Abstract
Sporothrix schenckii is a human pathogen that causes sporotrichosis, an important cutaneous mycosis with a worldwide distribution. It produces dark-brown conidia, which infect the host. We found that S. schenckii synthesizes melanin via the 1,8-dihydroxynaphthalene pentaketide pathway. Melanin biosynthesis in the wild type was inhibited by tricyclazole, and colonies of the fungus were reddish brown instead of black on tricyclazole-amended medium. Two melanin-deficient mutant strains were analyzed in this study: an albino that produced normal-appearing melanin on scytalone-amended medium and a reddish brown mutant that accumulated and extruded melanin metabolites into its medium. Scytalone and flaviolin obtained from cultures of the reddish brown mutant were identified by thin-layer chromatography, high-performance liquid chromatography, and UV spectra. Transmission electron microscopy showed an electron-dense granular material believed to be melanin in wild-type conidial cell walls, and this was absent in conidial walls of the albino mutant unless the albino was grown on a scytalone-amended medium. Melanized cells of wild-type S. schenckii and the albino grown on scytalone-amended medium were less susceptible to killing by chemically generated oxygen- and nitrogen-derived radicals and by UV light than were conidia of the mutant strains. Melanized conidia of the wild type and the scytalone-treated albino were also more resistant to phagocytosis and killing by human monocytes and murine macrophages than were unmelanized conidia of the two mutants. These results demonstrate that melanin protects S. schenckii against certain oxidative antimicrobial compounds and against attack by macrophages.
A wide variety of fungi synthesize distinctive dark brown or black pigments called melanins (3, 5). Fungal melanins are complex pigments which are produced by at least two different synthetic pathways, known as the 1,8-dihydroxynaphthalene (DHN) and dihydroxyphenylalanine pathways, depending on the species (5, 46). Some pathogenic brown to black fungi, i.e., Exophiala (Wangiella) dermatitidis, Cladosporium carrioni, and Fonsecaea pedrosoi (34), synthesize melanin via the DHN melanin pathway, where multiple enzymatic steps take place. The first known product in the pathway is 1,3,6,8-tetrahydroxynaphthalene (1,3,6,8-THN), which is synthesized from acetate via polyketide synthase. Thereafter, sequential reductions and dehydrations take place (Fig. 1). The last step is the polymerization of DHN to form DHN melanin (3, 5, 33, 45). Another pathogen, like Cryptococcus neoformans, produces melanin in media containing phenolic compounds, i.e., l-dopa and catecholamines (28, 29), and the synthesis of this pigment is catalyzed by a phenoloxidase. With C. neoformans, only the phenoloxidase is needed for the synthesis of melanin from l-dopa. Most of the reactions that occur are fast and probably nonenzymatic (25), leading to intermediates that combine and form melanin polymers (22). DHN melanin and dihydroxyphenylalanine melanin are different in their synthesis and structure; however, their redox function has been shown to be the same (7, 14). Melanins are not essential for fungal growth but appear to be important for the virulence of several pathogens (6, 16, 38). The mechanism by which pigments enhance virulence in fungi is not known, but it has been reported that pigmented cells of Aspergillus fumigatus (37), E. dermatitidis (6), and C. neoformans (16) are more virulent than hyaline cells in murine models. In vitro, melanized cells of C. neoformans are less susceptible to killing by ionizing radiation (42) and free radicals (13) than hyaline cells. Melanized cells of C. neoformans (31) and bluish green pigmented conidia of A. fumigatus (38) are also less susceptible to phagocytosis by macrophages than nonmelanized cells. These studies suggest that melanins confer tolerance against certain environmental stresses and protect against antimicrobial oxidants that are produced during the host defense response (17, 38). Sporothrix schenckii is a dimorphic fungus which is frequently associated with plants and soil (21). In these environments, the mycelial phase predominates, with hyphal and conidial cell types. In contrast, the yeast-like form develops in infected human and animal tissue (24). The dark pigment of S. schenckii has been found exclusively in the conidia, which are the infecting structures of this organism. In this paper we present evidence which demonstrates that S. schenckii synthesizes melanin by the DHN pathway and that melanized cells are less susceptible than nonmelanized cells to oxidant killing in vitro and to phagocytosis by human monocytes and murine macrophages.
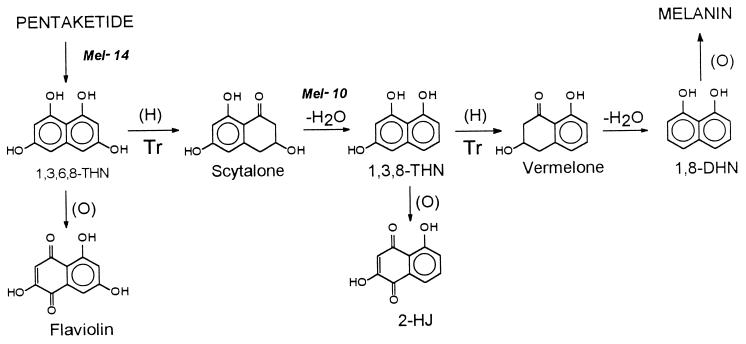
FIG. 1.
Pentaketide pathway of melanin biosynthesis. Sites of tricyclazole inhibition (Tr) are as proposed by Tokusbalides and Sisler (35) for V. dahliae. Mel−14 and Mel−10, proposed sites of inhibition by the mutants of S. schenckii.
MATERIALS AND METHODS
Chemicals.
Scytalone [3,4-dihydro-3,6,8-trihydroxy-1(2H)-naphthalenone] was purified from cultures of the brm-1 mutant of Verticillium dahliae (2). Tricyclazole [5-methyl-1,2,4-triazolo(3,4-b)benzothiazole] was kindly provided by Dow Agrosciences (Indianapolis, Ind.).
Strains.
The wild-type strain EH-217 is a clinical isolate from a patient with sporotrichosis and was provided by Jorge Mayorga, Instituto Dermatologico, Jalisco, Mexico. It produces black conidial colonies on potato dextrose agar (PDA) by 10 days. The mutants Mel−10 and Mel−14 were made by UV irradiation of the wild-type strain. Conidia from yeast extract-peptone-dextrose (YEPD) slants of the wild-type strain were spread on YEPD plates and exposed to 300 ergs of UV light per mm2 (36). This amount of irradiation usually gave a 10% survival rate in this isolate. The plates were incubated in the dark at 28°C to avoid photoreactivation repair. After 10 days at 28°C, mutagenized colonies were screened for melanin-deficient mutants.
Culture conditions.
Stock cultures of S. schenckii were maintained on YEPD agar medium. The solid cultures were incubated at 28°C in culture tubes or in petri dishes, and transfers were made with a bacterial loop. Melanin induction by wild-type S. schenckii was done on PDA. For this purpose, wild-type conidia from 7-day-old YEPD agar slants were washed with water and grown on this medium for 10 days at 28°C. The melanized conidial population was formed by lateral and sympodial conidia. Most of the conidia were oval and pigmented when they were grown on PDA; this was determined by light microscopy and transmission electron microscopy (TEM). The whole population of conidia was used in the various tests.
Melanin biosynthesis.
To study melanin biosynthesis, the wild type and Mel−10 and Mel−14 mutants were grown on 20 ml of PDA in 9-cm-diameter petri dishes or on the surface of 200 ml of potato dextrose broth (PDB) in 1-liter Erlenmeyer flasks. The PDA was inoculated with 106 conidia per petri dish. The cultures were grown in the dark for 10 days at 25°C. The PDB medium was inoculated with 5 × 107 conidia and grown under static conditions in the light for 14 days at 25°C. To demonstrate that the effects detected in the various tests were the result of the presence or absence of melanin, the mutant strain Mel−14 was also grown under the same conditions on PDA amended with 1 mM scytalone. Most conidia of the strain were melanized on scytalone-amended PDA, and the scytalone-treated strain is hereafter referred to as Mel−14p. The viabilities of conidia from Mel−14p and the wild-type strain were 89 and 90%, respectively. This showed that exogenous scytalone did not harm Mel−14 conidia. The wild-type strain was grown on PDB or PDA containing 8 or 16 μg of tricyclazole per ml, respectively. Scytalone and tricyclazole were added to PDA and PDB in ethanol (EtOH), and the final concentration of EtOH in the cultures did not exceed 0.6%. Controls without scytalone or tricyclazole were used to test the effects of EtOH on melanin synthesis.
Isolation and identification of metabolites from PDA and PDB cultures.
Two volumes of acetone was added to PDA and PDB cultures of the three strains at the end of 10 or 14 days, respectively. After 4 to 16 h, the acetone-treated media were filtered over no. 1 filter paper (Whatman Ltd., Maidstone, Kent, United Kingdom) to remove agar and the acetone-treated cells. The acetone was then removed under vacuum, and the remaining aqueous solution was examined for the presence of melanin metabolites by ethyl acetate extraction and thin-layer-chromatography procedures as described previously (34, 47). Flaviolin, 2-hydroxyjuglone (2-HJ), and scytalone were also identified by high-performance liquid chromatography (HPLC) (10) and their characteristic UV spectra were compared with standards by using a diode array detector.
TEM.
Conidia from the surface of PDA plates were examined with a transmission electron microscope (JEOL model JEM-1200EX-II). Comparisons were made between conidia of the wild-type, Mel−14, and Mel−14p strains. Blocks (2 mm2) were cut out from the agar cultures and placed for 1 h in fixative solution containing 2.5% glutaraldehyde in 10 mM sodium phosphate (pH 7.2). Postfixation was in 1% osmium tetraoxide–1.5% potassium ferricyanide in 0.1 M sodium cacodylate for 2 h at 4°C. The samples were dehydrated, embedded in Poly/bed 812 resin (Polyscience, Inc., Warrington, Pa.), and polymerized for 24 h at 65°C. Thin sections were stained with uranyl acetate and lead citrate.
Susceptibility of melanin-deficient and melanized cells to killing by UV light and oxidants (H2O2 and nitric oxide).
S. schenckii survival after exposure to UV light or reactive nitrogen and oxygen species was determined. For UV light exposure, conidia from PDA slants cultured for 7 days were washed with water, and the suspension was adjusted to 106 cells ml−1. Appropriate dilutions of cells were spread on YEPD plates and exposed to UV light (254 nm) generated in a Stratalinker 1800 (Stratagene, La Jolla, Calif.) at various energy settings. Percent survival was determined by comparing the number of colonies on irradiated plates to those on nonirradiated plates. For H2O2 assays, conidial suspensions were adjusted to 106 cells ml−1 in 100 mM potassium phosphate buffer (PBS) (pH 7.0) containing 25 mM oxidant. At 20-min intervals, aliquots were taken, diluted in 100 mM PBS, and plated on YEPD agar plates (19). For nitric oxide assays, 106 cells ml−1 were suspended in 25 mM succinic acid (Sigma Chemical Co., St. Louis, Mo.) (pH 4.0). Nitric oxide and reactive nitrogen intermediates were generated in a solution that initially contained 0.5 mM NaNO2 (Sigma Chemical Co.) and 25 mM succinic acid (pH 4.0) (1). Aliquots were taken at 20-min intervals, diluted in 50 mM PBS, and plated on YEPD agar plates.
Isolation of human monocytes.
Heparinized blood (10 IU of heparin ml−1) from healthy, fasting, nonsmoking adult donors was diluted 1:2 with PBS (pH 7.2). Samples of 10 ml were layered over a 4-ml Ficoll-Hypaque gradient (density = 1.077) (Sigma Chemical Co.) and centrifuged for 40 min at 400 × g at 18°C. The cells in the interface were removed and washed three times with PBS. Pelleted cells were treated with Tris-buffered 0.83% NH4Cl (pH 7.2) at 37°C to lyse contaminating red blood cells. The treated cells were washed with PBS and suspended in RPMI 1640 medium (Sigma Chemical Co.). A cell suspension of 3 × 105 monocytes in 0.2 ml of RPMI 1640 medium containing 10% fetal calf serum was added to each well of an eight-chamber tissue culture slide (Lab Tel Products, Naperville, Ill.). The cells were allowed to settle and adhere for 3 h at 37°C in a moist chamber of 5% CO2 and air. After this incubation, the medium was removed from each well and nonadherent cells were eliminated. Viability of the cells was over 95%; this was measured by trypan blue dye exclusion.
Isolation of peritoneal murine macrophages.
Resident peritoneal cells were collected from male BALB/c mice by washing the peritoneal cavities with cold RPMI 1640 medium. A cell suspension of 3 × 105 macrophages in 0.2 ml of RPMI 1640 medium containing 10% fetal calf serum was added to each well of an eight-chamber tissue culture slide. The cells were allowed to settle and adhere for 3 h at 37°C in a moist chamber of 5% CO2 and air. After this incubation, the medium was removed from each well and nonadherent cells were eliminated.
ConA coating of conidal cells.
S. schenckii conidia were opsonized as described by Oda et al. (27). Briefly, conidia at 107 cells ml−1 were incubated with 60 μg of concanavalin A (ConA) (type IV; Sigma Chemical Co.) per ml in sterile PBS for 30 min at 28°C. Conidial suspensions were vortexed every 10 min to prevent agglutination. Suspensions were washed three times by centrifugation (500 × g, 5 min) to remove ConA, and the cells were resuspended in PBS (107 cells ml−1).
Phagocytosis.
Opsonized conidia (1.5 × 107 cells ml−1) were mixed with human monocytes or murine macrophages (phagocytes) in a ratio of 5:1. The cultures were incubated at 37°C for 10 and 30 min. The medium from the wells was carefully removed and washed three times with 0.5 ml of sterile PBS to remove conidia that were not attached to or engulfed by phagocytes. Conidia and the two types of phagocytic cells attached to slides were fixed in absolute methanol for 1 min and stained with Giemsa stain (Sigma Chemical Co.) for 10 min. After the slides were air dried, the cells were observed microscopically at a magnification of ×100. An average of 200 monocytes or macrophages were counted in several microscope fields to determine the percentage of cells phagocytizing at least one conidial cell (P) and the average number of conidia in these monocytes or macrophages (F). The phagocytic index (I) was determined as P × F (27).
Oxidative burst.
A luminol-dependent chemiluminescence assay was carried out to measure the release of reactive oxygen species (oxygen burst). Freshly isolated human monocytes and murine macrophages were suspended in PBS to give a final concentration of 107 cells ml−1. To 100 μl of monocytes or macrophages (106 cells), 100 μl of opsonized conidia (5 × 107 cells) was added and incubated for 15 min at 37°C. After the incubation, 700 μl of 10−6 M luminol (Eastman Kodak, Rochester, N.Y.) and 200 μl of opsonized zymosan (Sigma) (12.5 mg/ml) were added to the cellular suspension. Cells treated with zymosan served as positive controls, and cells incubated without conidia served as background controls. Reactive oxygen intermediates associated with oxygen burst (ROIs) were measured at 1-min intervals for 30 min. Photon emission was detected in a Biorbit 1250 chemiluminometer (LKB-Pharmacia, Uppsala, Sweden). Results were expressed as millivolts per 106 phagocytic cells.
Antifungal activity of macrophage cells.
Opsonized conidia were mixed with human monocytes or murine macrophages in a ratio of 5:1. This suspension was incubated at 37°C for 30 min while being stirred. At different times during this period, aliquots of the incubation mixture were removed and diluted in ice-cold distilled water. Numbers of CFU were then determined by plating 100 μl of appropriate dilutions on YEPD plates. Each reaction mixture was plated in triplicate. For each experiment, two sets of tubes containing opsonized conidia and RPMI 1640 medium were included. The first tube was diluted and plated immediately (time zero). The second tube was incubated at 37°C for the same amount of time as the experimental tubes before being processed and plated (control). The percentage of killing was determined by the formula [100 − (experimental CFU/control CFU)] × 100 (30).
RESULTS
Identification of the pigment in S. schenckii as a DHN melanin.
The wild-type strain of S. schenckii developed dark-brown colonies on PDA in the absence of tricyclazole but was reddish brown on PDA amended with 8 or 16 μg of tricyclazole per ml (Fig. 2a and d). The culture medium also turned reddish brown when the wild type was grown in the presence of tricyclazole. Small but detectable amounts of 2-HJ, a shunt product of the DHN melanin pigment pathway, were identified in extracts from tricyclazole-amended PDA and PDB cultures of the wild type.
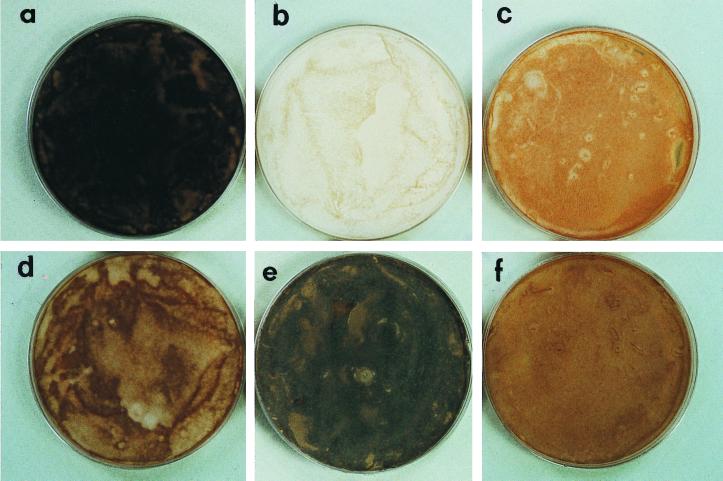
FIG. 2.
Appearance of S. schenckii growth on PDA. (a) Wild type; (b) Mel−14; (c) Mel−10; (d) wild type grown on PDA with tricyclazole; (e and f) Mel−14 and Mel−10, respectively, on medium amended with 1 mM scytalone.
Melanin-deficient mutants.
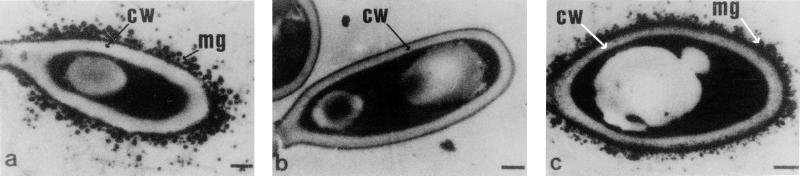
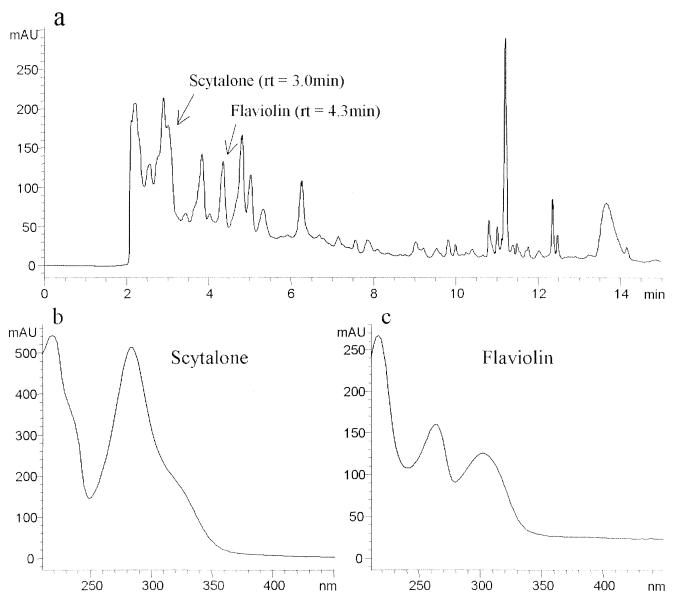
Mutagenesis of the wild type by exposure to UV light led to the isolation of two mutants which lack the ability to produce dark-brown conida. Strain Mel−14 is a colorless albino (Fig. 2b), and Mel−10 is light reddish brown (Fig. 2c). When the mutants were grown on PDA containing scytalone, only the albino (Mel−14) synthesized a dark-brown pigment (Fig. 2e) that was similar in appearance to that in the wild type (Fig. 2a). Mel−10 remained nearly the same color when grown on scytalone-amended PDA (Fig. 2c and f). TEM profiles showed that melanin in the scytalone-treated albino appeared as electron-dense granules and looked identical to melanin located at the cell wall surface of the pigmented wild type (Fig. 3a and c). Untreated albino conidia from Mel−14 lacked the electron-dense material (Fig. 3b). Extracts obtained from the PDA and PDB cultures of the Mel−10 mutant accumulated small amounts of scytalone and flaviolin (Fig. 4). In contrast, metabolites of the melanin pathway were not found in cultures of the albino mutant Mel−14 (data not shown). Small amounts of scytalone were also detected in PDA and PDB cultures of the wild-type strain (data not shown). UV, proton nuclear magnetic resonance, and mass spectral values for flaviolin, 2-HJ, and scytalone were reported elsewhere (2).
FIG. 3.
TEM of S. schenckii conidia grown on PDA. (a) Wild type; (b) Mel−14; (c) Mel−14 supplemented with scytalone. Cell wall (cw) and melanin granules (mg) are indicated. Bars, 0.5 μm.
FIG. 4.
HPLC separation of scytalone, flaviolin, and a number of unidentified compounds, extracted with ethyl acetate from a 14-day-old PDB culture of Mel−10. (a) Chromatogram obtained at 254 nm as described previously (10). rt, retention time. (b and c) UV-visible spectra of compounds identified as scytalone and flaviolin, respectively, obtained with a diode array detector. The retention times and spectra of the two compounds are identical to those of known standards of scytalone and flaviolin. AU, absorbance units.
Fungal susceptibility to ionizing irradiation and oxidants.
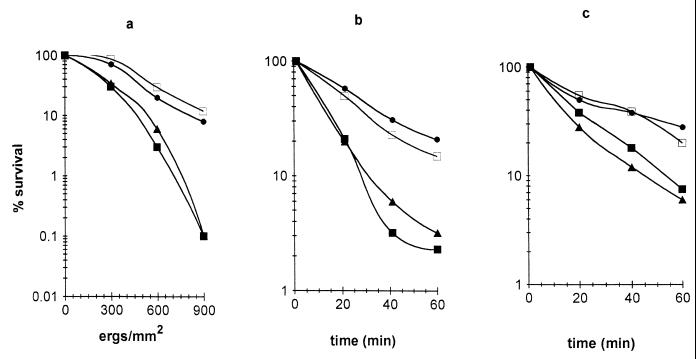
The viability of melanized and nonmelanized conidia after treatment with UV light, H2O2, and NaNO2 was determined. The percentage of cells capable of forming visible colonies was plotted as a function of UV light dose or time of incubation in the presence of the oxidants (Fig. 5). Treatments allowing survival of 50% of the cells (SD50) were compared between the strains. Melanized wild-type and scytalone-treated Mel−14p conidia were consistently the most resistant, compared with the melanin-deficient mutants Mel−14 and Mel−10. For UV light exposure, the SD50s for conidia were 380 ergs/mm2 for the wild type, 460 ergs/mm2 for Mel−14p, and 190 ergs/mm2 for Mel−10 and Mel−14 (Fig. 5a). Incubation with NaNO2 gave SD50s of 27 min for the wild type, 22 min for Mel−14p, and 9 and 10 min for Mel−10 and Mel−14, respectively (Fig. 5b). Similar results were obtained with H2O2, where the SD50s for the wild type and Mel−14p were 20 and 25 min, respectively, and those for Mel−10 and Mel−14 were 11 and 15 min, respectively (Fig. 5c).
FIG. 5.
Survival of 7-day-old melanized and nonmelanized S. schenckii after treatment with UV light (a), sodium nitrite (b), or H2O2 (c). Aliquots were plated on YEPD in duplicate to monitor cell viability. Values are averages from three experiments. ●, wild type; ▴, Mel−10; ■, Mel−14; □, Mel−14 supplemented with scytalone.
Phagocytosis of conidia by human monocytes and murine macrophages.
Opsonization of the conidia (wild type, Mel−14p, Mel−14, and Mel−10) with 60 μg of ConA per ml was sufficient to produce an important cell interaction with human monocytes and murine macrophages and to avoid conidial agglutination. Phagocytosis by human monocytes and murine macrophages of opsonized conidia of the Mel−10 and Mel−14 mutants was rapid and increased with time (Table 1). At 10 min, 85 to 91% of the human monocytes and murine macrophages ingested two to five melanin-deficient conidia; this increased to 90 to 93% at 30 min. The wild-type and Mel−14p conidia were phagocytized less efficiently than the melanin-deficient conidia. At 10 min only 69 and 55% of the monocytes ingested one conidium of the wild type and Mel−14p, respectively; this increased to 77 and 65% at 30 min. The phagocytic index values of the Mel−10 and Mel−14 mutants were three to four times higher than those of the wild-type in human monocytes and two times higher in murine macrophages; values for Mel−14p were less than those for the wild type for monocytes and macrophages.
TABLE 1.
Phagocytosis of lectin-coated conidia of S. schenckiia
| Strain and time (min) | Human
monocytes
|
Murine macrophages
|
||||
|---|---|---|---|---|---|---|
| P | F | I | P | F | I | |
| Wild type | ||||||
| 10 | 69 ± 1 | 1.26 ± 0.2 | 87 ± 6 | 68 ± 1 | 2 ± 0.5 | 136 ± 10 |
| 30 | 77 ± 3 | 2 ± 0.5 | 154 ± 20 | 69 ± 1 | 5 ± 0.6 | 345 ± 40 |
| Mel−14b | ||||||
| 10 | 55 ± 5 | 1 ± 0.3 | 55 ± 6 | 50 ± 1 | 2 ± 0.5 | 100 ± 9 |
| 30 | 65 ± 4 | 2 ± 0.5 | 130 ± 15 | 60 ± 1 | 4 ± 1 | 240 ± 30 |
| Mel−10 | ||||||
| 10 | 85 ± 6 | 4 ± 0.5 | 340 ± 17 | 86 ± 5 | 3 ± 0.5 | 258 ± 19 |
| 30 | 91 ± 10 | 5 ± 0.6 | 455 ± 20 | 90 ± 9 | 8 ± 2 | 720 ± 50 |
| Mel−14 | ||||||
| 10 | 90 ± 6 | 4 ± 1 | 360 ± 22 | 91 ± 4 | 5 ± 1 | 455 ± 13 |
| 30 | 92 ± 5 | 7 ± 3 | 644 ± 82 | 93 ± 1 | 9 ± 1 | 837 ± 40 |
I (phagocytic index) = P × F (see Materials and Methods). Values are the means and standard deviations from three independent experiments. Values of P, F, and I for Mel−10 and Mel−14 were significantly different from the corresponding values for the wild type, using the Mann-Whitney U test (P < 0.05).
Mel−14 was grown in medium amended with scytalone.
Oxidative burst in response to conidia.
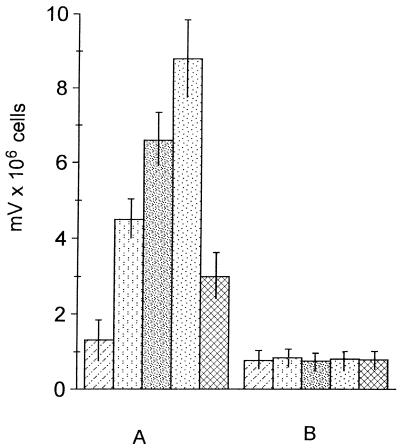
Levels of ROIs released were measured with human monocytes and murine macrophages after challenge with the respective conidia. This was done to compare the oxidative burst by conidia of the wild type and Mel−14p with that of Mel−10 and Mel−14. Incubation of human monocytes with the wild-type, Mel−14p, Mel−10, and Mel−14 conidia resulted in an increase in the amount of detectable ROIs compared with that for zymosan-stimulated monocytes (Fig. 6); however, the amount of stimulation differed with the strain. Wild-type and Mel−14p conidia caused 3.4- and 2.2-fold increases, respectively, of detectable ROIs over those produced by zymosan-stimulated monocytes, while Mel−10 and Mel−14 conidia caused 5- and 7-fold increases, respectively in ROIs. Murine macrophages did not stimulate the release of ROIs above that of zymosan-stimulated macrophages. Increasing the ratio of opsonized conidia to murine macrophages from 5:1 to 20:1 also did not result in a significant release of ROIs above that of the control (data not shown).
FIG. 6.
Release of ROIs by S. schenckii conidia.
Human monocytes (A) or murine macrophages (B) were incubated with
either the wild type
( ),
Mel−10 (░⃞), Mel−14
(
), or Mel−14 supplemented with scytalone
(
). Stimulation of cells with zymosan was employed as a
positive control (▨). Values are means and standard
deviations.
),
Mel−10 (░⃞), Mel−14
(
), or Mel−14 supplemented with scytalone
(
). Stimulation of cells with zymosan was employed as a
positive control (▨). Values are means and standard
deviations.
Antifungal activity of human monocytes and murine macrophages.
The antifungal activities of human monocytes on the melanized and melanin-deficient strains were different. Killing of melanized conidia by human cells was 40% ± 5% and 36% ± 7% for the wild-type strain and Mel−14p, respectively, after 2 h of incubation; killing for Mel−10 and Mel−14 conidia was 67% ± 3% and 64% ± 4%, respectively (means and standard deviations; n = 3). Murine macrophages did not show appreciable growth inhibition of conidia by 3 h of incubation.
DISCUSSION
Melanin is suggested to play an important role in the pathogenesis of infections by certain human pathogenic fungi (6, 9, 13, 14, 16, 18, 30, 32, 37, 38). However, despite the biochemical characterization of the melanin biosynthetic pathway in different species and the fact that melanins appear to protect fungi against ROIs (12, 13, 14, 32), studies are only now being conducted with S. schenckii. We report here that the DHN pathway is responsible for cell wall melanization in S. schenckii. DHN melanin has been reported to be present in many other medically important fungi, and it has been shown to originate from acetate via the pentaketide pathway (34, 37, 45).
Tricyclazole inhibits two reductase reactions in the melanin pathways of V. dahliae (2), Pyricularia oryzae (49), and other fungi (Fig. 1). One of the reactions reduces 1,3,6,8-THN to scytalone, and the other reduces 1,3,8-THN to vermelone. Inhibition at these sites causes the accumulation of melanin intermediates and the shunt product flaviolin or 2-HJ (35). In the present study, when S. schenckii cultures were treated with tricyclazole to block melanin biosynthesis, the culture medium turned reddish brown and, in some experiments, accumulated small amounts of 2-HJ but no flaviolin. One explanation for the absence of flaviolin in the cultures of S. schenckii is that tricyclazole was unable to appreciably inhibit the enzymatic reduction of 1,3,6,8-THN to scytalone. Once scytalone was made, it was dehydrated to 1,3,8-THN and tricyclazole prevented the enzymatic reduction of 1,3,8-THN to vermelone. Since 1,3,8-THN is unstable under culture conditions, it was then autoxidized to 2-HJ (Fig. 1). Earlier studies with V. dahliae (33, 37) and P. oryzae (11, 49) have shown that larger concentrations of tricyclazole and other melanin inhibitors are required to inhibit the reduction of 1,3,6,8-THN than are required to inhibit the reduction of 1,3,8-THN. Our results with S. schenckii are consistent with the fact that flaviolin is often not found in cultures of some fungi treated with larger amounts of tricyclazole, although 2-HJ is usually found (unpublished data).
Two melanin-deficient mutants of S. schenckii were isolated in the present study. The albino Mel−14 was able to produce normal-appearing melanin from scytalone, and this melanin was ultrastructurally identical in appearance to melanin of the wild type. Similar ultrastructural results with scytalone have been obtained with albino mutants of various other fungi, including V. dahliae, E. dermatitidis, Thielaviopsis basicola, Curvularia protuberata, Bipolaris sorokiniana, and Pleospora infectoria (3, 46). Cultures of Mel−14 did not accumulate intermediates from the melanin pathway, and its ability to synthesize melanin from scytalone suggests that the mutation in this strain affects a very early step in the biosynthetic pathway (Fig. 1), probably at polyketide synthase. Cultures of the reddish brown mutant Mel−10 accumulated small amounts of flaviolin and scytalone. Also, Mel−10 did not appear to appreciably metabolize exogenous scytalone to melanin, indicating that this strain was unable to use scytalone and thus was unlike Mel−14 and the wild-type strain. Mutant strains that produce flaviolin and scytalone but which fail to make DHN melanin have been identified in other fungi, including V. dahliae (2), E. dermatitidis (8, 9), and A. fumigatus (39). The mutant strains of these three fungi lack a normal scytalone dehydratase and are unable to enzymatically dehydrate scytalone to 1,3,8-THN. Since cultures of the Mel−10 mutant strain accumulate flaviolin and are unable to metabolize scytalone, it appears that this strain may also have a defective scytalone dehydratase enzyme.
Pathogens must evolve strategies to circumvent the lethal effects of environmental stress such as irradiation and desiccation. Once the fungus enters the host it must contend with the host defense mechanisms, including activated phagocytes where nitric oxide and oxygen intermediates are produced (50). These intermediates have been shown to be fungicidal and fungistatic (1, 50). In the present study, the function of S. schenckii melanin was evaluated in media where the fungus was exposed to UV irradiation and where free radicals (nitrogen- and oxygen-derived species) were generated. Melanized conidia of S. schenckii were less susceptible to killing by ionizing irradiation and by reactive oxygen and nitrogen species. These results support the idea that melanin in S. schenckii is an important component that protects cells from chemical and physical damage, and they suggest that it probably acts as a free-radical scavenger in carrying out physiological defense mechanisms. The role of melanins as free-radical scavengers is described elsewhere (4, 20, 22, 23, 46, 48).
Phagocytosis of microorganisms by host monocytes and macrophages is a basic event in immunity to infection and disease pathogenesis. Ingestion can occur via opsonins deposited on the pathogen surface or via cell surface receptors. In S. schenckii, melanized cells were more resistant to lectin-mediated phagocytosis than nonmelanized cells. The mechanism by which melanin prevents phagocytosis is poorly understood; however, it has been suggested that melanized cells may resist phagocytosis by surface charge effects (14, 41), since melanins are charged polymers and phagocytosis is inversely correlated with cell charge (43).
A consequence of the phagocytosis of microorganisms by monocytes and macrophages is the stimulation of the cell's microbiocidal mechanisms, i.e., the respiratory burst response. This process involves the production of ROIs that are responsible for killing bacteria and fungi. We examined the ability of melanized and albino conidia of S. schenckii to induce the production of ROIs in human monocytes and murine macrophages. Stimulation of the respiratory burst by S. schenckii conidia apparently is not regulated the same way in human monocytes and mouse macrophages. The former stimulated the respiratory, burst while ROIs were not detected in murine macrophages. The reason for the different responses of the monocyte and macrophage populations used in the present study is not known, but similar behavior has been reported for the intracellular parasite Histoplasma capsulatum (26).
Melanized conidia from the wild type and Mel−14p produced fewer ROIs than conidia from the melanin-deficient mutants, Mel−10 and Mel−14. The melanized conidia were also more resistant to killing by human monocytes than the conidia of the two mutants. This could be due to the fact that the pigment present in the melanized conidia scavenged ROIs produced during macrophage stimulation, decreasing chemiluminescence and protecting the conidia.
The melanized Mel−14p strain behaved comparably to the wild type; it was affected significantly less by ROIs than the nonmelanized Mel−10 and Mel−14 strains. It was phagocytized less efficiently and induced fewer ROIs from human monocytes. These studies with Mel−14p demonstrate that the effects detected in the different tests with Mel−14 were the result of mutations that affected enzymes in the melanin biosynthetic pathway and were not the result of other randomly introduced mutations.
Our results suggest that melanin prevents S. schenckii from being killed, enhances protection from UV solar irradiation, and during infection affects host defense mechanisms by reducing phagocytosis and scavenging reactive oxygen and nitrogen species. These findings and earlier findings of others (6, 15, 18, 30, 32, 37, 40, 44) support the possibility that the dark fungal pigment in S. schenckii is a virulence factor. Further investigation of host interactions with whole animals are required to understand how S. schenckii melanin contributes to infection.
ACKNOWLEDGMENTS
This work was supported by grants 4330-M (CONACyT) and IN207296 (PAPIIT-UNAM). A.G.-P. was supported by a CONACyT scholarship.
We thank Marie-Therese Nancy de Merchant and Lilia Robert for electron microscopy work and Lorraine Puckhaber for help with the HPLC.
REFERENCES
- 1.Alspaugh J A, Granger D L. Inhibition of Cryptococcus neoformansreplication by nitrogen oxides supports the role of these molecules as effectors of macrophage-mediated cytostasis. Infect Immun. 1991;59:2291–2296. doi: 10.1128/iai.59.7.2291-2296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell A A, Stipanovic R D, Puhalla J E. Pentaketide metabolites of Verticillium dahliae: identification of (+)-scytalone as a natural precursor to melanin. Tetrahedron. 1976;32:1353–1356. [Google Scholar]
- 3.Bell A A, Wheeler M H. Biosynthesis and functions of fungal melanins. Annu Rev Phytopathol. 1986;24:411–451. [Google Scholar]
- 4.Bustamante J, Bredeston L, Malanga G, Mordoh J. Role of melanin as a scavenger of active oxygen species. Pigment Cell Res. 1993;6:348–353. doi: 10.1111/j.1600-0749.1993.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 5.Butler M J, Day A W. Fungal melanins: a review. Can J Microbiol. 1998;44:1115–1136. [Google Scholar]
- 6.Dixon D M, Migliozzi J, Cooper C R, Jr, Solis O, Breslin B, Szaniszlo P J. Melanized and non-melanized multicellular form mutants of Wangiella dermatitidisin mice: mortality and histopathological studies. Mycoses. 1992;35:17–21. doi: 10.1111/j.1439-0507.1992.tb00814.x. [DOI] [PubMed] [Google Scholar]
- 7.Fogarty R V, Tobin J M. Fungal melanins and their interactions with metals. Enzyme Microb Technol. 1996;19:311–317. doi: 10.1016/0141-0229(96)00002-6. [DOI] [PubMed] [Google Scholar]
- 8.Geis P A, Wheeler M H, Szaniszlo P J. Pentaketide metabolites of melanin synthesis in the dematiaceous fungus Wangiella dermatitidis. Arch Microbiol. 1984;137:324–328. doi: 10.1007/BF00410729. [DOI] [PubMed] [Google Scholar]
- 9.Geis P A, Szaniszlo P J. Carotenoid pigments of the dematiaceous fungus Wangiella dermatitidis. Mycologia. 1984;76:268–273. doi: 10.1007/BF00410729. [DOI] [PubMed] [Google Scholar]
- 10.Greenblatt G A, Wheeler M H. HPLC analysis of fungal melanin intermediates and related metabolites. J Liquid Chomatogr. 1986;9:971–981. [Google Scholar]
- 11.Ishida M, Sumi H, Oku H. Pentachlorobenzyl alcohol, a rice blast control agent. Residue Rev. 1969;25:139–148. doi: 10.1007/978-1-4615-8443-8_12. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson E S, Emery H S. Catecholamine uptake, melanization, and oxygen toxicity in Cryptococcus neoformans. J Bacteriol. 1991;173:401–403. doi: 10.1128/jb.173.1.401-403.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson E S, Tinnell S B. Antioxidant function of fungal melanin. J Bacteriol. 1993;175:7102–7104. doi: 10.1128/jb.175.21.7102-7104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobson E S, Hove E, Emery H S. Antioxidant function of melanin in black fungi. Infect Immun. 1995;63:4944–4945. doi: 10.1128/iai.63.12.4944-4945.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jahn B, Koch A, Schmidt A, Wanner G, Gehinger H, Bhakdi S, Brakhage A. Isolation and characterization of a pigmentless-conidium mutant of Aspergillus fumigatuswith altered conidial surface and reduced virulence. Infect Immun. 1997;65:5110–5117. doi: 10.1128/iai.65.12.5110-5117.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon-Chung K J, Polacheck I, Popkin T J. Melanin-lacking mutants of Cryptococcus neoformansand their virulence for mice. J Bacteriol. 1982;150:1414–1421. doi: 10.1128/jb.150.3.1414-1421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon-Chung K J, Rhodes J C. Encapsulation and melanin formation as indicators of virulence in Cryptococcus neoformans. Infect Immun. 1986;51:218–223. doi: 10.1128/iai.51.1.218-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langfelder K, Jahn B, Gehinger H, Schmidt A, Wanner G, Brakhage A A. Identification of a polyketide synthase gene (pks P) of Aspergillus fumigatusinvolved in conidial pigment biosynthesis and virulence. Med Microbiol Immunol. 1998;187:79–89. doi: 10.1007/s004300050077. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Dawes I W, Roe J H. Adaptive response of Schizosaccharomyces pombeto hydrogen peroxide and menadione. Microbiology. 1995;141:3127–3132. doi: 10.1099/13500872-141-12-3127. [DOI] [PubMed] [Google Scholar]
- 20.Longuet-Higgins H C. On the origin of the free radical property of melanins. Arch Biochem Biophys. 1960;86:231–232. doi: 10.1016/0003-9861(60)90410-0. [DOI] [PubMed] [Google Scholar]
- 21.Mariat F. Observations sur l'ecologie de Sporothrix schenckii et de Ceraticystis stenoceras en Corse et en Alsace, provinces françaises indemnes de sporothicose. Sabouraudia. 1975;13:217–225. [PubMed] [Google Scholar]
- 22.Mason H S, Ingram D J E, Allen B. The free-radical property of melanins. Arch Biochem Biophys. 1960;86:225–230. doi: 10.1016/0003-9861(60)90409-4. [DOI] [PubMed] [Google Scholar]
- 23.Mason H S. The structure of melanin. In: Montagna W, Hu F, editors. Advances in biology of the skin. 8. The pigmentory system. New York, N.Y: Pergamon Press; 1967. pp. 293–312. [Google Scholar]
- 24.Mendonça-Previato L, Gorin P A J, Travassos L R. Galactose-containing polysaccharides from the human pathogens Sporothrix schenckii and Ceratocystis stenoceras. Infect Immun. 1980;29:934–939. doi: 10.1128/iai.29.3.934-939.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagatsu T, Levitt M, Udenfiriend S. Tyrosine hydroxylase. The initial site in norepinephrine biosynthesis. J Biol Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- 26.Newman S L. Macrophages in host defense against Histoplasma capsulatum. Trends Microbiol. 1999;7:67–71. doi: 10.1016/s0966-842x(98)01431-0. [DOI] [PubMed] [Google Scholar]
- 27.Oda L M, Kubelka C F, Alviano C S, Travassos L R. Ingestion of yeast forms of Sporothrix schenckiiby mouse peritoneal macrophages. Infect Immun. 1983;39:497–504. doi: 10.1128/iai.39.2.497-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polacheck I, Hearing V J, Kwon-Chung K J. Biochemical studies of phenoloxidase and utilization of catecholamines in Cryptococcus neoformans. J Bacteriol. 1982;150:1212–1220. doi: 10.1128/jb.150.3.1212-1220.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polacheck I, Kwon-Chung K J. Melanogenesis in Cryptococcus neoformans. J Gen Microbiol. 1988;134:1037–1041. doi: 10.1099/00221287-134-4-1037. [DOI] [PubMed] [Google Scholar]
- 30.Polak A. Melanin as a virulence factor in pathogenic fungi. Mycoses. 1990;33:215–224. doi: 10.1111/myc.1990.33.5.215. [DOI] [PubMed] [Google Scholar]
- 31.Rossi G R, Sastre D A, Rubinstein H R, Masih D T. Biochemical basis for the killing of Cryptococcus neoformansby rat peritoneal cells. J Med Vet Mycol. 1994;32:405–414. [PubMed] [Google Scholar]
- 32.Schnitzler N, Peltroche-Llacsahuanga H, Bestier N, Zündorf J, Lütticken R, Haase G. Effect of melanin and carotenoids of Exophiala (Wangiella) dermatitidison phagocytosis, oxidative burst, and killing by human neutrophils. Infect Immun. 1999;67:94–101. doi: 10.1128/iai.67.1.94-101.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stipanovic R D, Bell A A. Pentaketide metabolites of Verticillium dahliae. 3. Identification of (−)-3,4-dihydro-3,8-dihydroxy-1(2H)-naphthalenone [(−)-vermelone] as a precursor to melanin. J Org Chem. 1976;41:2468–2469. doi: 10.1021/jo00876a026. [DOI] [PubMed] [Google Scholar]
- 34.Taylor B E, Wheeler M H, Szaniszlo P J. Evidence for pentaketide melanin biosynthesis in dematiaceous human pathogenic fungi. Mycologia. 1987;79:320–322. [Google Scholar]
- 35.Tokousbalides M C, Sisler H D. Sites of inhibition by tricyclazole in the melanin biosynthetic pathway of Verticillium dahliae. Pestic Biochem Physiol. 1979;11:64–73. [Google Scholar]
- 36.Torres-Guerrero H, Arenas-Lopez G. UV irradiation induced high frequency of colonial variants with altered morphology in Sporothrix schenckii. Med Mycol. 1998;36:81–87. [PubMed] [Google Scholar]
- 37.Tsai H-F, Washburn R G, Chang Y C, Kwon-Chung K J. Aspergillus fumigatus arp1modulates conidial pigmentation and complement deposition. Mol Microbiol. 1997;26:175–183. doi: 10.1046/j.1365-2958.1997.5681921.x. [DOI] [PubMed] [Google Scholar]
- 38.Tsai H-F, Chang Y C, Washburn R G, Wheeler M H, Kwon-Chung K J. The developmentally regulated alb-1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. J Bacteriol. 1998;180:3031–3038. doi: 10.1128/jb.180.12.3031-3038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai H-F, Wheeler M H, Chang Y C, Kwon-Chung K J. A developmentally related gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J Bacteriol. 1999;181:6469–6477. doi: 10.1128/jb.181.20.6469-6477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vartivarian S E. Virulence properties and nonimmune pathogenetic mechanisms of fungi. Clin Infect Dis. 1992;14(Suppl. 1):s30–s36. doi: 10.1093/clinids/14.supplement_1.s30. [DOI] [PubMed] [Google Scholar]
- 41.Walter H, Graham L L, Krob E J, Hill M. Correlation between phagocytic and membrane surface properties reflected by partitioning of human peripheral blood monocytes in two-polymer aqueous phases. Biochem Biophys Acta. 1980;602:309–322. doi: 10.1016/0005-2736(80)90314-4. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Casadevall A. Decreased susceptibility of melanized Cryptococcus neoformansto UV light. Appl Environ Microbiol. 1994;60:3864–3866. doi: 10.1128/aem.60.10.3864-3866.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Aisen P, Casadevall A. Cryptococcus neoformansmelanin and virulence mechanism of action. Infect Immun. 1995;63:3131–3136. doi: 10.1128/iai.63.8.3131-3136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watanabe A, Ono Y, Fujii I, Sankawa U, Mayorga M E, Timberlake W E, Ebizuka Y. Product identification of polyketide synthase coded by Aspergillus nidulans wAgene. Tetrahedron Lett. 1998;39:7733–7736. [Google Scholar]
- 45.Wheeler M H, Stipanovic R D. Melanin biosynthesis and metabolism of flaviolin and 2-hydroxyjuglone in Wangiella dermatitidis. Arch Microbiol. 1985;142:234–241. doi: 10.1007/BF00693396. [DOI] [PubMed] [Google Scholar]
- 46.Wheeler M H, Bell A A. Melanins and their importance in pathogenic fungi. Curr Top Med Mycol. 1988;2:338–387. doi: 10.1007/978-1-4612-3730-3_10. [DOI] [PubMed] [Google Scholar]
- 47.Wheeler M H, Klich M A. The effects of tricyclazole, pyroquilon, phthalide, and related fungicides on the production of conidial wall pigments by Penicillium and Aspergillusspecies. Pestic Biochem Physiol. 1995;52:125–136. [Google Scholar]
- 48.White L P. Melanin: a naturally occurring cation exchange material. Nature. 1958;182:1427–1428. doi: 10.1038/1821427a0. [DOI] [PubMed] [Google Scholar]
- 49.Woloshuk C P, Sisler H D, Tokousbalides M C, Dutky S R. Melanin biosynthesis in Pyricularia oryzae: site of tricyclazole inhibition and pathogenicity of melanin deficient mutants. Pestic Biochem Physiol. 1980;14:256–264. [Google Scholar]
- 50.Yoshida K, Akaike T, Doi T, Sato K, Ijiri S, Suga M, Ando M, Maeda H. Pronounced enhancement of ṄO-dependent antimicrobial action by an ṄO-oxidizing agent, imidazolineoxyl N-oxide. Infect Immun. 1993;61:3552–3555. doi: 10.1128/iai.61.8.3552-3555.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]