Abstract
Purpose
Voriconazole (VRC) is an antifungal agent which is used for treatment and prophylaxis of invasive fungal infections. The common clinical adverse reactions mainly include central nervous system (CNS) toxicity and abnormal liver function. These adverse reactions limit the clinical use of voriconazole to a certain extent. Therefore, the aim of this study was to analyze the risk factors of voriconazole neurotoxic side effects and to determine the plasma trough concentration (Cmin) threshold of voriconazole-induced CNS toxicity, so as to improve the safety of voriconazole treatment.
Patients and Methods
This study retrospectively collected the clinical data of 165 patients who received voriconazole and underwent therapeutic drug monitoring (TDM). CNS toxicity was defined using the National Cancer Institute (NCI) criteria, logistic regression was used to analyze the risk factors of CNS toxicity, classification and Regression tree (CART) model was used to determine the Cmin threshold for CNS toxicity.
Results
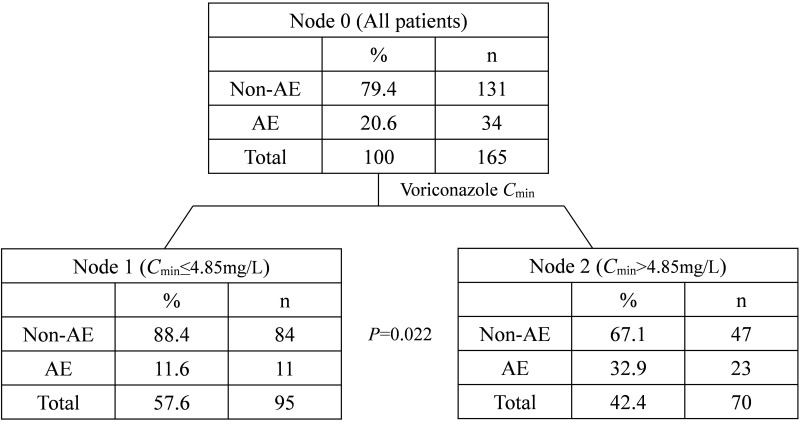
Voriconazole-related CNS toxicity occurred during treatment in 34 of 165 patients (20.6%) and the median time from administration to onset of CNS toxicity was 6 days (range, 2–19 days). The overall incidence of CNS toxicity was 20.6% (34/165), including visual disturbances in 4.8% (8/165) and nervous system disorders in 15.8% (26/165). Cmin significantly affects the occurrence of CNS toxicity and the threshold of Cmin for voriconazole CNS toxicity was determined to be 4.85 mg/L, when Cmin >4.85 mg/L and ≤4.85 mg/L, the incidence of CNS was 32.9% and 11.6%, respectively.
Conclusion
Voriconazole trough concentration of Cmin is an independent risk factor for CNS toxicity, and the threshold of Cmin for CNS toxicity is 4.85mg/L. TDM should be routinely performed in patients with clinical use of voriconazole to reduce the occurrence of CNS toxicity of voriconazole.
Keywords: voriconazole, TDM, C min , CNS toxicity, cohort study
Introduction
Voriconazole (VRC) is a triazole antifungal agent with a broad spectrum of activity, which is often recommended as primary therapy for invasive fungal diseases (IFDs).1 Voriconazole is mainly metabolized by CYP2C19 in liver, and it exhibits non-linear pharmacokinetics. CYP2C19 gene polymorphism, as well as enzyme inhibitors and inducers, will cause drug–drug interactions, leading to great differences in the pharmacokinetic parameters of voriconazole between individuals and within individuals, which is the main reason for the large individual differences in voriconazole blood concentration.2 Therefore, it is necessary to perform therapeutic drug monitoring (TDM) for voriconazole to optimize its efficacy and avoid the occurrence of adverse reactions.
Voriconazole often causes various adverse reactions, mainly including abnormal liver function, central nervous system (CNS) toxicity (including visual disturbances and nervous system disorders).3–7 According to the French Pharmacovigilance Database, the incidence of voriconazole adverse reactions accounted for 18% of visual disturbances and 14% of nervous system disorders.8 CNS toxicity is due to the fact that voriconazole can enter through blood-eye barrier, the blood-brain barrier into the vitreous body, aqueous humor and cerebrospinal fluid (concentrations in the cerebrospinal fluid are approximately 50% of plasma concentrations).1 Thereby causing changes in the retina and central nervous system, resulting in visual disturbances, hallucinations, mental state changes and confusion, headaches.
Meanwhile, previous studies demonstrated that the clinical efficacy and adverse reactions of voriconazole treatment are related to plasma trough concentration (Cmin).9,10 In addition, studies have pointed out that when the blood concentration of voriconazole is >5.5 mg/L, it will lead to the occurrence of neurotoxicity.11–13 The British Society for Medical Mycology recommends targeting voriconazole trough concentrations of <4–6 mg/L to minimize drug-related toxicity.14 However, at present, the risk factors of CNS toxicity by voriconazole have not been fully explored, whether the threshold for the occurrence of CNS toxicity caused by voriconazole use in the Chinese patient population is within the range reported in the study needs to be further verified.
Therefore, the present study aimed to: (i) analyze the risk factors of CNS toxicity in the treatment and prevention of fungal infections with voriconazole; (ii) establish the prediction model of CNS toxicity, and determine the Cmin threshold of CNS toxicity caused by voriconazole to reduce the occurrence of adverse reactions.
Patients and Methods
Patients
This study retrospectively reviewed the hospital information at the First Affiliated Hospital of Xi ‘an Jiaotong University. Patients who were treated with voriconazole treatment and prophylaxis and had TDM during antifungal therapy were identified and then screened by applying inclusion criteria of: (1) Age ≥18 years; (2) Voriconazole treatment >14 days; (3) At least one steady-state trough concentration blood sample was taken from each patient. Documented CNS toxicity prior to voriconazole treatment, and blood concentrations below the lower limit of detection were excluded. The study was approved by the ethics committee of the First Affiliated Hospital of Xi ‘an Jiaotong University.
Data Collection
The clinical data of patients were extracted retrospectively through the electronic medical record system and the nursing system of the hospital. The main patients information included is as follows: (1) Demographic data, such as gender, age, and weight; (2) The underlying diseases of the patients and the diagnosis at admission; (3) Voriconazole dosage schedules and concomitant medications (defined as other drugs given on the same day or at the same time as voriconazole before voriconazole TDM); (4) Adverse reaction information: patients with symptoms of CNS toxicity; (5) Plasma trough concentrations of voriconazole Cmin and CYP2C19 gene phenotype.
Determination of CNS Toxicity Induced by Voriconazole
Clinicians assessed the causality of voriconazole ADRs according to WHO-UMC criteria (World Health Organization, Uppsala Surveillance Center).15 According to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE),16 patient data were queried through electronic medical records, and central nervous system symptoms of patients during Voriconazole use were collected to identify and evaluate the types of CNS toxicity, and a series of subsequent retrospective studies and analyses were conducted.
CNS toxicity during voriconazole treatment usually includes: visual disturbances (blurred vision, photophobia, chromatism, color blindness, ocular halo, decreased vision) and nervous system disorders, neurological disorders are divided into mental disorders (such as hallucination, depression, anxiety, insomnia, confusion) and the nervous system abnormalities (including headache, seizures, tremor, muscle tension increases, paresthesia, drowsiness, dizziness, hepatic encephalopathy).
Blood Sampling and Analytical Assays
To evaluate the relationship between voriconazole exposure and adverse drug reactions during treatment, studies have shown that voriconazole Cmin can be used as a reliable predictor of voriconazole efficacy and safety.9 Therefore, the distribution characteristics of Cmin can be used to analyze the correlation between Cmin and CNS toxicity as well as the threshold of occurrence.
Plasma samples were collected from the patients, and plasma concentrations were determined by HPLC, the lower limit of quantification was 0.06 mg/L, the linear range was 0.06–8 mg/L, and the correlation coefficient R2= 0.9998.17 In patients receiving intravenous or oral loading doses of voriconazole, trough concentrations measured on or after day 2 of administration were included in the analysis. In patients who did not receive a loading dose, trough concentrations measured on or after days 5–7 post-dose were included.
CYP2C19 Genotyping
For voriconazole CYP2C19 genotyping, the CYP2C19*1, CYP2C19*2, CYP2C19*3 and CYP2C19*17 alleles were detected using a multi-channel fluorescence quantitative analyzer and a digoxin staining solution kit. The CYP2C19 genotype was classified as ultra-rapid metabolizer (*1/*17), extensive metabolizer (*1/*1), intermediate metabolizer (*1/*2, *1/*3), or poor metabolizer (*2/*2, *2/*3, *3/*3).
Statistical Analysis
SPSS 19.0 and GraphPad Prism 8.0 were used for statistical analysis and graphing. Continuous variables were described using mean ± standard deviation or median (interquartile range), and categorical variables were described using percentage or frequency. Differences between the two groups were compared with the T-test, and the χ2 test or Fisher’s exact test was used to compare categorical variables.
Univariate and multivariate logistic analyses were used to screen the risk factors of voriconazole-related CNS toxicity and reduce the occurrence of CNS toxicity. Variables with P<0.05 in Logistic regression analysis were included in CART analysis. Establish a CART model to predict the Cmin threshold of voriconazole toxicity in central nervous system. P<0.05 was considered statistically significant.
Results
Demographic Characteristics of the Patients
A total of 165 patients were screened in this study, and the results are shown in Table 1. Of the 165 patients, 127 (77%) were male and 38 (23%) were female, with a median age (interquartile range) of 54 years (45–65 years). And the median weight (interquartile range) was 63kg (56 to 70 kg). The main reason for receiving voriconazole treatment was severe fungal infection (51.5%, 85/165), among which pulmonary infection was more common (41.2%, 68/165). The rest of the patients received voriconazole for the prevention of fungal infections. Viral hepatitis was the most common comorbidities in the study population (57%).
Table 1.
Demographic and Clinical Characteristics of Patients with and without CNS Neurotoxicity
| Neurotoxicity (n = 34) | Non-Neurotoxicity (n = 131) | Total (N = 165) n% | |
|---|---|---|---|
| Demographics | |||
| Sex, female (male) | 5 (29) | 33 (98) | 38 (127) |
| Age(years), median (IQR) | 56 (45, 64) | 53 (44.5, 65) | 54 (45, 65) |
| Weight(kg), median (IQR) | 63 (59, 69) | 63 (56, 70) | 63 (56, 70) |
| Underlying condition | |||
| Viral hepatitis | 19 (55.9) | 76 (58.0) | 95 (57.6) |
| Liver cirrhosis | 14 (41.2) | 47 (35.9) | 61 (37.0) |
| Liver failure | 14 (41.2) | 37 (28.2) | 51 (30.9) |
| Hypertension | 2 (5.9) | 17 (13.0) | 19 (11.5) |
| Others | 8 (23.5) | 33 (25.2) | 41 (24.8) |
| Route of administration | |||
| Intravenous | 10 (29.4) | 51 (38.9) | 61 (37) |
| Oral | 24 (70.6) | 80 (61.1) | 104 (63) |
| Site of infection | |||
| Lung | 11 (32.4) | 57 (43.5) | 68 (41.2) |
| Abdominal cavity | 3 (8.8) | 4 (3.1) | 7 (4.2) |
| Intestinal tract | 1 (3) | 3 (2.3) | 4 (2.4) |
| Others | 2 (5.9) | 5 (3.8) | 7 (4.2) |
| Number of concomitant use of neurotoxic drugs | |||
| Overall | 31 (91.2) | 110 (84.0) | 141 (85.5) |
| Fluoroquinolone | 11 (32.4) | 30 (23.0) | 41 (24.8) |
| Cephalosporins | 4 (11.8) | 15 (11.5) | 19 (11.5) |
| Carbapenems | 18 (52.9) | 61 (46.6) | 79 (47.9) |
| Linezolid | 9 (26.5) | 23 (17.6) | 32 (19.4) |
| Others | 15 (44.1) | 73 (55.7) | 88 (53.3) |
| Central nervous system toxicity symptoms | |||
| Visual impairment | |||
| Blurred vision | 4 (11.8) | - | 4 (2.4) |
| Othersa | 4 (11.8) | - | 4 (2.4) |
| Nervous system disorder | |||
| Hallucinations | 9 (26.5) | - | 9 (5.5) |
| Altered mental state | 4 (11.8) | - | 4 (2.4) |
| Dizzy | 3 (8.8) | - | 3 (1.8) |
| Hepatic encephalopathy | 2 (5.9) | - | 2 (1.2) |
| Othersb | 8 (23.5) | - | 8 (4.8) |
| Metabolizer phenotype | |||
| UM | 0 | 1 (7.6) | 1 (6.1) |
| EM | 12 (35.3) | 35 (26.7) | 47 (28.5) |
| IM | 9 (26.5) | 43 (32.8) | 52 (31.5) |
| PM | 5 (14.7) | 17 (13.0) | 22 (13.3) |
Notes: aOthers include dazzled (n = 2), altered color perception (n = 2). bOthers include tremor (n = 2), drowsiness (n = 2), confusion (n = 1), paresthesia (n = 1), tinnitus (n = 2).
Abbreviations: IQR, interquartile range; EM, extensive metabolizer; IM, intermediate metabolizer; PM, poor metabolizer; UM, ultrarapid metabolizer.
Voriconazole-induced CNS toxicity occurred in 34 of 165 patients and subsequent CNS toxicity was detected at a median of 6 days (range, 2–19 days) after VRC administration. The overall incidence of CNS toxicity was 20.6% (34/165), and the most common CNS toxicity included visual impairment in 8 cases (4.8%). Neurological disorders occurred in 26 cases, with an incidence of 15.8% (see Table 1). All symptoms of CNS toxicity improved and resolved following voriconazole was discontinued or the dose was reduced.
Distribution Characteristics of Voriconazole Cmin
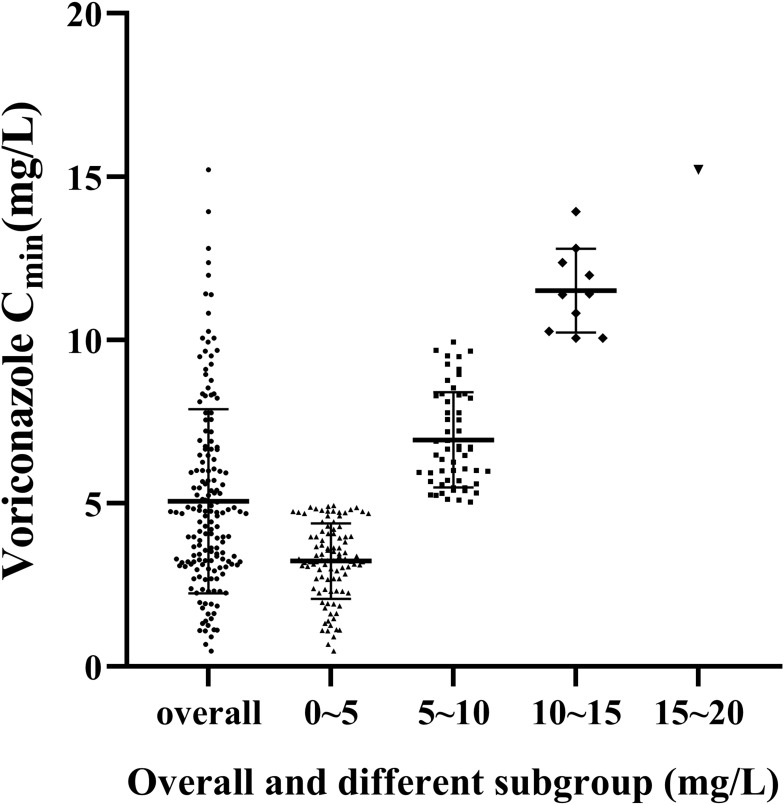
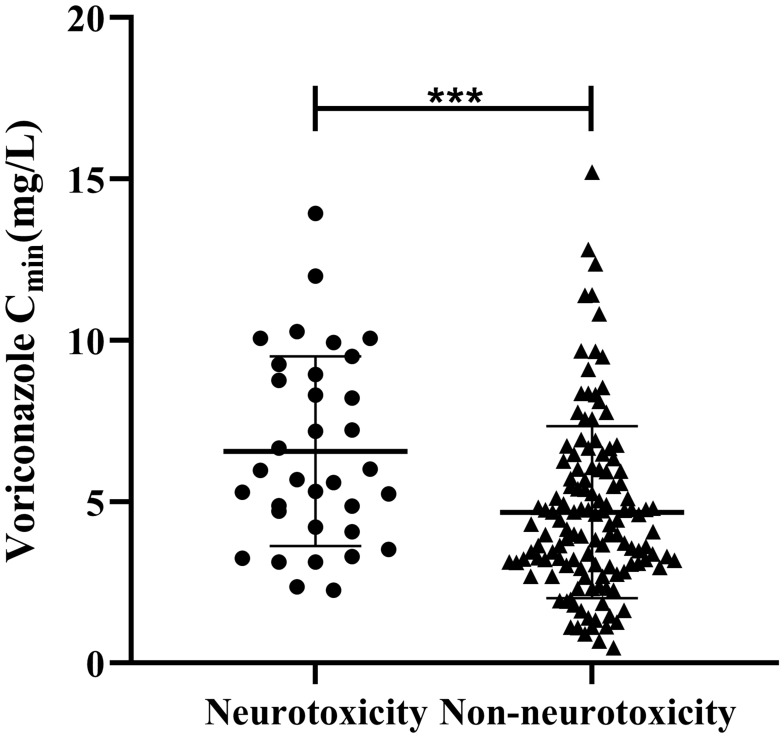
Voriconazole Cmin from 165 patients were included in this study, and their distribution is shown in Figure 1. The median Cmin (interquartile range) was 4.69 mg/L (3.14–6.47 mg/L). Among them, voriconazole Cmin was the most distributed in the range of 0–5 mg/L, accounting for 59.4%. Followed by 5–10 mg/L, accounting for 34%; Only 6.1% of voriconazole Cmin>10 mg/L. The Cmin distribution of voriconazole with and without CNS toxicity is shown in Figure 2. Compared with no CNS toxicity, the average Cmin with CNS toxicity was significantly higher (6.56 mg/L vs 4.68 mg/L, P<0.001).
Figure 1.
The Cmin distribution of voriconazole in whole and different concentrations.
Figure 2.
Distribution of voriconazole Cmin in patients with and without CNS toxicity. ***P<0.001.
Univariate and Multivariate Logistic Regression Analysis
Univariate analysis was used to determine the risk factors for CNS toxicity of voriconazole. There was no significant correlation between body weight, age, gender, and combined medication and CNS toxicity (data not shown). However, there was a significant correlation between Cmin and CNS toxicity (P<0.05). Therefore, Cmin and clinically significant variables were included in the multivariate Logistic regression analysis, and the results also showed that Cmin was an independent risk factor for CNS toxicity (Table 2).
Table 2.
Univariate and Multivariate Logistic Regression Analysis to Identify Risk Factors
| Univariate | P-value | Multivariate | P-value | |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||
| Voriconazole trough concentration, mg/L | 1.25 (1.09–1.42) | 0.001 | 1.28 (1.11–1.46) | 0.001 |
| Age, years | n.s | 0.708 | 1.02 (0.99–1.05) | 0.254 |
| Weight, kg | n.s | 0.807 | - | |
| Gender, female (male) | 0.51 (0.18–1.43) | 0.202 | - | |
| Concomitant use of neurotoxic drugs | ||||
| Overall | 0.51 (0.14–1.81) | 0.296 | 0.41 (1.00–1.79) | 0.236 |
| Fluoroquinolone | 0.62 (0.27–1.42) | 0.258 | - | |
| Cephalosporins | 0.97 (0.3–3.14) | 0.96 | - | |
| Carbapenems | 0.78 (0.36–1.65) | 0.508 | - | |
| Linezolid | 0.69 (0.28–1.72) | 0.429 | - | |
| Others | 1.59 (0.75–3.41) | 0.229 | - | |
| ALT(U/L) | n.s | 0.221 | n.s | 0.074 |
| AST(U/L) | n.s | 0.177 | - | |
| ALP(U/L) | n.s | 0.467 | - | |
| TBIL (mol/L) | n.s | 0.592 | - | |
| DBIL (mol/L) | n.s | 0.136 | - | |
| ALB(g/L) | 1.02 (0.97–1.08) | 0.444 | - |
Abbreviations: OR, odds ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; TBIL, total bilirubin; DBIL, direct bilirubin; ALB, albumin; n.s, not significant at the 5% concentration.
Threshold of Voriconazole Cmin for CNS Toxicity
Voriconazole Cmin is an independent risk factor for adverse reactions of CNS toxicity. A CART model was established to investigate the relationship between voriconazole Cmin and adverse events of CNS toxicity, to predict the incidence and toxicity threshold of voriconazole in the central nervous system. The results showed that when the patient’s voriconazole Cmin was >4.85 mg/L, the patients had a higher risk of CNS toxicity (Figure 3). In contrast, voriconazole Cmin ≤4.85 mg/L was associated with a lower risk of CNS toxicity.
Figure 3.
Categorical regression tree model predicting CNS toxicity of voriconazole (AE refers to adverse events).
Effect of CYP2C19 Gene Polymorphism on CNS Toxicity
As shown in Table 3, 122 patients were classified according to their CYP2C19 genetic polymorphisms. EM and IM patients accounted for a higher proportion, 38.5% (47/122) and 42.6% (52/122), respectively, PM patients accounted for 18.0% (22/122), and UM patients were less. Among the patients with CNS toxicity, the proportion of EM patients was higher (46.2%). At the same time, the Cmin/DMD of voriconazole in PM patients was higher than that in EM and IM patients, but there was no significant difference between EM and IM patients (P>0.05). There was no significant effect of CYP2C19 gene polymorphism on CNS toxicity of voriconazole tested by χ2 test.
Table 3.
Analysis of CNS Toxicity in CYP2C19 Subgroups in Patients with Voriconazole
| Neurotoxicity n (%) | Non-Neurotoxicity n (%) | Cmin (mg/L) | Cmin/DMD | |
|---|---|---|---|---|
| Gene states | ||||
| UM | 0 | 1 (1.0%) | 2.69 | 0.027 |
| EM | 12 (46.2%) | 35 (36.5%) | 4.78±2.84 | 0.029±0.017 |
| IM | 9 (34.6%) | 43 (44.8%) | 4.71±2.32 | 0.030±0.016 |
| PM | 5 (19.2%) | 17 (17.7%) | 4.80±2.73 | 0.034±0.022 |
Abbreviations: Cmin/DMD, the ratio between the voriconazole Cmin at steady state and the maintenance dose; EM, extensive metabolizer; IM, intermediate metabolizer; PM, poor metabolizer; UM, ultrarapid metabolizer.
Discussion
In this study, trough plasma concentration of voriconazole Cmin is a significant risk factor for toxic adverse reactions in the central nervous system. The threshold of Cmin was 4.85 mg/L when the CNS toxicity of voriconazole occurred, and when the Cmin was >4.85 and ≤4.85 mg/L, the incidence of CNS toxicity was 32.9% and 11.6%, respectively, close to the therapeutic target reported by Andres Pascual et al.18 We found a 20.6% (34/165) overall incidence of adverse reactions to CNS toxicity in patients receiving voriconazole, which is consistent with previous clinical studies showing that a wide variation in the incidence of CNS toxicity among patients treated with voriconazole, ranging from 9% to 31%.19 In previous studies, voriconazole-induced visual disturbances occurred more frequently than in patients reporting neurologic disturbances, but in this study the frequency of voriconazole-induced visual disturbances was lower than that of neurologic disturbances (4.8% vs 15.8%). On the one hand, the sample size of our study is not very large. In addition, because hallucinations are usually classified as symptoms of neurotoxicity, hallucinations (visual hallucinations, auditory hallucinations) are also listed as neurological disorders in our study. Also, we must be aware of the fact that it can be challenging to distinguish between visual hallucinations and visual disturbances.20 The mechanism of voriconazole-induced hallucinations and visual impairment is still unclear. Whether it was caused by ophthalmic toxicity or psychiatric disturbance, we attempted to clarify the source of these hallucinations using ophthalmic evaluation or imaging examination. However, considering the differences in study design, as a retrospective study, we could only detect clinically obvious and documented central nervous system toxicity. A more thorough neurological examination may be required in a prospective study.
Several factors have been found to be associated with greater variability in voriconazole exposure after standardized dose administration, such as nonlinear saturated pharmacokinetics, drug–drug interactions, liver disease, patient age and weight, and genetic polymorphisms in CYP2C19.21–24 In this study, univariate and multivariate correlation analyses were conducted between the above factors and neurotoxicity, respectively. The univariate and multivariate results showed that only voriconazole Cmin had a significant relationship with CNS toxicity. And there was no significant difference in age between patients with CNS toxicity and those without CNS toxicity (P>0.05). During the antifungal therapy and prophylaxis with voriconazole, the use of one or more concomitant neurotoxic drugs, such as fluoroquinolones, carbapenems, cephalosporins, linezolid, etc., were more common in patients with subsequent CNS toxicity than those without (n = 31, 91.2%). For antifungal management in patients with adverse CNS toxicity, both visual and psychiatric symptoms improved in almost all patients as the dose was reduced or discontinued.
There are large individual differences in voriconazole plasma concentrations, which are mainly caused by factors such as CYP2C19 gene polymorphism, patient gender, age, weight, physiological and pathological conditions, as well as concomitant medication.2,25 Voriconazole is mainly metabolized in vivo by liver drug enzymes CYP2C19, CYP3A4 and CYP2C9, among which CYP2C19 plays a major role.26–28 Significant genetic polymorphism of CYP2C19 gene encoding CYP2C19 enzyme can lead to rapid or slow metabolism of voriconazole, resulting in a 30–50% change in blood concentration.29 Therefore, the metabolic ability of CYP2C19 will affect the increase or decrease of blood drug concentration. Low concentration of voriconazole is related to treatment failure, while high concentration is related to serious adverse reactions such as neurotoxicity. In patients with liver disease, due to the decreased liver metabolic capacity and the decreased activity of CYP2C19 enzyme, the plasma clearance rate of drugs will decrease, and voriconazole exposure will be higher, which exceed the range of its target trough concentration, thus leading to the increased risk of adverse events and side effects (including hepatotoxicity and neurotoxicity).30
However, few previous studies have not included the influence of CYP2C19 gene polymorphism on CNS toxicity in patient population. We collected the information of patients with genotype determination and classified the patients into genotypes to explore the effect of CYP2C19 gene polymorphism on CNS toxicity, found that voriconazole Cmin/DMD in PM patients was higher than that in EM and IM patients, and there was no significant difference in Cmin/DMD between EM and IM patients. At the same time, the incidence of CNS toxicity in patients with EM after using voriconazole was higher than that in patients with other metabolic types (46.2%). Theoretically, PM patients should have higher Cmin than EM and IM patients and should be more prone to adverse effects, which is less consistent with the findings of Levin et al and Matsumoto et al.31 In addition, the proportion of EM and IM patients in this study was relatively high (38.5% and 42.6%, respectively), the proportion of PM patients was 18%, and only 1 case of UM patients. This is roughly in line with the frequency reported in other studies in the Chinese population.32 The present study showed that there was no significant relationship between voriconazole CNS toxicity and CYP2C19 gene polymorphism. Further population studies are needed to fully understand the impact of CYP2C19 polymorphisms on voriconazole efficacy and CNS toxicity.
When voriconazole is used for the treatment of identified invasive infections, the VRC Cmin target for TDM is between 1 and 6 mg/L, most studies and meta-analyses use Cmin>1 mg/L or a Cmin/MIC ratio of 2–5 as the efficacy targets, <4–6 mg/L as safety targets to minimize toxicity.33,34 As for how voriconazole causes CNS toxicity, there are few reports on the mechanism of CNS toxicity, some studies have proposed that the cause of transient visual impairment reported in patients receiving voriconazole treatment may be due to the direct inhibition of TRPM1 current in ON-bipolar cells by voriconazole. Furthermore, inhibition of TRPM3 by voriconazole may also underlie some of the other transient neurologic toxicities associated with voriconazole treatment, such as visual and auditory hallucinations.35 However, the specific mechanism of central nervous system toxicity needs further research to prove.
Of course, there are several limitations to this study. First, this study was a retrospective study, and the homogeneity of the population may cause the results not applicable to a larger patient population; In addition, the relationship between drug exposure and efficacy has not been explored because there are few pathogenic bacterial infections recorded by microbiology in this study. The limited number of PM and UM in the study may also be one of the reasons why the relationship between CYP2C19 polymorphism and CNS toxicity may not be found. Therefore, large-scale, multicenter prospective studies are needed to further validate the Cmin threshold for CNS toxicity induced by voriconazole.
Conclusion
This study was a retrospective study of TDM in patients with fungal infections treated and prophylaxis by voriconazole. Voriconazole Cmin is a significant risk factor associated with CNS toxicity. The Cmin threshold of voriconazole causing CNS toxicity during treatment is 4.85 mg/L, and the incidence of CNS toxicity will be significantly increased when it is greater than 4.85 mg/L. CYP2C19 gene polymorphism may not have a significant effect on CNS toxicity. It is recommended to further verify in future prospective studies to ensure the efficacy and safety of voriconazole.
Funding Statement
There is no funding to report.
Ethics Approval and Informed Consent
The study complied with the Declaration of Helsinki and was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Xi ‘an Jiaotong University School of Medicine [XJTU1AF2019LSK-087]. Waiving of informed consent was given due to the retrospective, non-interventional study design. All patient data were collected anonymously and ensured about the confidentiality of their information.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Theuretzbacher U, Ihle F, Derendorf H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet. 2006;45(7):649–663. doi: 10.2165/00003088-200645070-00002 [DOI] [PubMed] [Google Scholar]
- 2.Wang T, Sun JY, Chen SY, et al. Population pharmacokinetics study of voriconazole and dosing regimen optimization in patients with invasive fungus infections. Chin Pharm J. 2014;24:227–233. [Google Scholar]
- 3.Boyd AE, Modi S, Howard SJ, et al. Adverse reactions to voriconazole. Clin Infect Dis. 2004;39(8):1241–1244. doi: 10.1086/424662 [DOI] [PubMed] [Google Scholar]
- 4.Imhof A, Schaer DJ, Schanz U, et al. Neurological adverse events to voriconazole: evidence for therapeutic drug monitoring. Swiss Med Wkly. 2006;136(45–46):739–742. [DOI] [PubMed] [Google Scholar]
- 5.Luong ML, Hosseini-Moghaddam SM, Singer LG, et al. Risk factors for voriconazole hepatotoxicity at 12 weeks in lung transplant recipients. Am J Transplant. 2012;12(7):1929–1935. doi: 10.1111/j.1600-6143.2012.04042.x [DOI] [PubMed] [Google Scholar]
- 6.Saito T, Fujiuchi S, Tao Y, et al. Efficacy and safety of voriconazole in the treatment of chronic pulmonary aspergillosis: experience in Japan. Infection. 2012;40(6):661–667. doi: 10.1007/s15010-012-0322-x [DOI] [PubMed] [Google Scholar]
- 7.Zonios DI, Gea-Banacloche J, Childs R, et al. Hallucinations during voriconazole therapy. Clin Infect Dis. 2008;47:1. doi: 10.1086/588844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eiden C, Peyrière H, Cociglio M, et al. Adverse effects of voriconazole: analysis of the French pharmacovigilance database. Ann Pharmacother. 2007;41(5):755–763. doi: 10.1345/aph.1H671 [DOI] [PubMed] [Google Scholar]
- 9.Dolton MJ, McLachlan AJ. Voriconazole pharmacokinetics and exposure-response relationships: assessing the links between exposure, efficacy and toxicity. Int J Antimicrob Agents. 2014;44(3):183–193. doi: 10.1016/j.ijantimicag.2014.05.019 [DOI] [PubMed] [Google Scholar]
- 10.Lutsar I, Hodges MR, Tomaszewski K, et al. Safety of voriconazole and dose individualization. Clin Infect Dis. 2003;36(8):1087–1088. doi: 10.1086/374248 [DOI] [PubMed] [Google Scholar]
- 11.Chu HY, Jain R, Xie H, et al. Voriconazole therapeutic drug monitoring: retrospective cohort study of the relationship to clinical outcomes and adverse events. BMC Infect Dis. 2013;13:105. doi: 10.1186/1471-2334-13-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heo ST, Aitken SL, Tverdek FP, et al. How common is subsequent central nervous system toxicity in asymptomatic patients with haematologic malignancy and supratherapeutic voriconazole serum levels? Clin Microbiol Infect. 2017;23(6):387–390. doi: 10.1016/j.cmi.2016.12.031 [DOI] [PubMed] [Google Scholar]
- 13.Koselke E, Kraft S, Smith J, et al. Evaluation of the effect of obesity on voriconazole serum concentrations. J Antimicrob Chemother. 2012;67(12):2957–2962. doi: 10.1093/jac/dks312 [DOI] [PubMed] [Google Scholar]
- 14.Patterson TF, Thompson GR, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;63:4. doi: 10.1093/cid/ciw326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. The Uppsala monitoring centre. The use of the WHO-UMC system for standardised case causality assessment, 2018; 2020.
- 16.Cancer. NIo. Common Terminology Criteria for Adverse Events (CTCAE). Waltham, MA. UpToDate; 2017:1–9. [Google Scholar]
- 17.Wang T, Zhu H, Sun J, et al. Efficacy and safety of voriconazole and CYP2C19 polymorphism for optimised dosage regimens in patients with invasive fungal infections. Int J Antimicrob Agents. 2014;44(5):436–442. doi: 10.1016/j.ijantimicag.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 18.Pascual A, Csajka C, Buclin T, et al. Challenging recommended oral and intravenous voriconazole doses for improved efficacy and safety: population pharmacokinetics-based analysis of adult patients with invasive fungal infections. Clin Infect Dis. 2012;55(3):381–390. doi: 10.1093/cid/cis437 [DOI] [PubMed] [Google Scholar]
- 19.Pascual A, Calandra T, Bolay S, et al. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46(2):201–211. doi: 10.1086/524669 [DOI] [PubMed] [Google Scholar]
- 20.Hamada Y, Ueda T, Miyazaki Y, et al. Effects of antifungal stewardship using therapeutic drug monitoring in voriconazole therapy on the prevention and control of hepatotoxicity and visual symptoms: a multicentre study conducted in Japan. Mycoses. 2020;63(8):779–786. doi: 10.1111/myc.13129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashley ESD, Lewis R, Lewis JS, et al. Pharmacology of systemic antifungal agents. Clin Infect Dis. 2006;43(Supplement_1):S28–S39. doi: 10.1086/504492 [DOI] [Google Scholar]
- 22.Hyland R, Jones BC, Smith DA. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab Dispos. 2003;31(5):540–547. doi: 10.1124/dmd.31.5.540 [DOI] [PubMed] [Google Scholar]
- 23.Johnson LB, Kauffman CA. Voriconazole: a new triazole antifungal agent. Clin Infect Dis. 2003;36(5):630–637. doi: 10.1086/367933 [DOI] [PubMed] [Google Scholar]
- 24.Walsh TJ, Karlsson MO, Driscoll T, et al. Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration. Antimicrob Agents Chemother. 2004;48(6):2166–2172. doi: 10.1128/AAC.48.6.2166-2172.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seyedmousavi S, Mouton JW, Verweij PE, et al. Therapeutic drug monitoring of voriconazole and posaconazole for invasive aspergillosis. Expert Rev Anti Infect Ther. 2013;11(9):931–941. doi: 10.1586/14787210.2013.826989 [DOI] [PubMed] [Google Scholar]
- 26.Mikus G, Scholz IM, Weiss J. Pharmacogenomics of the triazole antifungal agent voriconazole. Pharmacogenomics. 2011;12(6):861–872. doi: 10.2217/pgs.11.18 [DOI] [PubMed] [Google Scholar]
- 27.Wang G, Lei H-P, Li Z, et al. The CYP2C19 ultra-rapid metabolizer genotype influences the pharmacokinetics of voriconazole in healthy male volunteers. Eur J Clin Pharmacol. 2009;65(3):281–285. doi: 10.1007/s00228-008-0574-7 [DOI] [PubMed] [Google Scholar]
- 28.Weiss J, Ten Hoevel MM, Burhenne J, et al. CYP2C19 genotype is a major factor contributing to the highly variable pharmacokinetics of voriconazole. J Clin Pharmacol. 2009;49(2):196–204. doi: 10.1177/0091270008327537 [DOI] [PubMed] [Google Scholar]
- 29.Sienkiewicz B, Urbaniak-Kujda D, Dybko J, et al. Influence of CYP2C19 genotypes on the occurrence of adverse drug reactions of voriconazole among hematological patients after Allo-HSCT. Pathology & Oncology Research. 2018;24(3):541–545. doi: 10.1007/s12253-017-0264-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moriyama B, Obeng AO, Barbarino J, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP2C19 and voriconazole therapy. Clin Pharmacol Ther. 2017;102(1):45–51. doi: 10.1002/cpt.583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto K, Ikawa K, Abematsu K, et al. Correlation between voriconazole trough plasma concentration and hepatotoxicity in patients with different CYP2C19 genotypes. Int J Antimicrob Agents. 2009;34(1):91–94. doi: 10.1016/j.ijantimicag.2009.01.008 [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Xu MA, Wang Y, et al. Analysis of genetic polymorphisms and metabolic phenotype of CYP2C19 in 604 senile patients with coronary heart disease. J Cardiovasc and Pulmonary Dis. 2015;23: 6186–6192. [Google Scholar]
- 33.Jin H, Wang T, Falcione BA, et al. Trough concentration of voriconazole and its relationship with efficacy and safety: a systematic review and meta-analysis. J Antimicrob Chemother. 2016;71(7):1772–1785. doi: 10.1093/jac/dkw045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gómez-López A. Antifungal therapeutic drug monitoring: focus on drugs without a clear recommendation. Clin Microbiol Infect. 2020;26(11):1481–1487. doi: 10.1016/j.cmi.2020.05.037 [DOI] [PubMed] [Google Scholar]
- 35.Xiong W-H, Brown RL, Reed B, et al. Voriconazole, an antifungal triazol that causes visual side effects, is an inhibitor of TRPM1 and TRPM3 channels. Invest Ophthalmol Vis Sci. 2015;56(2):1367–1373. doi: 10.1167/iovs.14-15270 [DOI] [PMC free article] [PubMed] [Google Scholar]