ABSTRACT
China has regulated its hepatitis B vaccination policy. However, data on the prevalence of hepatitis B virus (HBV) infection have not been updated since 2014. In addition, the impact of the policy on awareness of hepatitis B is limited, especially in Fujian Province where HBV infection is highly prevalent. We conducted a sero-epidemiological survey in five national monitoring counties to address these concerns. A total of 5,873 subjects were included and classified into four birth cohorts according to the policy time nodes (1981, 1992, and 2002). The HBsAg carrier rate for the general population was 8.6% (95% confidence interval [CI]: 7.9–9.3). Compared with those born before 1981, adjusted odds ratios (OR) for HBV infection were 0.51 (95% CI: 0.43–0.62), 0.10 (0.08–0.12), and 0.015 (0.01–0.023) among the 1981–1991, 1992–2001, and ≥2002 birth cohorts, respectively; while the OR was 1.26 (1.00–1.57), 0.39 (0.26–0.58), and 0.019 (0.006–0.06) for HBsAg carriage, respectively. Among the 4865 residents aged ≥15 years, hepatitis B awareness has been declining since the introduction of the hepatitis B vaccine into the immunization program (β = −0.25, SE = 0.08, P = .001, and β = −0.20, SE = 0.08, P = .017 for 1992–2001 and ≥2002 birth cohort, respectively). This decline was obvious for the initiation time of the first dose of the hepatitis B vaccine. Although the hepatitis B vaccination policies have helped reduce the infection, the awareness has declined. More measures on the target population are warranted to improve the public’s awareness of hepatitis B vaccination in the context of great achievements.
KEYWORDS: Hepatitis B, vaccine, infection, policy, awareness
Introduction
Hepatitis B vaccination is the most effective measure to prevent hepatitis B virus (HBV) infection.1,2 In China, the government has implemented two critical strategies to prevent and control HBV infection after the hepatitis B vaccine became available in 1981.3 In 1992, hepatitis B vaccine was introduced into the national immunization program and further included as a national immunization program vaccine series for newborns and infants in 2002,3 which meant the vaccine was free for infants with appropriate age. In the meantime, relevant measures (e.g., routine coverage surveillance and assessment,4,5 supplementary immunization activities,6 and supply of free hepatitis B immunoglobulin for mothers positive for HBV surface antigen (HBsAg)7) were taken to emphasize a timely initiation of the first dose (e.g., within 24 h after birth) and to improve coverage of the hepatitis B vaccine series completion.
In our previous study, we identified that the free hepatitis B vaccine policy implemented in 2002 significantly improved the timely initiation and completion of the hepatitis B vaccine series and thereby resulted in a decline of the HBV infection rate in Fujian province, China.8 According to three hepatitis B sero-epidemiological surveys carried out in 1992, 2006, and 2014, respectively, the prevalence of HBV infection in children <5 years old hugely decreased from 21.7% in 1992 to 0.2% in 2014.9 Remarkable achievement for targeted children was obtained; however, the HBsAg positivity prevalence remained high in China. According to a recent meta-analysis in which most included studies enrolled adult residents only, the estimated HBsAg positivity rate was 6.9% between 2013 and 2017.10 This rate was slightly declined compared with a 7.2% prevalence for the general population (residents aged 1–59 years) in 2006.11
In addition, updated evaluation data on hepatitis B may help assess the current prevalence and burden of the disease,10 especially in areas with high HBV infection prevalence.8 However, little is known on the recent HBV infection of the entire population in Fujian province since 2014. Furthermore, literature on the influence of hepatitis policy on HBV infection and hepatitis B awareness was largely absent in this area. Addressing these concerns is critical to further break down the barriers of hepatitis B vaccination and HBV prevention in the context of high coverage of routine hepatitis B vaccination.4,8,12
We conducted a fourth seroepidemiological survey in 2020 to update the prevalence of HBV infection and to evaluate the impact of hepatitis B policy on HBV infection and awareness of hepatitis B for residents aged 1–69 years old in Fujian, China. We hypothesized that the policy would decrease HBV infection by improvement of vaccination coverage and that the hepatitis-B-related knowledge might be lowered accompanied by a decline in HBV infection.
Materials and methods
Study design and data collection
This survey was part of a National Hepatitis Serosurvey conducted in October 2020. Local residents aged 1–69 years old in the five national disease surveillance counties (residence duration ≥6 months) were selected by the stratified 3-stage cluster random sampling method according to the survey protocol.13 The selected participants or their guardians were surveyed by trained professionals using a unified questionnaire. Basic characteristics (e.g., age, gender, ethnicity, and education level) and history of hepatitis B vaccination (yes or no) were collected for all subjects. Awareness of hepatitis B was further evaluated for all participants aged ≥15 years old at the survey. In the present study, a history of hepatitis B vaccination was defined as having completed three doses of hepatitis B vaccine series. This study was approved by the ethics committee of the Chinese Center for Disease Control and Prevention (CDC) (No. 2018-11). Written informed consent was obtained before the survey.
Birth cohorts
Surveyed subjects were classified into four birth cohorts according to the time nodes for hepatitis B vaccine accessibility and the implementation of hepatitis B vaccination policy (e.g., 1981, 1992, and 2002). The four birth cohorts were residents born before 1981, 1981–1991, 1991–2001, and ≥2002. They correspond to vaccines that were not available, vaccine accessibility, vaccines included in the immunization programs, and the free vaccine policy.
Outcomes
There were two outcomes in the present study. The primary outcome was HBV infection, which was defined according to the history of hepatitis B vaccination. For those with a history of hepatitis B vaccination, positive for HBsAg or antibody to hepatitis B core antigen (anti-HBc) was recognized as HBV infection.8 For people without vaccination, positive for HBsAg, anti-HBs, or anti-HBc, neither in isolation or in combination, was considered HBV infection.8 For those with unknown use of hepatitis B vaccination, subjects born since 1992 were deemed to have a vaccination since large-scale leak detection and revaccination was carried out for these residents in Fujian and the hepatitis B vaccination coverage was >97.4% after the campaign.14 Otherwise, they were considered an unvaccinated group.
HBV infection and clearance may have occurred prior to the vaccination, leading to anti-HBc-positive status. It is important to distinguish these individuals as those who cleared HBV infection from those who have not cleared it. In addition, although HBV infection might be a reasonable indicator in assessing overall infection risk in the general population, this marker might be confusing in the context of high vaccination coverage. Therefore, we further separately analyzed HBsAg carriage and anti-HBc positivity as secondary outcomes.
The second outcome was awareness of hepatitis B, which was evaluated by the scores of five hepatitis B and HBV transmission items, including (1) chronic hepatitis B will progress to cirrhosis and liver cancer; (2) HBV can be transmitted by shaking hands and hugging; (3) HBV can be mother-to-child transmission; (4) the first dose of hepatitis B should be initiated within 24 hours after birth; and (5) antiviral treatment can delay the progress of hepatitis B. Subjects obtained 1 point for each correct question. No point would be given for wrong or unknown answers (Table S1).
Laboratory test and covariates
Blood samples of the subjects were collected by negative vessel collection method after the questionnaire survey. Matched bar identification codes were pasted on each subject investigation form and collection vessel. Separated serums were stored at a temperature of −80°C or lower and were transported to the laboratory of Fujian CDC. The sample was firstly tested by enzyme-linked immunosorbent assay (ELISA) at Fujian CDC using detection reagents issued by China CDC (e.g., HBsAg, anti-HBs, or anti-HBc). The Institute of Viral Diseases of China CDC was responsible for rechecking the serum samples (e.g., anti-HBc) and for the detection of HBeAg and anti-HBe for those positive for HBsAg using Abbott microparticle ELISA.13
In the present study, we collected covariates that would be potential confounders affecting exposure and outcomes. Covariates included gender (male or female), ethnicity (Han or non-Han), education level (primary and below, secondary or high school, college and above, or students), hepatitis B vaccination (yes, no, or unknown), and mother’s HBsAg status (positive, negative, or unknown). Five counties were further divided into two categories as urban (Jiaocheng and Meilie) or rural (Huian, Yongding, and Jianou) according to the administrative division.8
Data analysis
We estimated the prevalence and 95% confidence interval (95% CI) of HBV infection, HBsAg carriage, and anti-HBc positivity in the general population. The prevalence and reported hepatitis B vaccination coverage were displayed by birth year to understand their trends. Chi-square or Fisher’s exact test was conducted to compare differences of category variables across the birth cohorts, while ANOVA was used for knowledge-level scores. If a significant difference was found for the scores, the Bonferroni method was further taken for pairwise comparison.
We constructed multivariate logistic regression models to probe the association between birth cohorts and the risk of HBV infection, HBsAg carriage, or anti-HBc positivity. Odds ratio (OR) and 95% CI were estimated. Coefficients and their standard errors for the differences of awareness scores across the birth cohorts were calculated by using multivariable linear regression. Hepatitis B awareness changes in each item across the birth cohorts were further compared to provide more details for policy improvement. All covariates mentioned above were included in the adjusted model to control for their potential impacts.
Sensitivity analysis restricted to Han ethnicity was run to test the robustness of the results. Difference of outcomes (e.g., infection and awareness) between residents (urban vs. rural) and gender (male vs. female) was compared. We further conducted stratification analyses (e.g., urban vs. rural; people with vaccination vs. those without or with unknown vaccination) to compare the disparity in risk reduction for viral infection (HBV infection or HBsAg carriage) and the change in hepatitis B awareness level. The heterogeneity between the parameters was compared.15
All analyses were conducted by using IBM SPSS Statistics version 24.0 (IBM Corp., Armonk, NY, USA). A two-sided P value <.05 was considered statistically significant.
Results
Basic characteristics and infection trend
According to the protocol, 5873 subjects were investigated and included into the analysis (Figure 1). Among them, 2665 were born before 1981 (45.4%), and 1037, 693, and 1478 were classified as 1981–1991, 1991–2001, and ≥2002 birth cohort, respectively. The majority of the participants were of Han ethnicity (98.7%), and 38.8% of them were males and 30.4% lived in urban areas. All characteristics except for ethnicity were unbalanced across the birth cohorts (Table 1).
Figure 1.
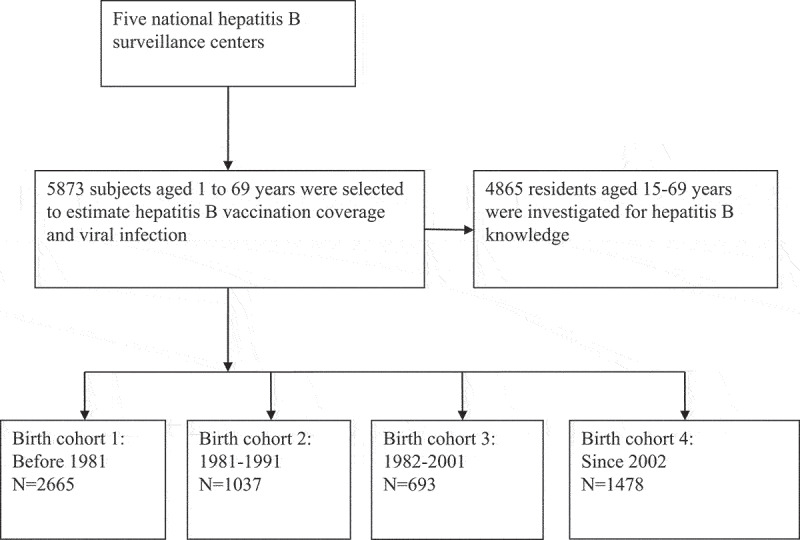
Flow chart of study.
Table 1.
Basic characteristics and hepatitis B viral infection outcomes by birth cohorts.
| Characteristics or outcomes* | Birth year |
P values | |||
|---|---|---|---|---|---|
| Before 1981 (N = 2665) | 1981–1991 (N = 1037) | 1992–2001 (N = 693) | Since 2002 (N = 1478) | ||
| HBV infection | 2209 (82.9) | 673 (64.9) | 167 (24.1) | 35 (2.4) | <.001 |
| HBsAg carriage | 332 (12.5) | 141 (13.6) | 30 (4.3) | 3 (0.2) | <.001 |
| Anti-HBc positive | 1829 (68.6) | 561 (54.1) | 124 (17.9) | 19 (1.3) | <.001 |
| Gender | <.001 | ||||
| Male | 998 (37.4) | 300 (28.9) | 236 (34.1) | 747 (50.5) | |
| Female | 1667 (62.6) | 737 (71.1) | 457 (65.9) | 731 (49.5) | |
| Mean ages (SD) | 53.8 (7.9) | 33.7 (3.4) | 24.9 (2.8) | 10.1 (5.5) | <.001 |
| Han ethnicity | 2639 (99.0) | 1023 (98.6) | 679 (98.0) | 1457 (98.6) | .16 |
| Education level | <.001 | ||||
| Primary and below | 1164 (43.7) | 73 (7.0) | 20 (2.9) | 3 (0.2) | |
| Secondary or high school | 1214 (45.6) | 623 (60.1) | 283 (40.8) | 455 (30.8) | |
| College and above | 287 (10.8) | 341 (32.9) | 390 (56.3) | 12 (0.8) | |
| Students | 0 (0) | 0 (0) | 0 (0) | 1008 (68.2) | |
| Resident | <.001 | ||||
| Urban | 750 (28.1) | 305 (29.4) | 243 (35.1) | 489 (33.1) | |
| Rural | 1915 (71.9) | 732 (70.6) | 450 (64.9) | 989 (66.9) | |
| History of hepatitis B vaccination | <.001 | ||||
| Yes | 333 (12.5) | 372 (35.9) | 366 (52.8) | 1304 (88.2) | |
| No | 1524 (57.2) | 311 (30.0) | 113 (16.3) | 55 (3.7) | |
| Unknown | 808 (30.3) | 354 (34.1) | 214 (30.9) | 119 (8.1) | |
* Present as n (%).
Figure 2 shows the reported hepatitis B vaccination coverage and the viral infection trend according to birth year. HBV infection rate remained above 80.0% before 1973 and began to decline after the hepatitis B vaccine became available in 1981. This rate dropped to less than 5.0% after 2004 and <1.0% after 2010. A similar trend was found for anti-HBc positivity. The HBsAg carriage peaked in 1985 with a prevalence around 16.5% and continued to decline since then. This prevalence has been <1% since 1999. The HBV infection, HBsAg carrier, and anti-HBc positivity rate in these study subjects was 52.5% (95% CI: 51.2–53.8), 8.6% (95% CI: 7.9–9.3), and 43.1% (95% CI: 41.9–44.4), respectively.
Figure 2.
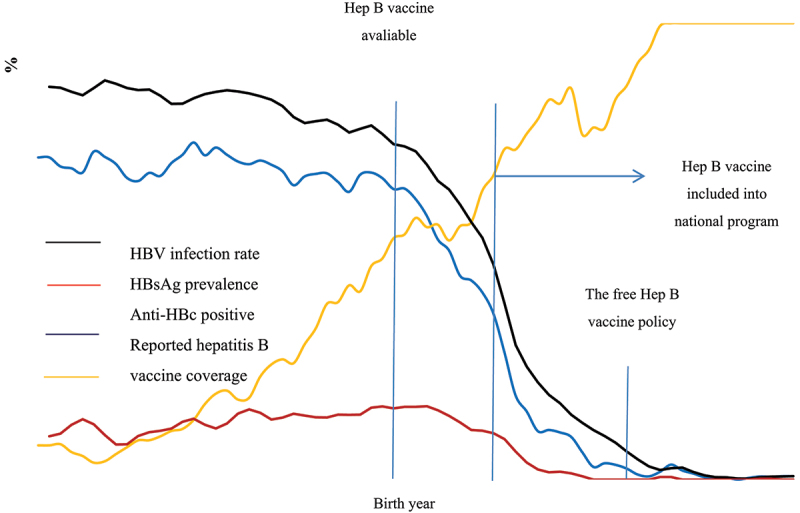
Prevalence of HBV infection, HBsAg carriage, and anti-HBc for the general population and reported hepatitis B vaccination rate at the survey period by birth year.
The reported hepatitis B vaccine coverage was calculated among residents with a clear history of hepatitis B vaccination (yes and no).
Association between policies and risk of infection
With the improvement of hepatitis B vaccination policies, hepatitis B vaccine coverage was 88.2% for those born after 2002. As a consequence, HBV infection rate also showed a continuous decline, from 82.9% for the before 1981 cohort to 2.4% among subjects born after 2002. Similarly, the HBsAg carriage rate, after a slight increase in the 1981–1991 birth cohort (from 12.5% to 13.6%), began to decline (Table 1).
Logistic regression models indicated that, compared with subjects who born before 1981 when the hepatitis B vaccine was not yet available, after controlling for potential confounders, adjusted ORs of HBV infection were 0.51 (95% CI: 0.43–0.62), 0.10 (95% CI: 0.08–0.12), and 0.015 (95% CI: 0.01–0.02) for the 1981–1991, 1992–2001, and ≥2002 birth cohorts, respectively (Figure 3). Similar associations were identified for the risk of anti-HBc positivity. For those born between 1981 and 1991, when hepatitis B vaccine was not introduced into the immunization program, the risk for HBsAg carriage increased, with an adjusted OR of 1.26 (95% CI: 1.00–1.57). This risk significantly declined after the vaccine was integrated into the project in 1992 (adjusted OR = 0.39, 95% CI: 0.26–0.58) and achieved a minimum value after the free vaccine policy implemented in 2002 (adjusted OR = 0.019, 95% CI: 0.006–0.06) (Figure 3).
Figure 3.
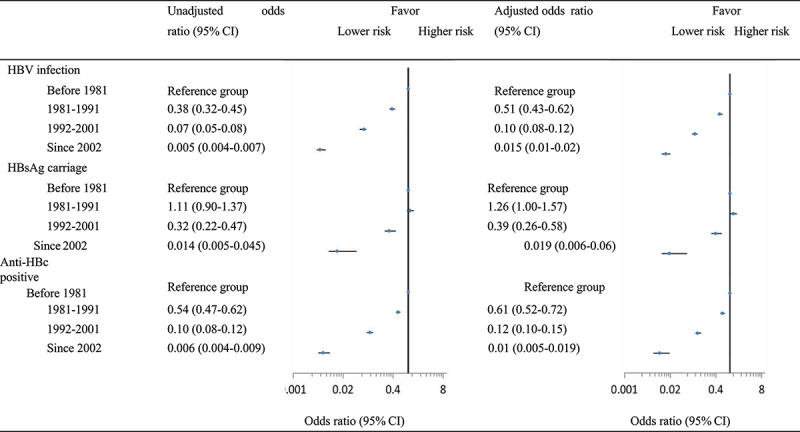
Association of birth cohort with risk of HBV infection, HBsAg carriage, and anti-HBc positivity.
Adjusted factors included gender (male or female), ethnicity (Han or non-Han), education level (primary and below, secondary or high school, college and above, or students), resident (urban or rural), and hepatitis B vaccination (yes or no).
Analyses in which we restricted to Han ethnicity or stratified by residence (urban or rural) did not produce significantly different results for HBV infection. The outcome prevalence in urban residents was significantly lower than that in rural residents, while only the gender difference of HBV infection rate was found (Tables S2, S3). However, the risk reduction for urban residents slightly differed from that for rural residents in the 1981–1991 and 1992–2001 cohorts (P for heterogeneity was 0.09 and 0.07, respectively, Table S4). In addition, the association between 1981 and 1991 birth cohort and the risk of HBsAg carriage disappeared in the sensitivity analyses (Table S5). No difference was identified in the sensitivity analysis for the risk of anti-HBc positive (Table S6).
Awareness of hepatitis B across birth cohorts
Subjects born between 1981 and 1991 had the highest accuracy for four related items (61.0%, 70.2%, 66.8%, and 61.4% for item 1, 2, 3, and 4, respectively) and the top total scores (3.19, full score 5 points), the knowledge level became weakened after the implementation of hepatitis B policy in 1992 (Table 2 and Figure 4).
Table 2.
Knowledge levels of hepatitis B across the four birth cohorts.
| Items | Before 1981 (N = 2665) | 1981–1991 (N = 1037) | 1992–2001 (N = 693) | Since 2002 (N = 470) | P values |
|---|---|---|---|---|---|
| 1 | 1272 (47.7) | 633 (61.0) | 414 (59.7) | 240 (51.1) | <.001 |
| 2 | 1352 (50.7) | 728 (70.2) | 482 (69.6) | 306 (65.1) | <.001 |
| 3 | 1297 (48.7) | 693 (66.8) | 427 (61.6) | 252 (53.6) | <.001 |
| 4 | 1039 (39.0) | 637 (61.4) | 337 (48.6) | 138 (29.4) | <.001 |
| 5 | 1081 (40.6) | 616 (59.4) | 438 (63.2) | 266 (56.6) | <.001 |
| Total scores, mean (SD)a | 2.27 (1.83) | 3.19 (1.60) | 3.03 (1.60) | 2.56 (1.62) | <.001 |
Item 1: Chronic hepatitis B will progress to cirrhosis and liver cancer; Item 2: HBV can be transmitted by shaking hands and hugging; Item 3: HBV can be mother-to-child transmission; Item 4: The first dose of hepatitis B should be initiated within 24 hours after birth; Item 5: Antiviral treatment can delay the progress of hepatitis B.
aFull score was 5 points.
Figure 4.
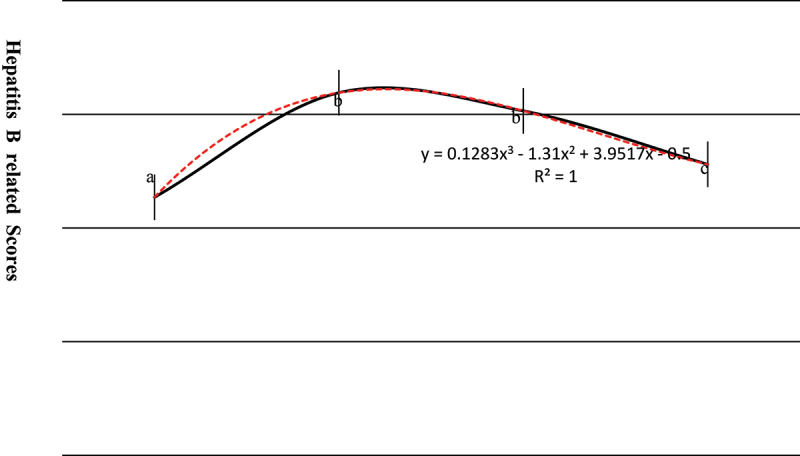
Knowledge levels of hepatitis B across four birth cohorts.
Bonferroni P value > .05 for the scores between birth cohort marked with the same letter (b), otherwise P < .05 (e.g., birth cohort marked with different letters).
In the multivariable linear regression model, after controlling for potential confounders, the score was higher (β = 0.25, P < .001) in the 1981–1991 birth cohort and significantly lower in the 1992–2001 (β = −0.25, P < .001) and ≥2002 (β = −0.20, P = .017) birth-cohorts, relative to those born before 1981 (Table 3).
Table 3.
Coefficients estimated in the multivariable linear regression.
| Unstandardized coefficients |
|||||
|---|---|---|---|---|---|
| Birth year |
B |
Std. Error |
Standardized coefficients |
||
| Before 1981 | Reference | Reference | T value | P value | |
| 1981–1991 | 0.25 | 0.06 | 0.06 | 3.88 | <.001 |
| 1992–2001 | −0.25 | 0.08 | −0.05 | −3.23 | .001 |
| Since 2002 | −0.20 | 0.08 | −0.03 | −2.39 | .017 |
Adjusted factors included gender (male or female), ethnicity (Han or non-Han), education level (primary and below, secondary or high school, college and above, or students), resident (urban or rural), and hepatitis B vaccination (yes or no).
The stratification analyses indicated that, compared with residents born before 1981, scores for hepatitis B awareness started to decline since 1981 for those with hepatitis B vaccination, while increased in 1981–1991 births and then reduced since 1992 for subjects without or with unknown hepatitis B vaccination (Table S7). Subjects born between 1992 and 2001 and since 2002 made lower scores for items 1 and 4 but varied for items 2, 3, and 5 (Tables S8–12).
Discussion
In the present study, we found that hepatitis B vaccine accessibility and related vaccination policies implemented in China had a large impact on reducing the HBV infection, HBsAg carriage, and anti-HBc positivity rate in Fujian. However, compared with subjects born before 1981, those born on and after 1992 when the vaccine was included into the national immunization program had lower hepatitis B awareness.
Hepatitis B vaccination is a priority of public health works in China.16 The government included hepatitis B vaccine in the routine vaccination program for infants on 1 January 1992.16 According to the recommended schedules, newborns should receive the first dose within 24 hours of birth and take subsequent doses at 1 and 6 months of age. The free vaccine policy implemented in 2002 discharged the fees for the vaccine and its administration.8 Based on the policy, many measurements were taken to achieve the HBV infection control and prevention target. From 2007 to 2010, Fujian had carried out two rounds of HBV infection detection and vaccine catch-up campaigns for children under 15 years old. A total of 4,858,713 children were vaccinated in the program, with a hepatitis B vaccine coverage of 97.4%.14 In 2011, 100 units of hepatitis B immunoglobulin were provided for newborns whose mothers were positive for HBsAg. In 2013, the dose of hepatitis B vaccine supplied for newborns was increased from 5 µg to 10 µg. In 2018, The Implementation Plan of Fujian Province for The Implementation of China Viral Hepatitis Prevention and Control Program (2017–2020) was issued.
After 3 decades of escalating vaccination policy, the prevalence of infection declined and maintained at a low level in Fujian. The HBsAg carriage rate was lowered to 0.2% for residents aged less than 18 years old in the present study. The anti-HBc positivity rate has maintained less than 2% for children born since 2010. By comparison, the HBV infection risk decreased by more than 98% in children born since 2002 relative to residents born before 1981. This means that more than 6.7 million residents have been protected by hepatitis B vaccination between 2002 and 2019 assuming an annual birth rate of 1.1% and total residents of 35 million in Fujian Province. The substantial achievement may be largely attributed to high hepatitis B vaccine coverage as a consequence of the vaccine strategies. Hepatitis B vaccine induced protective antibody levels and persisted for long term after primary immunization was critical for HBV prevention.17 Vaccine policies and related measures improved the vaccine series vaccination and stimulated a stronger response in the body and persisted for long term with a higher anti-HBs titer,18 thereby protecting children from HBV infection.
Awareness of hepatitis B
The achievement of prevention against HBV infection in children is very encouraging. However, there are still some barriers in improving the coverage and effectiveness of hepatitis B vaccination, such as a delay of the hepatitis B vaccine first dose, the impact of substitutable vaccine, immune failure, and cost of maternal antiviral treatment.16–19,20,21,22,23 In addition, the decline in hepatitis B awareness is one of the challenges for the prevention and control of HBV infection in China.16,24 In the present study, we identified that hepatitis B awareness has declined since the inclusion of hepatitis B into the national immunization program in 1992. Although the accuracy of hepatitis B awareness reached the target requirement of the National Health and Family Planning Commission of China (>50%),25 the scores of other birth cohorts in 1992–2001 and ≥2002 were lower, compared with those born before 1981. The decline in awareness was particularly prominent in the initiation time of hepatitis B vaccination. Only 29.4% (138/470) of subjects born since 2002 knew the time for the first dose of the hepatitis B vaccine. In addition, the reduction of awareness had some specific characteristics, including resident (e.g., urban vs. rural) and item (more obvious to the disease progress and initiation time of the first dose) disparity.
Level of knowledge and awareness impact on the five core interventions of a cascade of care for HBV to minimize the burden of viral hepatitis: testing, linkage to care, treatment, long-term care, and prevention. Testing for HBV infection and vaccination against it depends on the level of knowledge in the population and the awareness of healthcare personnel.26,27 In addition, awareness of the problem, but inadequate knowledge and information in a community, might result in stigmatization and discrimination.28
According to a cross-sectional study in Beijing, among the surveyed 35,956 subjects aged ≥15 years old, 13,659 (38%) did not have the awareness that HBV infection could be prevented by hepatitis B vaccination.29 This unawareness affected the willingness to receive the vaccine. Thus, the publicity of hepatitis-B-related knowledge deserved more attention. By improving knowledge of hepatitis B, they not only can guide the development of future interventions to improve effectiveness in changing HBV-related behaviors but also promote changes in beliefs and attitudes that can ultimately play a role in stigma reduction and behavior change.30 Therefore, on the premise of maintaining a high vaccine coverage for newborns and children, it is necessary to strengthen publicity to improve the public’s awareness of hepatitis B and increase the vaccination rate for adults.
Strengthen and limitation
The strengths of the study are as follows: (1) we identified that the hepatitis B vaccine policy plays a significant role in improving hepatitis B vaccination and in reducing HBV infection through a large sample size in Fujian, and (2) we further found the existing problems in the current situation, such as the decline of hepatitis B awareness. The findings may provide evidence for the supplement of future policies. Policy-makers may pay more attentions to improve awareness of targeted populations, such as students in schools and rural residents. Furthermore, the publicity should focus more on the importance of timely initiation (<24 hours) of the vaccine and on the improvement of the confidence of the vaccination.
There are some limitations in the present study. Firstly, we classified the birth cohort according to the year of policy implementation, which might generally reflect the impact of the policy on HBV infection. However, in the present study, HBV infection, HBsAg and anti-HBc positivity rates are decreasing earlier than the implementation of two major HBV policies (1991 and 2002). This suggests that HBV vaccination (without the aid of national support) and other favorable environments that prevent HBV transmission were widely accepted in the cohort before the adoption of vaccination policies. This may be verified by the reported hepatitis B vaccination coverage, although many of the residents who were born before the vaccine was available possibly received the vaccine through a supplementary program.
In addition, some other conditions, such as cost and knowledge,31 access to health care, vaccine dose and availability, and the cost-economic status of the child’s family,8 were not controlled in the present study. These merits might result in a potential overestimate of the impact of the policies. Secondly, the markers selected in the present study might have some deficiencies. For example, although the detection was rechecked by China CDC, there might be potential false positivity of anti-HBc. In addition, HBV infection covered past resolved and chronic infections, which might confuse and induce inconsistent trends between HBV infection and HBsAg carriage in the early period after the vaccine was available. Finally, the findings were powered by a large sample size; however, the study subjects were located at five monitoring points in a high hepatitis B prevalence area. Therefore, the universality of the findings might be limited to areas of moderate and low prevalence of the disease.
Conclusion
In summary, the implementation of hepatitis B vaccination policies achieved large success in improving vaccine coverage and in declining HBV infection in Fujian, China. However, a declined awareness of hepatitis B existed in the context of great achievements. More measures on the target population are warranted to improve the public’s awareness of hepatitis B vaccination.
Acknowledgments
The authors gratefully thank the assistance and cooperation of the local field investigators and the surveyed subjects. We appreciate staff in the China CDC for technical guidance in field epidemiological investigation and support of laboratory testing.
Funding Statement
This research was supported by the Prevention and Treatment of major infectious diseases (e.g., AIDS and viral hepatitis) under the National Science and Technology Support Projects of China [No. 2012ZX10002001-002-002] and Construction of Fujian Provincial Scientific and Technological Innovation Platform [No. 2019Y2001]. Young and Middle-aged Backbone Personnel Training Project of Health Science and Technology Program in Fujian Province (2020GGB020).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Data availability statement
The datasets used in the present study are available from the corresponding author (fjcdczhouyong@126.com or wjnhmm@126.com) on reasonable request only.
References
- 1.Zhao H, Zhou X, Zhou YH.. Hepatitis B vaccine development and implementation. Hum Vaccin Immunother. 2020;16(7):1533–8. doi: 10.1080/21645515.2020.1732166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krugman S. The newly licensed hepatitis B vaccine. Characteristics and indications for use. JAMA. 1982;247(14):2012–15. doi: 10.1001/jama.1982.03320390074052. [DOI] [PubMed] [Google Scholar]
- 3.Lu FM, Zhuang H. Management of hepatitis B in China. Chin Med J. 2009;122:3–4. doi: 10.1111/j.1365-2893.2010.01266.x. [DOI] [PubMed] [Google Scholar]
- 4.Wu JN, Zhou Y, Zhang DJ, Zheng JF, Pan WY, Cai ZK, YS Yan. Study on the authenticity of immunization coverage of the routine immunization coverage surveillance system of Fujian. Chin J Epidemiol. 2011;32(9):946–48. [PubMed] [Google Scholar]
- 5.Zhang M, Zheng JS, Cao L. Performance and methods of quality assessment of routine immunization coverage surveillance. Chin J Vaccin Immun. 2015;21:450–57. [Google Scholar]
- 6.Hutton DW, So SK, Brandeau ML. Cost-effectiveness of nationwide hepatitis B catch-up vaccination among children and adolescents in China. Hepatology. 2010;51(2):405–14. doi: 10.1002/hep.23310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang J, Luo YQ, Zhou YH. Elimination of hepatitis B virus infection in children: experience and challenge in China. Chin Med J. 2021;134(23):2818–24. doi: 10.1097/CM9.0000000000001791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu JN, Wen XZ, Zhou Y, Lin D, Zhang SY, Yan YS. Impact of the free-vaccine policy on timely initiation and completion of hepatitis B vaccination in Fujian, China. J Viral Hepat. 2015;22(6):551–60. doi: 10.1111/jvh.12359. [DOI] [PubMed] [Google Scholar]
- 9.Huang L, Zhou Y, Yang X. Epidemiological characteristics of hepatitis B infection in 1-29 year old population in Fujian Province. Strait J Pre Med. 2018;24:32–33. [Google Scholar]
- 10.Wang H, Men P, Xiao Y, Gao P, Lv M, Yuan Q, Chen W, Bai S, Wu J. Hepatitis B infection in the general population of China: a systematic review and meta-analysis. BMC Infect Dis. 2019;19(1):811. doi: 10.1186/s12879-019-4428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang XF, Bi SL, Yang WZ, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Epidemiological serosurvey of hepatitis B in China—declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27(47):6550–57. doi: 10.1016/j.vaccine.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 12.Wu JN, Li DJ, Zhou Y. Association between timely initiation of hepatitis B vaccine and completion of the hepatitis B vaccine and national immunization program vaccine series. Int J Infect Dis. 2016;51:62–65. doi: 10.1016/j.ijid.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Cui F, Shen L, Li L, Wang H, Wang F, Bi S, Liu J, Zhang G, Wang F, Zheng H, et al. Prevention of chronic hepatitis B after 3 decades of escalating vaccination policy, China. Emerg Infect Dis. 2017;23(5):765–72. doi: 10.3201/eid2305.161477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujian Provincial Health Commission, Fujian Center for Disease Control and Prevention . 40th anniversary of immunization program in Fujian province. Fujian Science and Technology Press;2019. Sept. [Google Scholar]
- 15.Rothman KJ, Greenland S. Modern epidemiology. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 16.Liao X, Liang Z. Strategy vaccination against hepatitis B in China. Hum Vaccin Immunother. 2015;11(6):1534–39. doi: 10.4161/21645515.2014.980206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, Bulkow L, Fiore A, Bell B, Hennessy T. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis. 2009;200(9):1390–96. doi: 10.1086/606119. [DOI] [PubMed] [Google Scholar]
- 18.Wu JN, Huang LF, Lin ZQ, Zhou Y. Association between vaccine dose and risk of hepatitis B virus infection in Fujian Province, China. Hum Vaccin Immunother. 2022;18(7):2153533. doi: 10.1080/21645515.2022.2153533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu JN, Zhou Y. Factors associated with and screening models of national immunization program completion for preschool children in Fujian, China. J Infect Public Health. 2019;12(2):236–41. doi: 10.1016/j.jiph.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Wu JN, Li DJ, Zhou Y, Du MR, Piao HL. Relationship between receipt of substitutable for free vaccines and completion of the expanded program on immunization: a cross-sectional study in Fujian, China. BMJ Open. 2017;7(7):e015666. doi: 10.1136/bmjopen-2016-015666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu J, Zhou Y, Huang L, He A. Study on the effects of timely initiation and completion of hepatitis B vaccine on hepatitis B virus prevention in Fujian, China. China J Epidemiol. 2014;35(4):471–72. [PubMed] [Google Scholar]
- 22.Huang C, Zeng S, Chen X. Epidemiological characteristics of immunoprophylaxis failure in the infants born to HBsAg positive mothers in Quanzhou, Fujian. J China Trop Med. 2020;20:245–58. [Google Scholar]
- 23.Bierhoff M, Angkurawaranon C, Rijken M, Sriprawa K, Kobphan P, Nosten FN, van Vugt M, McGready R, Devine A. Tenofovir disoproxil fumarate in pregnancy for prevention of mother to child transmission of hepatitis B in a rural setting on the Thailand-Myanmar border: a cost-effectiveness analysis. BMC Pregnancy Childbirth. 2021;21(1):157. doi: 10.1186/s12884-021-03612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyuregyan KK, Kichatova VS, Isaeva O, Potemkin IA, Malinnikova EY, Lopatukhina MA, Karlsen AA, Asadi Mobarhan FA, Mullin EV, Slukinova OS, et al. Coverage with timely administered vaccination against hepatitis B virus and its influence on the prevalence of HBV infection in the regions of different endemicity. Vaccines. 2021;9(2):82. doi: 10.3390/vaccines9020082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Health and Family Planning Commission of China . Notice on the issuance of China’s viral hepatitis prevention and control program (2017–2020). No.53; 2017.
- 26.Gürakar M, Malik M, Keskin O, Idilman R. Public awareness of hepatitis B infection in Turkey as a model of universal effectiveness in health care policy. Turk J Gastroenterol. 2014;25(3):304–08. doi: 10.5152/tjg.2014.6718. [DOI] [PubMed] [Google Scholar]
- 27.Ganczak M, Dmytrzyk-Danilow G, Korzen M, Drozd-Dąbrowska M, Szych Z. Prevalence of HBV infection and knowledge of hepatitis B among patients attending primary care clinics in Poland. J Commun Health. 2016;41(3):635–44. doi: 10.1007/s10900-015-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nwokediuko SC. Chronic hepatitis B: management challenges in resource-poor countries. Hepat Mon. 2011;11(10):786–93. doi: 10.5812/kowsar.1735143X.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang Y, Bai X, Liu X, Zhang Z, Pang X, Nie L, Qiu W, Zhao W, Hu G. Hepatitis B vaccination coverage rates and associated factors: a community-based, cross-sectional study conducted in Beijing, 2019–2020. Vaccines. 2021;9(10):1070. doi: 10.3390/vaccines9101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen C, Evans AA, Huang P, London WT, Block JM, Chen G. Hepatitis B knowledge among key stakeholders in Haimen City, China: implications for addressing chronic HBV infection. Hepatol Med Policy. 2016;1(1):4. doi: 10.1186/s41124-016-0004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan SHS, Wang D, Tan WJ, Allameen NA, Fong NP. Facilitators and barriers of hepatitis B screening and vaccination. Vaccine. 2020;38(34):5447–53. doi: 10.1016/j.vaccine.2020.06.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used in the present study are available from the corresponding author (fjcdczhouyong@126.com or wjnhmm@126.com) on reasonable request only.


