Abstract
Streptococcus pyogenes expresses a highly conserved extracellular cysteine protease that is a virulence factor for invasive disease, including soft tissue infection. Site-directed mutagenesis was used to generate a His340Ala recombinant mutant protein that was made as a stable 40-kDa zymogen by Escherichia coli. Purified His340Ala protein was proteolytically inactive when bovine casein and human fibronectin were used as substrates. Wild-type 28-kDa streptococcal protease purified from S. pyogenes processed the 40-kDa mutant zymogen to a 28-kDa mature form, a result suggesting that the derivative protein retained structural integrity. The data are consistent with the hypothesis that His340 is an enzyme active site residue, an idea confirmed by recent solution of the zymogen crystal structure (T. F. Kagawa, J. C. Cooney, H. M. Baker, S. McSweeney, M. Liu, S. Gubba, J. M. Musser, and E. N. Baker, Proc. Natl. Acad. Sci. USA 97:2235–2240, 2000). The data provide additional insight into structure-function relationships in this S. pyogenes virulence factor.
Streptococcus pyogenes produces a highly conserved extracellular protease (streptococcal pyrogenic exotoxin B [SpeB]) that is expressed as a 40-kDa zymogen and subsequently converted to a 28-kDa active enzyme (4, 5, 19, 24). Genetic, biochemical, and recent crystallographic studies have shown that amino acid residue Cys192 is involved in active site formation (9, 25).
Several laboratories have documented that the streptococcal protease is an important virulence factor in animal models of human invasive disease (8, 16, 20–22). The enzyme cleaves human fibronectin (FN), degrades vitronectin, cleaves interleukin-1β (IL-1β) precursor to form active IL-1β, and causes cytopathic effect on human endothelial cells (2, 12, 13). The enzyme activates a 66-kDa human matrix metalloprotease, a process that results in increased type IV collagenase activity (3). It has also been shown that the protease directly releases biologically active kinins from their purified precursor protein, H-kininogen, in vitro, and from kininogens present in human plasma, ex vivo (8).
Biochemical evidence suggests that a histidine residue is located at the active site of the streptococcal protease (6, 18). Although earlier biochemical data suggested that His159 was an active site residue, sequence alignments with several other cysteine proteases (14, 18, 26) led us to hypothesize that His340 participated in active-site formation. The goal of the present study was to test this idea by replacing His340 with an alanine residue.
Site-directed mutagenesis was used to create a mutant cysteine protease with the His340Ala amino acid replacement (7, 25). The mutagenesis was conducted using a previously described recombinant plasmid that encodes a His-tagged 40-kDa zymogen. The following synthetic oligonucleotides were used: SG13-FOR (5′-TGTCGGTAAAGTAGGCGGAGCAGCCTTTGTTATCGATGGTGC-3′) and SG2-REV (5′-GCACCATCGATAACAAAGGC TGC TCCGCC TAC T T TACCGACA-3′). The procedure resulted in construction of plasmid pGM-16, which was used for the expression of the His340Ala mutant protein. Automated DNA sequence analysis confirmed that the gene had been cloned in the correct reading frame and lacked spurious mutations. The recombinant protein made from this plasmid will have a 23-amino-acid His tag located at the amino terminus.
Recombinant His340Ala mutant protein was purified from Escherichia coli BL21 (Stratagene, La Jolla, Calif.) containing pGM-16 after isopropyl-β-d-thiogalactopyranoside induction, cell lysis with a French press, and chromatography with a Ni-nitrilotriacetic acid resin column (7). Recombinant proteins without the His tag were made by cleaving the His tag from the column-bound protein with approximately 1,000 U of recombinant TEV (rTEV) protease per 3 mg of bound fusion protein (7). The resulting mutant zymogen molecules contain two additional amino acid residues at the amino terminus.
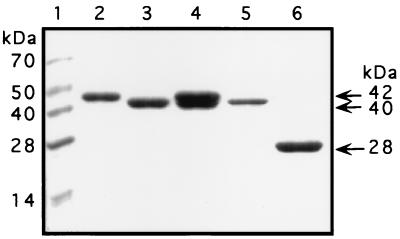
The protein was concentrated to 0.5 ml with Centrisep 3 or Centrisep 10 concentrators (Amicon, Beverly, Mass.), reconstituted to 3.0 ml with phosphate-buffered saline (pH 7.4) (PBS), and desalted with either desalting columns (Econopac 10 DG columns; Bio-Rad) or by dialysis against PBS with Slide-A-Lyser cassettes (Pierce, Rockford, Ill.). The Cys192Ser mutant mature form of the streptococcal protease was obtained from the 40-kDa Cys192Ser mutant zymogen after cleavage with trypsin (7, 28). These Cys192Ser mutant proteins were used as controls in several assays. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie brilliant blue staining showed that the recombinant His340Ala protein was expressed and purified as a stable zymogen (Fig. 1).
FIG. 1.
SDS-PAGE analysis and Coomassie brilliant blue staining of mutant cysteine protease made by E. coli. Lanes: 1, molecular mass standards of the sizes indicated at the left; 2, purified recombinant His340Ala zymogen with His tag; 3, purified recombinant His340Ala zymogen with His tag removed with rTEV protease; 4, purified recombinant Cys192Ser zymogen with His tag; 5, purified recombinant Cys192Ser zymogen with His tag removed with rTEV protease; 6, purified recombinant Cys192Ser 28-kDa mature form.
Wild-type 28-kDa streptococcal cysteine protease was purified to apparent homogeneity from the culture supernatant of strain MGAS1719, as described previously (12).
The purified mutant 40-kDa His340Ala zymogen and 28-kDa mature form were then assayed for their ability to degrade bovine casein embedded in an agarose gel matrix (13). No proteolytic activity against this substrate was identified, whereas wild-type streptococcal protease was very active in this assay (data not shown).
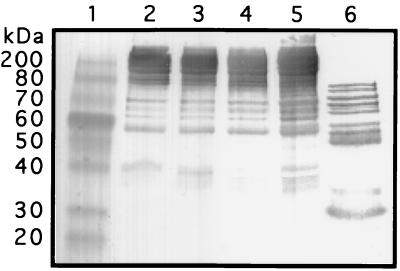
We next tested the ability of the 28-kDa His340Ala mutant to cleave human FN. The mutant protein and wild-type mature protease were incubated with human FN overnight, and the reaction products were analyzed by SDS-PAGE and Western immunoblot staining as described previously (7, 8). The Cys192Ser and His340Ala mutants lacked detectable protease activity, whereas wild-type mature streptococcal cysteine protease degraded FN to several lower-molecular-weight products (Fig. 2).
FIG. 2.
SDS-PAGE and immunoblot staining showing lack of human FN cleavage by the 28-kDa His340Ala mutant made by E. coli. Lanes: 1, molecular mass standards of the sizes indicated at the left; 2, FN without incubation; 3, FN incubated without streptococcal mutant enzyme; 4, FN incubated with His340Ala mutant protein; 5, FN incubated with Cys192Ser mutant protein (control); 6, FN incubated with 28-kDa wild-type streptococcal cysteine protease purified from strain MGAS 1719.
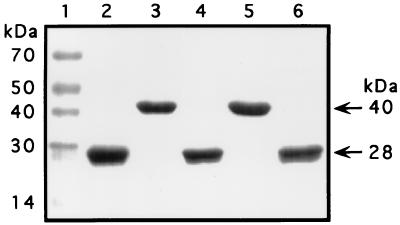
Previous investigators (4, 5, 15, 19) have shown that purified wild-type cysteine protease can cleave the 40-kDa zymogen to generate a 28-kDa mature form. We next tested if the His340Ala mutant zymogen was processed by wild-type 28-kDa streptococcal protease. Active protease was incubated at 37°C for 30 min with purified Cys192Ser or His340Ala zymogens at molar ratios of 1:10 (7). The digestion products were analyzed by SDS-PAGE and Coomassie brilliant blue staining. The His340Ala mutant zymogen was processed to a 28-kDa product that comigrated with wild-type mature streptococcal cysteine protease (Fig. 3). These results indicated that the His340Ala mutant zymogen retained the appropriate structure at the processing site to allow efficient cleavage by active streptococcal protease.
FIG. 3.
Processing of purified recombinant His340Ala mutant zymogen by wild-type streptococcal cysteine protease. Active purified streptococcal protease was incubated at 37°C for 30 min with purified mutant zymogen at a molar ratio of 1:10. The samples were analyzed by SDS-PAGE, and the products were visualized by staining with Coomassie brilliant blue. Lanes: 1, molecular mass standards of the sizes indicated at the left; 2, purified wild-type streptococcal cysteine protease; 3, purified Cys192Ser mutant zymogen; 4, purified Cys192Ser mutant zymogen incubated with wild-type streptococcal cysteine protease; 5, purified His340Ala mutant zymogen; 6, purified His340Ala mutant zymogen incubated with wild-type streptococcal cysteine protease.
The wild-type 40-kDa zymogen undergoes autocatalytic truncation under reducing conditions (4, 5, 19). To test whether reducing conditions would stimulate the transformation of the zymogen to a 28-kDa mature form, 5 μg of the mutant His340Ala zymogen was treated with 10 mM 2-mercaptoethanol for 30 min at room temperature. The samples were analyzed by SDS-PAGE and Coomassie brilliant blue staining (7, 25). The results showed that the His340Ala mutant was not autocatalytically processed (data not shown).
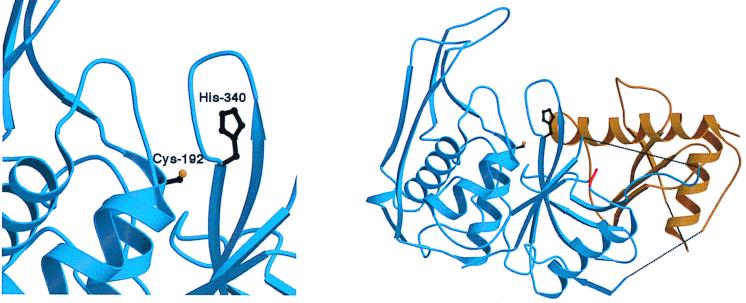
Many cysteine proteases have a catalytic triad of amino acid residues that participate in enzyme active-site formation (10, 14). For example, crystal structure analysis and site-directed mutagenesis studies have demonstrated that the active site of papain (the canonical cysteine protease) consists of Cys25, His159, and Asn175 (10, 30). By showing that replacement of His340 inactivates the group A Streptococcus cysteine protease, our results add to knowledge about structure-activity relationships in this virulence factor. After this work was completed and while the manuscript was in review, the crystal structure of the 40-kDa SpeB zymogen form was solved at 1.6 Å (9). The crystal structure confirmed that His340 is the critical active site His residue (Fig. 4). Interestingly, the crystal structure also demonstrated that SpeB is unusual among cysteine proteases in that it lacks a catalytic triad, having instead a Cys192-His340 interaction.
FIG. 4.
Crystal structure of the SpeB zymogen showing His340 to be located at the enzyme active site. The figure is based on the crystallographic structure recently presented by Kagawa et al. (9), solved at 1.6 Å resolution. (Left panel) Enlargement of active site showing Cys-192 and His-340 amino acid residues. (Right panel) Overview of SpeB zymogen crystal structure, with blue denoting the 28-kDa mature form, gold denoting the propeptide region, and red denoting the surface-exposed loop containing an Arg-Gly-Asp motif that binds human integrins (9, 28).
Inasmuch as the streptococcal cysteine protease has emerged as an area of considerable research interest in recent years (1–3, 7, 8, 11–13, 16, 17, 20–23, 27–29), it will be important to identify additional amino acid residues that participate in biomedically relevant functions. For example, additional site-specific mutagenesis studies may help to identify amino acid residues that participate in interaction with host molecules such as integrins (28). Our studies may also provide insight into the structure-activity relationships of related proteases, for example, those made by the peridontal pathogen Porphyromonas gingivalis (26).
Acknowledgments
This work was supported by Public Health Service Grant AI-33119 and Texas Advanced Research Program Technology Development and Transfer grant 004949-036 (J.M.M.).
We thank H. M. Baker for generously providing the SpeB crystal structure figure.
REFERENCES
- 1.Berge A, Bjorck L. Streptococcal cysteine proteinase releases biologically active fragments of streptococcal surface proteins. J Biol Chem. 1995;270:9862–9867. doi: 10.1074/jbc.270.17.9862. [DOI] [PubMed] [Google Scholar]
- 2.Burns E H, Jr, Lukomski S, Rurangirwa J, Podbielski A, Musser J M. Genetic inactivation of the extracellular cysteine protease enhances in vitro internalization of group A streptococci by human epithelial and endothelial cells. Microb Pathog. 1998;24:333–339. doi: 10.1006/mpat.1998.0204. [DOI] [PubMed] [Google Scholar]
- 3.Burns E H, Jr, Marciel A M, Musser J M. Activation of a 66-kilodalton human endothelial cell matrix metalloprotease by Streptococcus pyogenes extracellular cysteine protease. Infect Immun. 1996;64:4744–4750. doi: 10.1128/iai.64.11.4744-4750.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elliott S D. A proteolytic enzyme produced by group A streptococci with special reference to its effect on the type-specific M antigen. J Exp Med. 1945;81:573–592. doi: 10.1084/jem.81.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott S D, Dole V P. An inactive precursor of streptococcal proteinase. J Exp Med. 1947;85:305–320. doi: 10.1084/jem.85.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerwin B I, Stein W H, Moore S. On the specificity of streptococcal proteinase. J Biol Chem. 1966;241:3331–3339. [PubMed] [Google Scholar]
- 7.Gubba S, Low D E, Musser J M. Expression and characterization of group A Streptococcus extracellular cysteine protease recombinant mutant proteins and documentation of seroconversion during human disease episodes. Infect Immun. 1998;66:765–770. doi: 10.1128/iai.66.2.765-770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herwald H, Collin M, Muller-Ester W, Bjorck L. Streptococcal cysteine proteinase releases kinins: a novel virulence mechanism. J Exp Med. 1996;184:665–673. doi: 10.1084/jem.184.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kagawa T F, Cooney J C, Baker H M, McSweeney S, Liu M, Gubba S, Musser J M, Baker E N. Crystal structure of the zymogen form of the streptococcal virulence factor SpeB: an integrin-binding cysteine protease. Proc Natl Acad Sci USA. 2000;97:2235–2240. doi: 10.1073/pnas.040549997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamphuis I G, Kalk K H, Swarte M B A, Drenth J. Structure of papain refined at 1.65 Å resolution. J Mol Biol. 1984;179:233–256. doi: 10.1016/0022-2836(84)90467-4. [DOI] [PubMed] [Google Scholar]
- 11.Kapur V, Maffei J T, Greer R S, Li L L, Adams G J, Musser J M. Vaccination with streptococcal extracellular cysteine protease (interleukin-1β convertase) protects mice against challenge with heterologous group A streptococci. Microb Pathog. 1994;16:443–450. doi: 10.1006/mpat.1994.1044. [DOI] [PubMed] [Google Scholar]
- 12.Kapur V, Majesky M W, Li L-L, Black R A, Musser J M. Cleavage of interleukin 1β precursor to produce active IL-1β by a conserved extracellular cysteine protease from Streptococcus pyogenes. Proc Natl Acad Sci USA. 1993;90:7676–7680. doi: 10.1073/pnas.90.16.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapur V, Topouzis S, Majesky M W, Li L-L, Hamrick M R, Hamill R J, Patti J M, Musser J M. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb Pathog. 1993;15:327–346. doi: 10.1006/mpat.1993.1083. [DOI] [PubMed] [Google Scholar]
- 14.Karrer K M, Peiffer S L, DiTomas M E. Two distinct gene subfamilies within the family of cysteine protease genes. Proc Natl Acad Sci USA. 1993;90:3063–3067. doi: 10.1073/pnas.90.7.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kortt A A, Liu T Y. On the mechanism of action of streptococcal proteinase. I. Active-site titration. Biochemistry. 1973;12:320–327. doi: 10.1021/bi00726a023. [DOI] [PubMed] [Google Scholar]
- 16.Kuo C-F, Wu J-J, Lin K-Y, Tsai P-J, Lee S-C, Jin Y-T, Lei H-Y, Lin Y-S. Role of streptococcal pyrogenic exotoxin B in the mouse model of group A streptococcal infection. Infect Immun. 1998;66:3931–3935. doi: 10.1128/iai.66.8.3931-3935.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo C-F, Wu J-J, Tsai P-J, Kao F-J, Lei H-Y, Lin M T, Lin Y-S. Streptococcal pyrogenic exotoxin B induces apoptosis and reduces phagocytic activity in U937 cells. Infect Immun. 1999;67:126–130. doi: 10.1128/iai.67.1.126-130.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu T-Y. Demonstration of the presence of a histidine residue at the active site of streptococcal proteinase. J Biol Chem. 1967;242:4029–4032. [PubMed] [Google Scholar]
- 19.Liu T-Y, Elliott S D. Streptococcal proteinase: the zymogen to enzyme transformation. J Biol Chem. 1965;240:1138–1142. [PubMed] [Google Scholar]
- 20.Lukomski S, Burns E H, Jr, Wyde P R, Podbielski A, Rurangirwa J, Moore-Poveda D K, Musser J M. Genetic inactivation of an extracellular cysteine protease (SpeB) expressed by Streptococcus pyogenes decreases resistance to phagocytosis and dissemination to organs. Infect Immun. 1998;66:771–776. doi: 10.1128/iai.66.2.771-776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lukomski S, Montgomery C A, Rurangirwa J, Geske R S, Barrish J P, Adams G J, Musser J M. Extracellular cysteine protease produced by Streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice. Infect Immun. 1999;67:1779–1788. doi: 10.1128/iai.67.4.1779-1788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukomski S, Sreevatsan S, Amberg C, Reichardt W, Woischnik M, Podbielski A, Musser J M. Inactivation of Streptococcus pyogenes extracellular cysteine protease significantly decreases mouse lethality of serotype M3 and M49 strains. J Clin Investig. 1997;99:2574–2580. doi: 10.1172/JCI119445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuka Y V, Pillai S, Gubba S, Musser J M, Olmsted S B. Fibrinogen cleavage by the Streptococcus pyogenes extracellular cysteine protease and generation of antibodies that inhibit enzyme proteolytic activity. Infect Immun. 1999;67:4326–4333. doi: 10.1128/iai.67.9.4326-4333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musser J M. Streptococcal superantigen, mitogenic factor, and pyrogenic exotoxin B expressed by Streptococcus pyogenes. In: Leung D Y M, Huber B T, Schlievert P M, editors. Superantigens. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 281–310. [DOI] [PubMed] [Google Scholar]
- 25.Musser J M, Stockbauer K, Kapur V, Rudgers G W. Substitution of cysteine 192 in a highly conserved Streptococcus pyogenes extracellular cysteine protease (interleukin-1β convertase) alters proteolytic activity and ablates zymogen processing. Infect Immun. 1996;64:1913–1917. doi: 10.1128/iai.64.6.1913-1917.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson D, Potempa J, Kordula T, Travis J. Purification and characterization of a novel cysteine proteinase (periodontain) from Porphyromonas gingivalis: evidence for a role in the inactivation of human alpha-1-proteinase inhibitor. J Biol Chem. 1999;274:12245–12251. doi: 10.1074/jbc.274.18.12245. [DOI] [PubMed] [Google Scholar]
- 27.Raeder R, Woischnik M, Podbielski A, Boyle M D. A secreted streptococcal cysteine protease can cleave a surface-expressed M1 protein and alter the immunoglobulin binding properties. Res Microbiol. 1998;149:539–548. doi: 10.1016/s0923-2508(99)80001-1. [DOI] [PubMed] [Google Scholar]
- 28.Stockbauer K E, Magoun L, Liu M, Burns E H, Jr, Gubba S, Renish S, Pan X, Bodary S C, Baker E, Coburn J, Leong J M, Musser J M. A natural variant of the cysteine protease virulence factor of group A Streptococcus with an arginine-glycine-aspartic acid (RGD) motif preferentially binds human integrins αvβ3 and αIIbβ3. Proc Natl Acad Sci USA. 1998;95:3128–3133. doi: 10.1073/pnas.96.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai P-J, Kuo C-F, Lin K-Y, Lin Y-S, Lei H-Y, Chen F-F, Wang J-R, Wu J-J. Effect of group A streptococcal cysteine protease on invasion by epithelial cells. Infect Immun. 1998;66:1460–1466. doi: 10.1128/iai.66.4.1460-1466.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vernet T, Khouri H E, Laflamme P, Tessier D C, Musil R, Gour-Salin B J, Storer A C, Thomas D Y. Processing of the papain precursor. Purification of the zymogen and characterization of its mechanism of processing. J Biol Chem. 1991;266:21451–21457. [PubMed] [Google Scholar]