Abstract
SGLT2i therapy is well suited for initiation during the HF hospitalization, owing to clinical benefits that accrue rapidly within days to weeks, a strong safety and tolerability profile, minimal to no effects on blood pressure, and no excess risk of adverse kidney events. There is no evidence to suggest that deferring initiation to the outpatient setting accomplishes anything beneficial. Instead, there is compelling evidence that deferring in-hospital initiation exposes patients to excess risk of early post-discharge clinical worsening and death. Lessons from other HFrEF therapies highlight that deferring initiation of guideline-recommended medications to the US outpatient setting carries a >75% chance they will not be initiated within the next year. Recognizing that 1 in 4 patients hospitalized for worsening HF die or are readmitted within 30 days, clinicians should embrace the in-hospital period as an optimal time to initiate SGLT2i therapy and treat this population with the urgency it deserves.
Keywords: Heart failure, medical therapy, in-hospital prescribing, sodium glucose cotransporter-2 inhibitors, guideline-directed medical therapy
Many eligible patients with HFrEF never receive therapies proven to extend survival, prevent hospitalizations, and improve quality of life; or receive them with significant delay.(1,2) Hospitalizations for HF are seminal moments in a patient’s disease trajectory, but also provide a key opportunity to improve utilization of GDMT.(3) Currently, there are limited data directly assessing in-hospital initiation of SGLT2i for HFrEF, and the 2 initial cardiovascular outcomes trials of SGLT2i in HFrEF were conducted in outpatients with chronic HFrEF.(4,5) Herein, we present the case for routine in-hospital initiation of SGLT2i therapy for patients hospitalized for HFrEF.
OUTPATIENT HEART FAILURE TRIALS MAY GENERALIZE TO HOSPITALIZED PATIENTS WHO ARE STABILIZED
Although historically treated as separate entities in clinical trials, mounting evidence supports hospitalized and ambulatory patients with HFrEF as a common pathophysiology on a continuum.(6) Accumulating data suggest that location of care 1) is inherently subjective, 2) does not reliably distinguish biologic characteristics or clinical risk and 3) in and of itself, should not factor into decisions to withhold chronic HFrEF therapies, as compared with objective measures like vital signs and laboratories (Table 1).(6)
Table 1.
Rationale for Generalizing Data from Outpatient HFrEF Trials to Hospitalized Patients Who Are Stabilized and/or Pre-Discharge
| Location of care does not reliably distinguish biology or risk, and accumulating data suggest that “acute”/ hospitalized HFrEF and stable outpatient HFrEF are a single disease on a continuum. |
|
|
|
|
| To our knowledge, there are no examples where a chronic therapy improved outcomes among outpatients with HFrEF and was ineffective as chronic therapy when initiated among stabilized patients in the hospital |
|
|
| In-hospital initiation of GDMT for chronic HFrEF is consistent with regulatory labels and clinical practice guidelines. |
|
|
|
Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; ARNI, angiotensin receptor-neprilysin inhibitor; GDMT, guideline-directed medical therapy; HFrEF, heart failure with reduced ejection fraction; SGLT2i, sodium glucose cotransporter-2 inhibitor
To our knowledge, there are no examples of clinical trials where a drug proven to improve clinical outcomes in outpatient HFrEF has failed to improve clinical outcomes when initiated as long-term therapy among stable hospitalized patients.(7,8) Most recently, the SOLOIST-WHF trial confirmed efficacy and safety of sotagliflozin, a dual SGLT1/SGLT2 inhibitor, among patients hospitalized for HF (regardless of EF and with concomitant diabetes) with initiation either pre-discharge or early post-discharge.(9)
From a regulatory perspective, approved indications by the US FDA do not reference location of care, and in-hospital status does not impact eligibility criteria for SGLT2i or other HFrEF therapies. Decisions to initiate GDMT should be focused on objective clinical characteristics, regardless of hospitalization status, as highlighted in the ACC Expert Consensus document.(10) Further, there is an explicit ACC/AHA HF Guideline Class I recommendation for in-hospital or before discharge initiation of GDMT if not previously established, unless contraindicated.(11)
EARLY CLINICAL BENEFITS FOLLOWING INITIATION
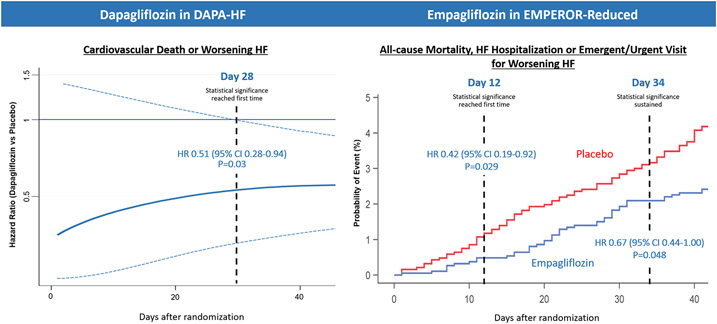
Among the strongest reasons for in-hospital initiation of SGLT2i therapy is the early and substantial clinical benefit that appears within days to weeks of initiation (Figure 1).(12-14) For example, empagliflozin demonstrated a statistically significant 58% relative reduction in mortality, HF hospitalization, or urgent HF visit at just 12 days after initiation.(13) These early benefits are reinforced by SOLOIST-WHF, where in-hospital or early post-discharge initiation of sotagliflozin demonstrated early separation of clinical event curves.(9) Not prescribing SGLT2i at discharge to an eligible patient exposes the patient to a statistically and clinically significant excess risk for death and readmission in the first days to weeks post-discharge.
Figure 1. Early Clinical Benefits Following Initiation of SGLT2i for HFrEF.
The benefit of SGLT2i on clinical event reduction reaches statistical significance <30 days after initiation. From Berg DD et al.(14) and Packer M et al.(13) Modified with permission from Packer M et al.(13)
IMPROVED TOLERANCE OF OTHER HEART FAILURE THERAPIES
SGLT2i’s decrease risk of hyperkalemia and slow progression of kidney dysfunction, features that favor short-term and/or long-term tolerance of ARNI and MRA therapy.(12,15) Paradoxical to their high clinical risk, patients hospitalized for HF commonly experience medication discontinuation both during and after hospitalization, with an associated increased risk of subsequent clinical events. In-hospital initiation of SGLT2i should be prioritized as part of a comprehensive approach to maximize medication tolerance and prevent withdrawal of other lifesaving medications.
DEFERRED IN-HOSPITAL INITIATION IS ASSOCIATED WITH NEVER INITIATING OR SUBSTANTIAL DELAYS
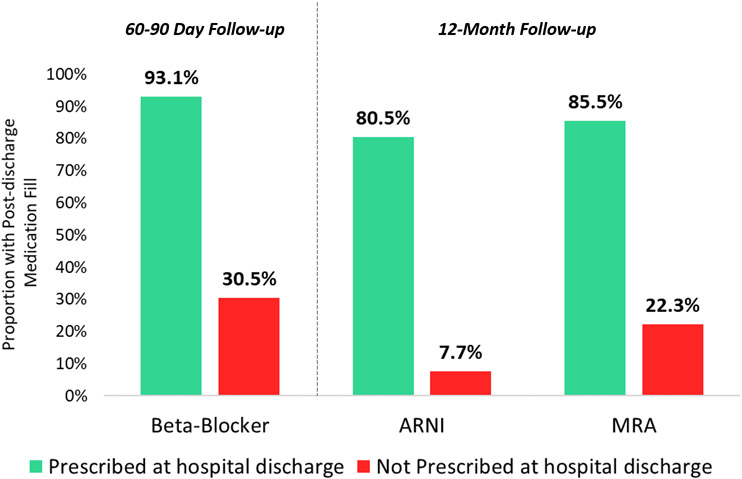
Multiple real-world studies show that failure to discharge eligible patients on GDMT significantly increases the chance therapies will never be started, or started with considerable delay.(12,16,17) Among US patients hospitalized for HFrEF and eligible for therapy, deferring initiation of ARNI or MRA therapies to the outpatient setting carries a >75% chance therapy will not be initiated within the next year (Figure 2).(12,16,17) Complementing real-world data, multiple randomized trials support the effectiveness of in-hospital initiation for improving post-discharge medication use, without higher risk of medication discontinuation.(18)
Figure 2. Post-discharge Use of GDMT by Prescription Status at Hospital Discharge.
Data from US clinical practice and reflect patients eligible each therapy at discharge. Not prescribing medication at discharge carries substantial risk patient will not receive therapy as outpatient. From Fonarow GC et al.(24), Curtis LH et al.(16), Carnicelli AP et al.(17)
ADDRESSING HESITATION WITH IN-HOSPITAL INITIATION OF SGLT2i
Tolerability
Clinicians may have concerns that patients hospitalized with HFrEF are clinically tenuous and that in-hospital initiation of SGLT2i will increase risk of adverse effects or medication intolerance. We highlight that SGLT2i’s have a particularly robust safety and tolerability profile, and distinguish themselves from traditional and overlapping potential side effects of other GDMTs (e.g., hypotension, worsening kidney function, hyperkalemia). In randomized trials, patients with systolic blood pressure as low as 95 mmHg were eligible, and SGLT2i’s showed minimal to no effect on blood pressure, and no excess risk of symptomatic hypotension.(4,5) Likewise, although a small initial drop in eGFR may be expected, there were no excess risks of acute kidney injury, even among patients with impaired baseline kidney function. Indeed, SGLT2i’s rarely cause symptomatic side effects in patients with HFrEF, and serious adverse events leading to drug discontinuation were numerically fewer with SGLT2i than placebo.(4,5) Although not a HFrEF population, the recent DARE-19 randomized trial (NCT04350593) among patients hospitalized for COVID-19 pneumonia with high cardiometabolic risk further supports the in-hospital safety profile of SGLT2i among clinically tenuous patients. While not achieving statistical significance, hospitalized COVID-19 patients randomized to in-hospital dapagliflozin were numerically less likely than placebo to have respiratory decompensation, cardiac decompensation, acute kidney injury, drug discontinuation, and death.
Glycemic Status
In both DAPA-HF and EMPEROR-Reduced, hypoglycemic and diabetic ketoacidosis (DKA) events were rare, and there was no excess risk with SGLT2i, irrespective of T2DM status.(4,5) Further reassuring, among patients hospitalized for HF and T2DM in SOLOIST-WHF, rates of DKA were nominally lower with sotagliflozin (0.3%) than placebo (0.7%).(9) In a broader assessment of ~50,000 patients across all SGLT2i trials, there was no significant risk of hypoglycemia, but a slightly higher absolute risk of DKA (0.23% vs 0.08%).(19) From the perspective of in-hospital T2DM management, a practical approach to SGLT2i initiation includes reducing daily insulin dose by 20% and stopping sulfonylureas if hemoglobin A1c <8.5%. Although decision-making regarding select patients requiring complex T2DM regimens or with recurrent hypoglycemic or DKA events can be challenging, there is no evidence for glycemic considerations as a routine barrier to in-hospital initiation of SGLT2i.
Patient Not Receiving Optimal Background Therapy for HFrEF
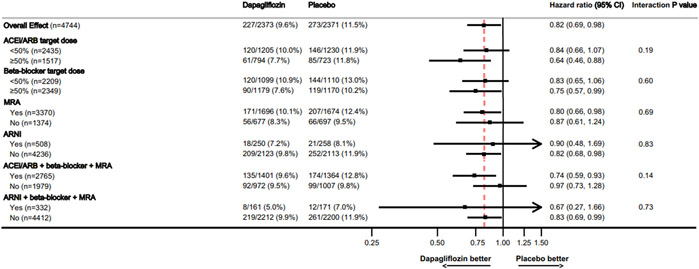
Some may question whether initiation of SGLT2i should be deferred in favor of in-hospital initiation or titration of other GDMTs. Data confirm that benefits of SGLT2i are fully additive to effects of other GDMTs, with consistent magnitude of incremental benefit regardless of quality of background therapy (Figure 3).(4,5) While every effort should be made to achieve target or maximally tolerated doses of ARNI, beta-blocker, and MRA, lower doses confer benefit. For purposes of maximizing clinical event reduction and chances of tolerance to the full quadruple therapy regimen, initiation of SGLT2i should be prioritized above dose escalation of other therapies.(12)
Figure 3. Clinical Effect of Dapagliflozin by Background Medical Therapy.
In the DAPA-HF trial, benefits of dapagliflozin on cardiovascular mortality or worsening HF were incremental and consistent regardless of background medical therapy for HFrEF. Modified with permission from Docherty KF et al.(25)
Costs
Cost-effectiveness analyses find SGLT2i therapy to provide intermediate to high value in HFrEF.(20,21) These estimates are based on outpatient initiation and are sensitive to the magnitude of mortality reduction. In-hospital initiation may be even more cost-effective, since higher absolute risk reductions would be expected with the higher clinical event rates following hospitalization for HFrEF. Nonetheless, formulary issues, prior authorization requirements, and out-of-pocket costs may remain barriers to SGLT2i for some patients. However, the multidisciplinary team and resources routinely available in many hospitals (as compared with outpatient clinic) may be optimally positioned to explore all possible strategies to secure access to medication.
In-hospital SGLT2i Trials Are Not Yet Completed
Some clinicians may feel uncomfortable with in-hospital initiation of SGLT2i because multiple dedicated clinical trials testing this strategy are not yet complete (Table 2). However, while results of ongoing trials will further contextualize in-hospital initiation, there is no available evidence to suggest that deferring initiation of SGLT2i for HFrEF to the outpatient setting accomplishes anything beneficial or improves medication tolerance. Instead, there is compelling evidence that deferring in-hospital initiation exposes patients to excess risk of early post-discharge clinical worsening and death, and the possibility of never having the medication prescribed.(12) We also re-emphasize that results from the 1222-patient SOLOIST-WHF trial are already available, supporting the strong efficacy and safety of in-hospital initiation of sotagliflozin.(9)
Table 2:
Recent and Ongoing Clinical Trials Evaluating In-hospital Initiation of SGLT2i Therapy for Patients Admitted for Acute Heart Failure
| Trial | Design | Follow-up | Outcomes |
|---|---|---|---|
| Completed | |||
| SOLOIST-WHF (NCT 03521934) | Randomized, double-blind multicenter trial. 1222 patients hospitalized with AHF were randomized 1:1 to sotagliflozin 200–400 mg or placebo during hospitalization or within 3 days of discharge. Included only patients with T2DM and eGFR ≥ 30 ml/min/1.73m2. Trial ended early because of loss of funding from sponsor. | Outpatient | Sotagliflozin reduced the total number of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure (primary endpoint). Diarrhea and severe hypoglycemia were more common in the sotagliflozin group. |
| EMPA-RESPONSE-AHF (NCT 03200860) | Randomized, double blind pilot study. 80 patients hospitalized with AHF were randomized in the first 24 hours of hospitalization 1:1 to either empagliflozin 10 mg daily or placebo for 30 days. Included patients with and without T2DM. All patients had eGFR ≥ 30 ml/min/1.73m2. Loop diuretic use was non-standardized. | In-hospital | There were no differences in the primary endpoints assessed on day 4 of hospitalization: change in VAS dyspnea score, weight change per 40 mg IV furosemide, length of stay, and change in NT-proBNP. Rates of adverse events were similar between groups. |
| Ongoing | |||
| DICTATE-AHF (NCT 04298229) | Randomized, open-label, blinded adjudication. 240 patients hospitalized with AHF randomized in the first 24 hours of hospitalization 1:1 to dapagliflozin or usual care until day 5 or hospital discharge. All patients have T2DM and receive diuretic and insulin therapies per protocol. All patients with eGFR ≥ 30. | In-hospital | Primary endpoint is weight change per 40 mg furosemide equivalent on day 5. Key safety endpoints include adverse diabetes-related events while hospitalized: incidence of hyper/ hypoglycemia, ketoacidosis, and hypovolemic hypotension. |
| Ertugliflozin in AHF (NCT 04438213) | Randomized, double-blind mechanistic study. 90 patients hospitalized with AHF randomized 1:1:1 to ertugliflozin, metolazone, or placebo. All patients receive non-standardized loop diuretic therapy. All patients have eGFR ≥ 30 ml/min/1.73m2 with or without T2DM. | In-hospital and outpatient | Primary endpoint is change in urine sodium and total body water at days 1, 7, and 42. |
| EMPULSE (NCT 04157751) | Randomized, double-blind study. 530 patients hospitalized for AHF on day 2–5 randomized 1:1 to empagliflozin 10 mg or placebo. Patients must be stabilized with SBP >100 mmHg, no IV vasodilators or increase in IV loop diuretics in 6 h and no IV inotropes in 24 h. Includes both HFpEF and HFrEF patients. eGFR ≥ 20 ml/min/1.73m2. | Outpatient | Primary endpoint is composite of death, number of heart failure events, time to first heart failure event, and ≥ 5 point KCCQ-TSS score change at 90 days using a “win-ratio” approach. |
| DAPA ACT HF-TIMI 68 (NCT 04363697) | Randomized, double-blind study. 2400 patients hospitalized with AHF randomized 1:1 to dapagliflozin 10 mg vs placebo on hospital day 2–7. Patients must be stabilized with no increase in IV loop diuretics in and no IV vasodilator or inotropes in 24 h. eGFR ≥ 30 ml/min/1.73m2. | Outpatient | Primary endpoint through 60 days is time to first cardiovascular death or worsening heart failure. |
Abbreviations: AHF, acute heart failure; T2DM, type 2 diabetes mellitus; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IV, intravenous; NT-proBNP, N-terminal-pro hormone brain natriuretic peptide; KCCQ-TSS, The Kansas City Cardiomyopathy Questionnaire Total Symptom Score.
IN-HOSPITAL INITIATION OF SGLT2i VERSUS THE STATUS QUO
In weighing the alternatives to in-hospital initiation, consider the current status of outpatient medical therapy for HFrEF in the US. There remains a strong culture of clinical inertia towards evidence-based medical therapy and initiation of lifesaving medications among eligible patients remains relatively rare.(2) Among US outpatients with HFrEF eligible for therapy, 1 in 4 are not prescribed a renin-angiotensin-system inhibitor, 1 in 3 are not prescribed a beta-blocker, and 2 in 3 are not prescribed a MRA.(1) Further, among outpatients with HFrEF and diabetes, SGLT2i use is <5% with no meaningful change during 12-month follow-up.(22) Challenges with implementation exist for both newer (e.g., ARNI) and older generic medications (e.g., MRA), strongly suggesting that drug prices are not the predominant explanation for widespread medication underuse.
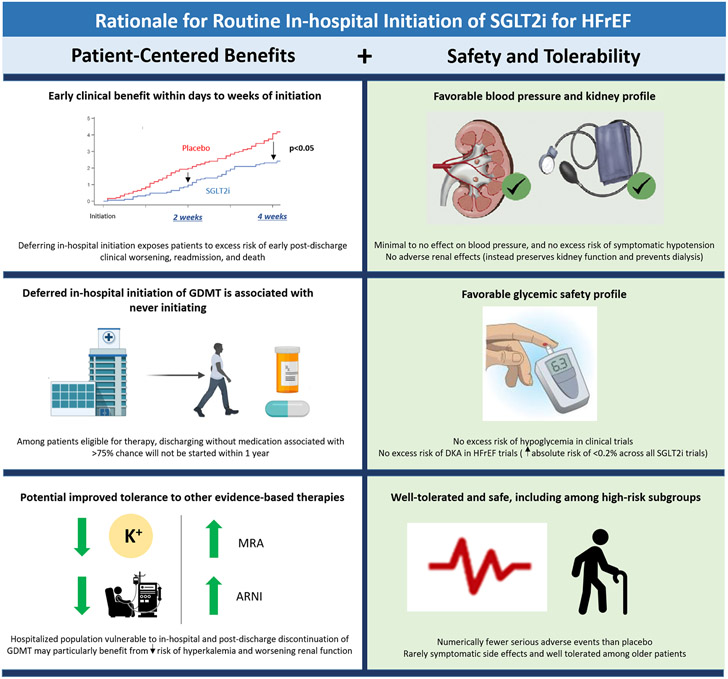
We must recognize that 1 in 4 patients hospitalized for worsening HFrEF are either re-hospitalized or dead within 30 days of discharge.(23) In-hospital initiation of SGLT2i seeks to break clinical inertia and treat this high-risk population with the sense of urgency it deserves. SGLT2i is particularly well suited for initiation during the HF hospitalization, owing to clinical benefits that accrue rapidly within days to weeks, a strong safety and tolerability profile, minimal to no effects on blood pressure, and no excess risk of adverse kidney events (Central Illustration). Failure to embrace routine in-hospital initiation of SGLT2i would be a significant missed opportunity, one that would lead to preventable deaths and readmissions, and the real possibility that the majority of these high-risk patients ultimately never receive this therapy.
Central Illustration. Rationale for Routine In-hospital Initiation of SGLT2i for HFrEF.
In-hospital initiation of SGLT2i among eligible patients is supported by multiple patient-centered benefits and a strong safety/tolerability profile well suited for the hospitalized population.
ACKNOWLEDGMENTS
The authors thank Ms. Christy Darnell for assisting in the coordination of this scientific meeting.
FUNDING SOURCES:
The manuscript summarizes proceedings of an independently organized academic meeting supported by an unrestricted educational grant from AstraZeneca. AstraZeneca did not have any role in the design, interpretation, or submission of the manuscripts content.
DISCLOSURES:
Dr. Rao is supported by a National Institutes of Health (NIH) Training Grant (NIH 5T32HL069749–17). Dr. Butler receives support from Boehringer Ingelheim, Cardior, CVRx, Foundry, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, Sequana Medical, V-Wave Ltd., and Vifor. Dr. Cooper receives consulting fees from AstraZeneca and research support from Abbott. Dr. Cox receives research support from AstraZeneca. Dr. Green receives research support from Boehringer Ingelheim/Lilly, Merck, Roche and Sanofi/Lexicon and consulting fees from AstraZeneca, Boehringer Ingelheim/Lilly, Hawthorne Effect/Omada, NovoNordisk, and Pfizer. Dr. Lindenfeld receives research support from AstraZeneca, Sensible Medical, and Volumetrix and consulting fees from Abbott, AstraZeneca, Boehringer Ingelheim, Boston Scientific, CVRx, Cytokinetics, Edwards Lifesciences, Impulse Dynamics, VWave, and Vifor. Dr. McGuire receives honoraria for clinical trial leadership from Boehringer Ingelheim, Sanofi, AstraZeneca, Merck & Co, Pfizer, Novo Nordisk, Esperion, Lilly USA, Lexicon, CSL Behring, and honoraria for consultancy from Lilly USA, Boehringer Ingelheim, Merck & Co, Novo Nordisk, Applied Therapeutics, Metavant, Sanofi, Afimmune, Lexicon. Dr. Pagidipati receives research support from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novartis, Novo Nordisk, Regeneron, Sanofi, Verily Life Sciences and consulting fees from Boehringer Ingelheim, Eli Lilly, AstraZeneca, and Novo Nordisk. Dr. Vaduganathan has received research grant support or served on advisory boards for American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, and Relypsa, speaker engagements for Novartis, and participates on clinical endpoint committees for studies sponsored by Galmed, Novartis, and the NIH. Dr. Fonarow reports serving as a consultant for Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Janssen, Medtronic, Merck, and Novartis. Dr. Mentz receives research support and honoraria from Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim/Eli Lilly, Boston Scientific, Cytokinetics, Fast BioMedical, Gilead, Innolife, Medtronic, Merck, Novartis, Relypsa, Respicardia, Roche, Sanofi, Vifor, and Windtree Therapeutics. Dr. Greene has received research support from the Duke University Department of Medicine Chair’s Research Award, American Heart Association, Amgen, AstraZeneca, Bristol-Myers Squibb, Cytokinetics, Merck, Novartis, and Pfizer; has served on advisory boards for Amgen, AstraZeneca and Cytokinetics; and has served as a consultant for Amgen, Bayer, Merck, and Vifor. All other authors report no disclosures.
ABBREVIATIONS
- ARNI
angiotensin receptor-neprilysin inhibitor
- DAPA-HF
Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure)
- eGFR
estimated glomerular filtration rate
- EMPEROR-Reduced
Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction
- FDA
Food and Drug Administration
- GDMT
guideline-directed medical therapy
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- MRA
mineralocorticoid receptor antagonists
- SGLT2i
sodium-glucose cotransporter-2 inhibitors
- SOLOIST-WHF
Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure
- T2DM
type 2 diabetes mellitus
REFERENCES
- 1.Greene SJ, Butler J, Albert NM et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J Am Coll Cardiol 2018;72:351–366. [DOI] [PubMed] [Google Scholar]
- 2.Greene SJ, Fonarow GC, DeVore AD et al. Titration of Medical Therapy for Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol 2019;73:2365–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhagat AA, Greene SJ, Vaduganathan M, Fonarow GC, Butler J. Initiation, Continuation, Switching, and Withdrawal of Heart Failure Medical Therapies During Hospitalization. J Am Coll Cardiol HF 2019;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMurray JJV, Solomon SD, Inzucchi SE et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 5.Packer M, Anker SD, Butler J et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 6.Greene SJ, Mentz RJ, Felker GM. Outpatient Worsening Heart Failure as a Target for Therapy: A Review. JAMA Cardiol 2018;3:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMurray JJ, Packer M, Desai AS et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 8.Velazquez EJ, Morrow DA, DeVore AD et al. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med 2019;380:539–548. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt DL, Szarek M, Steg PG et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med 2021;384:117–128. [DOI] [PubMed] [Google Scholar]
- 10.Hollenberg SM, Warner Stevenson L, Ahmad T et al. 2019 ACC Expert Consensus Decision Pathway on Risk Assessment, Management, and Clinical Trajectory of Patients Hospitalized With Heart Failure: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2019;74:1966–2011. [DOI] [PubMed] [Google Scholar]
- 11.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 12.Greene SJ, Butler J, Fonarow GC. Simultaneous or Rapid Sequence Initiation of Quadruple Medical Therapy for Heart Failure: Optimizing Therapy with the Need for Speed. JAMA Cardiol 2021. [DOI] [PubMed] [Google Scholar]
- 13.Packer M, Anker SD, Butler J et al. Effect of Empagliflozin on the Clinical Stability of Patients with Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial. Circulation 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg DD, Jhund PS, Docherty KF et al. Time to Clinical Benefit of Dapagliflozin and Significance of Prior Heart Failure Hospitalization in Patients With Heart Failure With Reduced Ejection Fraction. JAMA Cardiol 2021;6:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira JP, Zannad F, Pocock SJ et al. Interplay of Mineralocorticoid Receptor Antagonists and Empagliflozin in Heart Failure: EMPEROR-Reduced. J Am Coll Cardiol 2021;77:1397–1407. [DOI] [PubMed] [Google Scholar]
- 16.Curtis LH, Mi X, Qualls LG et al. Transitional adherence and persistence in the use of aldosterone antagonist therapy in patients with heart failure. American heart journal 2013;165:979–986. [DOI] [PubMed] [Google Scholar]
- 17.Carnicelli AP, Lippmann SJ, Greene SJ et al. Sacubitril/Valsartan Initiation and Post-discharge Adherence Among Patinets Hospitalized for Heart Failure. J Card Fail 2021;27:826–836. [DOI] [PubMed] [Google Scholar]
- 18.Gattis WA, O'Connor CM, Gallup DS, Hasselblad V, Gheorghiade M. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol 2004;43:1534–41. [DOI] [PubMed] [Google Scholar]
- 19.Salah HM, Al'Aref SJ, Khan MS et al. Effect of sodium-glucose cotransporter 2 inhibitors on cardiovascular and kidney outcomes-Systematic review and meta-analysis of randomized placebo-controlled trials. Am Heart J 2021;232:10–22. [DOI] [PubMed] [Google Scholar]
- 20.Parizo JT, Goldhaber-Fiebert JD, Salomon JA et al. Cost-effectiveness of Dapagliflozin for Treatment of Patients With Heart Failure With Reduced Ejection Fraction. JAMA Cardiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEwan P, Darlington O, McMurray JJV et al. Cost-effectiveness of dapagliflozin as a treatment for heart failure with reduced ejection fraction: a multinational health-economic analysis of DAPA-HF. Eur J Heart Fail 2020;22:2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaduganathan M, Fonarow GC, Greene SJ et al. Contemporary Treatment Patterns and Clinical Outcomes of Comorbid Diabetes Mellitus and HFrEF: The CHAMP-HF Registry. J Am Coll Cardiol HF 2020;8:469–480. [DOI] [PubMed] [Google Scholar]
- 23.Greene SJ, Triana TS, Ionescu-Ittu R et al. Patients Hospitalized for De Novo Versus Worsening Chronic Heart Failure in the United States. J Am Coll Cardiol 2021;77:1023–1025. [DOI] [PubMed] [Google Scholar]
- 24.Fonarow GC, Abraham WT, Albert NM et al. Prospective evaluation of beta-blocker use at the time of hospital discharge as a heart failure performance measure: results from OPTIMIZE-HF. Journal Card Fail 2007;13:722–31. [DOI] [PubMed] [Google Scholar]
- 25.Docherty KF, Jhund PS, Inzucchi SE et al. Effects of dapagliflozin in DAPA-HF according to background heart failure therapy. Eur Heart J 2020;41:2379–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greene SJ, Butler J, Fonarow GC et al. Pre-discharge and early post-discharge troponin elevation among patients hospitalized for heart failure with reduced ejection fraction: findings from the ASTRONAUT trial. Eur J Heart Fail 2018;20:281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]