Abstract
Factors determining the in vivo replication of the opportunistic pathogen Mycobacterium genavense are largely unknown. Following intravenous injection of a patient isolate, M. genavense could not be recovered by culture or detected by PCR in the livers or spleens of infected BALB/c mice. In contrast, M. genavense was found to chronically persist and multiply in the livers and spleens of intravenously infected syngeneic gamma-interferon-gene-deficient (GKO) mice as evidenced by acid-fast stains of infected tissues and recovery by both PCR and liquid culture following organ homogenization. In GKO mice, M. genavense elicited a chronic inflammatory response, resulting in marked splenomegaly and extensive lymphadenopathy. Granulomatous lesions in the livers of GKO mice were diffuse, were composed of monocytes, neutrophils, and CD3+ cells, and were histochemically negative for inducible nitric oxide synthase.
Mycobacterium genavense is an opportunistic mycobacterium frequently recovered from pet animals (16) that mainly infects immunocompromised patients, particularly those with AIDS (5, 22, 26). Recently, however, M. genavense has also been detected in the lymph nodes of patients with no apparent primary immune deficiency (2, 3, 19). M. genavense is difficult to grow in vitro (4, 7, 17). It has a very low growth rate in the standard liquid cultures used for diagnostic purposes, and growth on solid agar media is almost undetectable (7). Long-term incubation of liquid media, particularly under low oxygen tension (23), followed by acid-fast staining and PCR amplification and sequencing of 16S rRNA is needed to ascertain the presence of M. genavense in diagnostic specimens (1–3, 5). In vitro studies determining susceptibility to various antibiotic agents are severely compromised due to the very limited growth of this organism (4, 7).
The pathogenesis of M. genavense infection is not well understood due to the lack of an animal model of infection. For example, it is not known whether an immune deficiency (and if so, which type of deficiency) is truly necessary for infection to become established. In an effort to develop a robust and reproducible model of M. genavense infection in mice, we infected mouse strains (BALB/c or C57BL/6) carrying the susceptible allele of the bcg locus, because these mice are frequently used in studies involving other mycobacterial species, such as Mycobacterium avium (13, 14, 18). In order to address the question of whether the cytokine gamma interferon (IFN-γ), known to activate macrophages for mycobacteriostasis (6), is important for containing M. genavense infection in vivo, we also infected IFN-γ-gene-deficient (GKO) mice.
Recovery of M. genavense from the spleens of infected mice.
M. genavense was recovered from the blood of an AIDS patient by liquid culture (BACTEC 13A; Becton Dickinson Microbiology Systems, Cockeysville, Md.). Presence of M. genavense was assessed by acid-fast staining and PCR-mediated detection of mycobacterial 16S rRNA followed by sequencing. PCR for ribosomal DNA was performed by using primer A (10) and primer 264 (1) to amplify a DNA fragment of approximately 1,030 bp. Cycle sequencing was done with primer 9 (24) by using the BigDye Ready Reaction terminator sequencing kit (Applied Biosystems, Foster City, Calif.) on an automated DNA sequencer (ABI 377; Applied Biosystems).
C57BL/6, BALB/c, and syngeneic GKO mice (kindly provided by D. Dalton [8]) were raised under specific-pathogen-free conditions at the Animal Facilities of the Research Center Borstel, Borstel, Germany. Mice were infected intravenously with approximately 106 M. genavense organisms grown in liquid culture. For infection, mycobacteria were washed by centrifugation at 12,000 × g in an Eppendorf microcentrifuge and were counted microscopically in a Thoma chamber. Spleens and livers of infected mice were removed and subjected directly to PCR and/or liquid culture at different time intervals (Table 1). DNA from mouse tissues was isolated by using the QIAGEN tissue kit (QIAGEN, Hilden, Germany). PCR was performed with a modified nested PCR protocol (24) by using primer 9 and a novel reverse primer (primer 20, 5′-GGGCYCATCCCACACCGCWAAAG-3′). For culture, specimens were decontaminated by using the N-acetyl-l-cysteine–sodium hydroxide method and were inoculated into liquid media (MGIT 960; Becton Dickinson) and onto Löwenstein-Jensen and Middlebrook 7H10 solid agar supplemented with 10% oleic acid, dextrose, and catalase (Becton Dickinson). Infection experiments were performed at least twice, with similar results.
TABLE 1.
Detection of M. genavense DNA by PCR and sequencing in the spleens of infected mice
| Mouse strain | No. of M. genavense-positive micea (time postinfection [wk])
|
|||
|---|---|---|---|---|
| 4 | 12 | 20 | 26 | |
| BALB/c | 1/4 | 0/4 | ND | 0/4 |
| GKO | 2/4 | 4/4 | 4/4 | 4/4 |
Mice were infected with 106 M. genavense cells, and spleens were removed at indicated time points and processed for PCR-mediated amplification of DNA encoding 16S rRNA, followed by sequencing of amplicons. Numbers denote positive PCR-mediated detection of M. genavense DNA in the spleens of infected animals out of the total number of animals examined per time point. ND, not determined.
In several preliminary experiments, we were unable to recover M. genavense organisms from the organs of intravenously infected C57BL/6 or BALB/c mice. In contrast, GKO mice reproducibly had viable M. genavense in their livers and spleens 3 and 5 months after infection. A comparison of PCR-mediated detection rates of M. genavense in BALB/c and simultaneously infected GKO mice is shown in Table 1. Detection of M. genavense in the spleens of infected mice at 4 weeks postinfection was inconsistent in both immunocompetent and GKO mice, in that only one or two out of 4 samples, respectively, showed evidence of the presence of mycobacterial DNA (Table 1). No mycobacterial DNA was detected in any mice at 8 weeks postinfection. However, at 12, 20, and 26 weeks postinfection, all tissue samples (both liver and spleens) taken from GKO mice were positive for M. genavense DNA, while all tissue samples taken from BALB/c mice gave negative results by PCR (Table 1).
At 12 and 26 weeks postinfection, livers and spleens were also cultured for M. genavense growth. All samples from GKO mice showed a positive growth index and positive acid-fast stain in the liquid culture medium, while all the samples from immunocompetent mice remained negative. Organisms retrieved from cultured tissues of GKO mice were processed for 16S rRNA sequencing, and all were found to be M. genavense. Thus, live M. genavense was reproducibly detected in GKO mice following week 12 of infection.
In contrast to other investigators (27), we have consistently been unsuccessful at quantitating the growth of a number of different M. genavense isolates on solid Middlebrook agar supplemented with 10% oleic acid, dextrose, and catalase, even after extended incubation times of 12 weeks. In our experience, M. genavense isolates are difficult to expand, even in liquid culture. Since coinfection of AIDS patients with other organisms, such as M. avium, is quite common, growth of acid-fast bacilli may easily be mistaken for M. genavense (17).
Histopathology following M. genavense infection.
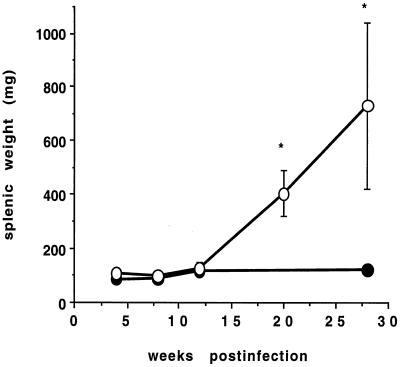
In all GKO mice, significant splenomegaly (splenic weight range at 26 weeks postinfection, 440 to 1,060 mg) and, in some GKO mice, dramatic enlargement of the axillary or mediastinal lymph nodes was noted following week 12 of infection with M. genavense. In some cases, enlargement of Peyer's patches was also evident. In contrast, no gross pathology or increase in spleen weight was observed in BALB/c mice at any point during infection (Fig. 1) or in uninfected GKO mice (data not shown).
FIG. 1.
Course of spleen weight following M. genavense infection of BALB/c and GKO mice. Mice were infected with approximately 106 M. genavense cells, and spleens were removed at indicated time intervals and weighed. Closed circles, BALB/c mice; open circles, GKO mice. Data represent the means of four mice ± standard deviations per time point. ∗, P < 0.001 by Student's t test.
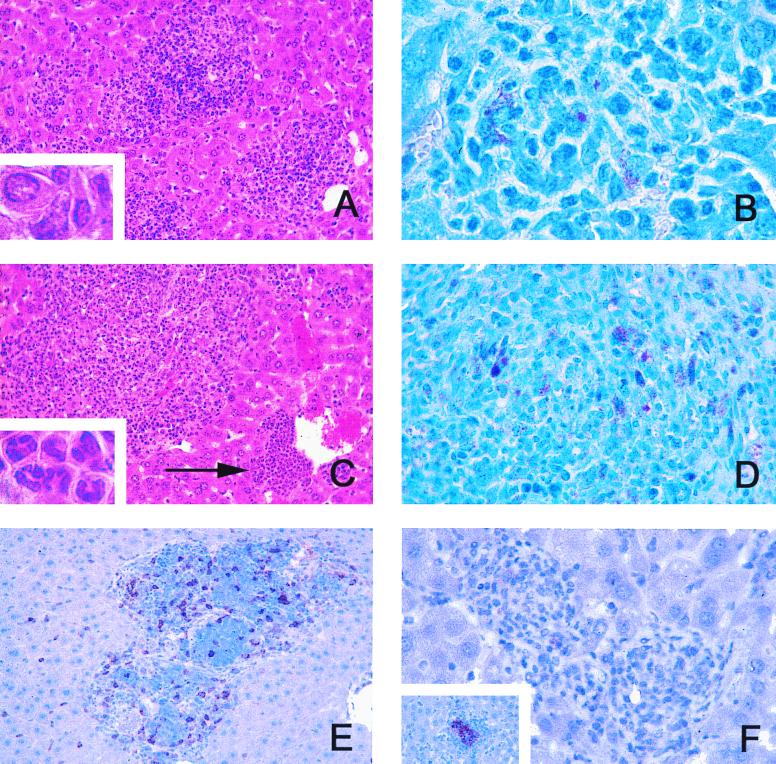
When livers, spleens, lungs, and lymph nodes of infected mice were examined histologically, immunocompetent mice showed only minimal inflammatory infiltrations in the liver and spleen early, i.e., at 4 weeks postinfection, but at no later time point. Acid-fast bacilli were never detected in the organs of BALB/c mice. In contrast, GKO mice showed numerous and increasingly large granulomatous infiltrations following week 12 of infection, particularly in the liver and spleen. These accumulations of inflammatory cells lacked the defined circumscript quality of granulomas evident in immunocompetent mice infected with other opportunistic mycobacteria, such as M. avium (11, 14), and appeared rather diffuse (Fig. 2A). In the liver, they were composed of granulocytes, foamy macrophages, and other mononuclear cells (Fig. 2A, inset). Frequently, perivascular infiltrations consisting almost exclusively of polymorphonuclear granulocytes were seen (Fig. 2C, arrow and inset), consistent with a previous report on mycobacterium-induced hematopoietic remodelling in GKO mice (21). Within granulomatous lesions, acid-fast bacilli were always detected following week 12 of infection, but not before that time point (Fig. 2B). Acid-fast bacilli were also prominent in enlarged lymph nodes (Fig. 2D), again suggesting that M. genavense had multiplied in GKO mice.
FIG. 2.
Granulomatous lesions in GKO mice infected with M. genavense. GKO mice were infected with 106 M. genavense cells, and livers and lymph nodes were removed for histological analysis at indicated times postinfection. (A) Malorganized inflammatory infiltrations in the liver composed predominantly of mononuclear cells at 20 weeks postinfection (hematoxylin and eosin stain; magnification, ×64). Inset, enlargement of intralesional mononuclear cells with little epithelioid transformation (magnification, ×320). (B) Granuloma in the liver with acid-fast M. genavense at 20 weeks postinfection (Ziehl-Neelsen stain; magnification, ×128). (C) Large diffuse mixed infiltrations and perivascular accumulation of predominantly polymorphonuclear cells (arrow) at 26 weeks postinfection (hematoxylin and eosin stain; magnification, ×64). Inset, enlargement of lesion indicated by arrow, demonstrating typical polymorphonuclear morphology (magnification, ×320). (D) Acid-fast M. genavense in mesenterial lymph node at 26 weeks postinfection (Ziehl-Neelsen stain; magnification, ×128). (E) CD3-positive cells in granulomatous lesions at 20 weeks postinfection (immunoperoxidase; magnification, ×64). (F) Granulomatous lesions negative for material reactive with an anti-iNOS-antiserum at 20 weeks postinfection (immunoperoxidase; magnification, ×128). Inset, iNOS-positive control in M. tuberculosis-infected BALB/c mice stained in parallel.
In order to determine whether GKO mice were deficient in recruiting T cells into granulomatous lesions, immunoperoxidase staining with a rat anti-mammalian-CD3 monoclonal antibody (clone CD3-12; Biotrend, Cologne, Germany) was performed. Inflammatory infiltrations in GKO mice always contained CD3+ cells, although these were not organized in a lymphocytic cuff around centrally located macrophages, as is the case in M. avium and Mycobacterium tuberculosis infection in immunocompetent mice (Fig. 2E). In view of the presence of intralesional T cells, we next asked whether granuloma macrophages in GKO mice were adequately activated, as determined by staining with a polyclonal rabbit anti-mouse-inducible nitric oxide synthase (iNOS) antiserum (Biomol, Hamburg, Germany). Macrophages in GKO mice were consistently negative for iNOS throughout the observation period (Fig. 2F) (12).
Our study clearly shows that immunocompetent mice are capable of clearing M. genavense infection. Furthermore, eradication of M. genavense is dependent on the presence of IFN-γ, as mice deficient for IFN-γ develop progressive mycobacterial growth. The mere presence of T cells within the lesions evident in GKO mice is not sufficient to contain M. genavense infection, and this may explain why patients with no apparent T-cell defects were also found to be infected with M. genavense (2, 3, 19).
IFN-γ has been shown to activate mycobacteriocidal mechanisms in macrophages, one of which is the production of nitrogen intermediates via iNOS (20). GKO mice did not express iNOS protein in their lesions at any point during infection. It remains to be determined whether the generation of nitric oxide via iNOS is necessary to inhibit multiplication of M. genavense (as is the case in murine infection with M. tuberculosis), because IFN-γ also inhibits bacterial growth in M. avium infection, but nitric oxide is not involved (11, 13).
IFN-γ is also a principal mediator of ordered, structured granuloma formation in response to mycobacterial infection. Thus, in GKO and SCID mice treated with a neutralizing anti-IFN-γ antibody and infected with M. avium, granuloma formation is greatly delayed, and isolated macrophages and Kupffer cells filled with acid-fast bacteria are frequently found (13, 25). In GKO mice infected with M. genavense, we also noted delayed development of inflammatory infiltrations which roughly corresponded to the time required for development of splenomegaly. It would seem that M. genavense needs time to establish infection even in GKO mice, and macrophages and/or T cells might only respond by releasing proinflammatory mediators after a certain threshold of bacterial numbers is reached. This would reflect the situation in humans, where a prolonged course of M. genavense infection with minor symptoms is followed by overgrowth in enlarged lymph nodes and spleens, leading to the requirement for medical attention (22, 26). In GKO mice, typically ordered granuloma formation with epithelioid cell differentiation did not occur, again reminiscent of the lesions present in immunodeficient patients with disseminated M. genavense infection (5, 22).
In summary, experimental M. genavense infection in GKO mice may be useful for studying the virulence factors of the microorganism that determine in vivo replication. Furthermore, because M. genavense infection in GKO mice closely resembles infection in immunodeficient patients, it may be a valuable experimental model for evaluating immunomodulatory and chemotherapeutic options. Finally, our studies indicate that in human subjects infected with M. genavense but not infected with human immunodeficiency virus (HIV) and with no apparent quantitative defect of T cells, some kind of involvement of the IFN-γ pathway (such as a deficiency of the IFN-γ receptor, the interleukin 12 receptor, or interleukin 12 secretion) should be suspected, similar to that recently described for disseminated M. avium infection in HIV-negative patients (9, 15).
Acknowledgments
We thank Claudia Hahn and Frauke Schaefer for expert technical assistance and Sven Mohr for breeding GKO mice.
REFERENCES
- 1.Böddinghaus B, Rogall T, Flohr T, Blöcker H, Böttger E C. Detection and identification of mycobacteria by amplification of rRNA. J Clin Microbiol. 1990;28:1751–1759. doi: 10.1128/jcm.28.8.1751-1759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogdan C, Kern P, Richter E, Tannapfel A, Rüsch-Gerdes S, Kirchner T, Solbach W. Systemic infection with Mycobacterium genavense following immunosuppressive therapy in a patient who was seronegative for human immunodeficiency virus. Clin Infect Dis. 1997;24:1245–1247. doi: 10.1086/513634. [DOI] [PubMed] [Google Scholar]
- 3.Bosquée L, Böttger E C, De Beenhouwer H, Fonteyne P A, Hirschel B, Larsson L, Meyers W M, Palomino J C, Realini L, Rigouts L, Silva M T, Teske A, Van der Auwera P, Portaels F. Cervical lymphadenitis caused by a fastidious mycobacterium closely related to Mycobacterium genavense in an apparently immunocompetent woman: diagnosis by culture-free microbiological methods. J Clin Microbiol. 1995;33:2670–2674. doi: 10.1128/jcm.33.10.2670-2674.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Böttger E C, Hirschel B, Coyle M B. Mycobacterium genavense sp. nov. Int J Syst Bacteriol. 1993;43:841–843. doi: 10.1099/00207713-43-4-841. [DOI] [PubMed] [Google Scholar]
- 5.Böttger E C, Teske A, Kirschner P, Bost S, Chang H R, Beer V, Hirschel B. Disseminated Mycobacterium genavense infection in patients with AIDS. Lancet. 1992;340:76–80. doi: 10.1016/0140-6736(92)90397-l. [DOI] [PubMed] [Google Scholar]
- 6.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyle M B, Carlson L C, Wallis C K, Leonard R B, Raisys V A, Kilburn J O, Samdadpour M, Böttger E C. Laboratory aspects of Mycobacterium genavense, a proposed species isolated from AIDS patients. J Clin Microbiol. 1992;30:3206–3212. doi: 10.1128/jcm.30.12.3206-3212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 9.Dorman S E, Holland S M. Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J Clin Investig. 1998;101:2364–2369. doi: 10.1172/JCI2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards U, Rogall T, Blöcker H, Emde M, Böttger E C. Isolation and direct complete nucleotide determination of entire genes: characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 1989;17:7843–7853. doi: 10.1093/nar/17.19.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehlers S, Kutsch S, Benini J, Cooper A, Hahn C, Gerdes J, Orme I M, Martin C, Rietschel E T. NOS2-derived nitric oxide regulates the size, quantity and quality of granuloma formation in M. avium-infected mice without affecting bacterial loads. Immunology. 1999;98:313–323. doi: 10.1046/j.1365-2567.1999.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehlers S, Seitzer U. Measuring immune responses in vivo. In: Kaufmann S H E, Kabelitz D, editors. Methods in microbiology. London, United Kingdom: Academic Press; 1998. pp. 365–387. [Google Scholar]
- 13.Florido M, Gonçalves A S, Silva R A, Ehlers S, Cooper A M, Appelberg R. Resistance of virulent Mycobacterium avium to gamma-interferon-mediated antimicrobial activity suggests additional signals for induction of mycobacteriostasis. Infect Immun. 1999;67:3610–3618. doi: 10.1128/iai.67.7.3610-3618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hänsch H C R, Smith D A, Mielke M E A, Bancroft G B, Ehlers S. Mechanisms of granuloma formation in murine M. avium infection: the role of CD4+ T cells. Int Immunol. 1996;8:1299–1310. doi: 10.1093/intimm/8.8.1299. [DOI] [PubMed] [Google Scholar]
- 15.Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile J-F, Newport M, Levin M, Blanche S, Seboun E, Fischer A, Casanova J-L. Interferon-γ-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N Engl J Med. 1996;335:1956–1962. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 16.Kiehn T E, Hoefer H, Böttger E C, Ross R, Wong M, Edwards F, Antinoff N, Armstrong D. Mycobacterium genavense infection in pet animals. J Clin Microbiol. 1996;34:1840–1842. doi: 10.1128/jcm.34.7.1840-1842.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirschner P, Vogel U, Hein R, Böttger E C. Bias of culture techniques for diagnosing mixed Mycobacterium genavense and Mycobacterium avium infection in AIDS. J Clin Microbiol. 1994;32:828–831. doi: 10.1128/jcm.32.3.828-831.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leitzke S, Bucke W, Borner K, Müller R, Hahn H, Ehlers S. Rationale for and efficacy of intermittent treatment with liposome-encapsulated amikacin in chronically lethal M. avium infection. Antimicrob Agents Chemother. 1998;42:459–461. doi: 10.1128/aac.42.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberek V, Soravia C, Ninet B, Hirschel B, Siegrist C A. Cervical lymphadenitis caused by Mycobacterium genavense in a healthy child. Pediatr Infect Dis J. 1996;15:269–270. doi: 10.1097/00006454-199603000-00021. [DOI] [PubMed] [Google Scholar]
- 20.MacMicking J, North R, LaCourse R, Mudgett J, Shah S, Nathan C F. Identification of NOS2 as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray P J, Young R A, Daley G Y. Hematopoietic remodeling in interferon-gamma-deficient mice infected with mycobacteria. Blood. 1998;91:2914–2924. [PubMed] [Google Scholar]
- 22.Pechere M, Opravil M, Wald A, Chave J P, Bessesen M, Sievers A, Hein R, von Overbeck J, Clark R A, Tortoli E, et al. Clinical and epidemiologic features of infection with Mycobacterium genavense. Swiss HIV Cohort Study. Arch Intern Med. 1995;155:400–404. doi: 10.1001/archinte.1995.00430040074009. [DOI] [PubMed] [Google Scholar]
- 23.Realini L, De Ridder K, Palomino J, Hirschel B, Portaels F. Microaerophilic conditions promote growth of Mycobacterium genavense. J Clin Microbiol. 1998;36:2565–2570. doi: 10.1128/jcm.36.9.2565-2570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richter E, Greinert U, Kirsten D, Rüsch-Gerdes S, Schlüter C, Duchrow M, Galle J, Magnussen H, Schlaak M, Flad H-D, Gerdes J. Assessment of mycobacterial DNA in cells and tissues of mycobacterial and sarcoid lesions. Am J Respir Crit Care Med. 1996;153:375–380. doi: 10.1164/ajrccm.153.1.8542146. [DOI] [PubMed] [Google Scholar]
- 25.Smith D A, Hänsch H C R, Bancroft G B, Ehlers S. T cell independent mechanisms of granuloma formation in M. avium infection: the role of TNFα and IFNγ. Immunology. 1997;92:413–419. doi: 10.1046/j.1365-2567.1997.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tortoli E, Brunello F, Cagni A E, Colombrita D, Dionisio D, Grisendi L, Manfrin V, Moroni M, Passerini Tosi C, Pinsi G, Scarparo C, Simonetti M T. Mycobacterium genavense in AIDS patients, report of 24 cases in Italy and review of the literature. Eur J Epidemiol. 1998;14:219–224. doi: 10.1023/a:1007401305708. [DOI] [PubMed] [Google Scholar]
- 27.Vrioni G, Nauciel C, Kerharo G, Matsiota-Bernard P. Treatment of disseminated Mycobacterium genavense infection in a murine model with ciprofloxacin, amikacin, ethambutol, clarithromycin and rifabutin. J Antimicrob Chemother. 1998;42:483–487. doi: 10.1093/jac/42.4.483. [DOI] [PubMed] [Google Scholar]