Abstract
Objective:
Surgical site infection (SSI) after open lower extremity revascularization is a relatively common complication associated with increased hospital stays, graft infection, and in severe cases, graft loss. Although the short-term effects of SSI can be significant, it has not been considered a complication that increases major limb amputation. The purpose of this study was to determine the association of SSI with outcomes in patients undergoing surgical revascularization for peripheral arterial disease.
Methods:
We analyzed nationwide Vascular Quality Initiative (VQI) data from the infrainguinal bypass module from 2003 to 2017. The cohort included adults who underwent open lower extremity bypass for symptomatic peripheral arterial disease and had at least one follow-up record. Weighted Kaplan-Meier curves and Cox proportional hazards regression were used to assess the association between SSI and 1-year mortality and major limb amputation. Inverse-probability of treatment weights were used to account for differences in demographics and patient characteristics and allow for ‘adjusted’ Kaplan-Meier curves.
Results:
The analysis included 21,639 patients, and 1155 (5%) had a reported SSI within 30 days of surgery. Patients with SSI were more likely be obese (41% vs 30%), but there were no other clinically relevant differences between demographics, comorbidities, and bypass details. After weighting, patients with SSI were almost twice as likely to undergo major amputation by 6 months (hazard ratio, 1.84; 95% confidence interval, 1.07-3.17). The association with SSI and increased amputation rates persisted at 1 year. The association of SSI on amputation was no different based on preoperative Rutherford class (P = .91). The association between SSI and 1-year mortality rate was not statistically significant (hazard ratio, 1.15; 95% confidence interval, 0.91-1.46).
Conclusions:
SSI is more common in obese patients, and patients who develop an SSI are observed to have a significantly increased rate of limb amputation after open lower extremity revascularization.
Keywords: Lower extremity bypass, Major limb amputation, Surgical site infection
Surgical site infection (SSI) is a well-documented complication in vascular surgery. Patients undergoing lower extremity bypass (LEB) are at a particularly high risk for surgical wound complications, given their underlying comorbidities and frequent utilization of the groin for surgical exposure.1 Rates of SSIs after infrainguinal bypass have been previously characterized in several large database reviews, ranging from 4.8% at the time of hospital discharge2 to 12.8% within 30 days postoperatively.1
Patient factors that have been shown to be associated with increased risk of SSI include obesity and metabolic syndrome.3 Many operative factors are also associated with SSI after LEB, including betadine-only prep, increased operative time, incisional hematoma, higher transfusion requirement, and adjunctive femoral endarterectomy.4 Although these perioperative variables associated with development of SSI have been identified in prior studies, the majority of research focusing on outcomes related to SSI has focused mainly on short-term, or 30-day, outcomes.5
SSI after LEB is a relatively common complication associated with significant short-term morbidity including increased risk of wound disruption, acute renal failure, pneumonia, and cardiac arrest5; however, the long-term outcomes are not well-described. Therefore, the purpose of this study was to examine a national quality registry to determine the association of SSI with 1-year outcomes in patients undergoing surgical revascularization for symptomatic peripheral arterial disease (PAD) with the hypothesis that there are likely underappreciated long-term outcomes in patients who develop SSI after LEB. Specifically, we chose to evaluate association of SSI with limb loss, graft patency, and mortality at 1 year.
METHODS
Data source.
This study utilized data from the Society for Vascular Surgery Vascular Quality Initiative (VQI) registry from the years 2003 through 2017 to evaluate the association of SSI with 1-year outcomes following infrainguinal lower extremity bypass. The VQI registry contains information on demographic traits, comorbidities, imaging studies, peri-procedural details, in-hospital complications, and long-term (9-21 months) outcomes. The VQI Research Advisory Committee approved this study, and the University of North Carolina Institutional Review Board waived patient informed consent as only de-identified data was used.
Study design and patient population.
We performed a secondary analysis of prospectively collected nationwide data from the infrainguinal bypass module from 2003 through 2017. The study cohort was comprised of adult patients (≥18 years old) who underwent lower extremity bypass operations for symptomatic PAD (n = 37,880). Symptomatic PAD was defined as those patients who had claudication, rest pain, or tissue loss. Therefore, patients were excluded if they did not have an indication of claudication, rest pain, or tissue loss for at least one limb (n = 5845) or if the limb being treated was not the limb diagnosed with PAD (n = 1280). Patients were also excluded if they did not have at least one long-term follow-up record in the VQI (n = 6947). Thirty-day SSI was defined as having either a postoperative SSI or SSI within 30 days of discharge. SSI included exposure sites and vein harvest sites (ipsilateral, contralateral, or arm). Patients missing data on postoperative SSI were also excluded (n = 32). All-cause mortality, primary patency, and postoperative amputation was reported to VQI. Follow-up data on primary patency was missing for 22% of patients (n = 5189), and patients with no record of major amputation, which was defined as above- or below-knee amputation, were assumed to have not undergone one. Long-term outcome data was obtained by merging the index infrainguinal dataset with the long-term follow-up VQI infrainguinal bypass dataset.
Statistical analysis.
χ2 and Wilcoxon tests were used to compare patient demographics and surgical characteristics between patients with and without a 30-day, postoperative SSI. Weighted Kaplan-Meier curves and Cox proportional hazards regression were used to assess the association between postoperative SSI and 1-year patency (primary vs primary assist/secondary/occluded) and 1-year all-cause mortality. For primary patency, patients were followed until loss of primary patency, death, 365 days after surgery, or the date of their last follow-up visit where patency was noted, whichever came first. Similarly, for major amputation, patients were followed until the date of their first major amputation, death, 365 days after surgery, or the date of their last follow-up visit, whichever came first. Inverse-probability of treatment weights (IPTW) were used to account for differences in demographics and patient characteristics and allow for ‘adjusted’ Kaplan-Meier curves to be generated.
Briefly, each patient’s propensity (ie, probability) of having a postoperative SSI was predicted using multivariable logistic regression, adjusting for patient age, sex, race, comorbidities (diabetes, preoperative smoking, hypertension, coronary artery disease, heart failure, chronic obstructive pulmonary disease, end-stage renal disease, obesity), indication (claudication, rest pain, tissue loss), preoperative creatinine, hemoglobin, statin use and aspirin use, graft artery recipient, material used for graft, and type of skin preparation used. Age, preoperative creatinine, and preoperative hemoglobin were treated as restricted quadratic splines. These propensity scores were then scaled by the overall probability of postoperative SSI in the cohort. Correlation between surgeries done at the same center was also accounted for using a cluster-specific random effect. Robust sandwich estimators were used to account for the weighting and random effects.
All analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
A total of 23,776 patients who underwent infrainguinal LEB for symptomatic PAD with long-term follow-up data were available for analysis. The majority of patients who underwent infrainguinal LEB were white (79.5%) and male (67.6%), with an average age of 66 years old. Only a small portion of the patients that underwent LEB in this cohort developed an SSI (5%; n = 1155). When comparing those patients who developed a postoperative SSI vs those that did not (Table I), there were no clinically meaningful differences between demographics, comorbidities, and perioperative factors between the groups. For both groups, hypertension was prevalent (88% vs 89%). There were no differences in the prevalence of diabetes, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, end-stage renal disease, or smoking history. Obesity was more common in those with SSI compared with no SSI (41% vs 30%). Additionally, there was no difference in type of skin preparation utilized or use of prosthetic conduit between the groups.
Table I.
Characteristics of patients undergoing infra-inguinal bypass by postoperative surgical site infection (SSI) status, before and after inverse probability of treatment weighting (IPTW)
| Crude/unweighted |
IPTWa |
|||||
|---|---|---|---|---|---|---|
| Postoperative SSI | No SSI | SDb | Postoperative SSI | No SSI | SDb | |
| Total, No. | 1155 | 22,621 | – | 985 | 19,288 | – |
|
| ||||||
| Male | 730 (63) | 15,340 (68) | 0.10 | 677 (69) | 13,158 (68) | 0.01 |
|
| ||||||
| Age, years | 66 (59-73) | 66 (59-74) | 0.07 | 66 (59-74) | 66 (59-74) | 0.01 |
|
| ||||||
| Race | ||||||
|
| ||||||
| White | 932 (84) | 17,970 (82) | 0.05 | 821 (83) | 16,045 (83) | 0.01 |
|
| ||||||
| Black/African American | 167 (15) | 3696 (17) | 0.05 | 152 (15) | 3039 (16) | 0.01 |
|
| ||||||
| Otherc | 12 (1) | 246 (1) | 0.00 | 11 (1) | 203 (1) | 0.00 |
|
| ||||||
| Comorbidities | ||||||
|
| ||||||
| Diabetes | 634 (55) | 11,292 (50) | 0.10 | 475 (48) | 9269 (48) | 0.00 |
|
| ||||||
| Hypertension | 1011 (88) | 20,043 (89) | 0.03 | 871 (89) | 16,989 (88) | 0.01 |
|
| ||||||
| Coronary artery disease | 372 (32) | 7009 (31) | 0.03 | 308 (31) | 5874 (30) | 0.02 |
|
| ||||||
| Congestive heart failure | 199 (17) | 3736 (17) | 0.02 | 152 (15) | 2898 (15) | 0.01 |
|
| ||||||
| COPD | 333 (29) | 5889 (26) | 0.06 | 255 (26) | 5071 (26) | 0.01 |
|
| ||||||
| ESRD | 87 (8) | 1363 (6) | 0.06 | 8 (1) | 162 (1) | 0.01 |
|
| ||||||
| Obesityd | 473 (41) | 6789 (30) | 0.23 | 305 (31) | 5953 (31) | 0.00 |
|
| ||||||
| Smoking status | ||||||
|
| ||||||
| Current | 462 (40) | 9292 (41) | 0.02 | 411 (42) | 8212 (43) | 0.02 |
|
| ||||||
| Former | 517 (45) | 9953 (44) | 0.02 | 441 (45) | 8454 (44) | 0.02 |
|
| ||||||
| Never | 176 (16) | 3360 (15) | 0.01 | 132 (13) | 2615 (14) | 0.00 |
|
| ||||||
| Indication | ||||||
|
| ||||||
| Claudication | 306 (26) | 7061 (31) | 0.10 | 319 (32) | 6260 (32) | 0.00 |
|
| ||||||
| Rest pain | 302 (26) | 6107 (27) | 0.02 | 269 (27) | 5333 (28) | 0.01 |
|
| ||||||
| Tissue loss | 547 (47) | 9453 (42) | 0.11 | 396 (40) | 7695 (40) | 0.01 |
|
| ||||||
| Elective admission | 908 (79) | 18,611 (82) | 0.09 | 813 (83) | 15,970 (83) | |
|
| ||||||
| Creatinine, mg/dL | 1.0 (0.8-1.2) | 1.0 (0.8-1.2) | 0.00 | 1.0 (0.8-1.2) | 1.0 (0.8-1.2) | 0.01 |
|
| ||||||
| Hemoglobin, g/dL | 12.1 (10.2-13.9) | 12.5 (10.8-14.0) | 0.12 | 12.7 (10.9-14.1) | 12.6 (10.9-14.1) | 0.00 |
|
| ||||||
| Preoperative medicationse | ||||||
|
| ||||||
| ASA | 842 (75) | 16,396 (75) | 0.01 | 740 (75) | 14,488 (75) | 0.00 |
|
| ||||||
| Statins | 862 (76) | 16,410 (74) | 0.05 | 730 (74) | 14,311 (74) | 0.00 |
|
| ||||||
| Below-knee graft | 855 (74) | 16,105 (71) | 0.06 | 708 (72) | 13,650 (71) | 0.03 |
|
| ||||||
| Prosthetic graft | 414 (36) | 9327 (41) | 0.11 | 403 (41) | 7876 (41) | 0.00 |
|
| ||||||
| Skin preparation | ||||||
|
| ||||||
| Chlorhexidine alone | 232 (20) | 4401 (20) | 0.02 | 192 (20) | 3819 (20) | 0.01 |
|
| ||||||
| Alcohol alone | <11 | 39 (<1) | 0.02 | <11 | 30 (<1) | 0.01 |
|
| ||||||
| Iodine alone | 60 (5) | 1283 (6) | 0.02 | 58 (6) | 1093 (6) | 0.01 |
|
| ||||||
| Chlorhexidine and iodine | 32 (3) | 542 (2) | 0.02 | 23 (2) | 461 (2) | 0.00 |
|
| ||||||
| Chlorhexidine and alcohol | 713 (62) | 13,990 (62) | 0.00 | 611 (62) | 11,980 (62) | 0.00 |
|
| ||||||
| Iodine and alcohol | 70 (6) | 1572 (7) | 0.04 | 68 (7) | 1312 (7) | 0.00 |
|
| ||||||
| Chlorhexidine, iodine, and alcohol | 41 (4) | 706 (3) | 0.02 | 29 (3) | 601 (3) | 0.01 |
ASA, acetylsalicylic acid (aspirin); COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; SD, standardized difference.
Data are presented as number (%) or median (interquartile range).
Multivariable logistic regression was used to estimate the probability of having a postoperative SSI, adjusting for gender, age, race, comorbidities, smoking status, indication, admission type, creatinine, hemoglobin, preoperative medications, graft type and location, and skin preparation; numbers are rounded for easier interpretation.
Absolute standardized difference; an absolute difference >0.20 was considered meaningfully different.
Includes Asian, American Indian/Alaskan Native, Native Hawaiian/Pacific Islander, and multiple races; race was collapsed due to small cell sizes.
Patients with a body mass index ≥30 were classified as obese, as per Centers for Disease Control cutoffs.
Taken within 36 hours of surgery; patients with medical contraindications are excluded (ASA n = 798; statins n = 419), and noncompliant patients are considered not having taken the medication.
Regarding primary and secondary patency, unadjusted analysis showed a 43% and 22% increased risk of loss of primary/secondary patency for patients with SSI at 6- and 12-month intervals respectively (6-month: hazard ratio [HR], 1.43; 95% confidence interval [CI], 1.08-1.90; 12-month: HR, 1.22; 95% CI, 1.05-1.41) (Table II). After IPTW, the observed difference was again no longer statistically significant, but did again show a trend towards higher risk for loss of primary/secondary patency for patient with SSI (6-month: HR, 1.34; 95% CI, 0.98-1.86; 12-month: HR, 1.14; 95% CI, 0.96-1.35).
Table II.
Crude and weighted association between postoperative surgical site infection (SSI) and primary assist or secondary patency
| Loss of primary patency, % |
||||
|---|---|---|---|---|
| SSI | No SSI | HR (95% CI) | P value | |
| Crude | ||||
|
| ||||
| 6-month | 6.2 | 3.6 | 1.43 (1.08-1.90) | .01 |
|
| ||||
| 12-month | 24.8 | 20.4 | 1.22 (1.05-1.41) | .01 |
|
| ||||
| IPTWa | ||||
|
| ||||
| 6-month | 5,6 | 3.4 | 1.34 (0.98-1.86) | .07 |
|
| ||||
| 12-month | 23.1 | 20.1 | 1.14 (0.96-1.35) | .13 |
CI, Confidence interval; HR, hazard ratio; IPTW, inverse probability of treatment weights.
IPTW were calculated using logistic regression; models included patient age, sex, race, comorbidities, indication, preoperative creatinine, hemoglobin, statin use and aspirin use, admission type, graft artery recipient, material used for graft, and type of skin preparation used.
Thirty-day incidence of SSI was also associated with a significantly higher risk of major limb amputation at 6- and 12-month intervals for both crude and weighted analyses. After weighting, SSI was associated with an 86% increased risk of major limb amputation at 6-months and a 49% increased risk of major limb amputation at 1 year (6-month: HR, 1.84; 95% CI, 1.07-3.17; 12-month: HR, 1.44; 95% CI, 1.10-1.90) (Table III). Fig 1 A and B shows similar curves for crude and IPTW incidence for major amputation, with rates increasing substantially for both groups (SSI and no SSI) at approximately postoperative day 280. The association between SSI and major amputation was consistent across indication (claudication: HR, 1.20; 95% CI, 0.49-2.93; tissue loss: HR, 1.47; 95% CI, 1.04-2.08; rest pain: HR, 1.48; 95% CI, 0.90-2.44; P = .91).
Table III.
Crude and weighted association between postoperative surgical site infection (SSI) and major amputation
| Amputation, % |
||||
|---|---|---|---|---|
| SSI | No SSI | HR (95% CI) | P value | |
| Crude | ||||
|
| ||||
| 6-month | 2.2 | 0.9 | 2.20 (1.43-3.38) | .0004 |
|
| ||||
| 12-month | 8.1 | 5.4 | 1.55 (1.22-1.96) | .0003 |
|
| ||||
| IPTWa | ||||
|
| ||||
| 6-month | 1.6 | 0.8 | 1.84 (1.07-3.17) | .03 |
|
| ||||
| 12-month | 7.1 | 5.0 | 1.44 (1.10-1.90) | .008 |
CI, Confidence interval; HR, hazard ratio; IPTW, inverse probability of treatment weights.
IPTW were calculated using logistic regression; models included patient age, sex, race, comorbidities, indication, preoperative creatinine, hemoglobin, statin use and aspirin use, admission type, graft artery recipient, material used for graft, and type of skin preparation used.
Fig 1.
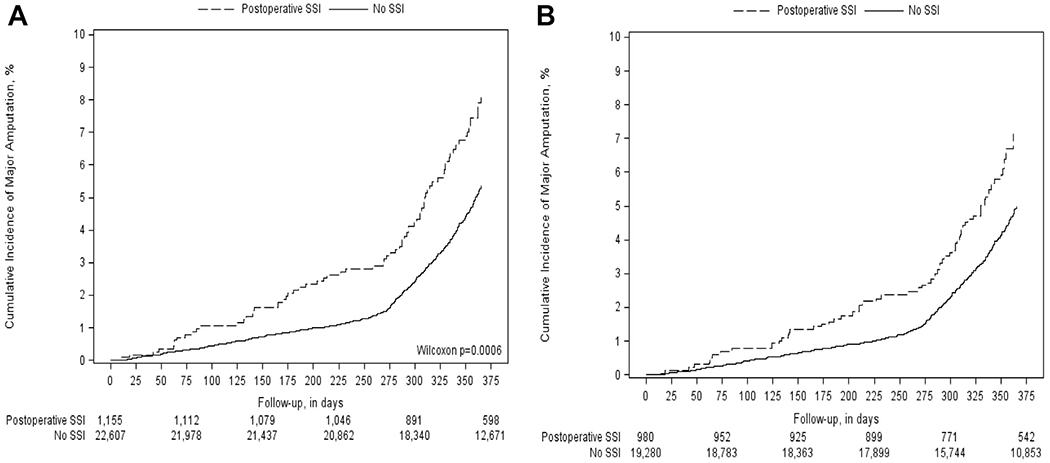
Figure depicts the crude (A) and weighted (B) incidence of major amputation stratified by postoperative surgical site infection (SSI) status. The crude and weighted cumulative incidence demonstrate an increased rate of major amputation in those patients that develop an SSI.
Prior to accounting for differences in patient demographics and clinical characteristics, there was a crude association of SSI with all-cause mortality at both 6- and 12-month intervals, with a statistically significant higher risk of mortality at 1 year for patients with a SSI as compared with no SSI (HR, 1.23; 95% CI, 1.01-1.50) (Table IV, Fig 2, A). After cohort weighting with IPTW, the association of SSI with all-cause mortality was no longer statistically significant (Table IV, Fig 2, B).
Table IV.
Crude and weighted association between postoperative surgical site infection (SSI) and all-cause mortality
| Mortality, % |
||||
|---|---|---|---|---|
| SSI | No SSI | HR (95% CI) | P value | |
| Crude | ||||
|
| ||||
| 6-month | 5.6 | 4.5 | 1.28 (0.99-1.65) | .06 |
|
| ||||
| 12-month | 9.2 | 7.5 | 1.23 (1.01-1.50) | .04 |
|
| ||||
| IPTWa | ||||
|
| ||||
| 6-month | 4.4 | 3.8 | 1.16 (0.85-1.58) | .36 |
|
| ||||
| 12-month | 7.2 | 6.5 | 1.13 (0.89-1.44) | .32 |
CI, Confidence interval; HR, hazard ratio; IPTW, inverse probability of treatment weights.
IPTW were calculated using logistic regression; models included patient age, sex, race, comorbidities, indication, preoperative creatinine, hemoglobin, statin use and aspirin use, admission type, graft artery recipient, material used for graft, and type of skin preparation used.
Fig 2.
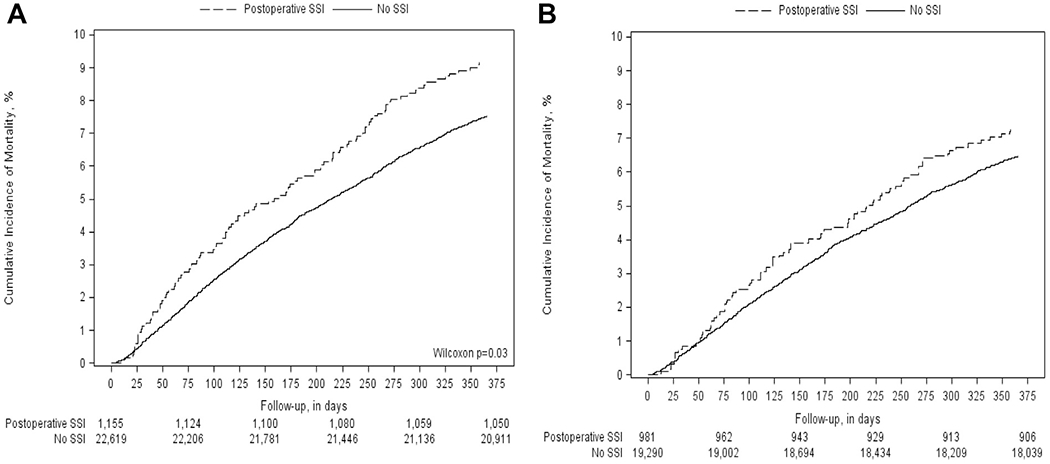
Figure demonstrates the crude (A) and weighted (B) incidence of all-cause mortality with respect to development of a postoperative surgical site infection (SSI).
DISCUSSION
Although SSIs after LEB have been shown to increase the incidence of short-term complications like acute renal failure, prolonged ventilation, and mortality within 30 days of the operation,5 there is a paucity of information on the potential impact of SSI on long-term patient outcomes. We hypothesized that there are likely underappreciated long-term patient outcomes associated with the development of SSI. We utilized the VQI registry to analyze 23,776 patients undergoing LEB and the effects of 30-day SSI on 6-month and 12-month patient outcomes. SSI was found to have an increased risk of amputation and decrease in primary and secondary patency.
SSI is a well-established cause of postoperative morbidity in the in the LEB population.5,6 Previous studies have focused on identification of preoperative risk factors and the 30-day outcomes associated with SSI after LEB using National Surgical Quality Improvement Program (NSQIP) and Department of Veterans Affairs databases.1,2,5,7,8 However, the VQI and NSQIP database registries have been compared and shown to capture different populations and outcomes in the LEB population.1 In our study, we found a 5% incidence of SSI, similar to a large review of the VQI database examining LEB from 2003 through 2012 that found an in-hospital SSI rate of 4.8%.2 Interestingly, the largest review of the NSQIP database found a wound infection rate of 11.1% at 30 days.1 Several randomized control trials describe even higher rates of SSI among the LEB cohort. The Project of Ex-Vivo Vein Engineering via Transfection (PREVENT III) trial cited an overall 30-day wound complication rate of 39.0% and wound infection rate of 20.2% among 1404 patients undergoing LEB.9 The Bypass vs Angioplasty in Severe Ischemia of the Leg (BASIL) trial reported a 22.8% and 15.6% in-hospital and 30-day wound infection rate.2,10 These differences in SSI rates are likely due to differences in patient populations and differing definitions of postoperative infection.1 When compared with previous large prospective randomized trials, studies in the VQI or NSQIP have been shown to underestimate the incidence of SSI. Diagnostic criteria for all infections in the VQI database include culture positivity, purulent drainage, intentional opening of the incision for infection, or declaration by the surgeon as to the presence of an SSI. Incisions that are opened and found to be culture-negative are not classified as an SSI. Incisions that are not opened or cultured but given antibiotics for presumed superficial infections are not necessarily classified as SSI. This may account for the lower rates of infection when compared with previous randomized trials.
Previous investigation in the NSQIP database has identified the increased risk of developing SSI in obese patients or those with metabolic syndrome.3,6 Our study of the VQI parallels this data as patients with SSI were more likely be obese (41% vs 30%; absolute standardized difference, 0.23). Within our VQI cohort, there were no other clinically relevant differences in demographics, comorbidities, or bypass details that differed between the SSI and no SSI groups. Previous studies in the VQI population have demonstrated an increase in SSI in patients with increased transfusion requirements, longer procedure time, and adding an adjunctive femoral endarterectomy.2,4
Our study demonstrates increased risk of loss of graft patency and major amputation in patients with SSI after LEB at 1 year, although it makes no distinction based on bypass conduit. Further investigation into the causative relationship of surgical site infection, graft failure, and major amputation is needed to determine the best method to interrupt this cycle. These data suggest the heightened follow-up is merited even after patients have recovered from the SSI.
Previous research in the field of SSI has focused on infection prevention. This includes previous analysis of perioperative antibiotic regimens and surgical skin preparation leading to SSI bundles.11–13 There was no difference in SSI with different surgical site preparation methods in our cohort, which is likely attributed to the overall low incidence of SSI and the overwhelming rate of chlorhexidine skin preparation, which has previously been identified as having the lowest SSI rate.11,14,15 Additionally, management of groin incisions, which are frequently associated with LEB operations, has gained attention. Augmentation of surgical closure with an incisional wound vacuum over the closed groin incision has offered some hope in decreasing the rate of groin SSI in high-risk patients. In a single-center retrospective review, among patients receiving a vacuum-assisted wound closure, only 6% developed an SSI in comparison with 30% of patients receiving a traditional closure.12 A prospective single-center study of 504 patients found the same significant reduction in SSI utilizing a negative-pressure wound dressing (9.8%) vs 19.0% with standard dressing. Interestingly, in this study there was also a significant decrease in mortality seen in patients treated with negative-pressure dressings (5.8% vs 11.2%).13 The focus of future investigation must center on prevention of SSI as postoperative infection can impact both short-and long-term patient outcomes. This includes optimizing risk factors, identification of high-risk patients, and possibly changes in traditional wound closure to decrease the rate of SSI.13,16
Although the correlation of SSI with worse 1-year outcomes is clearly seen in our research, the underlying causation for this association is uncertain. We believe there are two potential mechanisms for the correlation between SSI and worse 1-year outcomes. It is possible that the increased rate of SSI is an indicator of greater overall systemic illness or frailty that we are unable to measure in this database. It is also possible that SSI causes downstream complications that lead to decreased patency and lower rates of limb preservation at 1 year. Additional research is needed to elucidate the causal pathways between the short-term complication and the long-term effects.
This study has several limitations. Certainly, when compared with prospective trials, our study underestimates the SSI rates. This is likely due to the strict criteria used to define SSI and loss of patient data if their SSI was treated outside of a VQI center after discharge. We are also limited in the generalizability of our SSI estimates. The VQI registry does not include all centers where LEB is performed and is comprised of mostly tertiary care centers, which may not be representative of all practice locations. Also, due to the observational nature of our study the results of our study do not imply causality, only the association between SSI and long-term outcomes. There is also no reporting on why the bypass procedures were performed, as the VQI includes bypass procedures performed for multiple indications, including chronic limb-threatening ischemia as well as claudication, and possibly a more significant burden of disease could be responsible for the increased SSI rate.
Although these limitations are associated with database reviews, this study represents a real-world, multi-institutional (community and academic practices) assessment of SSI rates and the association between SSI and long-term patient outcomes among those undergoing LEB. The incidence of SSI in these patients and subsequent consequences would likely serve as a benchmark for surgeons across multiple practice settings as the follow-up and rate of complications seen in the VQI should be reflected in clinical practice. These findings reinforce the need for continued research into the field of SSI prevention. Given the significant negative outcomes seen in patients who develop an SSI and the possible effect that SSI has on these outcomes, a high degree of suspicion and prompt intervention could minimize this impact.
CONCLUSIONS
SSI is a well-known complication in patients undergoing lower extremity bypass. In addition to the known short-term consequences of SSI, there are possible long-term sequelae affecting patient outcomes. In this VQI analysis of LEB, patients that developed an SSI at 30 days had worse 1-year outcomes, including increased risk of major limb amputation and graft loss. Future investigation should focus on better identification of high-risk patients and mitigation of their risk factors, with attention to the role of increased follow-up and surveillance. Overall reduction in incisional complications and SSI in LEB patients should be aggressively pursued, given the associated long-lasting negative outcomes.
ARTICLE HIGHLIGHTS.
Type of Research: Retrospective, multi-institutional national quality registry cohort analysis
Key Findings: Surgical site infection (SSI) occurred in 5% of patients undergoing lower extremity bypass operations in this Vascular Quality Initiative analysis. Patients that developed SSI were more likely to be obese and to undergo major limb amputation at 6 and 12 months.
Take Home Message: SSI after open lower extremity revascularization almost doubles the rate of major limb amputation.
Author conflict of interest:
Dr. Paula Strassle performed this work while employed by the University of North Carolina; however, she is now supported by the Division of Intramural Research, National Institute of Minority Health and Health Disparities, National Institutes of Health. The contents and views in this manuscript are those of the authors and should not be construed to represent the views of the National Institutes of Health.
Footnotes
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
REFERENCES
- 1.Johnston LE, Robinson WP, Tracci MC, Kern JA, Cherry KJ, Kron IL, et al. Vascular Quality Initiative and National Surgical Quality Improvement Program registries capture different populations and outcomes in open infrainguinal bypass. J Vasc Surg 2016;64:629–37. [DOI] [PubMed] [Google Scholar]
- 2.Kalish JA, Farber A, Homa K, Trinidad M, Beck A, Davies MG, et al. ; Society for Vascular Surgery Patient Safety Organization Arterial Quality Committee. Factors associated with surgical site infection after lower extremity bypass in the Society for Vascular Surgery (SVS) Vascular Quality Initiative (VQI). J Vasc Surg 2014;60:1238–46. [DOI] [PubMed] [Google Scholar]
- 3.Arnaoutakis DJ, Selvarajah S, Mathioudakis N, Black JH 3rd, Freischlag JA, Abularrage CJ. Metabolic syndrome reduces the survival benefit of the obesity paradox after infrainguinal bypass. Ann Vasc Surg 2014;28:596–605. [DOI] [PubMed] [Google Scholar]
- 4.Soden PA, Zettervall SL, Shean KE, Deery SE, Kalish JA, Healey CT, et al. ; Vascular Study Group of New England (VSGNE). Effect of adjunct femoral endarterectomy in lower extremity bypass on perioperative and 1-year outcomes. J Vasc Surg 2017;65:711–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peacock MR, Shah NK, Farber A, Lee SY, Kalish JA, Rybin D, et al. Index complications predict secondary complications after infrainguinal lower extremity bypass for critical limb ischemia. J Vasc Surg 2017;65:1344–53. [DOI] [PubMed] [Google Scholar]
- 6.Wound infection after infrainguinal bypass operations: multivariate analysis of putative risk factors. Surg Infect (Larchmt) 2000;1:257–63. [DOI] [PubMed] [Google Scholar]
- 7.Bluemn EG, Flahive JM, Farber A, Bertges DJ, Goodney PP, Eldrup-Jorgensen J, et al. ; Vascular Study Group of New England. Analysis of thirty-day readmission after infrainguinal bypass. Ann Vasc Surg 2019;61:34–47. [DOI] [PubMed] [Google Scholar]
- 8.Brothers TE, Robison JG, Elliott BM. Predictors of prosthetic graft infection after infrainguinal bypass. J Am Coll Surg 2009;208:557–61. [DOI] [PubMed] [Google Scholar]
- 9.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, et al. PREVENT III Investigators. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg 2006;43:742–51; discussion: 751. [DOI] [PubMed] [Google Scholar]
- 10.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. ; BASIL trial participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005;366:1925–34. [DOI] [PubMed] [Google Scholar]
- 11.Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database Syst Rev 2015:CD004985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matatov T, Reddy KN, Doucet LD, Zhao CX, Zhang WW. Experience with a new negative pressure incision management system in prevention of groin wound infection in vascular surgery patients. J Vasc Surg 2013;57:791–5. [DOI] [PubMed] [Google Scholar]
- 13.Benrashid E, Youngwirth LM, Guest K, Cox MW, Shortell CK, Dillavou ED. Negative pressure wound therapy reduces surgical site infections. J Vasc Surg 2020;71:896–904. [DOI] [PubMed] [Google Scholar]
- 14.Darouiche RO, Wall MJ Jr, Itani KM, Otterson MF, Webb AL, Carrick MM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med 2010;362:18–26. [DOI] [PubMed] [Google Scholar]
- 15.Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev 2006:CD004122. [DOI] [PubMed] [Google Scholar]
- 16.Bennett KM, Levinson H, Scarborough JE, Shortell CK. Validated prediction model for severe groin wound infection after lower extremity revascularization procedures. J Vasc Surg 2016;63:414–9. [DOI] [PubMed] [Google Scholar]


