Abstract
Gingival fibroblasts produce proinflammatory cytokines in response to lipopolysaccharide (LPS) from periodontopathic bacteria. Recently it has become evident that the human homologue of Drosophila Toll can transduce intracellular signaling by LPS stimulation. Toll-like receptors (TLRs) have been identified in myeloid cells; however, their role in nonmyeloid cells such as gingival fibroblasts has not been fully elucidated. Here, we report that human gingival fibroblasts constitutively express TLR2 and TLR4 and that their levels of expression are increased by stimulation with LPS from Porphyromonas gingivalis. Upregulated expression of interleukin-6 gene and protein in fibroblasts stimulated with LPS is inhibited by anti-TLR4 antibody. These findings suggest that TLRs may confer responsiveness to LPS in gingival fibroblasts.
Gingival fibroblasts are the primary cell type present in the gingival connective tissue. In addition to maintaining gingival tissue integrity by regulating collagen and proteoglycan metabolism, they produce several proinflammatory cytokines such as interleukin-1 (IL-1), IL-6, and IL-8 in response to direct and indirect stimulations with lipopolysaccharide (LPS) from the major periodontopathic bacterium, Porphyromonas gingivalis (15, 17, 20). Hence, gingival fibroblasts are thought to play an important role in the pathogenesis of chronic inflammatory periodontal diseases.
LPS-induced signal transduction is thought to entail binding to specific cellular receptors, which trigger intracellular signaling cascades leading to activation of the transcription factor nuclear factor κB (NF-κB) (8). Generally, it is thought that myeloid cells primarily utilize membrane-bound CD14 (mCD14) whereas nonmyeloid cells utilize the soluble form of CD14 to bind LPS. However, CD14 lacks a transmembrane and cytoplasmic domain and is not believed to have intrinsic signaling capabilities. It is not likely that CD14 alone is responsible for directly transmitting a signal across the plasma membrane. However, the precise mechanisms by which LPS activates cells has not been elucidated (18).
Recent studies have implicated Toll receptor in LPS-mediated signaling. Toll receptor is the product of the toll gene, which controls dorsoventral pattern formation during the early embryonic development of Drosophila melanogaster (7). Toll is a type I transmembrane protein containing an extracellular domain with leucine-rich repeats and a cytoplasmic domain with sequence homology to the human IL-1 receptor. Since the first human homologue of Toll protein was cloned (9), six human Toll-like receptors (TLRs) have been identified (12, 16). Transfection of human embryonic kidney 293 cells with TLR2 confers on them the ability to respond to LPS with activation of NF-κB (21). Poltorak et al. (10) and Qureshi et al. (11) reported that LPS resistance in C3H/HeJ mice is mediated by a mutation in a gene coding for TLR4 (10). Together, TLR4 as well as TLR2 may represent the long-sought receptor for LPS.
Therefore, in this study, we investigated whether TLR2 and TLR4 are expressed and whether the molecules are involved in the production of proinflammatory cytokines in nonmyeloid (gingival) fibroblasts following LPS stimulation.
Human gingival fibroblasts were prepared from clinically normal gingival tissue obtained at extraction of a noninfected third molar from an 18-year-old female according to the standard method (14), with minor modification. Informed consent was obtained prior to inclusion in the study. The cells were maintained in 25 mM HEPES-buffered Dulbecco's modified Eagle's medium (GIBCO BRL, Gaithersburg, Md.) supplemented with 10% fetal calf serum (FCS), penicillin (100 U/ml), streptomycin (100 μg/ml), and 2 mM glutamine (complete medium) and used for the experiments at passages 5 to 8. For stimulation, cells adjusted to a concentration of 106/well in complete medium without FCS were incubated in Costar six-well culture plate (Corning Inc., Corning, N.Y.) with or without P. gingivalis LPS (1 μg/ml) for 4 and 24 h. To assess the effect of HTA125, a monoclonal antibody to human TLR4, the antibody (20 μg/ml) was added to the culture 1 h prior to LPS stimulation. P. gingivalis LPS was prepared as described previously (6). After incubation, the supernatants were removed, aliquoted, and stored at −80°C until analyzed for IL-6 levels by a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Endogen Inc., Woburn, Mass.). The cells were washed twice with phosphate-buffered saline and harvested by incubation with 0.25% trypsin-EDTA (GIBCO BRL) at 37°C for 5 min. Total RNA was separated by using Isogen (Nippon Gene, Tokyo, Japan) according to the manufacturer's instruction. The RNA samples were further purified by successive treatment with DNase I (GIBCO BRL), phenol-chloroform-isoamyl alcohol (GIBCO BRL), and ethanol sedimentation. The resultant RNA preparations were investigated for the expression of TLR2, TLR4, MD-2, CD14, and IL-6 by means of reverse transcription (RT)-PCR. The first-strand cDNA was synthesized using 200 U of Superscript II (GIBCO BRL) and 500 ng of oligo(dT)15 (Promega Co., Madison, Wis.) from 1.5 μg of total RNA in the reaction buffer, supplemented with 0.5 U of RNase inhibitor (GIBCO BRL), 0.01 M dithiothreitol, and deoxynucleoside triphosphate (each at 0.5 mM) in a total volume of 20 μl. The reaction mixture was incubated at 42°C for 50 min and then heated at 70°C for 15 min. PCR amplification of cDNA was performed using oligonucleotide primers specific for TLR2 (5′-GCCAAAGTCTTGATTGATTGG-3′ and 5′-TTGAAGTTCTCCAGCTCCTG-3′) (4), TLR4 (5′-TCCCTCCAGGTTCTTGATTA-3′ and 5′-GTAGTGAAGGCAGAGCTGAAA-3′), MD-2 (5′-TATTGGGTCTGCAACTCAT-3′ and 5′-CTCCCAGAAATAGCTTCAAC-3′), CD14 (5′-CTCAACCTAGAGCCGTTTCT-3′ and 5′-CAGGATTGTCAGACAGGTCT-3′) (14), and IL-6 (5′-GTGTTGCCTGCTGCCTTCCCTG-3′ and 5′-CTCTAGGTATACCTCAAACTCCAA-3′) (3). The primer sequences for TLR4 and MD-2 mRNA were determined based on the sequence data (GenBank accession no. U93091 and AB018549 [13], respectively) and by using Oligo primer analysis software (National Biosciences, Inc., Plymouth, Minn.). The reaction mixture prepared on ice contained 1× EXTaq buffer (Takara Shuzo Co., Ltd., Shiga, Japan), 0.2 mM each deoxynucleoside triphosphate, 0.42 μM each primer, 1.0 μl of cDNA, and 0.5 U of EXTaq DNA polymerase (Takara) in a total volume of 50 μl. The PCR was carried out using a DNA thermal cycler (PCR Thermal Cycler MP; Takara). The amplification cycle profile was as follows: for TLR2 and TLR4, denaturation at 94°C for 1 min, annealing at 58°C, and extension at 72°C for 1 min; for MD-2, denaturation at 94°C for 1 min, annealing at 55°C, and extension at 72°C for 1 min; and for CD14, denaturation at 94°C for 1 min, annealing at 58°C, and extension at 72°C for 1 min. The cycle profile for IL-6 consisted of denaturation at 94°C for 1 min followed by annealing and extension at 66°C for 1 min. The durations of denaturation at the first cycle and extension at the last cycle were extended for 3 min. After 30 cycles of amplification, 10 μl of each PCR product was electrophoresed on 2% agarose gel and visualized by ethidium bromide staining. To determine the amplification rate, the products of TLR2 and TLR4 were also sampled after 25, 27, and 33 cycles.
For immunohistochemistry, the cells at a concentration of 104/500 μl in FCS-depleted complete medium were seeded in Lab-Tek tissue culture chamber/slides (Miles Laboratories, Inc., Naperville, Ill.). On the next day, the cells were stimulated with or without P. gingivalis LPS (1 μg/ml) for 24 h. The slides were then fixed in equal parts of chloroform-acetone for 5 min. After washing in 0.05% Tris-buffered saline (pH 7.6) and blocking with normal horse serum (Vector Laboratories, Inc., Burlingame, Calif.), the slides were incubated with either anti-TLR4 (HTA125), MY4 (Coulter Co., Hialeah, Fla.), or anti-CD3 (Dako, Glostrup, Denmark) at predetermined dilutions followed by biotinylated goat anti-mouse immunoglobulin G (1:200; Vector) and finally with an avidin-biotin immunoperoxidase (ABC kit; Vector). The peroxidase was developed using 0.005% 3,3′-diaminobenzidine in Tris-HCl buffer (pH 7.2) containing 0.01% hydrogen peroxide.
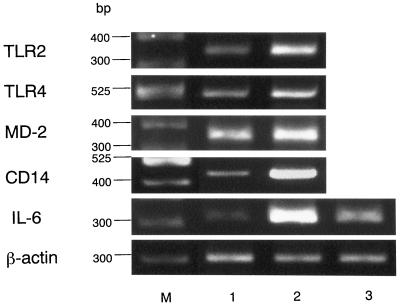
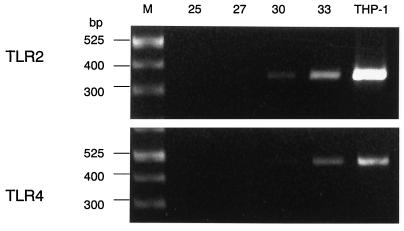
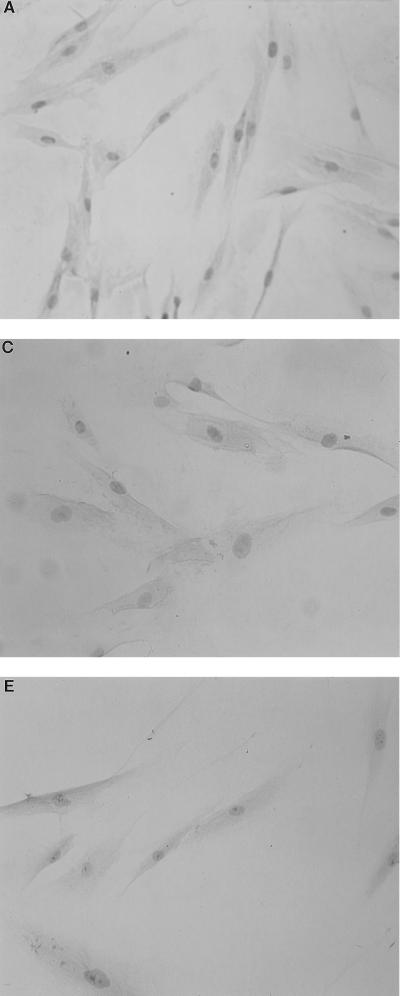
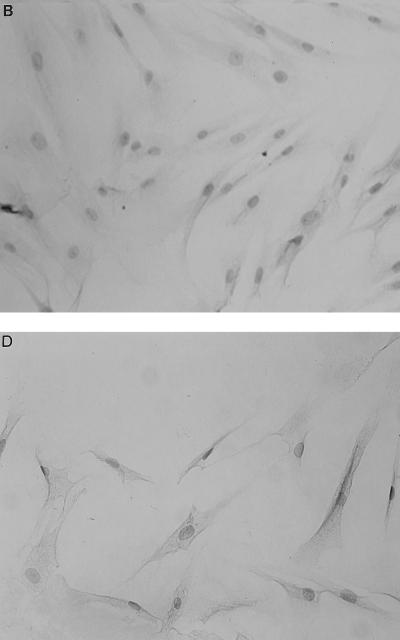
As shown in Fig. 1, gingival fibroblasts were found to constitutively express all of the mRNAs examined, and their expressions were clearly enhanced by LPS stimulation. This finding was particularly evident for CD14 and IL-6. In this regard, CD14 expression has been reported to be upregulated in the presence of LPS on myeloid as well as nonmyeloid cells (1). As RNA samples without RT did not give rise to any amplified products, it is concluded that the PCR products are in fact derived from expressed mRNA (data not shown). Whereas myeloid cells such as the myelomonocytic cell line THP-1 express TLR2 and TLR4 at the mRNA level (4, 13) and are reported to express them at the protein level (23), there has been no report of TLR expression in nonmyeloid cells. Expression of TLR2 appeared to be stronger than that of TLR4 in THP-1 cells, which is in accordance with the previous report (23); however, it was not evident for gingival fibroblasts even though the mRNA for TLR2 became visible at an earlier amplification cycle (Fig. 2). mRNA expression for TLR4 and CD14 in gingival fibroblasts was further confirmed by immunohistochemistry using monoclonal anti-human TLR4 antibody (HTA125) and anti-human CD14 (MY4), respectively (Fig. 3). In contrast to mRNA expression, cell surface expression of CD14 and TLR4 did not appear to be affected by LPS stimulation. HTA125 was generated by immunizing a mouse cell line expressing human TLR4 in BALB/c mice as described previously (13). This antibody recognizes TLR4 itself as well as coprecipitate MD-2, a molecule that is thought to confer LPS responsiveness to TLR4.
FIG. 1.
Effect of P. gingivalis LPS on expression of mRNAs for TLR2, TLR4, MD-2, CD14, and IL-6. Expression of TLR2 mRNA (347 bp), TLR4 mRNA (495 bp), MD-2 mRNA (359 bp), CD14 mRNA (426 bp), and IL-6 mRNA (321 bp) with (lane 2) or without (lane 1) LPS stimulation was analyzed by RT-PCR. Effect of the anti-TLR4 monoclonal antibody HTA125 on LPS stimulation was also analyzed (lane 3). β-Actin expression was used as a control. Sizes of molecular weight markers (M) are indicated.
FIG. 2.
Amplification profiles of TLR2 and TLR4 mRNAs. PCR products at various amplification cycles were analyzed. PCR products at 30 cycles of TLR2 and TLR4 in THP-1 were used as a control. Sizes of molecular weight markers (M) are indicated.
FIG. 3.
Immunoreactivity for TLR4 and CD14 in gingival fibroblasts. The cells cultured on Lab-Tek tissue culture slide were stimulated with (A and B) or without (C and D) P. gingivalis LPS for 24 h and subsequently stained for HTA125 (anti-TLR4; A and C) or MY4 (anti-CD14; B and D). Anti-CD3 was used as a negative control (E).
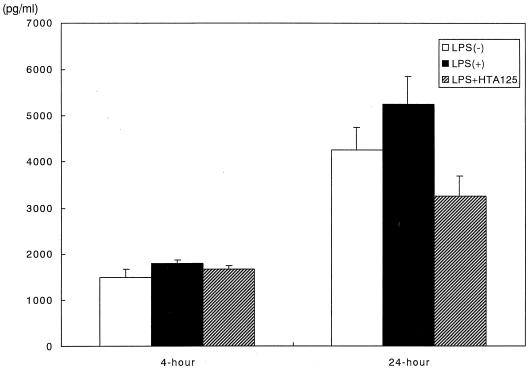
Upregulation of mRNA expression for IL-6 with LPS stimulation was significantly inhibited by the addition of HTA125 (Fig. 1). The effect of HTA125 on IL-6 production by gingival fibroblasts is rather complicated. Consistent with previous reports (3, 20), enhancement of IL-6 production by LPS stimulation was relatively weak irrespective of the culture period even though the longer stimulation showed more IL-6 production with or without stimulation. Interestingly, HTA125 strongly blocked the time course-dependent increase of basal IL-6 production, as measured by ELISA (Fig. 4). Although the reason for this inhibitory mechanism is unknown, TLR4 may play a complex regulatory role(s) in cytokine production mediated by a yet unknown function. Nevertheless, these findings, together with the fact that mRNA for CD14 was upregulated by LPS stimulation and MD-2 was expressed in gingival fibroblasts, suggest that the LPS-induced activation of gingival fibroblast is mediated at least in part by cell surface TLR4.
FIG. 4.
Effect of the monoclonal antibody HTA125 on the production of IL-6 by gingival fibroblasts stimulated with or without P. gingivalis LPS. The cells were incubated for 4 or 24 h. IL-6 levels in the culture supernatants were analyzed by ELISA. Data are expressed as mean ± standard deviation.
Several studies have demonstrated that P. gingivalis LPS enhances proinflammatory cytokine IL-1, IL-6, and IL-8 production in human gingival fibroblasts (15, 17, 20). Gene expression of these cytokines is regulated by the activation of NF-κB. Although the mechanism of the TLR-mediated LPS responsiveness has not been fully elucidated, both TLR2 and TLR4 have been shown to induce the activation of NF-κB via sequential activation of IRAK, TRAF6, and NIK (5).
Although TLRs are now believed to be critical signaling molecules mediating LPS responsiveness, CD14 is also important in LPS responsiveness. In the present study, we demonstrated that gingival fibroblasts expressed mCD14 (Fig. 3). The presence of mCD14 on gingival fibroblasts has been controversial. Whereas Watanabe et al. reported that mCD14 is expressed in gingival fibroblasts (19), Hayashi et al. failed to show mCD14 expression both at protein and mRNA levels (2). In this regard, heterogeneous expression of mCD14 has been demonstrated (14). A recent report has demonstrated that TLR2 associates with mCD14 and that LPS treatment enhances the oligomerization of TLR2, which leads to subsequent recruitment of IRAK (22). Furthermore, CD14 augments LPS responsiveness mediated by TLR2 (21). These reports further show the importance of mCD14 in LPS responsiveness.
Collectively, the available data suggest that gingival fibroblasts respond to periodontopathic bacterial LPS via TLR2 associated with mCD14 and TLR4–MD-2 complex, resulting in the production of proinflammatory cytokines, which in turn leads to periodontal tissue destruction. Although this study is the first to show that the expression of signaling molecules mediates LPS responsiveness in gingival fibroblasts, further study is obviously needed to clarify its complex regulatory role(s) in proinflammatory cytokine production.
Acknowledgments
We are indebted to Gregory J. Seymour (Oral Biology and Pathology, Department of Dentistry, The University of Queensland, Brisbane, Australia) for critical reading the manuscript.
This work was supported by grants from the Ministry of Education, Science Sports and Culture of Japan (10470458 and 10307054) and Special Grant for Development of Advanced Medical Technology.
REFERENCES
- 1.Fearns C, Kravchenko V V, Ulevitch R J, Loskutoff D J. Murine CD14 gene expression in vivo: extramyeloid synthesis and regulation by lipopolysaccharide. J Exp Med. 1995;181:857–866. doi: 10.1084/jem.181.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayashi J, Masaka T, Saito I, Ishikawa I. Soluble CD14 mediates lipopolysaccharide-induced intercellular adhesion molecule 1 expression in cultured human gingival fibroblasts. Infect Immun. 1996;64:4946–4951. doi: 10.1128/iai.64.12.4946-4951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kent L W, Rahemtulla F, Michalek S M. Interleukin (IL)-1 and Porphyromonas gingivalis lipopolysaccharide stimulation of IL-6 production by fibroblasts derived from healthy or periodontally diseased human gingival tissue. J Periodontol. 1999;70:274–282. doi: 10.1902/jop.1999.70.3.274. [DOI] [PubMed] [Google Scholar]
- 4.Kirschning C J, Wesche H, Ayres T M, Rothe M. Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopp E B, Medzhitov R. The toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–18. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 6.Kumada H, Watanabe K, Umemoto T, Haishima Y, Kondo S, Hisatsune K. Occurrence of O-phosphorylated 2-keto-3-deoxyoctanate in the lipopolysaccharide of Bacteroides gingivalis. FEMS Microbiol Lett. 1988;51:77–80. [Google Scholar]
- 7.Lemaitre B, Nicolas E, Michaut L, Reichhart J M, Hoffman J A. The Dorsoventral regulatory gene cassette spatzel/Toll/cactus controls the potent antifungal response in drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 8.May M J, Ghosh S. Signal transduction through NF-kB. Immunol Today. 1998;19:80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- 9.Medzhitov R, Preston-Hurlburt P, Janeway C A., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 10.Poltorak A, He X, Smirnova I, Liu M-Y, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in tlr4 gene. Science. 1998;181:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 11.Qureshi S T, Lariviere L, Leveque G, Clermont S, Moore K J, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rock F L, Hardiman G, Timans J C, Kastelein R A, Bazan J F. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugawara S, Sugiyama A, Nemoto E, Rikiishi H, Takada H. Heterogeneous expression and release of CD14 by human gingival fibroblasts: characterization and CD14-mediated interleukin-8 secretion in response to lipopolysaccharide. Infect Immun. 1998;66:3043–3049. doi: 10.1128/iai.66.7.3043-3049.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takada H, Mihara J, Morisaki I, Hamada S. Induction of interleukin-1 and -6 in human gingival fibroblast cultures stimulated with Bacteroides lipopolysaccharides. Infect Immun. 1991;59:295–301. doi: 10.1128/iai.59.1.295-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeuchi O, Kawai T, Sanjo H, Copeland N G, Gilbert D J, Jenkins N A, Takeda K, Akira S. TLR6: a novel member of an expanding Toll-like receptor family. Gene. 1999;231:59–65. doi: 10.1016/s0378-1119(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 17.Tamura M, Tokuda M, Nagaoka S, Takada H. Lipopolysaccharide of Bacteroides intermedius (Prevotella intermedia) and Bacteroides (Porphyromonas) gingivalis induce interleukin-8 gene expression in human gingival fibroblast cultures. Infect Immun. 1992;60:4932–4937. doi: 10.1128/iai.60.11.4932-4937.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe A, Takeshita A, Kitano S, Hanazawa S. CD14-mediated signal pathway of Porphyromonas gingivalis lipopolysaccharide in human gingival fibroblasts. Infect Immun. 1996;64:4488–4494. doi: 10.1128/iai.64.11.4488-4494.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamazaki K, Ikarashi F, Aoyagi T, Takahashi K, Nakajima T, Hara K, Seymour G J. Direct and indirect effects of Porphyromonas gingivalis lipopolysaccharide on interleukin-6 production by human gingival fibroblasts. Oral Microbiol Immunol. 1992;7:218–224. doi: 10.1111/j.1399-302x.1992.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 21.Yang R-B, Mark M R, Gray A, Huang A, Xie M H, Zhang M, Goddard A, Wood W I, Gurney A L, Godowski P J. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signaling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 22.Yang R-B, Mark M R, Gurney A L, Godowski P J. Signaling events induced by lipopolysaccharide-activated Toll-like receptor. J Immunol. 1999;163:639–643. [PubMed] [Google Scholar]
- 23.Zhang F X, Kirschning C J, Mancinelli R, Xu X-P, Jin Y, Faure E, Mantovani A, Rothes M, Muzio M, Arditi M. Bacterial lipopolysaccharide activates nuclear factor κB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999;274:7611–7614. doi: 10.1074/jbc.274.12.7611. [DOI] [PubMed] [Google Scholar]