Abstract
Enteropathogenic Escherichia coli (EPEC), a leading cause of diarrhea among infants in developing countries, induces dramatic alterations in host cell architecture that depend on a type III secretion system. EspB, one of the proteins secreted and translocated to the host cytoplasm via this system, is required for numerous alterations in host cell structure and function. To determine the role of EspB in virulence, we conducted a randomized, double-blind trial comparing the ability of wild-type EPEC and an isogenic ΔespB mutant strain to cause diarrhea in adult volunteers. Diarrhea developed in 9 of 10 volunteers who ingested the wild-type strain but in only 1 of 10 volunteers who ingested the ΔespB mutant strain. Marked destruction of the microvillous brush border adjacent to adherent organisms was observed in a jejunal biopsy from a volunteer who ingested the wild-type strain but not from two volunteers who ingested the ΔespB mutant strain. Humoral and cell-mediated immune responses to EPEC antigens were stronger among recipients of the wild-type strain. In addition, four of the volunteers who ingested the wild-type strain had lymphoproliferative responses to EspB. These results demonstrate that EspB is a critical virulence determinant of EPEC infections and suggest that EspB contributes to an immune response.
Enteropathogenic Escherichia coli (EPEC) strains cause serious diarrhea among infants in developing countries throughout the world (4). Because EPEC strains isolated from humans do not cause diarrhea in animals, EPEC pathogenicity and the role of EPEC virulence factors in disease can only be tested in volunteer studies (2, 5, 20, 21). During human infections, typical EPEC strains display two phenotypes, localized adherence and the attaching-and-effacing effect, which are reproduced in tissue culture. Localized adherence is dependent upon a type IV fimbria known as the bundle-forming pilus, which is encoded by a cluster of fourteen genes on a large plasmid common to EPEC strains (28, 30). The attaching-and-effacing effect is characterized by profound changes in the architecture of the host cell, with loss of microvilli and accumulations of cytoskeletal proteins within a cup-like pedestal upon which the bacteria rest (17, 18).
All of the genes required for the attaching-and-effacing effect are encoded by a pathogenicity island known as the locus of enterocyte effacement (LEE) (23). The LEE can be divided into three regions. At one end are the espA, espB (formerly known as eaeB), and espD genes. These genes encode secreted proteins required for attaching and effacing (8, 15, 16). At the other end lie many of the genes encoding a type III secretion apparatus (11). These genes are similar to loci from other pathogens, including Salmonella enterica serotype Typhimurium, Shigella flexneri, and Yersinia enterocolitica, which are required for the secretion of proteins that interact with host cells. Mutations in the escV and escN genes within this region result in the inability to secrete EspA, EspB, and EspD and, consequently, in the inability to cause attaching and effacing lesions. Between the genes encoding the secretion apparatus and those encoding the secreted proteins lie the eae and tir genes (13, 14). The eae gene encodes intimin, a 94-kDa outer membrane protein required for intimate attachment of EPEC to epithelial cells and for full virulence in experiments with volunteers (5). The tir gene encodes the translocated intimin receptor, which is secreted via the type III secretion apparatus and targeted to the host cell membrane, where it serves as the receptor for intimin (14).
The EspB protein is central to EPEC interactions with cells in vitro. In the absence of EspB, no alterations in the cytoskeleton are observed, Tir does not become localized to the host cell membrane, and fluxes of inositol phosphate are not observed in infected cells (8, 14). EspB is also required for changes in short circuit current across polarized intestinal epithelial cells mounted in Ussing chambers and for membrane depolarization in isolated patch-clamped Caco-2 cells (3, 29). These in vitro effects may reflect the ion fluxes that result in diarrhea in vivo. EspB is also required for induction of NF-κB activation, for interleukin-8 secretion, for transepithelial migration of neutrophils, and for a decrement in transepithelial electrical resistance, all of which may contribute to diarrhea (26, 27, 36). Furthermore, EspB is translocated by EPEC into the host cell cytoplasm, suggesting the possibility of a direct role in host cell damage (19, 33, 35). Moreover, the cytoplasmic location of the EspB protein suggests that, following processing and presentation to lymphocytes in the context of major histocompatibility complex (MHC) class I molecules, EspB might elicit cell-mediated immune responses. Recently, EspB of a rabbit EPEC strain was found to be required for attaching and effacing lesion formation and disease (1). Thus, a large body of evidence suggests that EspB is a critical protein required for many of the effects of EPEC infection. However, the relevance of these studies to human infection has not yet been validated.
The purpose of this study was to determine the role of EspB in the pathogenesis of EPEC infection in volunteers by giving volunteers wild-type EPEC and an isogenic mutant deleted in espB.
MATERIALS AND METHODS
Volunteers.
Twenty healthy adult volunteers aged 18 to 40 years, who had given informed, written consent, were admitted to the research isolation ward of the Center for Vaccine Development, located at Kernan Hospital, Baltimore, Md. The protocol was reviewed and approved by the Institutional Review Board, University of Maryland, Baltimore. Volunteers underwent an appropriate health screen which included a medical history, a physical examination, an interview by a clinical psychologist, and laboratory tests. A stool specimen was examined for ova and for parasites and bacterial pathogens. To ensure comprehension of the study and to document that informed consent had been elicited, the volunteers had to pass a written examination before inoculation.
Volunteers were randomly assigned to one of two groups in a double-blind manner to receive one of the following: (i) 1010 CFU of wild-type EPEC O127:H6 strain E2348/69 (20) with sodium bicarbonate buffer or (ii) 1010 CFU of UMD864 (an isogenic mutant of E2348/69 deleted in espB) with sodium bicarbonate buffer. This strain was constructed by allelic exchange from strain E2348/69 to have an in-frame deletion of 74% of the espB coding region and was verified to be nonpolar by complementation (6).
Subjects were randomized in blocks of two, whereby one individual received mutant and the other received the wild-type strain, as generated by a random number function using Microsoft Excel. The randomization assignment was unknown to the nurses and investigators with the exception of one individual responsible for administering the challenge organisms. This individual did not participate in assessing any of the outcome measures.
Preparation and administration of the challenge strains.
A vial of the challenge strains was thawed and streaked onto eosin methylene blue (EMB) and Trypticase soy agar (TSA) plates. After 24 h of incubation at 37°C, 10 colonies that agglutinated with anti-O127 antiserum were picked and used to heavily inoculate each of six TSA plates for incubation at 37°C. After 20 h, the cultures were harvested with saline (0.85%), and dilutions were made in saline. The optical density of the suspension was adjusted to equal that corresponding to the desired number of CFU per milliliter in the inoculum. Inoculum size was quantitated by plating serial dilutions on TSA before and after challenge. A sample of the final inoculum was examined by using Gram stain and agglutinated with specific antiserum before challenge.
The EPEC challenge strains were administered by the oral route with NaHCO3. Two grams of NaHCO3 were dissolved in 5.0 oz of distilled water. Volunteers drank 4.0 oz of the NaHCO3 water; 1 min later the volunteers ingested the E. coli suspended in the remaining 1.0 oz of NaHCO3 water. Volunteers took no food or water for 90 min before and after challenge.
Definitions.
Diarrhea was defined as the passage of two or more unformed (grades 3 to 5) stools over a 48-h period that equaled or exceeded 200 g or a single stool of 300 g or greater. Fever was defined as the oral temperature of ≥100.8°F.
Clinical-bacteriologic surveillance.
Volunteers resided in a research isolation ward and were under close clinical supervision throughout the period of the study. They were instructed in techniques to prevent person-to-person spread of the challenge organisms, including compulsive handwashing and use of gloves when handling specimens. Investigators interviewed and examined the volunteers at least once daily. Any volunteer who developed diarrhea received oral glucose-electrolyte solution or, if necessary, intravenous fluid. Volunteers with diarrhea were treated with ciprofloxacin (500 mg every 12 hours) for 5 days as soon as the stool volume exceeded 3 liters. All other volunteers received ciprofloxacin (500 mg every 12 h) for 5 days beginning 3 days after challenge to eradicate carriage of the challenge strain.
All stools were examined, graded, recorded, and, if loose, weighed. The following five grades were used: grade 1, firm; grade 2, soft; grade 3, thick liquid; grade 4, opaque watery; and grade 5, rice water. Grades 1 and 2 are variations of normal stools, while grades 3 to 5 are considered abnormal.
All stools were cultured for the presence of the challenge organisms. The challenge strains were nalidixic acid resistant. All stools and rectal swabs (if no stool was passed) were plated onto EMB agar. Five nalidixic acid-resistant colonies with a typical E. coli metallic sheen were picked and tested for agglutination with homologous antiserum.
PCR of recovered isolates for espB.
One colony from each of the first two cultures from each volunteer that was positive for the challenge strain by agglutination with O127 antiserum was tested to confirm the presence or absence of the espB deletion allele. PCR for espB was performed on fresh colonies as previously described using primers upstream and downstream of espB (6).
Endoscopy with biopsies for confocal microscopy.
Four volunteers, two from each group, underwent esophagogastroduodenoscopy (EGD) before challenge and on day 3 after challenge. These volunteers were chosen before challenge on the basis of their willingness to undergo the endoscopy procedures. EGD was performed by an experienced endoscopist using topical anesthesia applied to the throat and “conscious anesthesia” using a combination of intravenous Versed and Demerol. Fasting volunteers had multiple biopsies of duodenal and jejunal mucosa obtained through the flexible endoscope. Biopsy samples were coded and fixed for 2 h at room temperature with freshly prepared 4% paraformaldehyde in phosphate-buffered saline (PBS; pH 7.2) containing 0.1% sodium azide. Samples were washed twice with PBS and then incubated in 20% sucrose in PBS overnight at 4°C. Tissue samples were embedded in OCT Tissuetek and then frozen in cold 2-methylbutane (−50 to −60°C). The frozen tissues were cut into 20-μm sections on a cryostat. These sections were incubated in 10% normal goat serum in PBS for 10 min and then permeabilized with 100 μl of permeabilization buffer (0.2% saponin–10% normal goat serum in PBS) overnight at 4°C in the presence of rabbit antisera raised against the wild-type EPEC challenge strain (whole bacteria, 1:200). Sections were washed three times in PBS and then incubated in the permeabilization buffer containing phalloidin-Texas Red (1:400; Molecular Probes, Eugene, Oreg.) and Alexa 488 goat anti-rabbit immunoglobulin G (IgG) conjugate (1:400; Molecular Probes) as the secondary antibody for anti-EPEC antibody. Tissue samples were washed twice in PBS and then mounted on coverslips. Stained sections were visualized by a confocal laser scanning microscope (MRC-600; Bio-Rad, Hercules, Calif.). The resulting scanned images were analyzed by NIH Image (National Institutes of Health, Bethesda, Md.), and processed images were exported to Adobe Photoshop (Adobe Systems, Inc., San Jose, Calif.) to assign different fluorophore images into individual RGB channels. The samples were analyzed without knowledge of the group to which the volunteers were assigned.
Antigen preparations.
EspB was purified from transformants of BL21(DE3) containing a pET28a-based vector that encodes a His6-tagged EspB fusion protein (9). Purification of the fusion protein was carried out as described by the manufacturer (Novagen). Histidine-tagged EspB was purified to near homogeneity as determined by Coomassie blue staining of sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. The lot of EspB used for serum and stool antibody responses and antibody-secreting-cell (ASC) responses contained 0.04 μg of lipopolysaccharide (LPS) per mg of EspB. The lot used for proliferation and cytokine measurements contained 10 μg of LPS per mg of EspB protein. To prepare whole-cell homogenates, overnight cultures of wild-type EPEC strain E2348/69 or ΔespB mutant strain UMD864 grown in LB medium were diluted 100-fold in Dulbecco modified Eagle medium and grown with shaking (225 rpm) at 37°C until the A600 was approximately 0.5. The actual concentrations of bacteria were determined by plate dilution. The bacteria were centrifuged (4,000 × g, 10 min, 4°C), washed twice with PBS containing 0.02% sodium azide, resuspended in the same buffer, and held overnight at 4°C. The following day, when the actual bacterial concentrations were determined by colony count, the concentrations of the bacterial suspensions were adjusted to 108 CFU/ml.
Humoral immune responses.
Blood was collected before and 6, 20, and 27 days after challenge to provide sera for measurement of serum antibodies. Sera were tested for antibodies to O127 LPS, EspB, and whole cells by enzyme-linked immunosorbent assay (ELISA) (5). A response was defined as a fourfold rise in titer after challenge. Whole blood was collected for ASC assays on days 0 and 6 after challenge. These cells are believed to reflect immunologic priming of the gut mucosal immune system. ASCs producing antibody against O127 LPS, EspB, and whole cells were measured by ELISPOT assays (34). An ASC response was defined as a number of ASC > the mean plus 3 standard deviations of the number before challenge.
On days 0, 7, 13, 20, and 27, whole stool was collected and processed for measurement of sIgA and IgG against O127 LPS, EspB, and whole cells. Briefly, a 10% supernatant of stool was prepared, protease inhibitors were added (10 μl/ml of stool supernatant), and the supernatants were assayed by ELISA. A fourfold rise in the amount of specific antibody was considered a response.
Cell-mediated immune (CMI) responses.
Peripheral blood mononuclear cells (PBMC) were isolated from the blood of volunteers before or 14 and 28 days after the ingestion of wild-type EPEC O127:H6 strain E2348/69 or the ΔespB isogenic mutant UMD864 strain by density gradient centrifugation over LSM; they were then resuspended in medium (AIM-V containing 50 μg of gentamicin per ml) and used immediately for measuring proliferative responses and gamma interferon (IFN-γ) production to E. coli antigens.
Phytohemagglutinin (PHA) (HA-17; Wellcome Diagnostics, Beckenham, United Kingdom) and tetanus toxoid (TT; Wyeth, Marietta, Pa.) were used to confirm the ability of PBMC obtained from immunized volunteers to proliferate to mitogenic stimulation and antigenic stimulation, respectively (31). Bovine serum albumin (BSA; Fraction V; Sigma) was used as a control protein in these studies.
Measurement of lymphoproliferative responses.
A standard lymphocyte proliferation assay was used to examine the responses to whole-cell E. coli homogenates, recombinant EspB, TT, BSA, or PHA (31). Briefly, 1.5 × 105 PBMC were added in triplicate to the wells of 96-well plates in AIM-V medium. Whole-cell E. coli homogenates, recombinant EspB, and BSA antigens were added to a final concentration of 10 μg/ml. PHA and TT were used at 2 μg/ml. The final volume was 200 μl/well. Cells were cultured for 2 days (for PHA) or 6 days (for antigens) at 37°C in an atmosphere containing 5% CO2, and 1 μCi of tritiated thymidine ([3H]TdR) per well was added. Plates were harvested 20 h after the addition of [3H]TdR on a Wallac cell harvester (Wallac, Gaithersburg, Md.), and incorporated [3H]TdR (reported as counts per minute [cpm]) was measured on a Wallac Trilux Microbeta counter. Net counts per minute were calculated by subtracting the [3H]TdR incorporation of cells in the absence of antigens from [3H]TdR incorporation in antigen-containing cultures for that same day and subject. A positive lymphocyte proliferative response was defined as a difference (P < 0.05, one-tailed t test) in mean net counts per minute between triplicate pre- and postvaccination samples stimulated with each individual antigen (i.e., wild-type strain E2348/69 homogenate, ΔespB strain UMD864 homogenate, recombinant EspB, or BSA). Three volunteers (all immunized with the UMD864 ΔespB mutant) that exhibited very high background proliferation in the absence of antigenic stimulation (i.e., in excess of the mean ± 3 standard errors of the thymidine incorporation of cultures from all volunteers in the absence of stimulants) were excluded from the analysis of CMI responses.
Measurement of IFN-γ by chemiluminescence ELISA.
PBMC were incubated in 24-well plates with wild-type strain E2348/69 homogenate, ΔespB strain UMD864 homogenate, recombinant EspB, or BSA at the concentrations indicated above. Supernatants were collected after 3 days of incubation and kept at −70°C until analyzed. Chemiluminescence ELISAs were performed as previously described (24). Briefly, 96-well black opaque plates were coated with anti-IFN-γ monoclonal antibody (PharMingen, San Diego, Calif.) diluted in 0.1 M sodium carbonate buffer (pH 9.6) and incubated overnight. Plates were subsequently washed and blocked with PBS containing 3% BSA (PBS-BSA). After a washing, serial twofold dilutions of supernatants and recombinant human IFN-γ (standard) (PharMingen) were incubated in duplicate wells overnight at 4°C. Biotinylated anti-IFN-γ monoclonal antibody (PharMingen) was added, and plates were incubated for 1 h at 37°C. Wells were then washed, and avidin-peroxidase diluted 1:400 in PBST-BSA was added for 1 h at 37°C. Chemiluminescence ELISA reagent was used as substrate. Chemiluminescence (relative light units/second) was measured on a 1450 Microbeta Trilux plate reader (Wallac). The concentration of IFN-γ in each sample was calculated by interpolation on the standard curves. The limit of sensitivity was 2 pg/ml. Net IFN-γ production levels (in picograms per milliliter) were calculated by subtracting the IFN-γ produced by PBMC in the absence of antigens from the IFN-γ produced in antigen-containing cultures for that same day and subject. A positive IFN-γ response was defined as a difference of more than 36 pg/ml in the levels of net IFN-γ production between pre- and postvaccination PBMC stimulated with each individual antigen. The 36-pg/ml cutoff represents the mean ± 3 standard errors of the levels of IFN-γ released by PBMC from all volunteers before immunization.
Statistical analysis.
Rates of clinical, microbiologic, and immune responses were compared by Fisher's exact tests. Means were compared by Wilcoxon signed-rank tests and t tests.
RESULTS
Clinical and microbiologic responses.
Nine (90%) of ten volunteers who ingested wild-type EPEC strain E2348/69 developed diarrhea with a mean stool volume of 2.3 liters (range, 1.0 to 5.4 liters) (Table 1). In contrast, only 1 (10%) of 10 volunteers who ingested ΔespB derivative strain UMD864 developed diarrhea (P < 0.001, Fisher's exact test) (stool volume, 0.5 liters). The incubation periods for the illnesses in the two groups were similar, 8 and 7 h, respectively. Only one volunteer from the group that received the wild-type strain had fever. The mutant strain was shed in the stool less frequently than the wild-type strain, but when shedding of the mutant strain did occur, it persisted for the same number of days as the wild-type strain (Table 1).
TABLE 1.
Clinical and bacteriologic responses to challenge with wild-type EPEC strain E2348/69 or an isogenic ΔespB mutant strain (UMD864)
| Parameter | Results for:
|
Pb | |
|---|---|---|---|
| Recipients of wild-type EPEC | Recipients of ΔespB mutant EPEC | ||
| Attack rate for diarrheaa (%) | 9/10 (90) | 1/10 (10) | <0.001* |
| Attack rate for fevera (%) | 1/10 (10) | 0/10 (0) | NS* |
| Mean incubation period (h) | 8 | 7 | NS† |
| Mean diarrheal stool vol (liters [range]) | 2.3 (1.0–5.4) | 0.5 | 0.12† |
| Mean (±SD) no. of diarrheal stools | 11.3 ± 7.7 | 6 | NS† |
| Rate of sheddinga (%) | 10/10 (100) | 7/10 (70) | NS* |
| Mean duration of positive stool culture (days) | 3.2 | 1.6 (all) | 0.045 |
| 2.3 (shed) | NS | ||
Number of affected individuals/total number.
*, Fisher's exact test, two-tailed; †, Wilcoxon test, two-tailed, comparing all volunteers (all) or only volunteers who shed (shed). NS, not significant.
PCR for espB.
PCR was performed on one colony from the first two cultures positive for EPEC from each volunteer. The sizes of the PCR products were compared with the sizes of the fragments amplified from purified cultures of the wild-type and the espB mutant strains without knowledge of the group to which each volunteer was assigned. The PCR products amplified from the volunteers matched the size of the appropriate control in every case (not shown). Thus, there was no evidence of transmission of the challenge organisms between volunteers.
Analysis of jejunal biopsies by confocal microscopy.
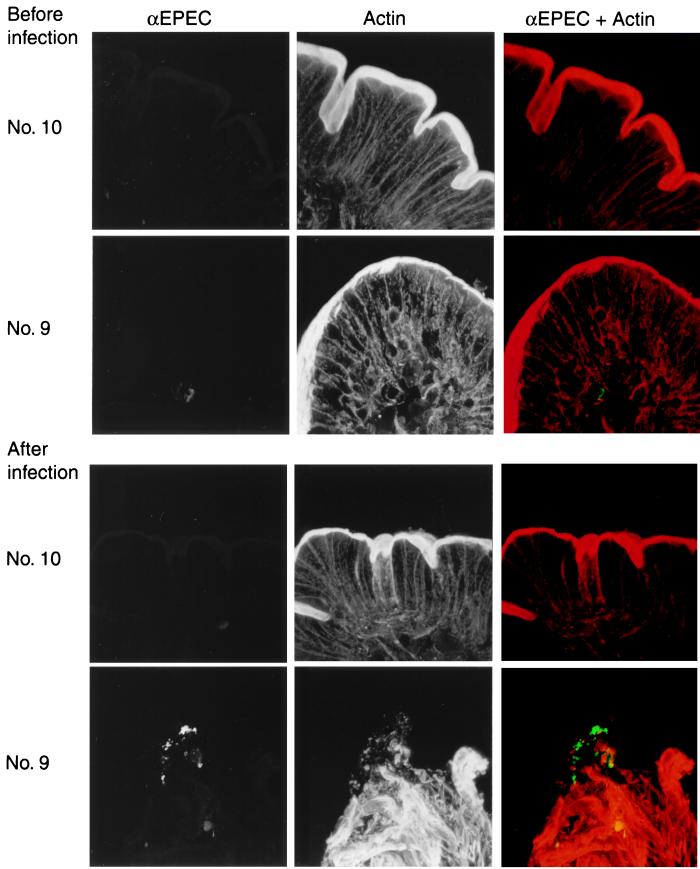
Four volunteers, two from each group, underwent prechallenge endoscopy, and three of these volunteers underwent a repeat procedure approximately 72 h after challenge (one volunteer who received the wild-type strain refused repeat endoscopy). In all prechallenge specimens, dense actin staining representing the microvillous brush border was observed lining the entire epithelial surface. No specific staining for organisms was detected (Fig. 1). Postchallenge specimens from both volunteers who received the espB mutant strain, one of whom had had mild diarrhea in the first 24 h only after challenge, revealed neither organisms nor damage to the epithelial brush border. Samples from volunteer number 9, who received the wild-type strain, revealed numerous EPEC organisms adjacent to the epithelium (Fig. 1). This volunteer did not develop diarrhea but did have three small-volume (total, 165 g) loose stools in the 72 h after challenge. This specific staining with the anti-EPEC antiserum was much more intense than the nonspecific staining seen in prechallenge samples. Staining for actin revealed that the brush border directly beneath the bacteria was completely destroyed. However, there was no detectable accumulation of actin beneath the adherent organisms corresponding to pedestal formation.
FIG. 1.
Confocal micrographs of jejunal biopsy samples from volunteers given wild-type or espB mutant strains of EPEC. The upper set of six images was from biopsies taken before challenge from volunteer number 10, who received the espB mutant strain, and volunteer 9, who received the wild-type strain. The lower set of images was from biopsies taken from the same volunteers approximately 72 h after infection. The samples were stained with an antibody against whole cells of the wild-type strain (αEPEC) and with phalloidin (Actin). The color images represent a merger of the anti-EPEC images in green and the phalloidin images in red.
Immunologic responses.
Immune responses were measured by detection of specific ASCs, serum and stool antibodies, and lymphocyte proliferation and IFN-γ production in response to EPEC antigens.
ASCs.
Seven (70%) of ten recipients of wild-type EPEC and two (20%) of ten recipients of the ΔespB mutant developed IgA ASC anti-EspB. All volunteers in both groups developed a significant number of IgA ASC against O127 LPS and whole-cell preparations derived from both the parent and mutant strains (Table 2).
TABLE 2.
ASC responses at day 6 after challenge with wild-type EPEC strain E2348/69 or an isogenic ΔespB mutant strain (UMD864)
| Antibody group and parameter | Results for:
|
Pc | |
|---|---|---|---|
| Recipients of wild-type EPEC | Recipients of ΔespB mutant EPEC | ||
| IgA anti-EspB | 7/9 (78)d | 2/10 (20) | 0.02* |
| Response ratea (%) | |||
| Mean no. of ASCs/106 PBMCb | 18 | 4 | <0.01† |
| IgG anti-EspB | 7/9 (78) | 2/10 (20) | 0.02 |
| Response rate (%) | |||
| Mean no. of ASCs/106 PBMC | 48 | 32 | <0.001 |
| IgA anti-O127 LPS | 9/9 (100) | 10/10 (100) | NS |
| Response rate (%) | |||
| Mean no. of ASCs/106 PBMC | 201 | 59 | <0.01 |
| IgG anti-O127 LPS | 9/9 (100) | 10/10 (100) | NS |
| Response rate (%) | |||
| Mean no. of ASCs/106 PBMC | 34 | 9 | 0.02 |
| IgA anti-whole wild-type cells | |||
| Response rate (%) | 9/9 (100) | 10/10 (100) | NS |
| Mean no. of ASCs/106 PBMC | 95 | 26 | 0.03 |
| IgG anti-whole wild-type cells | |||
| Response rate (%) | 7/9 (78) | 8/10 (80) | NS |
| Mean no. of ASCs/106 PBMC | 6 | 3 | NS |
Number of affected individuals/total number.
Geometric mean of all volunteers regardless of whether they were responders.
*, Fisher's exact test; †, Student's t test, two-tailed. NS, not significant.
No sample was available for one volunteer.
Antibody responses.
Serum IgG and IgA anti-EspB measured by ELISA were rarely detected in volunteers in either group; for example, serum IgG anti-EspB was induced in only 10% of volunteers who received wild-type EPEC or mutant EPEC. Responses to O127 and whole cells were more frequent and generally of higher titer among recipients of the wild-type strain (Table 3).
TABLE 3.
Serum and stool antibody responses after challenge with wild-type EPEC strain E2348/69 or an isogenic ΔespB mutant strain (UMD864)
| Antibody group and parameter | Results for:
|
Pb | |
|---|---|---|---|
| Recipients of wild-type EPEC | Recipients of ΔespB mutant EPEC | ||
| Serum IgA anti-EspB | |||
| Response ratea (%) | 2/10 (20) | 0/10 | NS* |
| Peak GMTd among responders | 400 | NS† | |
| Serum IgG anti-EspB | |||
| Response rate (%) | 1/10 (10) | 1/10 (10) | NS |
| Peak GMT among responders | 400 | 800 | NS |
| Serum IgA anti-O127 LPS | |||
| Response rate (%) | 10/10 (100) | 5/10 (50) | 0.02 |
| Peak GMT among responders | 1,393 | 264 | <0.01 |
| Serum IgG anti-O127 LPS | |||
| Response rate (%) | 9/10 (90) | 2/10 (20) | <0.01 |
| Peak GMT among responders | 1,089 | 800 | NS |
| Serum IgA anti-whole wild-type cells | |||
| Response rate (%) | 10/10 (100) | 4/10 (40) | <0.01 |
| Peak GMT among responders | 3,200 | 476 | 0.01 |
| Serum IgG anti-whole wild-type cells | |||
| Response rate (%) | 5/10 (50) | 2/10 (20) | NS |
| Peak GMT among responders | 1,838 | 6,400 | NS |
| Stool sIgA anti-EspB | |||
| Response rate (%) | 4/8c (50) | 4/7c (57) | NS |
| Peak GMT among responders | 3.4 | 3.4 | NS |
| Stool sIgA anti-O127 | |||
| Response rate (%) | 7/8 (88) | 3/7 (43) | NS |
| Geometric mean and (ng/ml) among responders | 39 | 8 | 0.01 |
Number of individuals affected/total number.
∗, Fisher's exact test; †, Student's t test, two-tailed. NS, not significant.
Sample was not of sufficient quantity to assay some of the volunteers.
GMT, geometric mean titer.
Stool antibodies to EspB were detected in 50% of recipients of wild-type EPEC and 57% of recipients of ΔespB mutant EPEC. Stool antibody responses to O127 LPS were more frequent and of higher magnitude among recipients of the wild-type strain (Table 3).
Lymphocyte proliferation.
Lymphocyte proliferation in response to wild-type strain E2348/69 homogenate, ΔespB strain UMD864 homogenate, recombinant EspB, or BSA was determined (Table 4). Following challenge, four (40%) and three (30%) of the volunteers who ingested the wild-type EPEC strain E2348/69 exhibited significant increases in lymphoproliferative responses to whole E. coli E2348/69 and UMD864 ΔespB mutant E. coli homogenates, respectively. In contrast, only one (14%) of the seven evaluable volunteers who ingested the UMD864 ΔespB mutant EPEC strain developed positive proliferative responses to whole wild-type EPEC E2348/69 or UMD864 ΔespB mutant EPEC homogenates (Table 4). In addition, four (40%) of the volunteers who ingested the wild-type EPEC strain also exhibited significant increases in lymphoproliferative responses to purified recombinant EspB protein, while none of the volunteers challenged with the UMD864 ΔespB mutant showed significant increases (Table 4). No proliferative responses to BSA were observed in any of the volunteers. No significant differences were observed among the two cohorts for any of the antigens studied.
TABLE 4.
Lymphocyte proliferation responses after challenge with wild-type EPEC strain E2348/69 or an isogenic ΔespB mutant strain (UMD864)
| Antigen | Response rate (%)a
|
|
|---|---|---|
| Recipients of wild-type EPEC | Recipients of ΔespB mutant EPEC | |
| Lymphoproliferative responses | ||
| Recombinant EspB | 4/10 (40) | 0/7 (0)b |
| Wild-type EPEC homogenate | 4/10 (40) | 1/7 (14) |
| ΔespB mutant EPEC homogenate | 3/10 (30) | 1/7 (14) |
| BSA | 0/10 (0) | 0/7 (0) |
| IFN-γ production | ||
| Recombinant EspB | 2/10 (20) | 0/7 (0) |
| Wild-type EPEC homogenate | 2/10 (20) | 0/7 (0) |
| ΔespB mutant EPEC homogenate | 3/10 (30) | 0/7 (0) |
| BSA | 0/10 (0) | 0/7 (0) |
Number of individuals affected/total number.
Three volunteers had a very high background proliferation in the absence of antigenic stimulation and were excluded from the analysis.
IFN-γ production.
We observed that following immunization, two (20%) and three (30%) of the volunteers who ingested the wild-type EPEC strain exhibited significant increases in IFN-γ production following exposure to whole E2348/69 E. coli and UMD864 ΔespB mutant E. coli homogenates, respectively (Table 4). In contrast, none of the seven evaluable volunteers who ingested the UMD864 ΔespB mutant EPEC strain developed positive responses to whole E2348/69 E. coli or UMD864 ΔespB mutant EPEC homogenates (Table 4). In addition, two (20%) of the volunteers who ingested the wild-type E2348/69 EPEC strain also exhibited significant increases in IFN-γ production to purified recombinant EspB protein, while none of the volunteers challenged with the UMD864 ΔespB mutant showed significant increases (Table 4). No increases in IFN-γ production to BSA were observed in any of the volunteers. No significant differences were observed among the two cohorts for any of the antigens studied.
DISCUSSION
We conducted a randomized, double-blind trial to determine the role of the EspB protein in the pathogenesis of diarrhea caused by EPEC. The principal result of this trial was that diarrhea developed in only 1 of 10 volunteers who ingested a mutant strain that had an in-frame deletion of the espB gene in contrast to 9 of 10 volunteers who ingested the wild-type strain from which the mutant was derived. Thus, we conclude unequivocally that the EspB protein is a virulence factor for EPEC. This result confirms and extends those obtained in rabbits using an espB mutant of an E. coli strain specific for that species (1). It is now clear that EspB, intimin, BfpA, and BfpF are required for full EPEC virulence in humans (2, 5). In addition, this is the first demonstration that a protein exported by a type III secretion apparatus is a virulence factor in humans.
We found that EspB was not absolutely required for colonization of the bowel. Although the infecting bacteria were recovered from a smaller proportion of the volunteers who received the espB mutant strain than from those who received the wild-type strain, this difference was not statistically significant. Furthermore, when only those who were colonized were considered, there was no significant difference in the duration of colonization between the groups. Thus, it appears that other factors present in the human EPEC strain contribute to intestinal colonization in humans.
In an earlier study, we did not detect EPEC in jejunal biopsy sections examined by electron microscopy (5). The approach using confocal microscopy in the present study makes examination of a much thicker preparation possible, reducing the risk of sampling error. We were able to visualize the infecting bacteria adhering to the jejunal mucosa of one of three volunteers. By chance, this volunteer was the only member of the group who received the wild-type bacteria who had loose stools which did not meet the definition of diarrhea. The adherent bacteria were associated with a dramatic destruction of the enterocyte brush border corresponding to the effacement of microvilli characteristic of EPEC attaching and effacing activity. However, we did not detect the accumulation of actin beneath the adherent bacteria, the pedestal formation also seen when EPEC interact with epithelial cells. It is possible that full attaching and effacing lesions would have been detected had different time points or a volunteer with more severe illness been examined.
We did not detect differences between the groups receiving wild-type EPEC and the espB mutant strain in mucosal IgA production or ASC producing antibody to LPS and whole cells, immune responses representing mucosal priming. In contrast, serum IgA and IgG responses to O127 LPS and whole cells occurred more frequently in volunteers who received the wild-type strain. In addition, cellular immune responses to E. coli antigens and homogenates were detected in a higher percentage of volunteers who received wild-type EPEC strain E2348/69 than in volunteers who received the UMD864 ΔespB mutant. These results suggest that there is a correlation between EPEC virulence and the induction of stronger immunological responses, likely the result of increased exposure of the wild-type EPEC to the inductive sites in the gut. A similar correlation between virulence and immune response was observed in an earlier study investigating the role of intimin in diarrhea (5).
We also evaluated humoral and cell-mediated responses to purified recombinant EspB. Surprisingly, some recipients of the ΔespB mutant strain developed serum IgG and stool IgA responses to EspB. The fact that we detected a response to EspB in any volunteer who received the espB mutant raises concerns for cross-infection between volunteers during the study. However, we did not detect the wild-type strain by PCR in the stools of any volunteer who received the mutant strain. It is also possible that the volunteers developed immune responses to an antigen that shares epitopes with EspB. The best candidate for such an antigen would be EspD, with which EspB has a limited degree of homology (7). Lastly, it is conceivable that the immune response was directed to epitopes present within the 78-amino-acid EspB peptide that could be produced by the ΔespB mutant (6), although Western blots did not support this possibility (data not shown). Thus, the humoral immune responses to EspB must be considered inconclusive.
We had hypothesized that EspB would stimulate cell-mediated responses since it is targeted to the host cell cytoplasm. This hypothesis is supported by our observations of lymphoproliferative responses and IFN-γ production in response to EspB in some of the volunteers who received the wild-type strain. In contrast to humoral responses, cell-mediated responses to EspB were not detected in any volunteer who received the ΔespB mutant. The ability of proteins delivered to the cytoplasm by a type III secretion system to induce MHC class I-restricted responses has been demonstrated in mice infected with recombinant strains of S. enterica serovar Typhimurium (25). Our study is one of the first to demonstrate CMI responses in humans to proteins delivered by a type III secretion system. Serum antibody responses to EspB have previously been demonstrated in infants convalescing from natural EPEC infection and in a patient recovering from enterohemorrhagic E. coli infection (12, 22). These results, taken together with those demonstrating that EspB plays a significant role in the pathogenesis of EPEC-induced diarrhea in humans, suggest that EspB might be an important component to be considered in the development of EPEC vaccines.
The precise role of EspB in pathogenesis remains obscure. Proteins secreted via type III systems may themselves be effector molecules that interact with host cell proteins or may be components of a translocation apparatus that delivers other secreted proteins to the host cell. Since EspB has been detected in host cells and shown to alter cell shape when expressed in cells, it appears that EspB could be an effector protein, though its target is unknown (32, 33, 35). On the other hand, EspB is required for the targeting of Tir to the host cell and it has been proposed that EspB is part of the translocation apparatus (10, 14). Regardless of its precise function, the results of this study indicate that EspB is crucial for EPEC pathogenesis in humans.
ACKNOWLEDGMENTS
We gratefully acknowledge the contributions of our volunteers and the effort of the staff of the Adult Clinical Studies Section, Center for Vaccine Development. M.B.S. thanks Susan DiLorenzo for expert technical assistance.
This study was supported by National Institute of Allergy and Infectious Diseases contract NO1-AI-65299, by Public Health Services award AI32074, by the Medical Research Council of Canada, and by a Howard Hughes Medical Institute International Research Scholar award.
REFERENCES
- 1.Abe A, Heczko U, Hegele R G, Finlay B B. Two enteropathogenic Escherichia coli type III secreted proteins, EspA and EspB, are virulence factors. J Exp Med. 1998;188:1907–1916. doi: 10.1084/jem.188.10.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bieber D, Ramer S W, Wu C Y, Murray W J, Tobe T, Fernandez R, Schoolnik G K. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science. 1998;280:2114–2118. doi: 10.1126/science.280.5372.2114. [DOI] [PubMed] [Google Scholar]
- 3.Collington G K, Booth I W, Donnenberg M S, Kaper J B, Knutton S. Enteropathogenic Escherichia coli virulence genes encoding secreted signalling proteins are essential for modulation of Caco-2 cell electrolyte transport. Infect Immun. 1998;66:6049–6053. doi: 10.1128/iai.66.12.6049-6053.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnenberg M S. Enteropathogenic Escherichia coli. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press, Ltd.; 1995. pp. 709–726. [Google Scholar]
- 5.Donnenberg M S, Tacket C O, James S P, Losonsky G, Nataro J P, Wasserman S S, Kaper J B, Levine M M. The role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J Clin Investig. 1993;92:1412–1417. doi: 10.1172/JCI116717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnenberg M S, Yu J, Kaper J B. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J Bacteriol. 1993;175:4670–4680. doi: 10.1128/jb.175.15.4670-4680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott S J, Wainwright L A, McDaniel T K, Jarvis K G, Deng Y, Lai L-C, McNamara B P, Donnenberg M S, Kaper J B. The complete sequence of the locus of enterocyte effacement (LEE) of enteropathogenic E. coli E2348/69. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 8.Foubister V, Rosenshine I, Donnenberg M S, Finlay B B. The eaeB gene of enteropathogenic Escherichia coli is necessary for signal transduction in epithelial cells. Infect Immun. 1994;62:3038–3040. doi: 10.1128/iai.62.7.3038-3040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frankel G, Phillips A D, Novakova M, Field H, Candy D C, Schauer D B, Douce G, Dougan G. Intimin from enteropathogenic Escherichia coli restores murine virulence to a Citrobacter rodentium eaeA mutant: induction of an immunoglobulin A response to intimin and EspB. Infect Immun. 1996;64:5315–5325. doi: 10.1128/iai.64.12.5315-5325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frankel G, Phillips A D, Rosenshine I, Dougan G, Kaper J B, Knutton S. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol Microbiol. 1998;30:911–921. doi: 10.1046/j.1365-2958.1998.01144.x. [DOI] [PubMed] [Google Scholar]
- 11.Jarvis K G, Girón J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarvis K G, Kaper J B. Secretion of extracellular proteins by enterohemorrhagic Escherichia coli via a putative type III secretion system. Infect Immun. 1996;64:4826–4829. doi: 10.1128/iai.64.11.4826-4829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jerse A E, Yu J, Tall B D, Kaper J B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 15.Kenny B, Finlay B B. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals to epithelial cells. Proc Natl Acad Sci USA. 1995;92:7991–7995. doi: 10.1073/pnas.92.17.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenny B, Lai L-C, Finlay B B, Donnenberg M S. EspA, a protein secreted by enteropathogenic Escherichia coli (EPEC), is required to induce signals in epithelial cells. Mol Microbiol. 1996;20:313–323. doi: 10.1111/j.1365-2958.1996.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 17.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knutton S, Lloyd D R, McNeish A S. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987;55:69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knutton S, Rosenshine I, Pallen M J, Nisan I, Neves B C, Bain C, Wolff C, Dougan G, Frankel G. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 1998;17:2166–2176. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine M M, Bergquist E J, Nalin D R, Waterman D H, Hornick R B, Young C R, Sotman S, Rowe B. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet. 1978;i:1119–1122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- 21.Levine M M, Nataro J P, Karch H, Baldini M M, Kaper J B, Black R E, Clements M L, O'Brien A D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985;152:550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- 22.Martinez M B, Taddei C R, Ruiz-Tagle A, Trabulsi L R, Girón J A. Antibody response of children with enteropathogenic Escherichia coli infection to the bundle-forming pilus and locus of enterocyte effacement-encoded virulence determinants. J Infect Dis. 1999;179:269–274. doi: 10.1086/314549. [DOI] [PubMed] [Google Scholar]
- 23.McDaniel T K, Jarvis K G, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasetti M F, Anderson R J, Noriega F R, Levine M M, Sztein M B. Attenuated deltaguaBA Salmonella typhi vaccine strain CVD 915 as a live vector utilizing prokaryotic or eukaryotic expression systems to deliver foreign antigens and elicit immune responses. Clin Immunol. 1999;92:76–89. doi: 10.1006/clim.1999.4733. [DOI] [PubMed] [Google Scholar]
- 25.Rüssmann H, Shams H, Poblete F, Fu Y X, Galán J E, Donis R O. Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science. 1998;281:565–568. doi: 10.1126/science.281.5376.565. [DOI] [PubMed] [Google Scholar]
- 26.Savkovic S D, Koutsouris A, Hecht G. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect Immun. 1996;64:4480–4487. doi: 10.1128/iai.64.11.4480-4487.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savkovic S D, Koutsouris A, Hecht G. Activation of NF-kappaB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am J Physiol. 1997;273:C1160–C1167. doi: 10.1152/ajpcell.1997.273.4.C1160. [DOI] [PubMed] [Google Scholar]
- 28.Sohel I, Puente J L, Ramer S W, Bieber D, Wu C-Y, Schoolnik G K. Enteropathogenic Escherichia coli: identification of a gene cluster coding for bundle-forming pilus morphogenesis. J Bacteriol. 1996;178:2613–2628. doi: 10.1128/jb.178.9.2613-2628.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein M A, Mathers D A, Yan H, Baimbridge K G, Finlay B B. Enteropathogenic Escherichia coli (EPEC) markedly decreases the resting membrane potential of Caco-2 and HeLa human epithelial cells. Infect Immun. 1996;64:4820–4825. doi: 10.1128/iai.64.11.4820-4825.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone K D, Zhang H-Z, Carlson L K, Donnenberg M S. A cluster of fourteen genes from enteropathogenic Escherichia coli is sufficient for biogenesis of a type IV pilus. Mol Microbiol. 1996;20:325–337. doi: 10.1111/j.1365-2958.1996.tb02620.x. [DOI] [PubMed] [Google Scholar]
- 31.Sztein M B, Wasserman S S, Tacket C O, Edelman R, Hone D, Lindberg A A, Levine M M. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J Infect Dis. 1994;170:1508–1517. doi: 10.1093/infdis/170.6.1508. [DOI] [PubMed] [Google Scholar]
- 32.Taylor K A, Luther P W, Donnenberg M S. Expression of the EspB protein of enteropathogenic Escherichia coli within HeLa cells affects stress fibers and cellular morphology. Infect Immun. 1999;67:120–125. doi: 10.1128/iai.67.1.120-125.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor K A, O'Connell C O, Luther P W, Donnenberg M S. The EspB protein of enteropathogenic Escherichia coli is targeted to the cytoplasm of infected HeLa cells. Infect Immun. 1998;66:5501–5507. doi: 10.1128/iai.66.11.5501-5507.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van de Verg L, Herrington D A, Murphy J R, Wasserman S S, Formal S B, Levine M M. Specific immunoglobulin A-secreting cells in peripheral blood of humans following oral immunization with a bivalent Salmonella typhi-Shigella sonnei vaccine or infection by pathogenic S. sonnei. Infect Immun. 1990;58:2002–2004. doi: 10.1128/iai.58.6.2002-2004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolff C, Nisan I, Hanski E, Frankel G, Rosenshine I. Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1998;28:143–155. doi: 10.1046/j.1365-2958.1998.00782.x. [DOI] [PubMed] [Google Scholar]
- 36.Yuhan R, Koutsouris A, Savkovic S D, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]