Abstract
The internalization of the N-terminal catalytic domain of Bordetella pertussis adenylate cyclase toxin (ACT) across the cytoplasmic membrane has been considered to occur independently from protein-protein interactions which can lead to oligomerization required for hemolytic activity by its C-terminal hemolysin domain. Here we report that when added in excess, this hemolysin domain stimulates the internalization, suggesting the involvement of protein-protein interactions in cell-invasive activity of ACT, as well as its hemolytic activity.
The adenylate cyclase toxin (ACT) of Bordetella pertussis is a 1,706-amino-acid (aa) protein (6, 12) which enters mammalian cells and unregulatedly converts intracellular ATP to cyclic AMP (cAMP) (7, 13, 16, 28). The calmodulin-dependent catalytic activity of this toxin is located in the N-terminal 400-aa domain which internalizes into target cells across the cytoplasmic membrane in the presence of calcium (11, 12, 19, 23). The posttranslationally fatty-acylated C-terminal 1,300-aa RTX domain acts as a pore-forming hemolysin (3, 9, 15, 24, 25), and the integrity of this hemolysin domain is necessary for the internalization of the catalytic domain; even a small deletion of 60 aa in the hemolysin domain largely impairs the invasive activity (17, 25).
The mechanism of ACT internalization is still largely unknown. Internalization of ACT into sheep erythrocytes is reported to be a linear function of ACT concentration, in contrast to a higher-order power dependency for hemolytic activity which probably involves oligomer formation. Therefore, a single ACT molecule appears to be capable of internalizing its catalytic domain by itself (5, 14, 22, 27). On the other hand, it has been previously demonstrated that pairs of truncated mutant ACT molecules, which are individually incapable of cell binding, internalization, and hemolysis, form active complexes to partially recover invasive and hemolytic activities (17) and fully recover invasive activity (2). In addition, a fragment originated from the ACT hemolysin domain has been suggested to interact with the catalytic domain and to facilitate its entry (8, 20, 21). Thus, protein-protein interactions between ACT molecules could be involved not only in hemolysis but also in internalization of the catalytic domain. In this study, the role of such protein-protein interactions in invasive activity of ACT was investigated.
Preparation of toxins.
ACT was prepared from Escherichia coli cells overexpressing the structural gene cyaA and an accessory gene, cyaC, required for posttranslational fatty acylation, cloned on plasmid pCACT3 (5). A catalytically inactive point mutant, ACTK58Q, was prepared using plasmid pT7CT7ACT-K58Q (17) (both plasmids were constructed by Peter Šebo and were kind gifts from Agnes Ullmann). Plasmids for truncated ACTs (Fig. 1) were constructed by joining the DNA sequences corresponding to the catalytic domain of ACTK58Q and the hemolysin domain of catalytically active deletion mutant toxins (17). The recombinant proteins were extracted with buffered urea solution (8 M urea, 50 mM Tris-HCl, and 0.2 mM CaCl2 [pH 8.0]) from ultrasonically disrupted bacterial cell debris, as described previously (25). Toxins were then purified close to homogeneity by DEAE-Sepharose chromatography (25) and subsequent calmodulin-agarose chromatography (26) or phenyl-Sepharose chromatography (1, 10), as shown in Fig. 1.
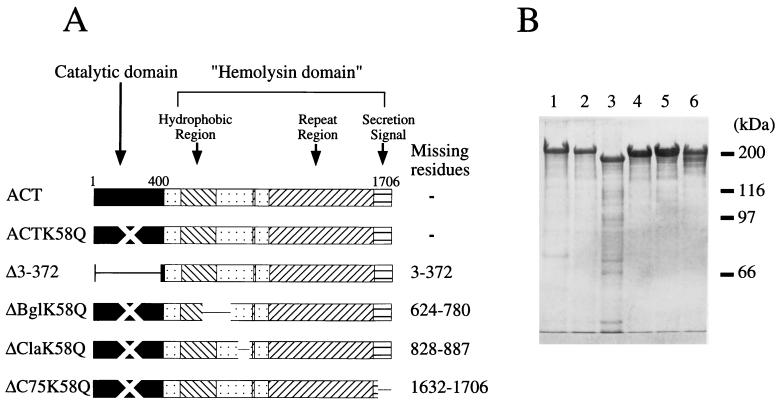
FIG. 1.
Toxins used in this study. (A) Schematic representation of the toxins. The mutant toxins were constructed and prepared as described in the text. K58Q toxins carry a mutation of lysine 58 to a glutamine, resulting in complete loss of catalytic activity (11). (B) Electrophoretic analysis of the toxin preparations. Toxin proteins were extracted by and purified in buffered urea solution, as described in the text, and 10 μg of each purified toxin was subjected to sodium dodecyl sulfate–8% polyacrylamide gel electrophoresis and visualized by Coomassie brilliant blue staining. Lanes: 1, DEAE- and calmodulin agarose-purified wild-type ACT; 2, DEAE-purified ACTK58Q; 3 to 6, DEAE- and phenyl-Sepharose-purified ACTΔ3-372, ΔBglK58Q, ΔClaK58Q, and ΔC75K58Q, respectively.
Stimulation of ACT internalization by ACTK58Q and ACTΔ3-372.
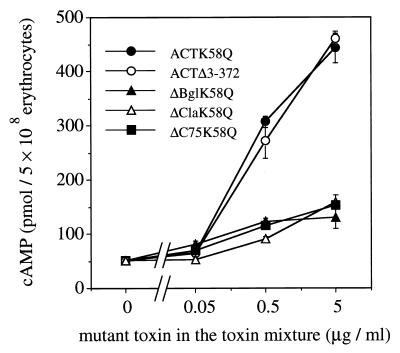
It has already been demonstrated that truncated ACT mutants with different nonoverlapping deletions in the hemolysin domain can complement each other to recover cell-invasive and hemolytic activities (2, 17), probably by forming dimeric or higher oligomeric complexes. This suggested that wild-type toxin could also form a complex active in internalization. For analyzing the role of such protein-protein interactions in cell-invasive activity of wild-type ACT, we first analyzed the dose dependency of internalization over a wide range of toxin concentrations. Internalization can be measured by intracellular cAMP determination (3) or by determination of trypsin-protected adenylate cyclase activity in ACT-treated cells (3, 18); however, neither the intracellular cAMP measurement nor the trypsin-protected enzyme assay is applicable for measurement of ACT internalization within a wide range of toxin concentrations. Intracellular cAMP levels do not exactly reflect the level of internalized ACT catalytic domain, especially at high toxin concentrations as a result of overconsuming of the intracellular substrate ATP. On the other hand, trypsin-protected enzyme activity measurements are not sufficiently reliable at a very low toxin concentration. Thus, as an alternative for dose dependency experiments, mixtures of wild-type ACT and mutant ACT were used to mimic increasing toxin concentrations. Mixtures contained wild-type ACT at a fixed low level (final concentration, 0.05 μg/ml), and the increase in toxin concentration was provided by mutant toxins (final concentration, 0.05 to 5 μg/ml). Toxin mixtures were prepared in buffered urea solution and added to sheep erythrocyte suspension (5 × 108/ml). Nonspecific adsorption of toxins to tube surfaces was prevented by adding 50 μg of bovine serum albumin (Wako Pure Chemical Industries, Osaka, Japan) per ml to toxin mixtures. The final urea concentration in erythrocyte suspension was kept below 40 mM. After incubation at 37°C for 30 min in TNC buffer (10 mM Tris [pH 8.0], 150 mM NaCl, 1 mM CaCl2), the intracellular cAMP level was measured by radioimmunoassay as described previously (3, 17), using rabbit anti-cAMP polyclonal antiserum (ICN Biochemicals, Costa Mesa, Calif.). Figure 2 shows intracellular cAMP levels in erythrocytes treated with toxin mixtures. Addition to the wild-type toxin of point mutant ACTK58Q, which is catalytically inactive but otherwise indistinguishable from the wild type, resulted in a marked increase of intracellular cAMP, proportional to increasing concentrations of this mutant toxin. About 10-fold stimulation was observed with a 100-fold excess (5 μg/ml) of ACTK58Q, although the wild-type ACT concentration was kept constant at a low level (0.05 μg/ml) throughout the experiment.
FIG. 2.
Effects of excess amounts of mutant toxins on ACT-mediated intracellular cAMP accumulation in sheep erythrocytes. Sheep erythrocytes (5 × 108/ml) were treated with mixtures containing wild-type (0.05 μg/ml) and mutant (0 to 5 μg/ml) toxins in TNC buffer at 37°C for 30 min. Intracellular cAMP was then extracted and measured as described in the text. Mean values and standard errors from triplicate assays are presented.
Similar stimulation was observed when ACTΔ3-372 was used instead of ACTK58Q (Fig. 2), indicating that the observed stimulation was independent of the presence of the catalytic domain but was rather due to the presence of excess ACT hemolysin domain, which binds to and forms pores on the erythrocyte membrane (25). As no significant hemolysis or amount of extracellular cAMP was detected during the experiment (data not shown), the measured cAMP represented exclusively intracellular levels, due to internalization of the ACT catalytic domain. Intermolecular complementation between active and inactive catalytic domains is not likely to contribute to the observed stimulation, because ACTΔ3-372 lacks the catalytic domain. ACTK58Q, ACTΔ3-372, and other truncated mutant toxins alone at 5 μg/ml did not show significant cAMP production compared to untreated controls and controls treated with buffered urea solution (data not shown), indicating that the observed stimulation was not due to residual activity of these mutants.
Truncated and catalytically inactive mutants ΔBglK58Q, ΔClaK58Q, and ΔC75K58Q, which are almost completely impaired in their ability to bind and internalize in erythrocytes (17), showed much-reduced stimulation (Fig. 2), indicating that the stimulation is specific to ACTK58Q and ACTΔ3-372 and suggesting that the stimulation involves an event which takes place on the erythrocyte membrane.
Stimulation of internalization of membrane-bound ACT by ACTΔ3-372.
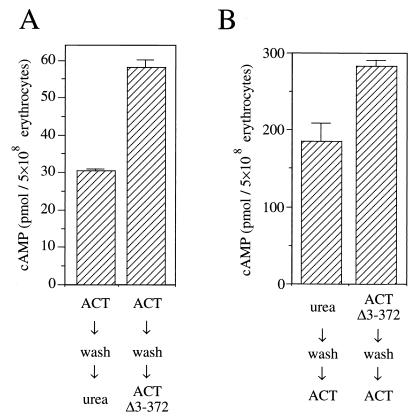
In order to further clarify the characteristics of the stimulation of ACT internalization by the hemolysin domain, we attempted to examine the stimulatory effect of ACTΔ3-372 on the internalization of membrane-bound ACT, in the absence of unbound ACT molecules in the incubation medium. Erythrocytes (5 × 108/ml in TNC buffer) were first treated with 0.05 μg of wild-type ACT per ml for 10 min on ice to allow binding but not internalization of its catalytic domain (23). Erythrocytes were then washed to eliminate unbound ACT as described previously (17) and resuspended in TNC buffer, and then ACTΔ3-372 (5 μg/ml) was added; after incubation at 37°C for 30 min, intracellular cAMP was measured. Figure 3A demonstrates the stimulatory effect of ACTΔ3-372 on internalization of membrane-bound ACT. Compared to the control treated with buffered urea solution, posttreatment by ACTΔ3-372 resulted in a twofold stimulation of intracellular cAMP accumulation. No significant release of adenylate cyclase activity into the incubation medium was observed during the 30 min of incubation at 37°C (data not shown), indicating that the stimulation was not accompanied by change in amount of membrane-bound wild-type ACT. This again indicates that the observed stimulation involves an event on the erythrocyte membrane and suggests the involvement of interaction between wild-type ACT and ACTΔ3-372 molecules on the membrane.
FIG. 3.
Effects of pre- and posttreatments by ACTΔ3-372 on ACT-mediated intracellular cAMP accumulation in sheep erythrocytes. (A) Posttreatment by ACTΔ3-372. Sheep erythrocytes were first treated with 0.05 μg of wild-type ACT per ml on ice for 10 min to allow binding but not internalization. Cells were then washed as described previously (17) and treated with buffered urea solution or 5 μg of ACTΔ3-372 per ml. After incubation at 37°C for 30 min, intracellular cAMP was measured. Mean values and standard errors from triplicate assays are presented. (B) Pretreatment by ACTΔ3-372. Sheep erythrocytes were first treated with buffered urea solution or 5 μg of ACTΔ3-372 per ml on ice for 10 min. Cells were then washed and treated with 0.05 μg of wild-type ACT per ml. Cells were further incubated at 37°C for 30 min, and then intracellular cAMP was measured. Mean values and standard errors from triplicate assays are presented.
Figure 3B shows the effects of ACTΔ3-372 pretreatment of cells prior to addition of wild-type ACT. Cells were treated with 5 μg of ACTΔ3-372 per ml or buffered urea solution (as a control) for 10 min on ice, washed, and subsequently treated with 0.05 μg of wild-type ACT per ml at 37°C for 30 min. ACTΔ3-372-pretreated erythrocytes showed significantly higher intracellular cAMP levels than the control. This may be due to increased efficiency of invasiveness and might additionally be due to an increase in the amount of wild-type ACT on the membrane through interaction between membrane-bound ACTΔ3-372 molecules and wild-type ACT molecules from the incubation medium.
The mechanism of ACT internalization still remains an open question. In aqueous solution, ACT forms self-aggregate (24), and on the membrane, ACT molecules undergo association-dissociation cycles and form unstable hemolytic channels (4, 27), indicating their strong tendency to interact with themselves. As ACTΔ3-372 is about as hemolytic as wild-type ACT (data not shown), the mutant toxin is probably equally capable of oligomerization. However, it has been repeatedly demonstrated that ACT internalization exhibits a linear dose dependency in contrast to a nonlinear pattern for hemolysis, suggesting that internalization of ACT could be a monomeric process (5, 14, 22). Contrary to these observations, our results indicate that protein-protein interaction may play a role in the mechanism of ACT internalization. Addition of ACTK58Q or ACTΔ3-372 to wild-type ACT could increase the frequency of encounter between membrane-bound toxin molecules through association-dissociation cycles, and if such encounters trigger or stimulate the internalization of individual ACT molecules in a still-unknown way, it could explain the observed increase in internalization. This increase could result in a nonlinear dose dependency of ACT internalization; however, the extent of nonlinearity may not be large enough to be significantly detectable under standard experimental conditions (10-fold stimulation by a 100-fold excess amount of hemolysin domain [Fig. 2]). This might be the reason that the stimulative effect has not been visible in previous studies (5, 14), and the mechanism underlying the observed stimulation may contribute, in part, to the internalization of ACT across the cytoplasmic membrane.
Acknowledgments
We thank Agnes Ullmann and Hiroko Sato for their critical reading of the manuscript. We are also grateful to Peter Sebo and Yoshichika Arakawa for their stimulating discussion and continuous encouragement throughout this work, respectively.
This work was financed in part by a Human Frontier Science Program Organization grant to Agnes Ullmann. Masaaki Iwaki was supported in part by the Yakult Bioscience Foundation.
REFERENCES
- 1.Basar T, Havlícěk V, Bezouskova S, Halada P, Hackett M, Šebo P. The conserved lysine 860 in the additional fatty-acylation site of Bordetella pertussis adenylate cyclase is crucial for toxin function independently of its acylation status. J Biol Chem. 1999;274:10777–10783. doi: 10.1074/jbc.274.16.10777. [DOI] [PubMed] [Google Scholar]
- 2.Bejarano M, Nisan I, Ludwig A, Goebel W, Hanski E. Characterization of the C-terminal domain essential for toxic activity of adenylate cyclase toxin. Mol Microbiol. 1999;31:381–392. doi: 10.1046/j.1365-2958.1999.01183.x. [DOI] [PubMed] [Google Scholar]
- 3.Bellalou J, Sakamoto H, Ladant D, Geoffroy C, Ullmann A. Deletions affecting hemolytic and toxin activities of Bordetella pertussis adenylate cyclase. Infect Immun. 1990;58:3242–3247. doi: 10.1128/iai.58.10.3242-3247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benz R, Maier E, Ladant D, Ullmann A, Šebo P. Adenylate cyclase toxin of Bordetella pertussis: evidence for the formation of small ion-permeable channels and comparison with HlyA of Escherichia coli. J Biol Chem. 1994;269:27231–27239. [PubMed] [Google Scholar]
- 5.Betsou F, Šebo P, Guiso N. CyaC-mediated activation is important not only for toxic but also for protective activities of Bordetella pertussis adenylate cyclase-hemolysin. Infect Immun. 1993;61:3583–3589. doi: 10.1128/iai.61.9.3583-3589.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brownlie R M, Coote J G, Parton R, Schultz J E, Rogel A, Hanski E. Cloning of the adenylate cyclase genetic determinant of Bordetella pertussis and its expression in Escherichia coli and B. pertussis. Microb Pathog. 1988;4:335–344. doi: 10.1016/0882-4010(88)90061-7. [DOI] [PubMed] [Google Scholar]
- 7.Confer D L, Eaton J W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982;217:948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- 8.Donovan M G, Masure H R, Storm D R. Isolation of a protein fraction from Bordetella pertussis that facilitates entry of the calmodulin-sensitive adenylate cyclase into animal cells. Biochemistry. 1989;28:8124–8129. doi: 10.1021/bi00446a024. [DOI] [PubMed] [Google Scholar]
- 9.Ehrmann I E, Gray M C, Gordon V M, Gray L S, Hewlett E L. Hemolytic activity of adenylate cyclase toxin from Bordetella pertussis. FEBS Lett. 1991;278:79–83. doi: 10.1016/0014-5793(91)80088-k. [DOI] [PubMed] [Google Scholar]
- 10.Fayolle C, Ladant D, Karimova G, Ullmann A, Leclerc C. Therapy of murine tumors with recombinant Bordetella pertussis adenylate cyclase carrying a cytotoxic T cell epitope. J Immunol. 1999;162:4157–4162. [PubMed] [Google Scholar]
- 11.Glaser P, Elmaoglou-Lazaridou A, Krin E, Ladant D, Bârzu O, Danchin A. Identification of residues essential for catalysis and binding of calmodulin in Bordetella pertussis adenylate cyclase by site-directed mutagenesis. EMBO J. 1989;8:967–972. doi: 10.1002/j.1460-2075.1989.tb03459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaser P, Ladant D, Sezer O, Pichot F, Ullmann A, Danchin A. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol. 1988;2:19–30. [PubMed] [Google Scholar]
- 13.Gordon V M, Young W W, Jr, Lechler S M, Gray M C, Leppla S H, Hewlett E L. Adenylate cyclase toxins from Bacillus anthracis and Bordetella pertussis. Different processes for interaction with and entry into target cells. J Biol Chem. 1989;264:14792–14796. [PubMed] [Google Scholar]
- 14.Gray M, Szabo G, Otero A S, Gray L, Hewlett E. Distinct mechanism for K+ efflux, intoxication, and hemolysis by Bordetella pertussis AC toxin. J Biol Chem. 1998;273:18260–18267. doi: 10.1074/jbc.273.29.18260. [DOI] [PubMed] [Google Scholar]
- 15.Gross M K, Au D C, Smith A L, Storm D R. Targeted mutations that ablate either the adenylate cyclase or hemolysin function of the bifunctional cyaA toxin of Bordetella pertussis abolish virulence. Proc Natl Acad Sci USA. 1992;89:4898–4902. doi: 10.1073/pnas.89.11.4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanski E, Farfel Z. Bordetella pertussis invasive adenylate cyclase: partial resolution and properties of its cellular penetration. J Biol Chem. 1985;290:5526–5532. [PubMed] [Google Scholar]
- 17.Iwaki M, Ullmann A, Šebo P. Identification by in vitro complementation of regions required for cell-invasive activity of Bordetella pertussis adenylate cyclase toxin. Mol Microbiol. 1995;17:1015–1024. doi: 10.1111/j.1365-2958.1995.mmi_17061015.x. [DOI] [PubMed] [Google Scholar]
- 18.Ladant D. Interaction of Bordetella pertussis adenylate cyclase with calmodulin: identification of two separated calmodulin-binding domains. J Biol Chem. 1988;263:2612–2618. [PubMed] [Google Scholar]
- 19.Ladant D, Michelson S, Sarfati R S, Gilles A-M, Predeleanu R, Bârzu O. Characterization of the calmodulin-binding and of the catalytic domains of Bordetella pertussis adenylate cyclase. J Biol Chem. 1989;264:4015–4020. [PubMed] [Google Scholar]
- 20.Masure H R, Oldenburg D J, Donovan M G, Shattuck R L, Storm D R. The interaction of Ca2+ with the calmodulin-sensitive adenylate cyclase from Bordetella pertussis. J Biol Chem. 1988;263:6933–6940. [PubMed] [Google Scholar]
- 21.Oldenburg D J, Storm D R. Identification of a domain in Bordetella pertussis adenylyl cyclase important for subunit interactions and cell invasion activity. Microb Pathog. 1993;15:153–157. doi: 10.1006/mpat.1993.1065. [DOI] [PubMed] [Google Scholar]
- 22.Osickova A, Osicka R, Maier E, Benz R, Šebo P. An amphipathic α-helix including glutamates 509 and 516 is crucial for membrane translocation of adenylate cyclase toxin and modulates formation and cation selectivity of its membrane channels. J Biol Chem. 1999;274:37644–37650. [PubMed] [Google Scholar]
- 23.Rogel A, Hanski E. Distinct steps in the penetration of adenylate cyclase toxin of Bordetella pertussis into sheep erythrocytes. Translocation of the toxin across the membrane. J Biol Chem. 1992;267:22599–22605. [PubMed] [Google Scholar]
- 24.Rogel A, Meller R, Hanski E. Adenylate cyclase toxin from Bordetella pertussis. The relationship between induction of cAMP and hemolysis. J Biol Chem. 1991;266:3154–3161. [PubMed] [Google Scholar]
- 25.Sakamoto H, Bellalou J, Sebo P, Ladant D. Bordetella pertussis adenylate cyclase toxin: structural and functional independence of the catalytic and hemolytic activities. J Biol Chem. 1992;267:13598–13602. [PubMed] [Google Scholar]
- 26.Šebo P, Glaser P, Sakamoto H, Ullmann A. High-level synthesis of active adenylate cyclase toxin of Bordetella pertussis in a reconstructed Escherichia coli system. Gene. 1991;104:19–24. doi: 10.1016/0378-1119(91)90459-o. [DOI] [PubMed] [Google Scholar]
- 27.Szabo G, Gray M C, Hewlett E L. Adenylate cyclase toxin from Bordetella pertussis produces ion conductance across artificial lipid bilayers in a calcium- and polarity-dependent manner. J Biol Chem. 1994;269:22496–22499. [PubMed] [Google Scholar]
- 28.Wolff J, Cook G H, Goldhammer A R, Berkowitz S A. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci USA. 1980;77:3841–3844. doi: 10.1073/pnas.77.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]