) mutant of L. monocytogenes resulted in an infection rate comparable to that of the wild type, as determined by Giemsa staining (Fig. 4C). Interestingly, the omission of human plasma or the use of fetal calf serum (10%) in the place of human plasma strongly decreased the uptake of L. monocytogenes, as determined by microscopy after Giemsa staining and by viable bacterial cell count (Fig. 4B and Fig. 5).
FIG. 5.
Comparison of the uptake of L. monocytogenes EGD in DCs with or without the presence of human plasma and after replacement of human plasma with fetal calf serum (10% FCS).
In contrast to uninfected DCs, infected cells became adherent and formed clusters at ca. 2 h p.i. The adherent cells then detached themselves from the tissue culture plate, and cell clusters disaggregated almost completely after 40 h. At this time the typical morphology of terminal differentiated DCs (extensive dendritic cytoplasmic processes) was observed by microscopy (Fig. 2B).
Response of DCs to infection with L. monocytogenes.
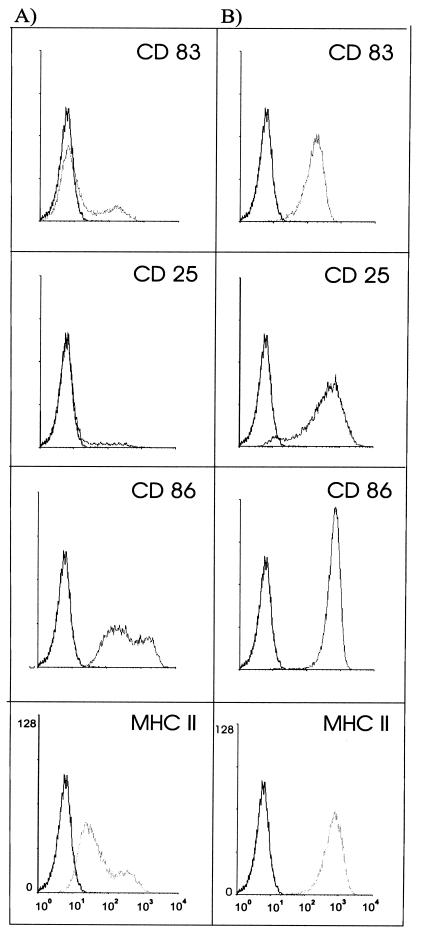
To investigate the host cell response after a L. monocytogenes infection, we assessed the surface marker expression of DCs (Fig. 6). All strains, including nonpathogenic L. innocua, had the same effect on the surface marker profile of DCs: CD83, a specific mature-phase marker for human DCs, was strongly upregulated 2 h after infection. Between 85 and 95% of DCs expressed CD83, indicating maturation of infected DCs. Infection also had an effect on MHC class II, costimulatory molecule CD86, and CD25 (interleukin-2 receptor, α chain) expression. All of these molecules were upregulated after infection with L. monocytogenes.
FIG. 6.
Flow cytometry profiles of surface marker expression of CD83, CD25, CD86, and MHC class II on human DCs of either uninfected cells (A) or L. monocytogenes EGD-infected cells (B) (MOI = 50, 16 h p.i.). The y axis of each histogram shows the relative cell number; the x axis shows the log fluorescence intensity. Black histograms represent staining with matched-isotype antibodies.
To test whether the maturation process depends on phagocytosis or is caused by the mere presence of bacteria and their soluble products, we used a double-chamber system with a membrane for the separation of bacteria and DCs (membrane pore size, 0.2 μm). As expected, coculture of DCs and bacteria in the same split-well chamber resulted in strong upregulation of CD83. However, even when separated from DCs, bacteria induced the upregulation of CD83 in up to 60% of DCs (data not shown). This result suggested that soluble products of L. monocytogenes that are smaller than 0.2 μm lead to maturation of human DCs.
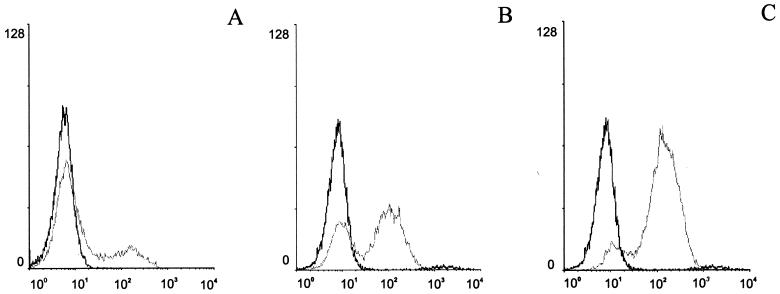
Therefore, the two forms of listerial LTA (22) were next assayed for their influence on maturation of DCs. LTA I (in the range of 2 to 20 μg/ml) gave a significant dose-dependent upregulation of the mature-phase surface markers CD83 (Fig. 7), MHC class II, and CD86 within 4 h of addition, whereby LTA II had a lower activity.
FIG. 7.
Flow cytometry profiles of maturation marker CD83 on human DCs incubated with LTA I fraction. The dose dependency of maturation was shown by using different amounts of LTA (A, without LTA; B, 2 μg/ml; C, 20 μl/ml). Black histograms represent staining with matched-isotype antibodies.
Cell death of DCs after infection with L. monocytogenes EGD and an hly deletion mutant.
After showing the efficient uptake and killing of L. monocytogenes by DCs, we asked whether this critical APC would survive the Listeria infection and be available for the induction of an immune response. Cell death by necrosis in L. monocytogenes-infected murine macrophages and induction of apoptosis by L. monocytogenes in murine DCs have been described (2, 21).
Therefore, we investigated infection-induced apoptosis and necrosis employing several complementary techniques. We first discriminated between apoptosis and necrosis by the assessment of DNA fragmentation and nuclear staining. In addition, at 3, 6, 12, and 24 h after infection with L. monocytogenes EGD and L. monocytogenes Δhly we carried out Cell Death Detection ELISA (Roche, Germany) and propidium iodide flow cytometry analyses. Results were compared with the cell death results of uninfected DCs (negative control); with DCs that had been treated with the apoptosis-inducing topoisomerase inhibitor camptothecine at 10 μM (apoptosis-positive control); and with distilled-water-killed DCs (necrosis-positive control).
Purified eukaryotic genomic DNA from L. monocytogenes-infected DCs resolved on an agarose gel did not show the typical apoptotic DNA degradation ladder pattern in comparison to the positive control of camptothecine-treated DCs. Furthermore, L. monocytogenes EGD failed to elicit apoptotic morphology, such as chromatin condensation and cell blebbing, as was observed by transmission electron microscopy (Fig. 1C) and nuclear staining with Hoechst 33342 and Giemsa. In addition, analysis of nucleosomes in the cytoplasmic fraction after infection with L. monocytogenes showed no significant difference from the uninfected control, except for the positive control treated with camptothecine (data not shown). Thus, infection with listeriae did not induce apoptosis in DCs.
In contrast, however, infection of DCs with the wild-type strains L. monocytogenes EGD and 10403S (25) resulted in a significant (P = 0.0001, Student's t test) increase of necrosis (up to 20%, MOI = 50, 6 h p.i.) compared to uninfected cells measured by propidium iodide flow cytometry and Cell Death Detection ELISA. As shown in Fig. 8, the number of dead DCs was dependent on the MOI. At 12 and 24 h after infection no further increase of cell death was detected.
This increase of necrosis could be induced by the presence of extracellular bacteria and/or their soluble products in cell culture medium, or it could require the uptake of listeriae. To inhibit the uptake of listeriae, we preincubated the cells with cytochalasin D (2 μg/ml). Under this condition, infection with the wild-type strain (MOI = 50) showed very little necrosis and was comparable to the cytochalasin D-treated uninfected control (Fig. 8). To characterize the product involved in mediating the cytotoxic effect, infection was performed using the isogenic Δhly mutant of the wild-type strain 10403S (25), which does not produce listeriolysin. Listeriolysin mediates the lysis of the phagocytic vacuole and liberation of bacteria into the cytoplasma (26). Infection of DCs with L. monocytogenes Δhly showed a slightly lower (but not significant) level of necrosis in comparison to the wild type (Fig. 8). This slight difference could be explained by a direct effect of extracellular listeriolysin. Therefore, we added the hemolytic supernatant of wild-type L. monocytogenes and the nonhemolytic supernatant of the Δhly mutant to a human DC culture. The hemolytic activity of both supernatants was controlled with sheep red blood cells. The addition of neither wild-type supernatant nor Δhly supernatant (up to 20% of the cell culture medium) triggered cell death of human DCs (Fig. 8).
In conclusion, the increased necrosis of DCs after L. monocytogenes infection required the uptake of bacteria, and induced necrosis is not primarily dependent on extracellular listeriolysin.
DISCUSSION
DCs play the most critical role in the activation of naive CD4 and CD8 T lymphocytes, and constitute the basis of efficient defense against infective agents (42). Only a few mature DCs are needed to provoke a potent T-cell immune response (38). Until recently, the difficulty of obtaining freshly isolated DCs and the lack of specific markers had impeded research into the interactions between primary DCs and microorganisms. Sufficient human DCs can now be prepared from blood progenitors (1).
In the present study, we demonstrated that human monocyte-derived DCs can efficiently phagocytose the gram-positive bacterium L. monocytogenes as detected by Giemsa staining and transmission electron microscopy. After internalization, L. monocytogenes is mainly found in membrane-bound phagosomes.
The presence of human plasma (containing immunoglobulins and complement factors) during the infection seemed to be conducive to the internalization, possibly via opsonins such as Fc receptors and complement receptors on the surface of DCs (1).
After incubation with a cytokine cocktail, DCs decreased their capacity to endocytose listeriae, confirming that maturation of DC decreases their antigen uptake ability.
Although transmission electron microscopy demonstrated the uptake of L. monocytogenes by DCs, it failed to discriminate between living and dead bacteria. Using a GFP-expressing L. monocytogenes strain (10), we monitored viable L. monocytogenes in the cytoplasm of DCs by microscopy. The gfp cDNA was put under the transcriptional control of the actA promoter, which is highly active when the bacteria reside inside the host cell cytoplasm. Therefore, GFP expression under the control of the actA promoter is a marker of cytoplasmically located bacteria. We found that listeriae showed fluorescence at approximately 4 h p.i.. This corresponds to the time that L. monocytogenes needs to escape from the phagosome, to activate the actA promoter, and to produce GFP. The number of listeria-infected DCs determined by viable counts after lysing the host cells at 1 h p.i. was only 20% of the total number of DCs and strongly decreased over the following time period.
In comparison to the efficient uptake shown by transmission electron microscopy and Giemsa staining, the low number of viable L. monocytogenes bacteria in DCs and the few bacteria expressing GFP suggest that only a minority of the internalized bacteria succeeded in escaping from the phagosome. The strong decrease of viable bacteria within the first few hours is therefore most likely due to the bactericidal efficiency of the DC phagosome, as shown by transmission electron microscopy.
As expected from other uptake studies with different mammalian cells (29), the pretreatment of DCs with cytochalasin D, which disturbs de novo actin polymerization, inhibits the uptake of L. monocytogenes. Thus, an intact actin cytoskeleton is essential for efficient DC uptake of L. monocytogenes.
Recent studies showed that internalin A and internalin B are critical for the uptake of L. monocytogenes into several mammalian cell types (4, 12, 17, 19). The process of L. monocytogenes internalization into human DCs appears to be independent of either internalin A or B. In addition, Guzman et al. (20) showed that the uptake of L. monocytogenes by a mouse dendritic cell line is also independent of these internalins.
The uptake of L. monocytogenes in DCs resulted in an altered surface marker expression and altered morphology of the cells, indicating maturation of DCs. DCs have been described as having two distinct functional stages: immature cells, with high antigen uptake and processing ability and a poor T-cell stimulatory function, and mature cells, with high stimulatory function and poor antigen uptake (38). To investigate cell maturation, we assessed the increased membrane expression of MHC class II, CD83, and costimulatory molecules. These surface markers were all upregulated, indicating maturation. CD83, a member of the immunoglobulin superfamily and a selective mature-phase marker (46) with a still unknown function, was strongly upregulated. Therefore, L. monocytogenes has an efficiency comparable to that of inflammatory cytokine cocktails in mediating maturation of DCs. This observation is in line with previous reports demonstrating that bacteria are efficient at inducing progression from immature to mature stage DCs (16, 23). L. monocytogenes EGD and nonpathogenic L. innocua led to maturation.
From the experiments with the double-chamber system, we concluded that the maturation of DCs is only partially dependent on the phagocytosis of bacteria and that the presence of bacterial products (<0.2 μm) is sufficient to mediate maturation. In the search for components present on the surface of L. monocytogenes, purified LTA preparations were tested for the ability to induce the maturation of DCs. All of the maturation effects after infection with the whole bacterium could be reproduced by using listerial LTA. Therefore, we suggest that LTA has a major role in listeria-induced DC maturation, with the LTA I fraction having more activity than the LTA II fraction. Recently, it was shown that L. monocytogenes infection of P388D1 macrophages results in an NF-κB activation induced by LTA and bacterial phospholipases (22). Interestingly, in contrast to our results, Hauf et al. (22) showed that the LTA I fraction was ineffective in NF-κB, whereas LTA II was strongly active. This difference may depend on different receptors for listerial LTA expressed by the host cell.
Eukaryotic cell death occurs by necrosis or apoptosis. Various pathogenic bacteria with the capacity to live within eukaryotic cells activate an apoptotic program in infected host cells (2). Induction of apoptosis by L. monocytogenes in murine DC lines has been described earlier (21). In contrast to that study, our data argue against the induction of apoptosis in human DCs by all strains of L. monocytogenes tested. L. monocytogenes failed to elicit features of apoptotic nuclear morphology such as chromatin condensation and DNA fragmentation. In contrast, this pathogen was mostly killed in the phagosome of DCs. After infection with the wild type, fewer than 20% of DCs were killed by necrosis, and this result was dose dependent. This increased necrosis required the uptake of listeriae.
In comparison to the wild type, infection of DCs with the Δhly mutant strain showed a lower level of necrosis. This difference could be due to either the lack of escape into the cytosol of the Δhly mutant or a direct trigger of cell death by listeriolysin. The addition of wild-type Listeria supernatant (with hemolytic activity) to the cell culture showed no difference in cell death from that of uninfected DCs.
Therefore, the entrance of wild-type L. monocytogenes into the cytosol rather than the mere presence of extracellular listeriolysin is probably responsible for the slightly increased necrosis compared to the Δhly mutant. However, it is still unclear if listeriolysin within the host cell plays a part in induction of cell death.
For induction of an immune response, DCs need to be stimulated by the pathogen, but if they die or downregulate their costimulatory molecules after infection, they fail to activate T lymphocytes. Our results suggest that effective DC-mediated immunity to L. monocytogenes infection hinges critically on the ability of the DC to take up, kill, and process bacteria for antigen presentation while avoiding cell death and productive infection. Both the ability of human DCs to resist death and the ability to undergo maturation by upregulation of costimulatory signals and MHC class II after infection with L. monocytogenes may be important factors in minimizing dissemination of the disease and in inducing an effective host immune response.
DCs play a critical role in the induction of an immune response and are important vehicles for vaccination strategies (7). Delivery of antigen in a manner that induces effective antigen-specific immunity is a critical challenge in vaccine design (6, 43, 44). From our data on the response of human DCs to Listeria infection, we conclude that L. monocytogenes represents a promising live carrier for DC-based vaccination strategies.
ACKNOWLEDGMENTS
This study was supported by a fellowship from the Bundesministerium für Bildung und Forschung (Az 01 KS9603) to A.K.-M. within the scope of IZKF Würzburg and by grants from the Deutsche Forschungsgemeinschaft (SFB479 and SFB465) and the Fond der Chemischen Industrie.
We thank J. Daniels, A. McLellan, and M. Mäurer for critical reading of the manuscript. We are grateful to A. Bubert for donating L. monocytogenes gfp strains and I. Karunasagar for helping with electron microscopy.
REFERENCES
- 1.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Barsig J, Kaufmann S. The mechanism of cell death in Listeria monocytogenes-infected murine macrophages is distinct from apoptosis. Infect Immun. 1997;65:4075–4081. doi: 10.1128/iai.65.10.4075-4081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender A, Sapp M, Schuler G, Steinman R M, Bhardwaj N. Improved methods for the generation of dendritic cells form nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 4.Braun L, Ohayon H, Cossart P. The InlB protein of Listeria monocytogenes is sufficient to promote entry into mammalian cells. Mol Microbiol. 1998;27:1077–1087. doi: 10.1046/j.1365-2958.1998.00750.x. [DOI] [PubMed] [Google Scholar]
- 5.Bubert A, Sokolovic Z, Chun S, Papatheodorou L, Simm A, Goebel W. Differential expression of Listeria monocytogenes virulence genes in mammalian host cells. Mol Gen Genet. 1999;261:323–336. doi: 10.1007/pl00008633. [DOI] [PubMed] [Google Scholar]
- 6.Condon C, Watkins S C, Celluzzi C M, Thompson K, Falo L D., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 7.Corinti S, Medaglini D, Cavani A, Rescigno M, Pozzi G, Ricciardi-Castagnoli P, Girolomoni G. Human dendritic cells very efficiently present a heterologous antigen expressed on the surface of recombinant gram-positive bacteria to CD4+ T lymphocytes. J Immunol. 1999;163:3029–3036. [PubMed] [Google Scholar]
- 8.Cormack B P, Valdivia R H, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1995;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 9.Cossart P, Lecuit M. Interactions of Listeria monocytogenes with mammalian cells during entry and actin-based movement: bacterial factors, cellular ligands and signalling. EMBO J. 1998;17:3797–3806. doi: 10.1093/emboj/17.14.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietrich G, Bubert A, Gentschev I, Sokolovic Z, Simm A, Katic A, Kaufmann S H E, Hess J, Szalay A A, Goebel W. Delivery of antigen-encoding plasmid DNA into the cytosol of macrophages by attenuated suicide in Listeria monocytogenes. Nat Biotechnol. 1997;16:181–185. doi: 10.1038/nbt0298-181. [DOI] [PubMed] [Google Scholar]
- 11.Dramsi S, Dehoux P, Cossart P. Common features of gram-positive bacterial proteins involved in cell recognition. Mol Microbiol. 1993;9:1119–1122. doi: 10.1111/j.1365-2958.1993.tb01241.x. [DOI] [PubMed] [Google Scholar]
- 12.Dramsi S, Biswas I, Maguin E, Braun L, Mastroeni P, Cossart P. Entry of Listeria monocytogenes requiers expression of InlB, a surface protein of the internalin multigene family. Mol Microbiol. 1995;16:251–261. doi: 10.1111/j.1365-2958.1995.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 13.Drevets D A, Sawyer R T, Potter T A, Campbell P A. Listeria monocytogenes infects human endothelial cells by two distinct mechanisms. Infect Immun. 1995;63:4268–4276. doi: 10.1128/iai.63.11.4268-4276.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelbrecht F, Chun S-K, Ochs C, Hess J, Lottspeich F, Goebel W, Sokolovic Z. A new PrfA-regulated gene of Listeria monocytogenes encoding a small, secreted protein which belongs to the family of internalins. Mol Microbiol. 1996;21:823–837. doi: 10.1046/j.1365-2958.1996.541414.x. [DOI] [PubMed] [Google Scholar]
- 15.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filgueira L, Nestle F O, Rittig M, Joller H I, Groscurth P. Human dendritic cells phagocytose and process Borrelia burgdorferi. J Immunol. 1996;157:2998–3005. [PubMed] [Google Scholar]
- 17.Gaillard J L, Berche P, Frehel C, Gouin E, Cossart P. Entry of Listeria monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991;65:1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 18.Greiffenberg L, Skolovic Z, Schnittler H-J, Spory A, Böckmann R, Goebel W, Kuhn M. Listeria monocytogenes-infected human umbilical vein endothelial cells: internalin-independent invasion, intracellular growth, movement, and host cell responses. FEMS Microbiol Lett. 1997;157:163–170. doi: 10.1111/j.1574-6968.1997.tb12768.x. [DOI] [PubMed] [Google Scholar]
- 19.Greiffenberg L, Goebel W, Kim K S, Weiglein I, Bubert A, Engelbrecht F, Stins M, Kuhn M. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infect Immun. 1998;66:5260–5267. doi: 10.1128/iai.66.11.5260-5267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guzman C A, Rohde M, Chakraborty T, Domann E, Hudel M, Wehland J, Timmis K N. Interaction of Listeria monocytogenes with mouse dendritic cells. Infect Immun. 1995;63:3665–3673. doi: 10.1128/iai.63.9.3665-3673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guzman C A, Domann E, Rohde M, Bruder D, Darji A, Weiss S, Wehland J, Chakraborty T, Timmis K N. Apoptosis of mouse dendritic cells is triggered by listeriolysin, the major virulence determinant of Listeria monocytogenes. Mol Microbiol. 1996;20:119–126. doi: 10.1111/j.1365-2958.1996.tb02494.x. [DOI] [PubMed] [Google Scholar]
- 22.Hauf N, Goebel W, Fiedler F, Skolovic Z, Kuhn M. Listeria monocytogenes infection of P388D1 macrophages results in a biphasic NF-κB (RelA/p50) activation induced by lipoteichoic acid and bacterial phospholipases and mediated by IκBα and IκBβ degradation. Proc Natl Acad Sci USA. 1997;94:9394–9399. doi: 10.1073/pnas.94.17.9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson R A, Watkins S C, Flynn J L. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J Immunol. 1997;159:635–643. [PubMed] [Google Scholar]
- 24.Jakob T, Walker P S, Krieg A M, Udey M C, Vogel J C. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J Immunol. 1998;161:3042–3049. [PubMed] [Google Scholar]
- 25.Jones S, Portnoy D A. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect Immun. 1994;62:5608–5613. doi: 10.1128/iai.62.12.5608-5613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhn M, Goebel W. Molecular studies on the virulence of Listeria monocytogenes. Genet Eng. 1995;17:31–51. [PubMed] [Google Scholar]
- 27.Kuhn M, Goebel W. Responses by murine macrophages infected with Listeria monocytogenes crucial for the development of immunity of this pathogen. Immunol Rev. 1997;158:57–67. doi: 10.1111/j.1600-065x.1997.tb00992.x. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn M, Goebel W. Host cell signalling during Listeria monocytogenes infection. Trends Microbiol. 1998;6:11–14. doi: 10.1016/S0966-842X(97)01139-6. [DOI] [PubMed] [Google Scholar]
- 29.Kuhn M. The microtubule depolymerizing drugs nocodazole and colchicine inhibit the uptake of Listeria monocytogenes by P388D1 macrophages. FEMS Microbiol Lett. 1998;160:87–90. doi: 10.1111/j.1574-6968.1998.tb12895.x. [DOI] [PubMed] [Google Scholar]
- 30.Larsson M, Majeed M, Ernst J D, Magnusson K E, Stendahl O, Forsum U. Role of annexins in endocytosis of antigens in immature human dendritic cells. Immunology. 1997;92:501–511. doi: 10.1046/j.1365-2567.1997.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine T P, Chain B M. Endocytosis by antigen presenting cell: dendritic cells are as endocytically active as other antigen presenting cells. Proc Natl Acad Sci USA. 1992;89:8342–8346. doi: 10.1073/pnas.89.17.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellman I, Turley S J, Steinman R M. Antigen processing for amateurs and professionals. Trends Cell Biol. 1998;8:231–237. doi: 10.1016/s0962-8924(98)01276-8. [DOI] [PubMed] [Google Scholar]
- 33.Mengaud J, Ohayon H, Gounon P, Mege R-M, Cossart P. E-cadherin is a receptor for internalin, a surface protein required for entry of Listeria monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 34.Ni K, O'Neill H C. The role of dendritic cells in T cell activation. Immunol Cell Biol. 1997;75:223–230. doi: 10.1038/icb.1997.35. [DOI] [PubMed] [Google Scholar]
- 35.Raffelsbauer D, Bubert A, Engelbrecht F, Scheinpflug J, Simm A, Hess J, Kaufmann S H, Goebel W. The gene cluster inlC2DE of Listeria monocytogenes contains additional new internalin genes and is important for virulence in mice. Mol Gen Genet. 1998;260:144–158. doi: 10.1007/s004380050880. [DOI] [PubMed] [Google Scholar]
- 36.Reddy A, Sapp M, Feldman M, Subklewe M, Bhardwaju N. A monocyte conditioned medium is more effective than defined cytokines in mediating the terminal maturation of human dendritic cells. Blood. 1997;90:3640–3646. [PubMed] [Google Scholar]
- 37.Reis e Sousa C, Sher A, Kaye P. The role of dendritic cells in the induction and regulation of immunity to microbial infection. Cur Opin Immunol. 1999;11:392–399. doi: 10.1016/S0952-7915(99)80066-1. [DOI] [PubMed] [Google Scholar]
- 38.Rescigno M, Granucci F, Citterio S, Foti M, Ricciardi-Castagnoli P. Coordinated events during bacteria-induced DC maturation. Immunol Today. 1999;20:200–203. doi: 10.1016/s0167-5699(98)01427-3. [DOI] [PubMed] [Google Scholar]
- 39.Ruhland G J, Fiedler F. Occurrence and structure of lipoteichoic acids in the genus Staphylococcus. Arch Microbiol. 1990;154:375–379. doi: 10.1007/BF00276534. [DOI] [PubMed] [Google Scholar]
- 40.Schnorr J-J, Xanthakos S, Keikavoussi P, Kämpgen E, Ter Meulen V, Schneider-Schaulies S. Induction of maturation of human blood dendritic cell precursors by measles virus is associated with immunosuppression. Proc Natl Acad Sci USA. 1997;94:5326–5331. doi: 10.1073/pnas.94.10.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheehan B, Kocks C, Dramsi S, Gouin E, Klarsfeld A D, Mengaut J, Cossart P. Molecular and genetic determinants of the Listeria monocytogenes infectious process. Curr Top Microbiol Immunol. 1994;192:187–216. doi: 10.1007/978-3-642-78624-2_9. [DOI] [PubMed] [Google Scholar]
- 42.Steinman R M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 43.Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today. 1998;19:89–97. doi: 10.1016/s0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- 44.Wan Y, Bramson J, Carter R, Graham F, Gauldie J. Dendritic cells transduced with an adenoviral vector encoding a model tumor-associated antigen for tumor vaccination. Hum Gene Ther. 1997;8:1355–1363. doi: 10.1089/hum.1997.8.11-1355. [DOI] [PubMed] [Google Scholar]
- 45.Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, Zimmermann V S, Davoust J, Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou L J, Tedder T F. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154:3821–3835. [PubMed] [Google Scholar]