Abstract
Background
Venovenous extracorporeal membrane oxygenation (ECMO) can be considered for patients with COVID-19-associated acute respiratory distress syndrome (ARDS) who continue to deteriorate despite evidence-based therapies and lung-protective ventilation. The Extracorporeal Life Support Organization has emphasised the importance of patient selection; however, to better inform these decisions, a comprehensive and evidence-based understanding of the risk factors associated with poor outcomes is necessary. We aimed to summarise the association between pre-cannulation prognostic factors and risk of mortality in adult patients requiring venovenous ECMO for the treatment of COVID-19.
Methods
In this systematic review and meta-analysis, we searched MEDLINE and Embase from Dec 1, 2019, to April 14, 2022, for randomised controlled trials and observational studies involving adult patients who required ECMO for COVID-19-associated ARDS and for whom pre-cannulation prognostic factors associated with in-hospital mortality were evaluated. We conducted separate meta-analyses of unadjusted and adjusted odds ratios (uORs), adjusted hazard ratios (aHRs), and mean differences, and excluded studies if these data could not be extracted. We assessed the risk of bias using the Quality in Prognosis Studies tool and certainty of evidence using the Grading of Recommendations Assessment, Development and Evaluation approach. Our protocol was registered with the Open Science Framework registry, osf.io/6gcy2.
Findings
Our search identified 2888 studies, of which 42 observational cohort studies involving 17 449 patients were included. Factors that had moderate or high certainty of association with increased mortality included patient factors, such as older age (adjusted hazard ratio [aHR] 2·27 [95% CI 1·63–3·16]), male sex (unadjusted odds ratio [uOR] 1·34 [1·20–1·49]), and chronic lung disease (aHR 1·55 [1·20–2·00]); pre-cannulation disease factors, such as longer duration of symptoms (mean difference 1·51 days [95% CI 0·36–2·65]), longer duration of invasive mechanical ventilation (uOR 1·94 [1·40–2·67]), higher partial pressure of arterial carbon dioxide (mean difference 4·04 mm Hg [1·64–6·44]), and higher driving pressure (aHR 2·36 [1·40–3·97]); and centre factors, such as less previous experience with ECMO (aOR 2·27 [1·28–4·05].
Interpretation
The prognostic factors identified highlight the importance of patient selection, the effect of injurious lung ventilation, and the potential opportunity for greater centralisation and collaboration in the use of ECMO for the treatment of COVID-19-associated ARDS. These factors should be carefully considered as part of a risk stratification framework when evaluating a patient for potential treatment with venovenous ECMO.
Funding
None.
Introduction
COVID-19 is an important cause of acute respiratory distress syndrome (ARDS)1, 2 and indication for invasive mechanical ventilation. However, despite best practice management, including the use of evidence-based therapies3 and lung-protective ventilation strategies,4, 5 some patients with COVID-19 will continue to deteriorate. Informed by existing evidence from studies of patients with ARDS unrelated to COVID-19,6, 7, 8, 9 several international medical organisations have recommended that venovenous extracorporeal membrane oxygenation (ECMO) is considered for such patients.10, 11
A meta-analysis including nearly 1900 patients with COVID-19 who were supported with ECMO during the first year of the pandemic showed that these patients had similar outcomes to those with non-COVID-19-associated ARDS,12 highlighting the potential effectiveness of ECMO in the treatment of carefully selected patients with COVID-19. However, this analysis represented only the initial experience with ECMO in the treatment of COVID-19, predominantly during the first wave of the pandemic. Later studies showed an increase in mortality of patients with COVID-19 who were treated with ECMO, possibly due to changes in concomitant treatments (such as corticosteroids, immunomodulators, and non-invasive ventilation), patient selection, and SARS-CoV-2 variants.13
Research in context.
Evidence before this study
Venovenous extracorporeal membrane oxygenation (ECMO) can be considered for patients with COVID-19-associated acute respiratory distress syndrome (ARDS) who continue to deteriorate despite evidence-based therapies and lung-protective ventilation. The Extracorporeal Life Support Organization has emphasised the importance of prioritising patients who are most likely to benefit from the treatment, requiring a comprehensive and evidence-based understanding of the risk factors associated with poor outcomes; however, to our knowledge, no systematic reviews have summarised the association between important prognostic factors and risk of mortality for this patient population. We searched MEDLINE and Embase from Dec 1, 2019, to April 14, 2022, using clinical content terms including “SARS-CoV-2”, “COVID-19”, “extracorporeal membrane oxygenation”, and “ECMO”, and prognostic methodology terms including “predict”, “model”, “risk”, and “mortality”. Randomised controlled trials and observational studies were eligible if they included adult patients with confirmed COVID-19 who required ECMO for COVID-associated ARDS and for whom pre-cannulation prognostic factors associated with in-hospital mortality were evaluated. We excluded case reports or case series with fewer than five patients, and studies that did not provide sufficient data to extract or calculate unadjusted odds ratios, adjusted odds ratios, adjusted hazard ratios, or mean differences.
Added value of this study
This systematic review and meta-analysis summarises the association between several pre-cannulation prognostic factors and the risk of mortality in patients requiring venovenous ECMO for COVID-19. Factors with moderate or high certainty of association with increased mortality included patient factors, such as older age, male sex, and chronic lung disease; pre-cannulation disease factors, such as longer duration of symptoms, longer duration of invasive mechanical ventilation, higher partial pressure of arterial carbon dioxide, and higher driving pressure; and centre factors, such as lower previous ECMO volume. Taken together, these findings provide the framework for evidence-based risk stratification of patients with COVID-19 who require venovenous ECMO.
Implications of all the available evidence
The prognostic factors identified highlight several well established principles of effective ARDS and ECMO care: the importance of patient selection, the effect of injurious lung ventilation, and the potential opportunity for greater centralisation and collaboration in the use of ECMO across centres and regions. We advocate for the careful consideration of these prognostic factors as part of a risk stratification framework when evaluating a patient's potential indication for venovenous ECMO for the treatment of COVID-19.
A 2022 multicontinental comparative effectiveness study showed that ECMO was associated with a reduction in mortality for some patients with COVID-19.14 However, the scarcity of ECMO resources during the COVID-19 pandemic15 and the high associated costs16 probably affected patient selection in many health-care systems throughout the world.17 Recognising the intensity of resources required and the potential for major complications associated with ECMO, the Extracorporeal Life Support Organization (ELSO) emphasised the importance of appropriate patient selection—ie, prioritising those most likely to benefit.10
To better inform appropriate patient selection, a comprehensive and evidence-based understanding of risk factors associated with poor outcomes is necessary. We conducted a systematic review and meta-analysis with the aim of summarising the association between pre-cannulation prognostic factors (including patient factors, disease factors, and centre factors) and the risk of in-hospital mortality in adult patients receiving venovenous ECMO for acute respiratory failure secondary to COVID-19.
Methods
Search strategy and selection criteria
For this systematic review and meta-analysis, we adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines,18 the Prognosis Research Strategy Group (PROGRESS) recommendations,19, 20, 21, 22 the Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modeling Studies (CHARMS) checklist,23 and guidelines for meta-analyses of prognostic factor studies.24
An experienced systematic review methodologist (BR) assisted in the development of the search strategy. We searched MEDLINE and Embase from Dec 1, 2019, to April 14, 2022, using clinical content terms combined with terms related to prognostic research, consistent with similar prognostic meta-analyses and recommendations from the PROGRESS group.25, 26, 27, 28 The full search strategy is included in the appendix (p 2).
We included randomised controlled trials and observational studies published in English that included adult patients (aged ≥16 years) with COVID-19 confirmed by PCR, patients requiring any configuration of ECMO for COVID-19-associated ARDS (at least 90% of studies included venovenous ECMO exclusively), and the evaluation of pre-cannulation prognostic factors associated with in-hospital mortality. We excluded case reports or case series with fewer than five patients, and studies that did not provide sufficient data to extract or calculate unadjusted odds ratios (uORs), adjusted odds ratios (aORs), adjusted hazard ratios (aHRs), or mean differences. ORs and HRs were considered to be adjusted if calculated with confounding adjustment in a multivariable logistic regression (OR) or Cox proportional hazards (HR) model. If these values could not be obtained from the reported data, we contacted the corresponding authors of the studies for clarification.
We screened studies using Covidence (Melbourne, VIC, Australia). We imported titles into Covidence directly from the databases and removed duplicates. Two reviewers (AT and SMF) independently screened the titles and abstracts of all identified studies and resolved disagreements by discussion; no third-party adjudication was necessary. The same two reviewers then independently assessed the full text of the selected studies, and disagreements were resolved by discussion.
Data analysis
Two investigators (AT and SMF) extracted the following variables: author information, year of publication, study design, study dates, eligibility criteria, prognostic factors available before cannulation for ECMO, and mortality as defined by study authors. Prognostic factors included patient factors, such as age, sex, pre-existing comorbidities, and obesity (as defined by study authors); disease factors, such as the duration of invasive mechanical ventilation before ECMO or the duration of symptoms (as defined by study authors), and physiological markers of oxygenation and ventilation, such as the ratio of partial pressure of arterial oxygen to fractional concentration of oxygen in inspired air (PaO2/FiO2) or respiratory system compliance; and centre factors, such as more previous experience (higher-volume centres) or less previous experience (lower-volume centres) with ECMO (as defined by study authors). For each prognostic factor, two investigators (AT and SMF) independently collected or calculated ORs and HRs for mortality. For mean differences, means and corresponding SDs were collected or estimated from medians and IQRs using Wan's method.29 In the event of overlapping patient cohorts, we preferentially included data from the larger patient cohort. We extracted data using a modified CHARMS checklist for prognostic factors.23
Two reviewers (AT and SMF) independently assessed the risk of bias in the included studies using the Quality in Prognosis Studies tool.30 Disagreements were resolved by consensus following discussion. Specific domains were judged to be at low, moderate, or high risk of bias. Funnel plots were constructed for analyses with at least ten included studies to evaluate for publication bias.
We extracted or calculated uORs, aORs, aHRs, and mean differences on the basis of available data and mortality as defined by the study authors. Specifically, the primary outcome of interest was in-hospital mortality, and alternative definitions are addressed in the sensitivity analysis. We conducted meta-analyses of uORs, aORs, aHRs, and mean differences separately using the random-effects method31 and the Review Manager software (version 5.3; Cochrane, Copenhagen, Denmark). In accordance with Cochrane guidance, given the high event rate for mortality in this review, we pooled aOR and aHR in separate analyses.32 We present results as pooled uORs, aORs, aHRs, and mean differences with 95% CIs. We assessed statistical heterogeneity using the I 2 statistic, the χ2 test for homogeneity, and visual inspection of the forest plots.
An investigator (BR) with expertise in the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology assessed overall certainty in pooled estimates using the GRADE approach for the meta-analyses of prognostic studies.33 All assessments were reviewed with the whole author group, discussed, and approved with unanimous consent. The overall certainties in estimates were categorised into one of four levels: high, moderate, low, or very low. Consistent with GRADE guidance for prognostic studies, observational data start as high-certainty evidence, but certainty can be downgraded as a result of concern for the precision, consistency, risk of bias, directness, or publication bias. A GRADE evidence profile was created with the GRADEpro Guideline Development Tool. If multiple analyses were available for the same prognostic factor, including those based on different categorisation thresholds and those with or without confounding adjustment, we highlighted results from the analysis with the highest certainty. Consistent with GRADE recommendations, high certainty was described as was associated, moderate certainty was described as was probably associated, low certainty was described as may have been associated, and very low certainty was described as uncertain.34
We conducted two pre-specified sensitivity analyses (appendix p 34–35). In the first analysis, we excluded studies evaluating intensive care unit (ICU) mortality or 28-day mortality,35, 36, 37, 38, 39 rather than the intended primary outcome of in-hospital mortality. In the second analysis, we excluded adjusted analyses from studies with a potential risk of bias due to poor adherence to best practice methodological guidelines for prediction model development.35, 36, 37, 39, 40, 41, 42, 43, 44, 45, 46 We considered the use of metaregression to further explore subgroups of interest, but did not have a sample size of ten or more studies per adjusted variable, as recommended by Cochrane guidance.47 For the unadjusted analyses, we decided not to use study-level metaregression because it was unlikely to further benefit the analysis.
Our protocol was registered with the Open Science Framework registry, osf.io/6gcy2.
Role of the funding source
There was no funding source for this study.
Results
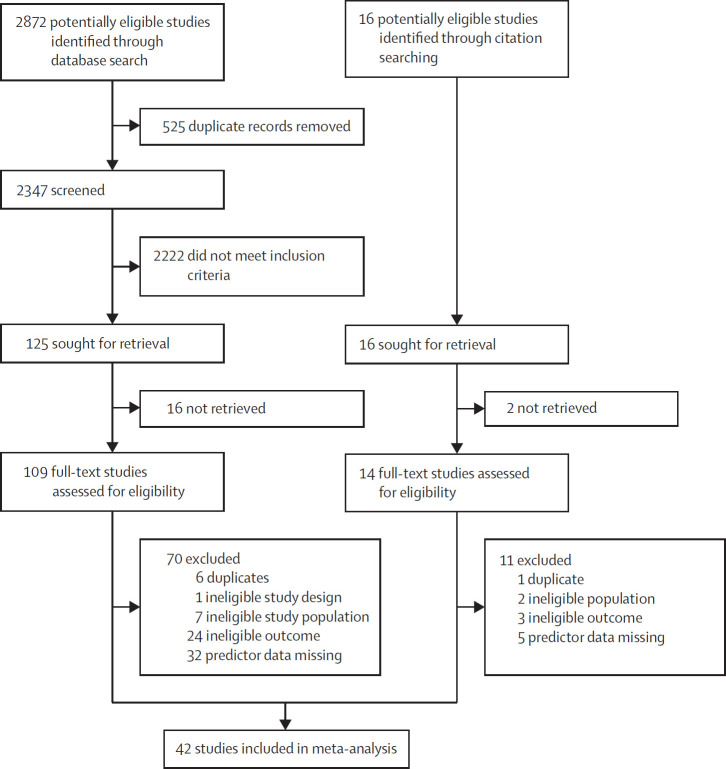
Of 2888 studies identified (figure ), we selected 123 for full-text review. We included 42 observational cohort studies13, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75 involving 17 449 patients (table 1 ), predominantly from North America or Europe. We did not identify any randomised controlled trials that met our eligibility criteria. In-hospital mortality was most commonly reported (54% across all included studies).
Figure.
Study selection
Table 1.
Characteristics of included studies
| Studies | |
|---|---|
| Location | |
| North America | 16 (38%) |
| Europe | 15 (36%) |
| Asia | 4 (10%) |
| South America | 1 (2%) |
| Multicontinental | 6 (14%) |
| Design | |
| Retrospective cohort | 38 (91%) |
| Prospective cohort | 4 (10%) |
| Patient population | |
| Overall sample size of meta-analysis, n | 17 449 |
| Median sample size (IQR) | 83 (41–294) |
| Overall mortality, % | 54% |
| Median mortality, % (IQR) | 47% (41–56%) |
| Measure of mortality | |
| In-hospital | 28 (67%) |
| 28-day or intensive care unit | 5 (12%) |
| 60-day | 5 (12%) |
| 90-day | 4 (10%) |
Data are n (%), unless otherwise stated.
Using the Quality in Prognosis Studies tool for the evaluation of prognostic studies,30 most studies were judged to be at low risk of bias in the domains of study participation, study attrition, prognostic factor measurement, and outcome measurement. Some studies were judged to be at moderate or high risk of bias for outcome measurement if only ICU mortality or 28-day mortality was reported.35, 36, 37, 38, 39 Studies were judged to be at some risk of bias for confounding adjustment and for statistical analysis and reporting if they did not account for clinically important confounders using either logistic regression or Cox proportional hazards models.15, 39, 44, 46, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66 Studies reporting adjusted analyses were judged to be at some risk of bias for statistical analysis35, 36, 37, 40, 41, 42, 43, 44, 45, 46 if they did not adhere to methodological standards set by the PROGRESS guidelines,19, 20, 21, 22 in particular with regards to a priori selection of clinically important confounders and consideration of an appropriate sample size to minimise potential overfitting.28, 76 The composition and methodological quality of individual prediction models for each study and funnel plots used to evaluate for publication bias among variables that were assessed in at least ten studies are included in the appendix (p 36–41). Forest plots for each prognostic factor and GRADE certainty assessments and rationale are included in the appendix (p 26–31).
The summary of findings is presented in table 2 . Of the patient factors, older age, as defined by the study authors (five studies;13, 38, 45, 70, 71 aHR 2·27 [95% CI 1·63–3·16]; high certainty), was associated with increased mortality. Studies most commonly differentiated between older and younger age at between 50 years and 59 years,38, 44, 49, 61, 62, 67, 68, 69 although age thresholds between 40 years and 49 years,43 60 years and 69 years,13, 52, 70, 71, 72 and over 70 years were also used.45 Similarly, when the effect of age was measured in 10-year increments in eight studies,35, 40, 41, 42, 44, 60, 69, 73 older age remained associated with mortality (aOR 1·19 [95% CI 1·11–1·27]). Of the 26 studies36, 37, 39, 40, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 58, 60, 62, 63, 64, 65, 66, 67, 68, 71, 74 that included sex as a prognostic factor, male sex was found to be probably associated with increased mortality (uOR 1·34 [95% CI 1·20–1·49]; moderate certainty) and of the three studies13, 35, 71 that included lung disease as a prognostic factor, chronic lung disease was found to be probably associated with increased mortality (aHR 1·55 [95% CI 1·20–2·00]; moderate certainty). In the 14 studies35, 41, 49, 51, 54, 55, 61, 62, 66, 67, 68, 72, 73, 74 that included obesity as a prognostic factor, obesity may have been associated with decreased mortality (uOR 0·84 [0·72–0·97]), although this finding was based on low-certainty evidence with a serious risk of bias and imprecision. Obesity was most commonly defined43, 44, 49, 54, 61, 66, 68, 72, 73 as a BMI of at least 30 kg/m2, although it was also defined as at least 40 kg/m2 in one study,71 and was not explicitly defined in others.35, 51, 55, 62, 67, 74 In the five studies35, 46, 50, 68, 71 that included immunocompromised status as a prognostic factor, immunocompromised status might have been associated with increased mortality (uOR 2·34 [1·19–4·61]; low certainty), but this finding was also limited by a high risk of bias and imprecision.
Table 2.
Prognostic factors associated with mortality
|
uOR analysis |
aOR analysis |
aHR analysis |
Mean difference analysis |
GRADE certainty | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies, n | Patients, n | uOR (95% CI) | Studies, n | Patients, n | aOR (95% CI) | Studies, n | Patients, n | aHR (95% CI) | Studies, n | Patients, n | Mean difference (95% CI) | ||
| Patient factors | |||||||||||||
| Older age | 1145, 48, 49, 52, 56, 61, 62, 68, 69, 71, 72 | 6275 | 2·51 (2·07 to 3·03) | 443, 44, 67, 68 | 1137 | 2·09 (1·22 to 3·59) | 513, 38, 45, 70, 71 | 5607 | 2·27 (1·63 to 3·16) | .. | .. | .. | High |
| Male sex | 266, 37, 39, 40, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 58, 60, 62, 63, 64, 65, 66, 67, 68, 71, 74 | 7947 | 1·34 (1·20 to 1·49) | 442, 43, 44, 67 | 887 | 1·42 (0·98 to 2·05) | 413, 44, 71, 75 | 5669 | 1·06 (0·96 to 1·16) | .. | .. | .. | Moderate |
| Chronic lung disease | 1935, 37, 39, 40, 46, 49, 50, 52, 55, 60, 62, 64, 66, 67, 68, 71, 72, 73, 74 | 9387 | 1·08 (0·92 to 1·26) | 335, 43, 44 | 431 | 2·50 (1·09 to 5·74) | 313, 35, 71 | 5103 | 1·55 (1·20 to 2·00) | .. | .. | .. | Moderate |
| Obesity | 1435, 41, 49, 51, 54, 55, 61, 62, 66, 67, 68, 72, 73, 74 | 5408 | 0·84 (0·72 to 0·97) | 243, 44 | 330 | 0·67 (0·36 to 1·27) | 271, 74 | 275 | 0·94 (0·75 to 1·19) | .. | .. | .. | Low |
| Immunocompromised | 535, 46, 50, 68, 71 | 666 | 2·34 (1·19 to 4·61) | 173 | 1985 | 4·35 (2·46 to 7·69) | 113 | 4812 | 1·06 (0·83 to 1·35) | .. | .. | .. | Low |
| Pre-cannulation disease factors | |||||||||||||
| Higher driving pressure | .. | .. | .. | .. | .. | .. | 238, 74 | 244 | 2·36 (1·40 to 3·97) | .. | .. | .. | High |
| Longer symptom duration | .. | .. | .. | .. | .. | .. | .. | .. | .. | 837, 42, 56, 58, 63, 65, 69, 71 | 463 | −1·51 days (−2·65 to −0·36) | Moderate |
| Higher PaCO2 | .. | .. | .. | .. | .. | .. | 238, 74 | 244 | 3·18 (1·41 to 7·15) | 1635, 37, 40, 41, 43, 46, 49, 51, 53, 54, 58, 60, 64, 71, 72, 74 | 1929 | −4·04 mm Hg (−6·44 to −1·64) | Moderate |
| Longer IMV duration | 635, 36, 45, 57, 59, 72 | 1482 | 1·94 (1·40 to 2·67) | 343, 67, 73 | 455 | 1·11 (0·65 to 1·92) | 145 | 127 | 1·66 (1·00 to 2·75) | 2037, 38, 40, 41, 42, 43, 46, 53, 54, 56, 58, 60, 63, 64, 65, 69, 71, 72, 73, 74 | 3897 | −1·15 days (−1·85 to −0·46) | Moderate |
| Lower PaO2/FiO2 ratio | .. | .. | .. | .. | .. | .. | 238, 71 | 349 | 1·28 (1·12 to 1·46) | 2035, 37, 38, 41, 43, 49, 51, 53, 54, 55, 58, 60, 63, 64, 65, 69, 71, 72, 73, 74 | 4189 | 1·52 (−1·16 to 4·20) | Low |
| Higher plateau pressure | .. | .. | .. | .. | .. | .. | .. | .. | .. | 1235, 38, 40, 41, 43, 49, 54, 58, 65, 71, 72, 74 | 1738 | −0·71 cm H2O (−1·15 to −0·06) | Low |
| Need for renal replacement therapy | 749, 51, 54, 60, 65, 71, 72 | 1145 | 3·18 (1·41 to 7·17) | .. | .. | .. | .. | .. | .. | .. | .. | .. | Low |
| Lower positive end expiratory pressure | .. | .. | .. | .. | .. | .. | .. | .. | .. | 1637, 40, 46, 49, 51, 54, 58, 60, 63, 64, 65, 68, 71, 72, 73, 74 | 3750 | −0·05 cm H2O (−0·37 to 0·26) | No effect (low) |
| Higher tidal volume | .. | .. | .. | .. | .. | .. | .. | .. | .. | 935, 38, 40, 41, 53, 54, 71, 72, 74 | 1304 | 0·03 mL/kg (−0·09 to 0·15) | No effect (low) |
| Lower compliance | .. | .. | .. | .. | .. | .. | .. | .. | .. | 1038, 41, 43, 53, 54, 60, 63, 65, 71, 74 | 1149 | 1·55 (−0·12 to 3·21) | Very low |
| Higher peak pressure | .. | .. | .. | .. | .. | .. | .. | .. | .. | 537, 49, 51, 64, 73 | 2235 | 1·20 (−1·51 to 3·90) cm H2O | Very low |
| Bacterial co-infection | .. | .. | .. | 240, 73 | 2059 | 0·58 (0·13 to 2·57) | 213, 70 | 5131 | 1·03 (0·88 to 1·20) | .. | .. | .. | Moderate |
| Pre-ECMO proning | .. | .. | .. | 267, 73 | 2490 | 0·97 (0·58 to 1·63) | 244, 75 | 667 | 1·18 (0·86 to 1·61) | .. | .. | .. | Low |
| Centre factors | |||||||||||||
| Lower volume | .. | .. | .. | 243, 68 | 609 | 2·27 (1·28 to 4·05) | 313, 70, 75 | 5526 | 1·57 (1·11 to 2·20) | .. | .. | .. | Moderate |
uOR=unadjusted odds ratio. aOR=adjusted odds ratio. aHR=adjusted hazard ratio. GRADE=Grading of Recommendations Assessment, Development and Evaluation. PaCO2=partial pressure of arterial carbon dioxide. IMV=invasive mechanical ventilation. PaO2=partial pressure of arterial oxygen. FiO2=fractional concentration of oxygen in inspired air. ECMO=extracorporeal membrane oxygenation.
Two studies38, 74 included pre-cannulation disease factors, and showed that higher driving pressure was associated with increased mortality (aHR 2·36 [95% CI 1·40 to 3·97]; high certainty). Higher driving pressure was defined as greater than 16 cm H2O in both studies that examined this variable.38, 74 Eight studies37, 42, 56, 58, 63, 65, 69, 71 included symptom duration before cannulation as a prognostic factor, and showed that longer symptom duration was probably associated with increased mortality (mean difference 1·51 days [95% CI 0·36 to 2·65]; moderate certainty). 16 studies35, 37, 40, 41, 43, 46, 49, 51, 53, 54, 58, 60, 64, 71, 72, 74 included partial pressure of arterial carbon dioxide (PaCO2) as a prognostic factor, and showed that higher PaCO2 was probably associated with increased mortality (mean difference 4·04 mm Hg [95% CI 1·64 to 6·44]; moderate certainty). Six studies35, 36, 45, 57, 59, 72 assessed duration of invasive mechanical ventilation before cannulation and showed that longer duration of ventilation was probably associated with increased mortality (uOR 1·94 [95% CI 1·40 to 2·67]; moderate certainty). However, adjusted analyses showed wide variation in effect sizes, suggesting increased risk when categories of longer duration were used but not when duration was assessed as a linear, per-day variable. Studies most commonly defined shorter duration as less than 7 days and longer duration as 7 days or more,35, 36, 45, 57, 72, 73 although 6 days,67 4 days,59 and 2·5 days43 were also used as predefined thresholds. Two studies13, 70 included bacterial co-infection as a prognostic factor, and showed that co-infection probably had no effect on mortality (aHR 1·03 [0·88 to 1·22]; moderate certainty). Analysis of studies that included physiological markers of oxygenation and ventilation as prognostic factors (PaO2/FiO2[n=20 studies35, 37, 38, 41, 43, 49, 51, 53, 54, 55, 58, 60, 63, 64, 65, 69, 71, 72, 73, 74],plateau airway pressure [n=12 studies35, 38, 40, 41, 43, 49, 54, 58, 65, 71, 72, 74], and pre-cannulation renal replacement therapy [n=seven studies49, 51, 54, 60, 65, 71, 72]) showed that a lower PaO2/FiO2 (mean difference 1·52 [95% CI −1·16 to 4·20 points]; low certainty), higher plateau airway pressure (mean difference 0·71 cm H2O [95% CI 0·26 to 1·15]; low certainty), and need for pre-cannulation renal replacement therapy (uOR 3·18 [95% CI 1·41 to 7·17]; low probability) might have been associated with increased mortality, although these data were limited by high risk of bias, imprecision, and unclear clinical significance. Two studies67, 73 showed that proning before ECMO might have had no effect on mortality (aOR 0·97 [0·58 to 1·63]; low certainty), although these data were limited by very serious imprecision. 16 studies37, 40, 46, 49, 51, 54, 58, 60, 63, 64, 65, 68, 71, 72, 73, 74 that included positive end-expiratory pressure and nine studies35, 38, 40, 41, 53, 54, 71, 72, 74 that included tidal volume showed that these factors might have had no effect on mortality (mean difference in positive end-expiratory pressure 0·05 cm H2O [95% CI −0·37 to 0·26]; low certainty; mean difference in tidal volume 0·03 mL/kg [95% CI −0·09 to 0·15]; low certainty), although the findings were limited by high risk of bias and imprecision. Ten studies38, 41, 43, 53, 54, 60, 63, 65, 71, 74 included respiratory system compliance and five studies37, 49, 51, 64, 73 included peak airway pressure, and showed that the effect of these prognostic factors on survival was uncertain (mean difference in respiratory system compliance 1·55 cm H2O [95% CI −0·12 to 3·21]; very low certainty; mean difference in peak airway pressure 1·20 cm H2O [−1·51 to 3·90]; very low certainty).
Two studies43, 68 that included centre volume showed that lower patient volume (or less experienced) was probably associated with increased mortality (aOR 2·27 [95% CI 1·28–4·05]; moderate certainty). The threshold for classification varied between studies, with centres being defined as high volume (or more experienced) if they had treated at least 30 patients with venovenous ECMO,13, 68 had treated 30 or more70 or 50 or more75 patients with venoarterial or venovenous ECMO, or if they had established ECMO services before January, 2020.43
We conducted two pre-specified sensitivity analyses (appendix p 34, 35). In the first analysis, we excluded studies evaluating ICU mortality or 28-day mortality.35, 36, 37, 38, 39 In the second analysis, we excluded adjusted analyses from studies with moderate or higher risk of bias in the domains of confounding adjustment or statistical analysis. Specifically, bias in these studies was associated with poor adherence to methodological guidelines for the development of prediction models.35, 36, 37, 39, 40, 41, 42, 43, 44, 45, 46 In this sensitivity analysis, the results from unadjusted analyses or mean difference analyses remained unchanged. In both sensitivity analyses, no meaningful effect on the overall results or conclusion was observed.
Discussion
In this systematic review and meta-analysis, we investigated the prognostic association between several pre-cannulation factors and in-hospital mortality for patients who received venovenous ECMO for COVID-19. Factors with moderate or high certainty of association with increased mortality included patient factors, such as older age, male sex, and chronic lung disease; pre-cannulation disease factors, such as longer duration of symptoms, longer duration of invasive mechanical ventilation, higher PaCO2, and higher driving pressure; and centre factors, such as less previous experience with ECMO.
The prognostic factors identified highlight several well established principles of effective ARDS and ECMO care: the importance of patient selection, the effect of pre-ECMO injurious lung ventilation on prognosis, and the potential benefit of greater centralisation in the use of ECMO across centres and regions.77 ECMO is highly invasive and is associated with a high risk of adverse events, including vascular injury, infection, major bleeding, and worsening systemic inflammation,78 many of which could be amplified in the context of COVID-19-associated ARDS.38 Acknowledging the associated risks, the high demand on resources, and the scarcity of ECMO, ELSO advocated for the importance of patient selection, in particular for the prioritisation of younger patients with fewer comorbidities.10, 79 This guidance is supported by our findings, in which we have shown, with moderate or high certainty, that older age and chronic lung disease are important patient-specific prognostic factors.
Of the pre-cannulation disease factors, we showed with moderate or high certainty that higher driving pressure, longer symptom duration before cannulation, longer duration of invasive mechanical ventilation before cannulation, and higher PaCO2 are associated with increased mortality. We also showed with moderate certainty that bacterial co-infection is probably not associated with a difference in mortality. These findings suggest the effect of injurious lung ventilation and indicate that the severity of pre-cannulation acute lung injury is a major determinant of outcome. Despite well established, evidence-based principles for lung protective ventilation,4, 5 wide variation in the management of ARDS across hospitals continues in practice, with up to half of patients not receiving care consistent with guideline recommendations during the first year of the COVID-19 pandemic.80 The identification of driving pressure as an important prognostic factor, based on high-certainty evidence, is consistent with existing evidence showing that this ventilation variable is strongly associated with mortality in patients with ARDS.81, 82 Additionally, we found that a longer duration of invasive mechanical ventilation (most commonly defined by a 7-day threshold) and a higher PaCO2 are poor prognostic signs. However, the potentially synergistic interaction between protracted and injurious lung ventilation could not be elucidated within the scope of this review. Only one study74 adjusted for both driving pressure and the duration of mechanical ventilation, and suggested a small increased risk of mortality per additional day of ventilation before cannulation. Specific to COVID-19, we showed with moderate certainty that longer duration of symptoms before ECMO cannulation was probably associated with increased mortality, further highlighting the importance of efficient referral pathways and early intervention.77
We showed with moderate certainty that lower case volume at medical centres is an important determinant of mortality risk. These findings are consistent with the well established association between higher case volume and better outcomes across a wide range of procedures and disease conditions,83 and particularly for ECMO,84 including in the setting of COVID-19.13 Specific to ARDS, a large nationwide cohort study85 in the USA found that higher hospital case volumes were associated with lower ARDS mortality at both the individual and hospital level. The identification of a case volume benchmark is not within the scope of this review; rather we aimed to emphasise the importance of the relationship between volume and outcome in principle. Additionally, we note that the discrepancy in ARDS and ECMO outcomes in different centres suggests that a meaningful opportunity exists to improve the coordination between centres, in addition to the optimisation and distribution of resources.13, 77, 86 Similar to other regionalised care models that have been used successfully for coronary revascularisation, complex cancer surgery, and major vascular procedures,83 the concentration of resources at specialised, high-volume ECMO centres in a hub-and-spoke model could offer a greater degree of efficiency and effectiveness.77
Appropriate use of ECMO should begin with well established clinical practice guidelines and incorporate more nuanced prognostic enrichment principles to develop an individualised harm–benefit profile for each patient.86 An improved understanding of individualised prognostication not only has meaningful implications for bedside care, but could also offer important insight for the enrolment and conduct of clinical trials.87 The fundamental basis of accurate prognostication begins with a comprehensive and evidence-based understanding of potentially important clinical factors,21 which are summarised in this review. However, we emphasise that although evidence-based prognostication is an important consideration for patient selection, it should not be solely responsible for identifying appropriate candidates for ECMO. Specifically, this review does not address how patients with high expected risk of mortality on ECMO would have fared without it and, as such, we are unable to make definitive recommendations regarding patient selection. Ultimately, ECMO has the largest effect when it is most likely to change an individual patient's risk of mortality, even if overall mortality on ECMO remains high.
This review was strengthened by a comprehensive search, adherence to recommendations for the meta-analysis of prognostic studies,24 and use of the GRADE approach to assess the certainty in the estimates and contextualise results.33 The face validity, consistency, precision, and generally robust effect sizes for the prognostic factors we identified justify their inclusion in any risk stratification framework. However, this review also has limitations. The prognostic factors identified reflect their importance for patients who ultimately received ECMO, rather than for all patients who are potentially eligible for ECMO. As such, despite our best efforts to specifically evaluate pre-cannulation factors and emphasise confounding adjustment, the potential for residual confounding and selection bias remains. Importantly, few studies provided appropriate adjustment for extrapulmonary organ failure, which could have affected patient selection and outcomes. We are also limited by variability in practice and quality of the prognostic modelling methodology used by the included studies, many of which did not adhere to prognostic guideline recommendations21, 22, 28, 88 and were therefore prone to overfitting.22, 28 Additionally, a scarcity of well established prognostic factors contributed to few studies prespecifying clinically important variables,21, 22, 88 and models therefore differed in terms of outcome definition, variable definition, categorisation thresholds, and composition. These limitations resulted in a GRADE certainty downgrade for risk of bias for confounding adjustment and statistical analysis.23, 30
We took a pragmatic approach to the inclusion of studies and grouping of variables, allowing for use of the definitions in the included studies to maximise data yield and utility. However, the appropriate interpretation of results is supported by confirmation after clinically important sensitivity analyses and characterisation of certainty using GRADE methodology.33
Data sharing
This manuscript makes use of publicly available data from the included studies and their supplementary information files; therefore, no original data are available for sharing.
Declaration of interests
RPB is Chair of the Extracorporeal Life Support Organization (ELSO) registry. GM serves on the Board of Directors for ELSO. KR reports honoraria from Baxter and Faesenius, outside of the submitted work. LJB reports research support from Medtronic and Draeger, and honoraria from Fisher Paykel, outside of the submitted work. NDF reports consulting fees from Baxter and Xenios, outside of the submitted work. ASS reports consulting fees from Baxter and Xenios, outside of the submitted work. He is the Chair of the Scientific Committee of the International ECMO Network (ECMONet). EF reports personal fees from ALung Technologies, Aerogen, Baxter, Boehringer-Ingelheim, GE Healthcare, Inspira, and Vasomune, outside of the submitted work. He is the Chair of the Data Committee of ECMONet. DB reports research support from Alung Technologies, outside of the submitted work. He has been on medical advisory boards for Abiomed, Xenios, Medtronic, LivaNova, Inspira, and Cellenkos, and is the President-Elect of ELSO and the Chair of the Executive Committee of ECMONet. All other authors declare no competing interests.
Contributors
AT, SMF, BR, EF, and DB conceived the idea. AT and SMF curated the data and did the investigation and formal analysis. BR, EF, and DB provided supervision. AT wrote the initial draft. All authors participated in methodology, data interpretation and the reviewing and editing of the manuscript. AT and SMF accessed and verified the data. AT, DB and EF were responsible for the decision to submit the manuscript.
Supplementary Material
References
- 1.Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan E, Beitler JR, Brochard L, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8:816–821. doi: 10.1016/S2213-2600(20)30304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siemieniuk RA, Bartoszko JJ, Zeraatkar D, et al. Drug treatments for COVID-19: living systematic review and network meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan E, Del Sorbo L, Goligher EC, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195:1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 5.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 6.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 7.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 8.Munshi L, Walkey A, Goligher E, Pham T, Uleryk EM, Fan E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med. 2019;7:163–172. doi: 10.1016/S2213-2600(18)30452-1. [DOI] [PubMed] [Google Scholar]
- 9.Goligher EC, Tomlinson G, Hajage D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc Bayesian analysis of a randomized clinical trial. JAMA. 2018;320:2251–2259. doi: 10.1001/jama.2018.14276. [DOI] [PubMed] [Google Scholar]
- 10.Bartlett RH, Ogino MT, Brodie D, et al. Initial ELSO guidance document: ECMO for COVID-19 patients with severe cardiopulmonary failure. ASAIO J. 2020;66:472–474. doi: 10.1097/MAT.0000000000001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramanathan K, Shekar K, Ling RR, et al. Extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Crit Care. 2021;25:211. doi: 10.1186/s13054-021-03634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet. 2021;398:1230–1238. doi: 10.1016/S0140-6736(21)01960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urner M, Barnett AG, Bassi GL, et al. Venovenous extracorporeal membrane oxygenation in patients with acute COVID-19 associated respiratory failure: comparative effectiveness study. BMJ. 2022;377 doi: 10.1136/bmj-2021-068723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Supady A, Badulak J, Evans L, Curtis JR, Brodie D. Should we ration extracorporeal membrane oxygenation during the COVID-19 pandemic? Lancet Respir Med. 2021;9:326–328. doi: 10.1016/S2213-2600(21)00131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernando SM, Qureshi D, Tanuseputro P, et al. Mortality and costs following extracorporeal membrane oxygenation in critically ill adults: a population-based cohort study. Intensive Care Med. 2019;45:1580–1589. doi: 10.1007/s00134-019-05766-z. [DOI] [PubMed] [Google Scholar]
- 17.Supady A, Curtis JR, Abrams D, et al. Allocating scarce intensive care resources during the COVID-19 pandemic: practical challenges to theoretical frameworks. Lancet Respir Med. 2021;9:430–434. doi: 10.1016/S2213-2600(20)30580-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 19.Hemingway H, Croft P, Perel P, et al. Prognosis research strategy (PROGRESS) 1: a framework for researching clinical outcomes. BMJ. 2013;346 doi: 10.1136/bmj.e5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hingorani AD, Windt DA, Riley RD, et al. Prognosis research strategy (PROGRESS) 4: stratified medicine research. BMJ. 2013;346 doi: 10.1136/bmj.e5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riley RD, Hayden JA, Steyerberg EW, et al. Prognosis research strategy (PROGRESS) 2: prognostic factor research. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steyerberg EW, Moons KG, van der Windt DA, et al. Prognosis research strategy (PROGRESS) 3: prognostic model research. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moons KG, de Groot JA, Bouwmeester W, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riley RD, Moons KGM, Snell KIE, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ. 2019;364 doi: 10.1136/bmj.k4597. [DOI] [PubMed] [Google Scholar]
- 25.Fernando SM, Tran A, Cheng W, et al. Pre-arrest and intra-arrest prognostic factors associated with survival after in-hospital cardiac arrest: systematic review and meta-analysis. BMJ. 2019;367 doi: 10.1136/bmj.l6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tran A, Fernando SM, Rochwerg B, et al. Prognostic factors associated with development of infected necrosis in patients with acute necrotizing or severe pancreatitis—a systematic review and meta-analysis. J Trauma Acute Care Surg. 2022;92:940–948. doi: 10.1097/TA.0000000000003502. [DOI] [PubMed] [Google Scholar]
- 27.Tran A, Fernando SM, Rochwerg B, et al. Pre-arrest and intra-arrest prognostic factors associated with survival following traumatic out-of-hospital cardiac arrest—a systematic review and meta-analysis. Resuscitation. 2020;153:119–135. doi: 10.1016/j.resuscitation.2020.05.052. [DOI] [PubMed] [Google Scholar]
- 28.Riley RD, Ensor J, Snell KIE, et al. Calculating the sample size required for developing a clinical prediction model. BMJ. 2020;368:m441. doi: 10.1136/bmj.m441. [DOI] [PubMed] [Google Scholar]
- 29.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 31.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 32.Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10 doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iorio A, Spencer FA, Falavigna M, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. doi: 10.1136/bmj.h870. [DOI] [PubMed] [Google Scholar]
- 34.Santesso N, Glenton C, Dahm P, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126–135. doi: 10.1016/j.jclinepi.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Hermann M, Laxar D, Krall C, et al. Duration of invasive mechanical ventilation prior to extracorporeal membrane oxygenation is not associated with survival in acute respiratory distress syndrome caused by coronavirus disease 2019. Ann Intensive Care. 2022;12:6. doi: 10.1186/s13613-022-00980-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurihara C, Manerikar A, Gao CA, et al. Outcomes after extracorporeal membrane oxygenation support in COVID-19 and non-COVID-19 patients. Artif Organs. 2022;46:688–696. doi: 10.1111/aor.14090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raasveld SJ, Delnoij TSR, Broman LM, et al. Extracorporeal membrane oxygenation in patients with COVID-19: an international multicenter cohort study. J Intensive Care Med. 2021;36:910–917. doi: 10.1177/08850666211007063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt M, Langouet E, Hajage D, et al. Evolving outcomes of extracorporeal membrane oxygenation support for severe COVID-19 ARDS in Sorbonne hospitals, Paris. Crit Care. 2021;25:355. doi: 10.1186/s13054-021-03780-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trejnowska E, Drobiński D, Knapik P, et al. Extracorporeal membrane oxygenation for severe COVID-19-associated acute respiratory distress syndrome in Poland: a multicenter cohort study. Crit Care. 2022;26:97. doi: 10.1186/s13054-022-03959-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng W, Ma XD, Su LX, et al. Retrospective study of critically ill COVID-19 patients with and without extracorporeal membrane oxygenation support in Wuhan, China. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.659793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daviet F, Guilloux P, Hraiech S, et al. Impact of obesity on survival in COVID-19 ARDS patients receiving ECMO: results from an ambispective observational cohort. Ann Intensive Care. 2021;11:157. doi: 10.1186/s13613-021-00943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunavarapu C, Yeramaneni S, Melo J, et al. Clinical outcomes of severe COVID-19 patients receiving early VV-ECMO and the impact of pre-ECMO ventilator use. Int J Artif Organs. 2021;44:861–867. doi: 10.1177/03913988211047604. [DOI] [PubMed] [Google Scholar]
- 43.Rabie AA, Azzam MH, Al-Fares AA, et al. Implementation of new ECMO centers during the COVID-19 pandemic: experience and results from the Middle East and India. Intensive Care Med. 2021;47:887–895. doi: 10.1007/s00134-021-06451-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao A, Zaaqoq AM, Kang IG, et al. Palliative care for patients on extracorporeal membrane oxygenation for COVID-19 infection. Am J Hosp Palliat Care. 2021;38:854–860. doi: 10.1177/10499091211001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Supady A, Taccone FS, Lepper PM, Ziegeler S, Staudacher DL. Survival after extracorporeal membrane oxygenation in severe COVID-19 ARDS: results from an international multicenter registry. Crit Care. 2021;25:90. doi: 10.1186/s13054-021-03486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zayat R, Kalverkamp S, Grottke O, et al. Role of extracorporeal membrane oxygenation in critically Ill COVID-19 patients and predictors of mortality. Artif Organs. 2021;45:E158–E170. doi: 10.1111/aor.13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higgins JPT, Green S. Wiley-Blackwell; Chichester; Hoboken, NJ: 2008. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 48.Ahmad Q, Green A, Chandel A, et al. Impact of noninvasive respiratory support in patients with COVID-19 requiring V-V ECMO. ASAIO J. 2022;68:171–177. doi: 10.1097/MAT.0000000000001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bergman ZR, Wothe JK, Alwan FS, et al. Risk factors of mortality for patients receiving venovenous extracorporeal membrane oxygenation for COVID-19 acute respiratory distress syndrome. Surg Infect (Larchmt) 2021;22:1086–1092. doi: 10.1089/sur.2021.114. [DOI] [PubMed] [Google Scholar]
- 50.Blazoski C, Baram M, Hirose H. Outcomes of extracorporeal membrane oxygenation in acute respiratory distress syndrome due to COVID-19: the lessons learned from the first wave of COVID-19. J Card Surg. 2021;36:2219–2224. doi: 10.1111/jocs.15512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braaten JA, Bergman ZR, Wothe JK, et al. Increasing mortality in venovenous extracorporeal membrane oxygenation for COVID-19-associated acute respiratory distress syndrome. Crit Care Explor. 2022;4:e0655. doi: 10.1097/CCE.0000000000000655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friedrichson B, Kloka JA, Neef V, et al. Extracorporeal membrane oxygenation in coronavirus disease 2019: a nationwide cohort analysis of 4279 runs from Germany. Eur J Anaesthesiol. 2022;39:445–451. doi: 10.1097/EJA.0000000000001670. [DOI] [PubMed] [Google Scholar]
- 53.Giraud R, Legouis D, Assouline B, et al. Timing of VV-ECMO therapy implementation influences prognosis of COVID-19 patients. Physiol Rep. 2021;9 doi: 10.14814/phy2.14715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hajage D, Combes A, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: an emulated target trial analysis. Am J Respir Crit Care Med. 2022;206:281–294. doi: 10.1164/rccm.202111-2495OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacobs JP, Stammers AH, Louis JS, et al. Multi-institutional analysis of 100 consecutive patients with COVID-19 and severe pulmonary compromise treated with extracorporeal membrane oxygenation: outcomes and trends over time. ASAIO J. 2021;67:496–502. doi: 10.1097/MAT.0000000000001434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kakar V, North A, Bajwa G, Raposo N, Kumar PG. Long runs and higher incidence of bleeding complications in COVID-19 patients requiring venovenous extracorporeal membrane oxygenation: a case series from the United Arab Emirates. Indian J Crit Care Med. 2021;25:1452–1458. doi: 10.5005/jp-journals-10071-24054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karagiannidis C, Strassmann S, Merten M, et al. High in-hospital mortality rate in patients with COVID-19 receiving extracorporeal membrane oxygenation in Germany: a critical analysis. Am J Respir Crit Care Med. 2021;204:991–994. doi: 10.1164/rccm.202105-1145LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lai W, Li S, Du Z, et al. Severe patients with ARDS with COVID-19 treated with extracorporeal membrane oxygenation in China: a retrospective study. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.699227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X, Hu M, Zheng R, et al. Delayed initiation of ECMO is associated with poor outcomes in patients with severe COVID-19: a multicenter retrospective cohort study. Front Med. 2021;8 doi: 10.3389/fmed.2021.716086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loforte A, Di Mauro M, Pellegrini C, et al. Extracorporeal membrane oxygenation for COVID-19 respiratory distress syndrome: an Italian Society for Cardiac Surgery report. ASAIO J. 2021;67:385–391. doi: 10.1097/MAT.0000000000001399. [DOI] [PubMed] [Google Scholar]
- 61.Mustafa AK, Joshi DJ, Alexander PJ, et al. Comparative propensity matched outcomes in severe COVID-19 respiratory failure-extracorporeal membrane oxygenation or maximum ventilation alone. Ann Surg. 2021;274:e388–e394. doi: 10.1097/SLA.0000000000005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nguyen NT, Sullivan B, Sagebin F, Hohmann SF, Amin A, Nahmias J. Analysis of COVID-19 patients with acute respiratory distress syndrome managed with extracorporeal membrane oxygenation at US academic centers. Ann Surg. 2021;274:40–44. doi: 10.1097/SLA.0000000000004870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rajajee V, Fung CM, Seagly KS, et al. One-year functional, cognitive, and psychological outcomes following the use of extracorporeal membrane oxygenation in coronavirus disease 2019: a prospective study. Crit Care Explor. 2021;3:e0537. doi: 10.1097/CCE.0000000000000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tabatabai A, Ghneim MH, Kaczorowski DJ, et al. Mortality risk assessment in COVID-19 venovenous extracorporeal membrane oxygenation. Ann Thorac Surg. 2021;112:1983–1989. doi: 10.1016/j.athoracsur.2020.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Voicu S, Goury A, Lacoste-Palasset T, et al. Dismal survival in COVID-19 patients requiring ECMO as rescue therapy after corticosteroid failure. J Pers Med. 2021;11 doi: 10.3390/jpm11111238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang J, Merrick B, Correa GL, et al. Veno-venous extracorporeal membrane oxygenation in coronavirus disease 2019: a case series. ERJ Open Res. 2020;6 doi: 10.1183/23120541.00463-2020. 00463-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hall CA, Jacobs JP, Stammers AH, et al. Multi-institutional analysis of 505 COVID-19 patients supported with ECMO: predictors of survival. Ann Thorac Surg. 2022;114:61–68. doi: 10.1016/j.athoracsur.2022.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lebreton G, Schmidt M, Ponnaiah M, et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med. 2021;9:851–862. doi: 10.1016/S2213-2600(21)00096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raff LA, Gallaher JR, Johnson D, Raff EJ, Charles AG, Reid TS. Time to cannulation after ICU admission increases mortality for patients requiring veno-venous ECMO for COVID-19 associated acute respiratory distress syndrome. Ann Surg. 2020 doi: 10.1097/SLA.0000000000004683. published online Dec 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Riera J, Alcántara S, Bonilla C, et al. Risk factors for mortality in patients with COVID-19 needing extracorporeal respiratory support. Eur Respir J. 2022;59 doi: 10.1183/13993003.02463-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shaefi S, Brenner SK, Gupta S, et al. Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med. 2021;47:208–221. doi: 10.1007/s00134-020-06331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nesseler N, Fadel G, Mansour A, et al. Extracorporeal membrane oxygenation for respiratory failure related to COVID-19: a nationwide cohort study. Anesthesiology. 2022;136:732–748. doi: 10.1097/ALN.0000000000004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Joshi H, Flanagan M, Subramanian R, Drouin M. Respiratory ECMO survival prediction (RESP) score for COVID-19 patients treated with ECMO. ASAIO J. 2022;68:486–491. doi: 10.1097/MAT.0000000000001640. [DOI] [PubMed] [Google Scholar]
- 74.Diaz RA, Graf J, Zambrano JM, et al. Extracorporeal membrane oxygenation for COVID-19-associated severe acute respiratory distress syndrome in Chile: a nationwide incidence and cohort study. Am J Respir Crit Care Med. 2021;204:34–43. doi: 10.1164/rccm.202011-4166OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saeed O, Stein LH, Cavarocchi N, et al. Outcomes by cannulation methods for venovenous extracorporeal membrane oxygenation during COVID-19: a multicenter retrospective study. Artif Organs. 2022;46:1659–1668. doi: 10.1111/aor.14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leisman DE, Harhay MO, Lederer DJ, et al. Development and reporting of prediction models: guidance for authors from editors of respiratory, sleep, and critical care journals. Crit Care Med. 2020;48:623–633. doi: 10.1097/CCM.0000000000004246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brodie D, Abrams D, MacLaren G, et al. Extracorporeal membrane oxygenation during respiratory pandemics: past, present, and future. Am J Respir Crit Care Med. 2022;205:1382–1390. doi: 10.1164/rccm.202111-2661CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brodie D, Slutsky AS, Combes A. Extracorporeal life support for adults with respiratory failure and related indications: a review. JAMA. 2019;322:557–568. doi: 10.1001/jama.2019.9302. [DOI] [PubMed] [Google Scholar]
- 79.Badulak J, Antonini MV, Stead CM, et al. Extracorporeal membrane oxygenation for COVID-19: updated 2021 guidelines from the Extracorporeal Life Support Organization. ASAIO J. 2021;67:485–495. doi: 10.1097/MAT.0000000000001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson SW, Garcia MA, Sisson EKQ, et al. Hospital variation in management and outcomes of acute respiratory distress syndrome due to COVID-19. Crit Care Explor. 2022;10:e0638. doi: 10.1097/CCE.0000000000000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 82.Aoyama H, Pettenuzzo T, Aoyama K, Pinto R, Englesakis M, Fan E. Association of driving pressure with mortality among ventilated patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med. 2018;46:300–306. doi: 10.1097/CCM.0000000000002838. [DOI] [PubMed] [Google Scholar]
- 83.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511–520. doi: 10.7326/0003-4819-137-6-200209170-00012. [DOI] [PubMed] [Google Scholar]
- 84.Barbaro RP, Odetola FO, Kidwell KM, et al. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the Extracorporeal Life Support Organization registry. Am J Respir Crit Care Med. 2015;191:894–901. doi: 10.1164/rccm.201409-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ike JD, Kempker JA, Kramer MR, Martin GS. The association between acute respiratory distress syndrome hospital case volume and mortality in a U.S. cohort, 2002–2011. Crit Care Med. 2018;46:764–773. doi: 10.1097/CCM.0000000000003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.MacLaren G, Fisher D, Brodie D. Treating the most critically ill patients with COVID-19: the evolving role of extracorporeal membrane oxygenation. JAMA. 2022;327:31–32. doi: 10.1001/jama.2021.22580. [DOI] [PubMed] [Google Scholar]
- 87.Tran A, Fernando SM, Rochwerg B, Seymour CW, Cook DJ. Characterizing systematic challenges in sample size determination for sepsis trials. Intensive Care Med. 2022;48:750–752. doi: 10.1007/s00134-022-06691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. 2014;35:1925–1931. doi: 10.1093/eurheartj/ehu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This manuscript makes use of publicly available data from the included studies and their supplementary information files; therefore, no original data are available for sharing.