Abstract
Trehalose 6,6′-dimycolate (TDM) is a cell surface molecule of Mycobacterium tuberculosis. TDM induced a loss of body weight and prominent granulomas in the liver and lungs by the intravenous injection of TDM into rabbits. TDM also induced atrophy of the thymus and spleen due to apoptosis. By contrast, sulfolipid (2,3,6,6′-tetraacyl trehalose 2′-sulfate) induced neither toxicity, nor granuloma formation, nor atrophy of the thymus and spleen. In rabbits the histopathological changes were more dramatic than in mice. The rabbit model may be more sensitive and may provide more information on the beneficial or pathological effects of TDM.
Tuberculosis has recently been reemerging, owing to human immunodeficiency virus infection, overcrowded populations, poverty, and the appearance of drug-resistant mycobacteria. Granuloma formation followed by caseous necrosis, liquefaction, and subsequent cavity formation are central processes in the pathology of human pulmonary tuberculosis, and these changes are induced by cell-mediated immune responses to mycobacterial infection (4, 16, 17). However, precise mechanisms and processes for the development of tuberculosis have not been fully clarified, particularly concerning the role of each cellular component or product of Mycobacterium tuberculosis at the molecular level. Among mycobacterial components, only trehalose 6,6′-dimycolate (TDM) can induce granulomatous lesions in experimental animals without protein antigen. To explore the role of delayed-type hypersensitivity to mycobacterial antigens, most immunologic studies of tuberculosis have been carried out using mice and guinea pigs (4).
Mice cannot develop cavitary lesions induced by tuberculosis. It has been reported that granulomatous changes in rabbit lungs are followed by caseous necrosis, liquefaction, and finally cavity formation by direct intrapulmonary injection of live or heat-killed bacilli or their components, including lipoprotein antigen and glycolipid (12, 14, 22). In the process, hydrolytic enzymes, including nucleases, proteinases, and cathepsin D from activated macrophages, participate in liquefaction and cavity formation (3–6). Using mouse models, we previously demonstrated granuloma- and cytokine-inducing activities of mycolyl glycolipids such as TDM (10, 15, 18, 20, 23). In mice, TDM and related mycolyl glycolipids (glucose mycolate or trehalose monomycolate) can induce foreign-body-type granulomas in the absence of protein antigens, although TDM cannot induce further pathological changes, even in hyperimmune mice. The rabbit is the only animal that readily produces tuberculous cavities (3, 22). Furthermore, tuberculosis in rabbits resembles human disease more closely than does tuberculosis in any other animal species (4, 13). The present study is the first report describing granuloma formation in rabbits that has been induced by the intravenous administration of protein-free TDM and the absence of granulomas with related glycolipids such as sulfolipid (SL; 2,3,6,6′-tetraacyltrehalose 2′-sulfate), another virulence factor of M. tuberculosis.
Rabbits.
Specific-pathogen-free New Zealand White female rabbits, 8 weeks old (average body weight, 1.7 kg), were purchased from Japan KEARI Co. (Gifu, Japan).
Preparation of TDM and SL.
TDM and SL were extracted from the total lipid of heat-killed M. tuberculosis AOYAMA-B. Each glycolipid was finally separated by preparative thin-layer chromatography of silica gel (Uniplate; 20 by 20 cm, 250 μm; Analtech, Inc., Newark, Del.). The purity of glycolipid was confirmed by fast atom bombardment mass spectrometry of the intact molecule with a JMSSX102A double-focusing mass spectrometer (JEOL, Tokyo, Japan) before and after hydrolysis of the cord factor. The result showed that the only hydrolysis products were α-, methoxy-, and keto-mycolic acids and trehalose (7).
In vivo administration of water-oil-water (w/o/w) emulsion of glycolipids.
Purified TDM or SL was emulsified with 0.2% Tween 80 and 3.2% Freund's incomplete adjuvant (Difco, Detroit, Mich.) in 0.1 M phosphate-buffered saline to form w/o/w emulsion (18, 20). As controls, w/o/w emulsion micelles without glycolipids were used. Rabbits were injected intravenously with various doses of TDM or SL in the form of a w/o/w emulsion. Rabbits were sacrificed on day 2, 7, or 21 after the injection. For organ index determinations, lungs, liver, spleen, and thymus were removed. The organ index was calculated as the organ weight (in grams)/body weight (in grams) × 100. For histological examination, organs were fixed in 10% formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin (HE).
Histopathological examination.
To detect apoptotic changes, sections of the spleen and thymus were stained by TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) (TACSTM In Situ Apoptosis Detection Kit; Trevigen, Inc., Gaithersburg, Md.).
Statistical analysis.
Data were analyzed by using Statview 5.0 (SAS Institute, Inc., Cary, N.C.) and expressed as the mean ± the standard deviation (SD). Data that appeared to be statistically significant were compared by an analysis of variance designed for comparing the means of multiple groups and then considered significant if the P values were <0.05.
Toxicity of TDM and SL.
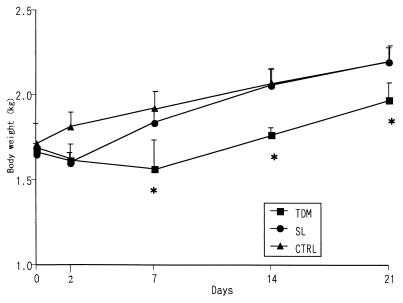
TDM induces a lethal toxicity in mice, when injected intravenously in w/o/w emulsion micelles (2). Intravenous injection of TDM into rabbits resulted in diarrhea and delay in the body weight increase in a dose-dependent fashion. Lethal toxicity was not observed in the rabbits administered a dose of 20 mg of TDM until 21 days after the challenge. The toxic effect on the rabbit body weight continued at least for 21 days after a single injection of TDM (Fig. 1). Administration of vehicle micelles served as a control, and SL induced neither lethal toxicity nor a delay in the increase in rabbit body weights.
FIG. 1.
Time-dependent changes in body weight in rabbits challenged with either TDM or SL derived from M. tuberculosis AOYAMA-B. The data represent the mean ± the SD compiled from experiments with three to nine rabbits per condition. ∗, significant difference (P < 0.05) compared to the SL or control (CTRL) group.
Induction of granuloma formation.
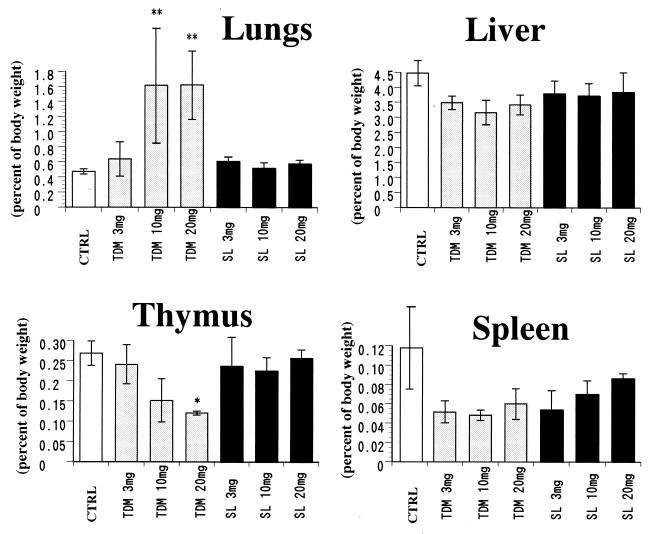
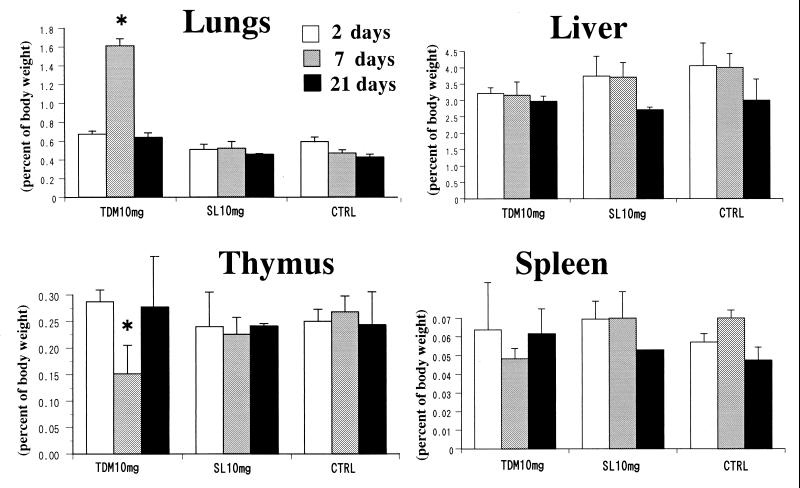
TDM induces foreign-body-type granulomas in mice by intravenous injection of w/o/w emulsion micelles (1). Intravenous injection of 3 to 20 mg of TDM per rabbit induced maximal granulomatous lesions in lungs 7 days after the injection. More than threefold increases in the lung weight and index were observed in a dose-dependent manner (Fig. 2). The lung weight and index returned to baseline levels 21 days after injection (Fig. 3). Grossly, lungs were enlarged and showed marked white granular surfaces, which in some parts fused with each other to form large and tightly infiltrated granulomas entirely in groups of rabbits given 10 to 20 mg of TDM. The weight of the liver and the liver index were not changed or rather decreased slightly for 7 to 21 days after injection of TDM, although small granulomas were clearly observed grossly 7 days after the injection. SL, however, did not induce significant granulomas in lungs and liver at 7 to 21 days after the challenge. Administration of w/o/w emulsion alone did not result in any significant changes in organ indices.
FIG. 2.
Dose-related changes in organ indices of rabbits 7 days after the challenge with either TDM or SL. A more than threefold increase in the lung index was observed in rabbits given TDM. SL did not have any effect. Following the administration of TDM, thymic and splenic weights decreased to one-half of those of the controls. The data indicate the mean ± the SD compiled from experiments with three to nine rabbits per condition. Asterisks indicate a significant difference (∗, P < 0.05; ∗∗, P < 0.01) compared to the SL or control (CTRL) group.
FIG. 3.
Time-related changes in organ indices of rabbits 2, 7, and 21 days after the injection with TDM or SL. The lung index and the atrophy of the thymus reached a peak at day 7 in rabbits given TDM. SL did not produce any changes. The data represent the mean ± the SD compiled from experiments with three to nine rabbits per condition. *, a significant difference (P < 0.05) compared to the SL or control (CTRL) group.
Induction of thymic and splenic atrophy.
TDM induces remarkable atrophy of the thymus in mice due to apoptosis when injected intravenously in w/o/w emulsion micelles (18). Intravenous injection of TDM into rabbits induced peak atrophy of both the thymus and the spleen by day 7. The weights of the thymus and spleen were reduced to one-half or less of those of the controls (Fig. 2). Atrophy of the thymus and spleen was dose responsive, and organ weights reached a minimum on day 7 after the injection and returned to the control level on day 21, paralleled by the lung granuloma formation and resolution (Fig. 3). Administration of emulsion without glycolipids or with SL showed neither atrophy nor a decrease in the organ weight of the rabbit thymus or spleen.
Histopathological changes.
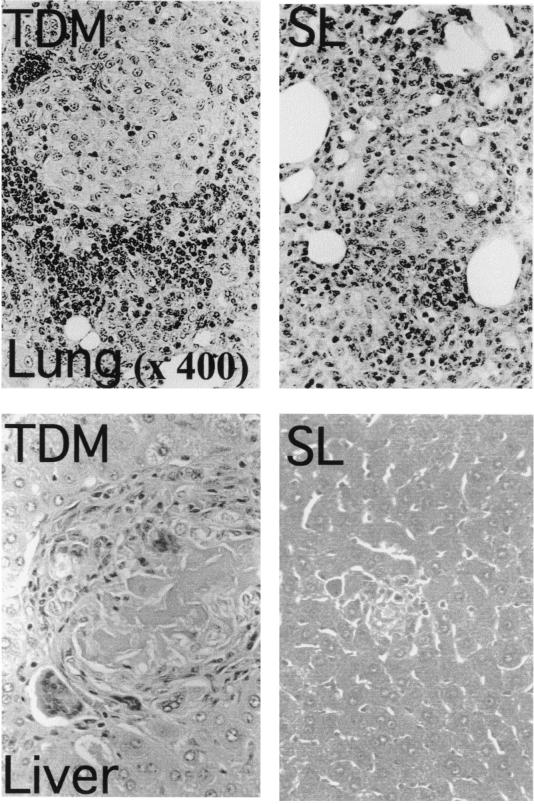
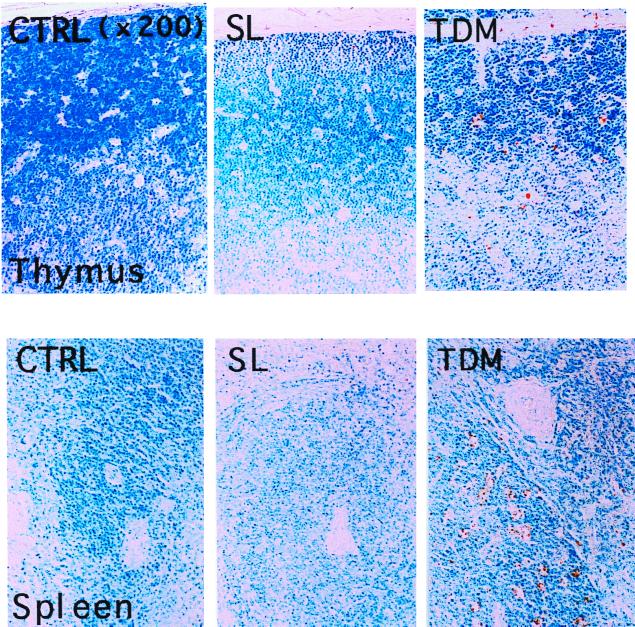
Histological changes were observed distinctively in the lungs, liver, thymus, and spleen 7 days after injection of TDM. In rabbits given 20 mg of TDM, the lungs showed solidification due to the loss of normal alveolar structure. Numerous lightly stained mature epithelioid cells and lymphocytes with fewer neutrophils infiltrated into the alveolar space to form well-organized granulomas, although no distinctive caseation and necrosis were observed (Fig. 4). Multinucleated giant cells could be observed occasionally. Scattered areas of pneumonia and perivasculitis were also seen in the lungs. SL did not induce granulomas in the lungs and liver, although focal pneumonia, mild perivasculitis, and slight macrophage infiltration were found. In the livers of rabbits injected with 10 to 20 mg of TDM, significant granuloma formation was observed (Fig. 4). These granulomas were solitary but highly organized and islet-like. In the livers of rabbits injected with 20 mg of TDM, foreign-body-type multinucleated giant cells and lymphocyte infiltration were observed mostly in the marginal region of granulomatous lesions. It was particularly noted that in the central region of liver granulomas, significant focal necrosis of hepatocytes was observed, suggesting that a large amount of TDM administration might induce liver damage due to its potent toxicity (11). In thymus tissues of rabbits injected with 10 to 20 mg of TDM, apoptotic nuclei or nuclear condensation was observed by HE and TUNEL staining (Fig. 5). Electron microscopically marked nuclear condensation and karyorrhectic changes in thymic cortical lymphocytes were observed (data not shown). In the spleens of rabbits given 10 to 20 mg of TDM, both granulomatous and apoptotic changes were observed in the white pulp. In addition, follicular hyperplasia and splenitis were observed in rabbits injected with TDM. Follicle formation, indicating the development of humoral immunity, was also observed in the spleens of TDM-injected rabbits. SL did not induce any significant changes such as apoptosis in the thymus and spleen (Table 1). Granulomatous changes in lungs and livers continued up to 21 days after the challenge, but the size and number of lung granulomas decreased significantly. By contrast, rabbits given SL did not show significant granulomatous changes in lungs and livers on days 7 to 21. The apoptotic changes in the thymus and spleen elicited by administration of TDM disappeared by day 21; however, granulomas of the spleen and splenitis induced by TDM or SL (10 mg) continued for a longer period of time.
FIG. 4.
Histopathology of lung and liver granulomas in a rabbit challenged with TDM or SL (HE stain). The rabbit was sacrificed 7 days after the challenge with 20 mg of TDM. In the lung, alveolar spaces were fully replaced with granulomatous lesions (>90% of alveolar space area), and the normal alveolar structure disappeared. The development of lung granulomas was dose responsive. Typical granulomas induced by TDM were characteristic in that they consisted of large macrophages with lightly stained round nuclei (epithelioid cells) infiltrated into the central region with lymphocytes with densely stained nuclei in the marginal region. In the livers of rabbits given 20 mg of TDM, solitary granulomas with occasional multinucleated giant cells were seen. The number of solitary granulomas was dependent on the dose of TDM injected. In the central region, macrophages, epithelioid cells, and hepatocytes were stained lightly, and their nuclei could hardly be seen, a result probably due to necrosis or apoptosis. Lymphocytes infiltrated into the marginal region of granulomas were observed.
FIG. 5.
Apoptosis of thymocytes and splenocytes in rabbits challenged with SL or TDM (TUNEL stain). Rabbits were sacrificed 7 days after the challenge. Cortical lymphocytes were decreased, and apoptosis was noted in the thymus of a rabbit given TDM. Foci of apoptotic lymphocytes could be seen in the white pulp of the spleen from a rabbit challenged with TDM.
TABLE 1.
Histopathological findings in New Zealand White rabbits given a single intravenous administration of glycolipids from M. tuberculosis AOYAMA-Ba
| Tissue | Finding(s) | Score with w/o/w emulsion control | Score with TDM dose of:
|
Score with SL dose of:
|
||||
|---|---|---|---|---|---|---|---|---|
| 3 mg | 10 mg | 20 mg | 3 mg | 10 mg | 20 mg | |||
| Lung | Granulomas | + | ++ | ++ | +++ | ± | − | − |
| Mature and immature macrophages | ± | ++ | ++ | +++ | − | + | + | |
| Epithelioid cells | − | + | +++ | +++ | − | − | − | |
| Lymphocytes | − | + | + | + | − | − | − | |
| Multinucleated giant cells | − | ± | + | + | − | − | − | |
| Focal pneumonia and/or perivasculitis | + | + | + | + | + | + | + | |
| Liver | Granulomas, interlobular | ± | ± | + | + | − | − | ± |
| Epithelioid cells | − | + | ++ | ++ | − | − | − | |
| Lymphocytes | − | + | + | + | − | − | − | |
| Multinucleated giant cells | − | ± | ++ | ++ | − | − | − | |
| Granulomas, portal tract | ± | + | + | + | − | − | − | |
| Necrosis, hepatocytes, focal | − | − | + | ++ | − | − | − | |
| Spleen | Apoptosis, white pulp | − | − | + | + | − | − | − |
| Granulomas, white pulp | + | ± | ± | + | − | + | + | |
| Follicular hyperplasia | − | + | + | + | − | − | − | |
| Splenitis | − | + | + | + | + | + | + | |
| Thymus | Apoptosis, cortex | − | − | + | + | − | − | − |
Grade: ±, very slight; +, slight; ++, moderate; +++, marked; −, not detected.
TDM is a toxic substance in mice, although it possesses potent adjuvant activity by stimulating cell-mediated immunity with coadministration of protein antigen (19). This adjuvant activity and granulomatogenic activity can be further potentiated by costimulation with other cellular components, such as cell wall skeleton derived from tubercle bacilli (19). Because mycobacterial TDM showed a potent toxicity and marked granuloma-inducing activity in rabbits, these highly organized granulomatous lesions may play an important role in bactericidal activity. On the other hand, since mycobacterial TDM has been reported to be toxic in mice due to the inhibition of liver mitochondrial electron transport systems (11), such a toxic effect may play a role in the liver damage in rabbits. At present, it is difficult to conclude whether such toxic effects induce tissue necrosis directly or indirectly, and we need more precise and immunological observation of histological changes of granulomatous tissues after TDM administration.
Thymic atrophy induced by TDM was closely related to granuloma formation in mice (18). Because thymectomized mice fail to develop Mycobacterium bovis BCG cell wall-induced lung granulomas but restore their capacity to respond after the transfer of thymic or splenic cells, thymocytes appeared to be necessary for T-cell-mediated granulomatous responses in mice (9). TDM-induced granulomas in rabbits were also paralleled by marked atrophy of the thymus and spleen, suggesting that thymus or T lymphocytes may play an important role in the development of lung granulomas. SL did not induce granuloma formation or cause thymic and splenic atrophy. Although TDM and SL possess a common structure in having a trehalose moiety, the different biological effects of these two compounds observed here must be derived from the differences in the fatty acid moieties (8). Such cell surface molecules make contact with host cells and stimulate them at the early stage of the infection. Mycobacterial TDM may contact a larger part of the host cell surface than SL does, thus causing extensive stimulation of host cells.
The exact physiological role of apoptosis induced by TDM must be clarified. The thymic and splenic atrophy produced by TDM could result in the negative selection, involving activation-induced T-cell death, before the specific cellular immunity against M. tuberculosis antigen develops. We have reported previously that abundant NK cells and extrathymic T lymphocytes are found in lung granulomas induced by TDM challenge in mice (20). The biological effect of TDM is a double-edged sword in tuberculosis. TDM shows granuloma-inducing activity, a benefit associated with gamma interferon production and intracellular killing (21). In contrast, TDM shows detrimental functions such as lethal toxicity, loss of body weight, liver damage, and temporal immunosuppression in mice and rabbits. Probably, the balance of these two potentials determines whether the disease progresses or regresses.
The challenge for the future will be to understand and clarify the precise role of TDM in the pathophysiology of mycobacterial infections in rabbits. Such an understanding may open new avenues for better interventions against tuberculosis in rabbits as well as humans.
Acknowledgments
We thank Tetsuo Kuroki for helpful suggestions.
This work was supported by grants of Research on Emerging and Re-emerging Infectious Diseases (Health Sciences Research Grants, Ministry of Health and Welfare) and The United States-Japan Cooperative Medical Science Program against Tuberculosis and Leprosy.
REFERENCES
- 1.Bekierkunst A, Levij I S, Yarkoni E, Vilkas E, Adam A, Lederer E. Granuloma formation induced in mice by chemically defined mycobacterial fractions. J Bacteriol. 1969;100:95–102. doi: 10.1128/jb.100.1.95-102.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloch H. Studies on the virulence of tubercle bacilli. Isolation and biological properties of a constituent of virulent organisms. J Exp Med. 1950;91:197–219. doi: 10.1084/jem.91.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Converse P J, Dannenberg A M, Jr, Estep J E, Sugisaki K, Abe Y, Schofield B H, Pitt M L M. Cavitary tuberculosis produced in rabbits by aerosolized virulent tubercle bacilli. Infect Immun. 1996;64:4776–4787. doi: 10.1128/iai.64.11.4776-4787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dannenberg A M., Jr Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunol Today. 1991;12:228–233. doi: 10.1016/0167-5699(91)90035-R. [DOI] [PubMed] [Google Scholar]
- 5.Dannenberg A M., Jr Immunopathogenesis of pulmonary tuberculosis. Hosp Pract. 1993;15:51–58. doi: 10.1080/21548331.1993.11442738. [DOI] [PubMed] [Google Scholar]
- 6.Dannenberg A M., Jr . Rabbit model of tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C.: ASM Press; 1994. pp. 149–156. [Google Scholar]
- 7.Fujiwara N, Pan J, Enomoto K, Terano Y, Honda T, Yano I. Production and partial characterization of anti-cord factor (trehalose-6,6′-dimycolate) IgG antibody in rabbits recognizing mycolic acid subclass of Mycobacterium tuberculosis or Mycobacterium avium. FEMS Immunol Med Microbiol. 1999;24:141–149. doi: 10.1111/j.1574-695X.1999.tb01275.x. [DOI] [PubMed] [Google Scholar]
- 8.Goren M B, Brokl O, Das B C. Sulfatides of Mycobacterium tuberculosis: the structure of the principal sulfatide (SL-1) Biochemistry. 1976;15:2728–2735. doi: 10.1021/bi00658a003. [DOI] [PubMed] [Google Scholar]
- 9.Kakimura M, Onoe K, Okada M, Kimura T, Kato K, Okuyama H, Morikawa K, Yamamoto K. Failure of C3H mice to develop lung granuloma after intravenous injection of BCG cell wall vaccine. Immunology. 1981;43:1–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Kaneda K, Sumi Y, Kurano F, Kato Y, Yano I. Granuloma formation and hemopoiesis induced by C36–48-mycolic acid-containing glycolipids from Nocardia rubra. Infect Immun. 1986;54:869–875. doi: 10.1128/iai.54.3.869-875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato M. Studies of a biochemical lesion in experimental tuberculosis in mice. VIII. Structural and functional damage in mouse liver mitochondria under the toxic action of cord factor. Am Rev Respir Dis. 1968;98:260–269. doi: 10.1164/arrd.1968.98.2.260. [DOI] [PubMed] [Google Scholar]
- 12.Lurie M B. The correlation between the histological changes and the fate of living tubercle bacilli in the organs of tuberculous rabbits. J Exp Med. 1932;55:31–54. doi: 10.1084/jem.55.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lurie M B. Resistance to tuberculosis: experimental studies in native and acquired defensive mechanisms. Cambridge, Mass: Harvard University Press; 1964. [Google Scholar]
- 14.Maeda H, Yamamura Y, Ogawa Y. Mycobacterial antigens relating to experimental pulmonary cavity formation. Am Rev Respir Dis. 1977;115:617–623. doi: 10.1164/arrd.1977.115.4.617. [DOI] [PubMed] [Google Scholar]
- 15.Matsunaga I, Oka S, Fujiwara N, Yano I. Relationship between induction of macrophage chemotactic factors and formation of granulomas caused by mycolyl glycolipids from Rhodococcus ruber (Nocardia rubra) J Biochem. 1996;120:663–670. doi: 10.1093/oxfordjournals.jbchem.a021463. [DOI] [PubMed] [Google Scholar]
- 16.Myrvik Q N. Immunopathology of pulmonary tuberculosis. Adv Exp Med Biol. 1982;155:649–657. doi: 10.1007/978-1-4684-4394-3_71. [DOI] [PubMed] [Google Scholar]
- 17.Myrvik Q N, Leake E S, Tenner-Racz K. Interaction of mycobacteria with normal and immunologically activated alveolar macrophages. Adv Exp Med Biol. 1983;162:83–98. doi: 10.1007/978-1-4684-4481-0_9. [DOI] [PubMed] [Google Scholar]
- 18.Ozeki Y, Kaneda K, Fujiwara N, Morimoto M, Oka S, Yano I. In vivo induction of apoptosis in the thymus by administration of mycobacterial cord factor (trehalose 6,6′-dimycolate) Infect Immun. 1997;65:1793–1799. doi: 10.1128/iai.65.5.1793-1799.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribi E, Granger D L, Milner K C, Yamamoto K, Strain S M, Parker R, Smith R W, Brehmer W, Azuma I. Induction of resistance to tuberculosis in mice with defined components of mycobacteria and with some unrelated materials. Immunology. 1982;46:297–305. [PMC free article] [PubMed] [Google Scholar]
- 20.Tabata T, Kaneda K, Watanabe H, Abo T, Yano I. Kinetics of organ-associated natural killer cells and intermediate CD3 cells during pulmonary and hepatic granulomatous inflammation induced by mycobacterial cord factor. Microbiol Immunol. 1996;40:651–658. doi: 10.1111/j.1348-0421.1996.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe K, Hasunuma R, Horikoshi T, Yamana H, Maruyama H, Fujiwara N, Kumazawa Y, Yano I. Induction of hypersensitivity to endotoxin lethality in mice by treatment with trehalose 6,6′-dimycolate but not with 2,3,6,6′-tetraacyl trehalose 2′-sulfate. J Endotoxin Res. 1999;5:23–30. [Google Scholar]
- 22.Yamamura Y, Maeda H, Ogawa Y. Experimental pulmonary cavity formation by mycobacterial components and synthetic adjuvants. Microbiol Immunol. 1986;30:1175–1187. doi: 10.1111/j.1348-0421.1986.tb03046.x. [DOI] [PubMed] [Google Scholar]
- 23.Yano I, Tomiyasu I, Kitabatake S, Kaneda K. Granuloma forming activity of mycolic acid-containing glycolipids in Nocardia and related taxa. Acta Leprol. 1984;2:341–349. [PubMed] [Google Scholar]