Abstract
The kinetics of the humoral response to Cryptococcus neoformans proteins were studied in outbred mice infected with isolate NIH52D. Future nonsurvivors had earlier and stronger (i.e., more bands recognized) humoral responses than survivors. In addition, antibodies to a 56- to 60-kDa membrane antigen and to a 39- to 40-kDa cytosolic antigen were detected more frequently in samples from future nonsurvivors and from survivors, respectively (P < 0.05).
Cryptococcus neoformans is an encapsulated yeast mainly responsible for meningitis, particularly in AIDS patients (2). Many clinical, histopathological, and experimental data prove that cellular immunity makes a critical contribution to host defense against C. neoformans (1). During the past decade, several laboratories have studied the role of humoral immunity, especially that elicited by capsular polysaccharide, and found some monoclonal antipolysaccharide antibodies to be protective against murine cryptococcosis (9, 11, 19). In contrast, very few studies have investigated the antibody response to protein antigens (4, 12–14), and no study has looked at the role of antibody response or its kinetics during the course of cryptococcosis.
We used a murine model of disseminated cryptococcosis to analyze the kinetics of the humoral response and to look for indicators predictive of the outcome (15). In this model, outbred mice exhibit individual patterns of susceptibility to C. neoformans infection, independently of the inoculum size. Some of the mice develop acute, disseminated and rapidly lethal infections, whereas others survive for several weeks with limited chronic infection, thus allowing comparison of the antibody responses as a function of outcome.
Outbred male OF1 mice (Iffa-Credo Laboratories, l'Arbresle, France; mean body weight, 18 to 20 g) were infected with C. neoformans isolate NIH52D (104 to 106 yeasts/animal in groups of 12 mice that could be identified individually, in three independent experiments). Survival was recorded daily until sacrifice by CO2 inhalation (up to day 84 after inoculation). Blood was drawn weekly from the lateral tail vein (34 μl) and immediately used for blood culture (10 μl), as previously reported (15), and for immunoblotting (24 μl). For mice that were sacrificed, blood was drawn by cardiac puncture, buffy coats were cultured, and plasma samples were stored at −20°C until assayed. For each experiment, noninfected control mice housed under the same conditions were used.
The cytosol and membrane extracts were prepared as previously described (4) from strain NIH52D and after heat stress (13). The resulting cytosol (C52D) extract and membrane (M52D) extract were aliquoted and stored at −20°C. The same procedure was used to obtain cytosol and membrane fractions from an equal (vol/vol) mixture of 11 epidemiologically unrelated recent clinical isolates of C. neoformans.
The electrophoreses were run on precast polyacrylamide preparative gels in the Mini-Protean II system, and proteins were transferred onto nitrocellulose membranes, as recommended by the manufacturer (Bio-Rad Laboratories, Ivry-sur-Seine, France). Immunoblotting was performed with a multiscreen apparatus (Bio-Rad), using either plasma or whole blood from infected and control mice, peroxidase-conjugated antimouse immunoglobulin G (heavy plus light chains) antibody (Bio-Sys, Compiègne, France), and a chemiluminescent substrate. The band patterns were analyzed after digitalization using Taxotron software (P. D. Grimont, Institut Pasteur, Paris, France). For each mouse, only bands that were observed on two separate days or bands that were as intense as those obtained with the positive control serum were considered positive. In addition, for the analysis of bands associated with survival or death, the decision regarding the existence of a given band was based on its detection in samples from at least 10 mice. Statistical analyses were performed using Statview II software (Abacus Concepts, Inc., Berkeley, Calif.) and nonparametric tests. The level of significance was 0.05.
Course of C. neoformans infection in OF1 mice.
The course of the infection was the same as that observed previously (15). All 25 mice that died of the infection had at least one positive blood culture during the study and died during the acute phase of the infection before day 32 after inoculation. In contrast, all survivors during the chronic phase of the infection had negative blood cultures at the time of the sacrifice.
Kinetics of the antibody response in C. neoformans-infected mice.
In preliminary experiments, it was determined that similar data were obtained when plasma samples diluted 1:100 or supernatants of the blood drawn on the same day from the same mice immediately diluted 1:50 in blocking buffer and centrifuged were used. Therefore, subsequent experiments were conducted with whole blood from living animals diluted 1:50 or with plasma from sacrificed animals diluted 1:100.
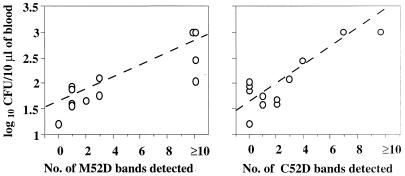
No antibody was detected in the samples from noninfected mice throughout the study. Antibodies were detected significantly earlier in M52D and C52D extracts from future nonsurvivors than from survivors (P < 0.0003). In addition, significantly more bands were recognized by future nonsurvivors' samples than by survivors' samples during the acute phase (P < 0.003). Finally, the number of positive bands obtained with the last blood sample from future nonsurvivors and the number of yeasts cultured from the same sample were significantly correlated (Fig. 1).
FIG. 1.
Correlation between fungemia and the magnitude of the antibody response to C. neoformans protein antigens (i.e., the number of bands) detected in the blood sample drawn before death of infected OF1 mice (M52D [rs = 0.848] and C52D [rs = 0.605]).
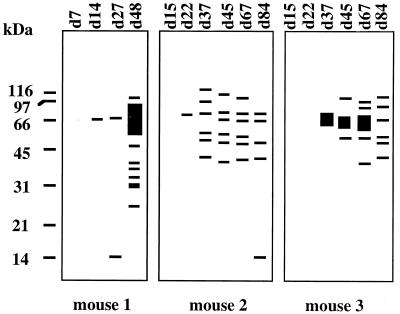
Most of the survivors (13 of 23 [56%]) mounted no antibody response during the acute phase. During the chronic phase of the infection, all but one of them had detectable antibodies. The numbers of positive bands increased during the course of infection (P < 0.001) (an example is presented in Fig. 2).
FIG. 2.
Evolution of the antibody response during the course of C. neoformans infection in three arbitrarily selected OF1 mice. The antibodies, detected sequentially by immunoblotting using the C. neoformans M52D extract, are represented as band patterns generated by Taxotron software. The day of the sampling is shown at the top of each lane.
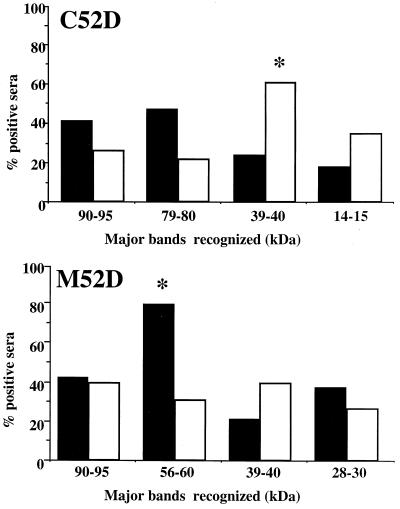
Thus, the antibody response to protein antigens was bimodal: the future nonsurvivors mounted strong humoral responses during the acute phase, and the majority of the survivors produced antibodies later, during the chronic phase of the infection. We then analyzed the humoral responses according to the outcome: survivors produced antibodies directed against the 39- to 40-kDa C52D antigen significantly more frequently than did the future nonsurvivors (P < 0.05) (Fig. 3), while the 56- to 60-kDa antigen in M52D extracts was recognized more frequently by antibodies from future nonsurvivors than from survivors (P < 0.05).
FIG. 3.
Comparison of percentages of antibody-positive sera from NIH52D-infected OF1 mice recognizing selected C. neoformans protein antigens according to the outcome of the infection. Sera obtained from survivors (□) and future nonsurvivors (■) during the acute phase of the infection were compared by immunoblotting on C52D and M52D protein extracts from C. neoformans. ∗, P < 0.05 versus the other group (Fisher's exact test).
For each of the three independent experiments, the kinetics of the antibody response were identical, thereby supporting the reproducibility of the model. In addition, the same antibody response patterns were obtained when plasma samples from survivors were tested against cytosol and membrane extracts of NIH52D or of a mixture of 11 epidemiologically unrelated isolates (data not shown).
Up to now, very little attention has been paid to the humoral response in animals infected with C. neoformans or other fungi. In this study, we demonstrated that the kinetics of the humoral response in NIH52D-infected animals were bimodal and seemed to reflect the outcome of the infection. To explain the quantitative and qualitative differences between the two responses, we envisaged several hypotheses. Indeed, yeast factors, host factors, or both could be responsible for the different humoral responses. In vitro, C. neoformans protein production has been shown to be influenced by various factors, including the culture medium (5). Thus, the yeasts may generate different antigens depending on their in vivo environment and/or growth stage, which probably changed during infection progression. In addition, genetic microevolutions sometimes occur in C. neoformans during the infection (3), and they have been shown to influence the inflammatory response in the lung and could also alter the humoral response.
In addition to the yeast factors, other properties specific to the host could be involved. Above all, the fact that the severity of the infection differs during acute and chronic phases could trigger different antibody responses. We already showed that the local cellular immune response differs according to the infected organs (16). The genetic background was also certainly critical. The antipolysaccharide antibody response has been reported to be controlled by several genes, including the immunoglobulin H complex-linked genes (10), which influence the clearance of the yeasts in the pulmonary model (17). In our model, survivors may be able to develop a strong early cellular response, but only a mild humoral response, whereas future nonsurvivors may not. This scenario does not mean that the antibodies produced early by the future nonsurvivors were deleterious or that the antibodies produced later by survivors were protective. Passive immunization using polyclonal sera from these animals or monoclonal antibodies is required to assess this aspect.
The detection of antibodies to the cytosol antigen during the course of cryptococcosis might predict recovery, and the appearance of antibodies to the membrane antigen might predict a relapse. Although serotherapy using monoclonal antipolysaccharide antibodies has been shown to either prolong or shorten the survival of infected mice depending on the isotypes and idiotypes of the antibodies (7, 11, 18, 20, 21), no study has monitored the evolution of the humoral response to the capsular polysaccharide or assessed its predictive value for infection outcome in mice. In humans, the presence of antipolysaccharide antibodies in the sera of human immunodeficiency virus-negative patients at the time of cryptococcosis diagnosis was associated with a good prognosis (6). The only attempt to correlate the evolution of antipolysaccharide antibodies with outcome was made with data from seven AIDS patients and failed to show any significant relationship (8).
We are aware that the clinical setting differs from the experimental infection by the diversity of the infecting isolates, by underlying diseases, and by previous contacts with the fungus. We already know that individuals without cryptococcosis have antibodies that recognize C. neoformans proteins and that the patterns of antibody reactivity against these proteins obtained with sera from infected patients are far more complex than those that we observed in mice (4, 12). However, the demonstrated reproducibility of the antibody-recognition profile, using extracts prepared with the infecting isolate or a mixture of epidemiologically unrelated clinical isolates, should allow us to test sera collected from patients with cryptococcosis during the prospective clinical Crypto A/D study ongoing in France (F. Dromer, O. Lortholory, S. Mathoulin-Pélissier, K. Sitbon, A. de Gouvello, B. Dupont, and the French Cryptococcosis Study Group. Epidemiological and clinical aspects of Cryptococcus neoformans infection in France. 4th Int. Conf. Cryptococcus Cryptococcosis, abstr. I33, 1999.) with the methodology used for the infected mice. Whether monitoring the antibody responses to protein antigens in patients with cryptococcosis and in those at risk for cryptococcosis will be useful for clinical management remains to be determined.
Acknowledgments
This work was supported by grants from the Pasteur Institute (Contrat Interne de Recherche Clinique) to Francoise Dromer, from the SmithKline Beecham Institute (Nanterre, France), and from the Association des Professeurs de Pathologie Infectieuse et Tropicale to Ségolène Neuville.
We thank Janet Jacobson for reviewing the English text.
REFERENCES
- 1.Buchanan K L, Murphy J W. What makes Cryptococcus neoformans a pathogen? Emerg Infect Dis. 1998;4:71–83. doi: 10.3201/eid0401.980109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadevall A, Perfect J R. Cryptococcus neoformans. Washington, D.C.: American Society for Microbiology; 1998. pp. 351–380. [Google Scholar]
- 3.Chen L-C, Casadevall A. Variants of a Cryptococcus neoformans strain elicit different inflammatory responses in mice. Clin Diagn Lab Immunol. 1999;6:266–268. doi: 10.1128/cdli.6.2.266-268.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L-C, Goldman D L, Doering T L, Pirofski L-A, Casadevall A. Antibody response to Cryptococcus neoformans proteins in rodents and humans. Infect Immun. 1999;67:2218–2224. doi: 10.1128/iai.67.5.2218-2224.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L-C, Pirofski L-A, Casadevall A. Extracellular proteins of Cryptococcus neoformans and host antibody response. Infect Immun. 1997;65:2599–2605. doi: 10.1128/iai.65.7.2599-2605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond R D, Bennett J E. Prognostic factors in cryptococcal meningitis: a study in 111 cases. Ann Intern Med. 1974;80:176–181. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- 7.Dromer F, Charreire J, Contrepois A, Carbon C, Yeni P. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect Immun. 1987;55:749–752. doi: 10.1128/iai.55.3.749-752.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dromer F, Denning D W, Stevens D A, Noble A, Hamilton J R. Anti-Cryptococcus neoformans antibodies in patients with AIDS and cryptococcosis. Serodiagn Immunother Infect Dis. 1995;7:181–188. [Google Scholar]
- 9.Dromer F, Perronne C, Barge J, Vildé J L, Yeni P. Role of specific IgG and complement during the initial course of experimental cryptococcosis. Clin Exp Immunol. 1989;78:412–417. [PMC free article] [PubMed] [Google Scholar]
- 10.Dromer F, Yeni P, Charreire J. Genetic control of the humoral response to cryptococcal polysaccharide in mice. Immunogenetics. 1988;28:417–424. doi: 10.1007/BF00355373. [DOI] [PubMed] [Google Scholar]
- 11.Eckert T F, Kozel T R. Production and characterization of monoclonal antibodies specific for Cryptococcus neoformans capsular polysaccharide. Infect Immun. 1987;55:1895–1899. doi: 10.1128/iai.55.8.1895-1899.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton A J, Figueroa J I, Jeavons L, Seaton R A. Recognition of cytoplasmic yeast antigens of Cryptococcus neoformans var. neoformans and Cryptococcus neoformans var. gattii by immune human sera. FEMS Immunol Med Microbiol. 1997;17:111–119. doi: 10.1111/j.1574-695X.1997.tb01003.x. [DOI] [PubMed] [Google Scholar]
- 13.Kakeya H, Udono H, Ikuno N, Yamamoto Y, Mitsutake K, Miyazaki T, Tomono K, Koga H, Tashiro T, Nakayama E, Kohno S. A 77-kilodalton protein of Cryptococcus neoformans, a member of the heat shock protein 70 family, is a major antigen detected in the sera of mice with pulmonary cryptococcosis. Infect Immun. 1997;65:1653–1658. doi: 10.1128/iai.65.5.1653-1658.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakeya H, Udono H, Maesaki S, Sasaki E, Kawamura S, Hossain M A, Yamamoto Y, Sawai T, Fukuda M, Mitsutake K, Miyazaki Y, Tomono K, Tashiro T, Nakayama E, Kohno S. Heat shock protein 70 (Hsp70) as a major target of the antibody response in patients with pulmonary cryptococcosis. Clin Exp Immunol. 1999;115:485–490. doi: 10.1046/j.1365-2249.1999.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lortholary O, Improvisi L, Nicolas M, Provost F, Dupont B, Dromer F. Fungemia during murine cryptococcosis sheds some light on pathophysiology. Med Mycol. 1999;37:169–174. [PubMed] [Google Scholar]
- 16.Lortholary O, Improvisi L, Rayhane N, Gray F, Fitting C, Cavaillon J M, Dromer F. Cytokine profiles of AIDS patients are similar to those of mice with disseminated Cryptococcus neoformans infection. Infect Immun. 1999;67:6314–6320. doi: 10.1128/iai.67.12.6314-6320.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovchik J A, Wilder J A, Huffnagle G B, Riblet R, Lyons C R, Lipscomb M F. Ig heavy chain complex-linked genes influence the immune response in a murine cryptococcal infection. J Immunol. 1999;163:3907–3913. [PubMed] [Google Scholar]
- 18.Mukherjee J, Nussbaum G, Scharff M D, Casadevall A. Protective and nonprotective monoclonal antibodies to Cryptococcus neoformans originating from one B cell. J Exp Med. 1995;181:405–409. doi: 10.1084/jem.181.1.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherjee S, Lee S C, Casadevall A. Antibodies to Cryptococcus neoformans glucuronoxylomannon enhance antifungal activity of murine macrophages. Infect Immun. 1995;63:573–579. doi: 10.1128/iai.63.2.573-579.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savoy A C, Lupan D M, Manalo P B, Roberts J S, Schlageter A M, Weinhold L C, Kozel T R. Acute lethal toxicity following passive immunization for treatment of murine cryptococcosis. Infect Immun. 1997;65:1800–1807. doi: 10.1128/iai.65.5.1800-1807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan R R, Spira G, Oh J, Paizi M, Casadevall A, Scharff M D. Isotype switching increases efficacy of antibody protection against Cryptococcus neoformans infection in mice. Infect Immun. 1998;66:1057–1062. doi: 10.1128/iai.66.3.1057-1062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]