Abstract
Objective.
To demonstrate the effects of rituximab (RTX) in patients with rheumatoid arthritis-related interstitial lung disease (RA-ILD).
Methods.
A total of 165 patients who used RTX for the management of rheumatoid arthritis were retrospectively scrutinised. Among these, 26 patients diagnosed with RA-ILD were analysed (61.5% male, mean age at RTX infusion 61.4 ± 6.5 years). To evaluate the efficacy of RTX on lung response, patients with pulmonary function test results and/or thorax computed tomography (chest-CT) of pre- and post-RTX were compared. Disease progression was defined as either a decline of ≥10% in forced vital capacity (FVC) and/or a decline of ≥15% in diffusion capacity of carbon monoxide (DLCO), or an increase of parenchymal involvement on chest-CT images according to the radiologists’ assessment.
Results.
Among 26 patients, the most common radiologic pattern was usual interstitial pneumonia (42.3%), followed by non-specific interstitial pneumonia (38.5%). Data for lung response was available in 20 patients. Median pre- and post- RTX DLCO values were 71.0% (60.0-77.0) and 63.0% (47.0-74.0), respectively (p= 0.06). Median pre- and post-RTX FVC values were 74.0% (61.0-99.0) and 84.0% (63.0-100.0), respectively (p= 0.28). Overall, stabilization or regression of RA-ILD was provided in 13 (65.0%) patients, whereas 7 patients had progressive RA-ILD. Post-RTX, 5 patients were diagnosed with RA-ILD.
Conclusion.
Our results suggest that RTX is effective in achieving stabilization or even improvement of RA-ILD. However, considering that it does not cause regression in every patient and some develop RA-ILD under RTX, we still need more effective treatment options.
Keywords: Rheumatoid arthritis, interstitial lung disease, progression, rituximab
Supplementary Table 1.
Characteristics of 20 patients whose pre- and post-RTX data were available
| Male sex, n (%) | 12 (60.0) |
| Age at first RTX infusion, mean (SD), years | 61.5 (5.8) |
| RA disease duration at first RTX, median (Q1-Q3), years | 12.2 (4.4-19.8) |
| ILD disease duration at first RTX, median (Q1-Q3), years | 2.6 (0.1-5.2) |
| Age at RA diagnosis, mean (SD), years | 48.6 (13.2) |
| Age at ILD diagnosis, mean (SD), years | 58.1 (7.0) |
| RF positivity, n (%) | 19 (95.0) |
| ACPA positivity*, n (%) | 15 (88.2) |
| ANA positivity†, n (%) | 9 (47.4) |
| Higher CRP (> 5 mg/L), n (%) | 14 (70.0) |
| CRP levels,mg/L, mean (SD) | 40.3 (58.9) |
| Higher ESR (>20 mm/hour), n (%) | 16 (80.0) |
| ESR levels, mean (SD) | 37.7 (18.2) |
| Smoking status, n (%) | |
| Never | 6 (30.0) |
| Ex-smoker | 12 (60.0) |
| Current | 2 (10.0) |
| Secondary Sjögren’s syndrome, n (%) | 1 (5.0) |
| Prior anti-TNF treatment, n (%) | 4 (20.0) |
| Concomitant DMARDs | |
| Methothrexate | 1 (5.0) |
| Azathioprine | 1 (5.0) |
| Leflunomide | 9 (45.0) |
| Mycophenolate mofetil | 2 (10.0) |
| Steroids | 18 (90.0) |
| DAS28 at first RTX infusion, mean (SD) | 5.1 (1.5) |
| Radiographic pattern, n (%) | |
| Usual interstitial pneumonia | 10 (50.0) |
| Non-specific interstitial pneumonia | 7 (35.0) |
| Organizing pneumonia | 2 (10.0) |
| Indeterminate | 1 (5.0) |
| Median pre-RTX DLCO ¥ , %, (Q1-Q3) | 71 (54-79) |
| Median pre-RTX FVC § , %, (Q1-Q3) | 79 (64-100) |
| Follow-up duration, median (Q1-Q3), years | 2.7 (2.1-3.8) |
RA= rheumatoid arthritis, ILD= interstitial lung disease, RTX= rituximab, RF= romatoid factor, ACPA= anti-citrullinated protein antibody, CRP= C reactive protein, ESR= erythrocyte sedimentation rate, anti-TNF= anti tumor necrosis factor, DMARDs= disease-modifying antirheumatic drugs, DAS28= disease activity score 28, DLCO= diffusion capacity of carbon monoxide, FVC= forced vital capacity
*Data of 17 patients were available, †Data of 19 patients were available, ¥Data of 15 patients were available, §Data of 13 patients were available
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory rheumatic disease which mainly affects small joints of the hands along with extra-articular manifestations (1). Lungs are one of the most common extra-articular involvement sites of RA (2). Lung involvement might occur in different forms, and is reported to be seen in 7.7% of RA patients (3). Interstitial lung disease (ILD) is the most common type of lung involvement in RA, and it is mostly detected within the first years of RA diagnosis (3-6). Older age at RA diagnosis, male sex and severe RA activity is associated with the development of RA-ILD (7, 8).
In contrast to other sites primarily affected by RA, the existence of RA-ILD is associated with worse survival (5, 8). Older age, radiologically excessive involvement at RA-ILD diagnosis and usual interstitial pneumonia (UIP) pattern are related to mortality (6, 9).
The introduction of more effective treatment options has resulted in less destructive articular disease. However, there is no mainstay treatment in the case of RA-ILD, as patients with RA-ILD are excluded from clinical trials. Nevertheless, recent data suggest that rituximab (RTX) might be an effective agent to prevent the progression of RA-ILD (10-12). In this observational retrospective study, we aimed to determine the effects of RTX in patients with RA-ILD from a single centre.
Methods
Patient selection and treatment
A retrospective chart review was conducted of patients with RA treated with RTX between January 2010 and December 2020 in our outpatient clinic. A total of 165 patients were using RTX for their RA diagnosis. Among them, 26 (15.8%) patients with RA-ILD were analysed. All the patients included in the study met 2010 ACR/EULAR criteria for RA (13). RA-ILD was diagnosed by computed tomography of the chest (chest-CT). RTX was applied on day 1 and 14 with a dosage of 1000 mg every 6 months, along with 40 mg methylprednisolone.
Data collection
Chest-CT images of patients with RA-ILD were re-evaluated by two experienced radiologists (A.C. and C.U.). The date of RA-ILD diagnosis was considered as the date of the first chest-CT detecting pathological changes related to ILD. RA-ILD was subclassified as UIP, non-specific interstitial pneumonia (NSIP), organizing pneumonia (OP), and indeterminate interstitial lung disease in compliance with the American Thoracic Society – European Respiratory Society classification (14). The severity of RA-ILD was determined by visual volumetric assessment. The presence/absence of multiple features of fibrosis such as architectural distortion, reticulation, honeycombing, and traction bronchiectasis/bronchiolectasis was considered when examining CT images. Volumes of all parenchymal abnormalities (ground-glass opacity, consolidation, reticular pattern, and honeycombing) were roughly converted into a percentage of the total lung volume based on density changes. Limited, moderate and extensive disease on chest-CT was defined as the involvement <10%, 10-30% and >30% of lung tissue, respectively. The patient’s demographic, clinical, laboratory and treatment characteristics with disease-modifying antirheumatic drugs (DMARDs) were also considered. The results of pulmonary function tests (PFTs) were also recorded, when available.
To assess lung response to RTX, we primarily compared PFTs in the 6 to 12 months prior to RTX with PFTs in the 6 to 12 months following RTX or, preferably with PFTs in the most recent follow-up. Disease progression was defined as either a decline of ≥10% in forced vital capacity (FVC) and/or a decline of ≥15% in diffusion capacity of carbon monoxide (DLCO). Disease improvement was defined as either an increase ≥10% in FVC and/or an increase of ≥15% in DLCO. Others who did not meet criteria for improvement or progression were classified as having stable lung response. In addition, when PFTs were not available, the percentage obtained by visual volumetric assessment was integrated into lung response so as to endorse data provided by PFTs via using the similar time points with PFTs. An increase/decrease in percentage was considered disease progression/improvement as well.
Follow-up duration was calculated by using the date on which RTX was first applied and the date on which last visit or death occurred. Any side effect which might be related to RTX infusion was recorded.
Statistical analysis
Data are shown in numbers and percentages for categorical variables. Quantitative variables are shown as median and 25th and 75th percentile (Q1-Q3) or as mean and standard deviation (SD). Comparisons of categorical variables were performed using the chi-square test or Fischer’s exact test. To demonstrate the differences in PFTs pre- and post- RTX, Wilcoxon rank test was performed. Independent sample t-test, or Mann-Whitney U test as a nonparametric substitute, were used to analyse the differences between the groups in which lung response was evaluated. All tests were 2-sided at the 0.05 significance level. Analyses were performed by using the SPSS software version 22.
Results
The frequency of RA-ILD was 15.8% among 165 patients who were initiated on RTX. Of 26 patients, 16 (61.5%) patients were male. Ages at RA and ILD diagnosis were 48.9 ± 14.0 and 58.4 ± 7.3 years, respectively. At the first RTX infusion, mean age of patients was 61.4 ± 6.5 years and median RA-ILD disease duration was 1.9 (0.1-4.5) years. Overall, 18 (69.2%) patients had erosive disease. Rheumatoid nodules of skin and/or lungs were documented in 14 (53.8%) patients. All patients, but one, were positive for rheumatoid factor. Two (7.7%) patients had secondary Sjögren’s syndrome. The characteristics of 26 patients are illustrated in Table 1.
Table 1.
Characteristics of patients with RA-ILD treated with RTX
| Male sex, n (%) | 16 (61.5) |
| Age at first RTX infusion, mean (SD), years | 61.4 (6.5) |
| RA disease duration at first RTX, median (Q1-Q3), years | 10.1 (4.3-29.7) |
| ILD disease duration at first RTX, median (Q1-Q3), years | 1.9 (0.1-4.5) |
| Age at RA diagnosis, mean (SD), years | 48.9 (14.0) |
| Age at ILD diagnosis, mean (SD), years | 58.4 (7.3) |
| RF positivity | 25 (96.2) |
| ACPA positivity* | 20 (90.9) |
| ANA positivity† | 11 (42.3) |
| Higher CRP (> 5 mg/L), n (%) | 19 (73.1) |
| CRP levels,mg/L, mean (SD) | 35.7 (52.9) |
| Higher ESR (>20 mm/hour), n (%) | 21 (80.8) |
| ESR levels, mean (SD) | 39.4 (18.0) |
| Smoking status‡, n (%) | 16 (64.0) |
| Never | 9 (37.5) |
| Ex-smoker | 11 (45.8) |
| Current | 4 (16.7) |
| Secondary Sjögren’s syndrome, n (%) | 2 (7.7) |
| Prior anti-TNF treatment, n (%) | 4 (15.4) |
| Concomitant DMARDs | |
| Methothrexate | 3 (11.5) |
| Azathioprine | 1 (3.8) |
| Leflunomide | 12 (46.2) |
| Mycophenolate mofetil | 3 (11.5) |
| Steroids | 24 (92.3) |
| DAS28 at first RTX infusion, mean (SD) | 5.2 (1.5) |
| Radiographic pattern, n (%) | |
| Usual interstitial pneumonia | 11 (42.3) |
| Non-specific interstitial pneumonia | 10 (38.5) |
| Organizing pneumonia | 2 (7.7) |
| Indeterminate | 3 (11.5) |
| Median pre-RTX DLCO ¥ , %, (Q1-Q3) | 71.5 (51.0-79.8) |
| Median pre-RTX FVC § , %, (Q1-Q3) | 76.5 (61.0-97.5) |
| Follow-up duration, median (Q1-Q3), years | 2.5 (1.2-3.7) |
RA= rheumatoid arthritis, ILD= interstitial lung disease, RTX= rituximab, RF= romatoid factor, ACPA= anti-citrullinated protein antibody, CRP= C reactive protein, ESR= erythrocyte sedimentation rate, anti-TNF= anti tumor necrosis factor, DMARDs= disease-modifying antirheumatic drugs, DAS28= disease activity score 28, DLCO= diffusion capacity of carbon monoxide, FVC= forced vital capacity *Data of 22 patients were available, †Data of 24 patients were available, ‡Data of 25 patients were available, ¥Data of 18 patients were available, §Data of 16 patients were available
Six patients (23.1%) received 7 biological or targeted synthetic DMARDs prior to RTX (infliximab= 3, adalimumab= 1, etanercept= 1, tofacitinib= 2). In 20 patients (76.9%), RTX was the first biological agent selected. RTX treatment was initiated either due to articular symptoms in 12 patients (46.2%), or due to newly diagnosed RA-ILD or worsening of existent ILD in 12 patients (46.2%), or due to both articular symptoms and RA-ILD in 2 patients (7.7%). The most common interstitial pattern detected on chest-CT was UIP in 11 patients (42.3%), followed by NSIP in 10 (38.5%), indeterminate in 3 (11.5%), and OP in 2 (7.7%). Pre-RTX chest-CT images were available for 24 (92.3%) patients. Limited disease extent was observed in 11 (45.8%) patients, whereas moderate or extensive disease was seen in 9 (37.5%) and 4 (16.7%) patients, respectively.
A total of 121 cycles of RTX was applied to the patients (median 4.5 (2.0-6.0)). In the second cycle of RTX, there was a significant reduction in DAS28 scores (pre-RTX 5.1 vs. post-RTX 3.4, p<0.001). In the entire cohort, the most commonly used concomitant conventional synthetic DMARDs were leflunomide (46.2%), followed by mycophenolate mofetil (11.5%), methotrexate (11.5%) and azathioprine (3.8%). Median glucocorticoid dosage was 10.0 mg/day (5.0-16.25) at the initiation of RTX, whereas it was 5.0 mg/day (5.0-10.0) at the last RTX infusion (p= 0.012). Twenty-four (92.3%) patients continued using steroids during follow-up.
Lung response to RTX
For the evaluation of lung response, 20 patients who had follow-up PFTs and/or chest-CT were compared. The detailed data of these 20 patients showed in supplementary Table 1. Twelve patients had pre- and post PFT results available. Median DLCO values for pre- and post- RTX were 71.0% (60.0-77.0) and 63.0% (47.0-74.0), respectively (p= 0.06). Median pre- and post-RTX FVC values were 74.0% (61.0-99.0) and 84.0% (63.0-100.0), respectively (p= 0.28). Extensive disease on chest-CT was detected in 3 patients prior to RTX, whereas 4 patients had extensive disease at the last visit. The features on chest-CT scans are shown in Table 2. After a median of 2.4 years, 11 (55.0%) patients had stable disease, 2 (10.0%) patients had improvement of RA-ILD (Figure 1) and 7 (35.0%) patients had progressive disease. Among those with progressive disease, either UIP pattern (n= 4) or NSIP pattern (n= 3) were recorded. Sex, age and radiographic patterns were not associated with progression (Table 3). There was a weak correlation between articular response and overall lung response (r= 0.28, p= 0.258). Among 20 patients, only 1 patient with progressive disease died of respiratory failure related to lung disease.
Table 2.
Pre- and post- RTX chest-CT images’ findings of 20 patients analysed
| Pre-RTX | Post-RTX | |
|---|---|---|
| Irregular reticulation | 20 (100.0) | 20 (100.0) |
| Honeycombing | 11 (55.0) | 11 (55.0) |
| Traction bronchiectasis | 17 (85.0) | 17 (85.0) |
| Ground glass opacities | 20 (100.0) | 20 (100.0) |
| Emphysema | 12 (60.0) | 12 (60.0) |
| Pleural effusion | 2 (10.0) | 3 (15.0) |
| Bronchiectasis (tubular/cystic) | 2 (10.0) | 2 (10.0) |
| Extensive disease | 3 (15.0) | 4 (20.0) |
RTX= rituximab, chest-CT= computed tomography of the chest
Figure 1.
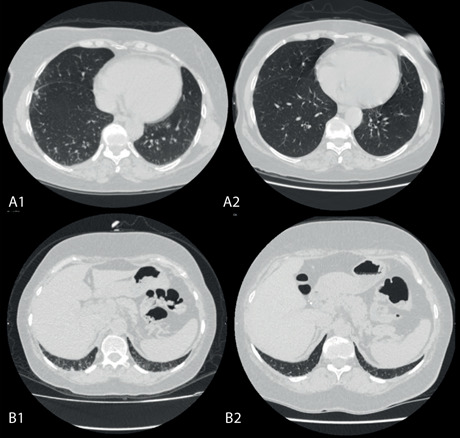
CT images of two separate patients (A-B) obtained at mid-lung and basal levels show visual improvement of subpleural reticulations in a 1-year interval. A1 and B1 images show the involvement of lung tissue pre-RTX, A2 and B2 images illustrate the involvement of lung tissue post-RTX.
Table 3.
The clinical features of the patients with data for lung response assessment
| Progressed n= 7 (35%) | Not progressed* n= 13 (65%) | |
|---|---|---|
| Male sex, n (%) | 5 (71.4) | 7 (53.8) |
| Age at RA diagnosis, mean (SD), years | 46.1 (15.9) | 49.9 (11.9) |
| Age at ILD diagnosis, mean (SD), years | 57.1 (9.9) | 58.7 (5.2) |
| Age at first RTX infusion, years, mean (SD) | 61.5 (7.8) | 61.4 (4.8) |
| ILD duration at first RTX infusion, years, median (Q1-Q3) | 4.2 (0.06-7.03) | 1.6 (0.06-3.98) |
| Smoking, n (%) | 7 (100.0) | 7 (53.8) |
| Never | 0 (0.0) | 6 (46.2) |
| Ex-smoker | 7 (100.0) | 5 (38.5) |
| Current | 0 (0.0) | 2 (15.4) |
| Pre-RTX progression, n (%) | 2 (28.6) | 3 (23.1) |
| Pre-RTX extensive disease ‡ , n (%) | 0 (0.0) | 3 (23.1) |
| Pre-RTX moderate disease ‡ , n (%) | 4 (57.1) | 4 (30.8) |
| Pre- and post-RTX PFT availability, n (%) | 6 (85.7) | 6 (46.2) |
| Pre-RTX FVC, %, median (Q1-Q3) | 74 (64-90) | 82 (63-109) |
| Pre-RTX DLCO, %, median (Q1-Q3) | 71 (65-74) | 68 (48-77) |
| Pre-RTX DLCO ≤ 60%, n (%) | 1 (16.7) | 4 (44.4) |
| DAS28 at first RTX infusion, mean (SD) | 4.6 (1.5) | 5.3 (1.5) |
| Erosive disease, n (%) | 5 (71.4) | 8 (61.5) |
| Subcutaneous rheumatoid nodules n (%) | 2 (28.6) | 6 (46.2) |
| UIP pattern, n (%) | 4 (57.1) | 6 (46.2) |
| Emphysema, n (%) | 5 (71.4) | 7 (53.8) |
| Bronchiectasis (tubular/cystic), n (%) | 1 (14.3) | 0 (0.0) |
| Death, n (%) | 1 (14.3) † | 0 (0.0) |
RA= rheumatoid arthritis, ILD= interstitial lung disease, RTX= rituximab, PFT= pulmonary function test, FVC= forced vital capacity, DLCO= diffusion capacity of carbon monoxide, DAS28= disease activity score 28, UIP= usual interstitial pneumonia
*This group includes the patients with stabilization or improvement of RA-ILD after the usage of RTX.
‡According to the HRCT extension
†This patient died of respiratory failure.
In the remaining 6 patients whose data were incomplete for lung response assessment, no adverse affects were detected related to RTX and RTX was continued during the follow-up. After a median duration of 1.7 years (0.24-4.13), 2 out of 6 patients died of cardiac events.
Safety and adverse events
Among 165 patients, 5 (3%) patients were diagnosed with RA-ILD during RTX treatment. All 5 patients did not have any symptoms and/or radiological changes related to ILD before RTX initiation. One out of 5 patients died of viral pneumonia and the rest continued RTX without a progression of their RA-ILD.
Considering 26 patients treated with RTX for their RA-ILD, 23 adverse events were detected in 11 (42.3%) patients. Five of them were serious infections requiring hospitalization, mostly pneumonia. RTX was stopped in 4 (15.4%) patients due to side-effects: infections secondary to hypogammaglobulinaemia (2), malignancy (1) and allergic reaction (1).
During a median follow-up of 2.5 (1.2-3.7) years, 3 (11.5%) exitus were noted among 26 patients. The causes of death were respiratory failure related to ILD in 1 patient and atherosclerotic heart disease in 2 patients.
Discussion
The exact mechanism by which RA-ILD occurs is poorly understood. There are several hypotheses suggesting that lungs are the first part of autoimmunity in RA development (15, 16). It was documented that higher articular disease activity increases the risk of RA-ILD development, which clinically supports the interaction between joints and lungs (8, 17). As well as active articular disease, male gender, seropositivity, older age, and smoking are also shown to be associated with RA-ILD development (6, 18). Even though our cohort does not reflect all patients with RA-ILD, most patients were male and seropositive in our study group, and more than 60% of them were smokers.
The usage of methotrexate has been controversial in the setting of RA-ILD. Previous recommendations did not support the usage of methotrexate when RA-ILD was evident, even though it is considered to be the anchor drug for RA treatment (19). However, the most recent American College of Rheumatology guideline for RA treatment conditionally recommends methotrexate usage in the existence of mild or asymptomatic RA-ILD (20). Nevertheless, the percentage of methotrexate users in our cohort was very low (11.5% in the last visit), but no respiratory adverse event was detected during follow-up related to methotrexate.
RA-ILD is a progressive disease in which up to 50% of patients deteriorate within the first two years of the disease (12, 21). Extensive lung involvement and low basal DLCO portend disease progression (21). However, due to the lack of clinical trials in the setting of RA-ILD and concerns about aggravation of the disease with conventional or biological DMARDS, there is no standard care for patients with RA-ILD. The demonstration of follicular B-cell hyperplasia in the lung tissue of patients with RA-ILD has paved the way for the usage of RTX in RA-ILD (22). Clinically, data regarding RTX efficacy for ILD mostly come from scleroderma trials. In some studies conducted in patients with connective tissue disease-associated ILD, which comprise RA patients as well, the efficacy of RTX on functional status was demonstrated, especially in patients with a NSIP pattern and even in severely affected patients (23, 24). In cases with RA-ILD, RTX seems to stabilize or even ameliorate functional status of patients (3, 10, 12). In addition, it was shown that the overall mortality rate is lower in RA-ILD patients using RTX as their first biological agent than those using anti-TNF agents (11, 25). Besides, RTX decreases respiratory infection rates and results in better survival rates compared to anti-TNF agents in patients with RA-related bronchiectasis (26). These reports support the benefit of RTX in RA-related lung diseases. In favour of these results, in this study, more than half of the patients showed sustainable stable or improved lung response to RTX, which might also be justified by the presence of many patients with mild RA-ILD. However, RTX caused stabilization of the disease in 4 patients with radiologically extensive involvement and/or with a DLCO less than 40%, who were supposed to have poor prognosis.
In this study, an additional 5 patients developed RA-ILD after the initiation of RTX. In the literature, the development of ILD during RTX treatment was also reported (3, 10).
There are some limitations to the current study. Firstly, due to its retrospective nature, treatment decisions for RA-ILD patients were not standardized which might cause selection bias. Secondly, we did not have follow-up data for 6 patients about the evaluation of lung response, which might affect the study results. Lastly, the limited number of the patients prevented us from doing further analyses.
Conclusion
In this single-centre, observational, retrospective study, we presented our rituximab experience in RA-ILD patients. Our results suggest that RTX might stabilize or even improve RA-ILD with fewer adverse events. However, given that RA-ILD may develop during RTX treatment, it is not a panacea; thus, we need more effective treatment options.
Declarations:
We obtained approval from the ethics committee of Ankara University (approval number: I1-50-21). The study was conducted in accordance with the Declaration of Helsinki.
Conflict of interests:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
Authors’ contribution:
DSE, MT and AA designed the study; MEY, SS, TMT and GK did literature search; DSE, SB, MEY, SS and OOK collected the data; AC and CU re-evaluated the images; AB and OOK evaluated the PFTs results; MT, DSE and AC did statistical analyses; DSE and MT wrote the paper; all authors reviewed and approved the final version of the paper.
References
- 1.Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. doi: 10.1038/nrdp.2018.1. [DOI] [PubMed] [Google Scholar]
- 2.Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis. 2003;62(8):722–7. doi: 10.1136/ard.62.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duarte AC, Porter JC, Leandro MJ. The lung in a cohort of rheumatoid arthritis patients-an overview of different types of involvement and treatment. Rheumatology (Oxford) 2019;58(11):2031–8. doi: 10.1093/rheumatology/kez177. [DOI] [PubMed] [Google Scholar]
- 4.Huang S, Doyle TJ, Hammer MM, Byrne SC, Huang W, Marshall AA, et al. Rheumatoid arthritis-related lung disease detected on clinical chest computed tomography imaging: Prevalence, risk factors, and impact on mortality. Semin Arthritis Rheum. 2020;50(6):1216–25. doi: 10.1016/j.semarthrit.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyldgaard C, Hilberg O, Pedersen AB, Ulrichsen SP, L⊘kke A, Bendstrup E, et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis. 2017;76(10):1700–6. doi: 10.1136/annrheumdis-2017-211138. [DOI] [PubMed] [Google Scholar]
- 6.Kelly CA, Saravanan V, Nisar M, Arthanari S, Woodhead FA, Price-Forbes AN, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics--a large multicentre UK study. Rheumatology (Oxford) 2014;53(9):1676–82. doi: 10.1093/rheumatology/keu165. [DOI] [PubMed] [Google Scholar]
- 7.Koduri G, Norton S, Young A, Cox N, Davies P, Devlin J, et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology (Oxford) 2010;49(8):1483–9. doi: 10.1093/rheumatology/keq035. [DOI] [PubMed] [Google Scholar]
- 8.Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62(6):1583–91. doi: 10.1002/art.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Liu R, Zhang Y, Zhou J, Li Y, Xu Y, et al. A retrospective study on the predictive implications of clinical characteristics and therapeutic management in patients with rheumatoid arthritis-associated interstitial lung disease. Clinical rheumatology. 2020;39(5):1457–70. doi: 10.1007/s10067-019-04846-1. [DOI] [PubMed] [Google Scholar]
- 10.Md Yusof MY, Kabia A, Darby M, Lettieri G, Beirne P, Vital EM, et al. Effect of rituximab on the progression of rheumatoid arthritis-related interstitial lung disease: 10 years’ experience at a single centre. Rheumatology (Oxford) 2017;56(8):1348–57. doi: 10.1093/rheumatology/kex072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly CA, Nisar M, Arthanari S, Carty S, Woodhead FA, Price-Forbes A, et al. Rheumatoid arthritis related interstitial lung disease - improving outcomes over 25 years: a large multicentre UK study. Rheumatology (Oxford) 2020 doi: 10.1093/rheumatology/keaa577. [DOI] [PubMed] [Google Scholar]
- 12.Vadillo C, Nieto MA, Romero-Bueno F, Leon L, Sanchez-Pernaute O, Rodriguez-Nieto MJ, et al. Efficacy of rituximab in slowing down progression of rheumatoid arthritis-related interstitial lung disease: data from the NEREA Registry. Rheumatology (Oxford) 2020;59(8):2099–108. doi: 10.1093/rheumatology/kez673. [DOI] [PubMed] [Google Scholar]
- 13.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 14.Sverzellati N, Lynch DA, Hansell DM, Johkoh T, King TE, Jr, Travis WD. American Thoracic Society-European Respiratory Society Classification of the Idiopathic Interstitial Pneumonias: Advances in Knowledge since 2002. Radiographics. 2015;35(7):1849–71. doi: 10.1148/rg.2015140334. [DOI] [PubMed] [Google Scholar]
- 15.Demoruelle MK, Weisman MH, Simonian PL, Lynch DA, Sachs PB, Pedraza IF, et al. Brief report: airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis Rheum. 2012;64(6):1756–61. doi: 10.1002/art.34344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scher JU, Joshua V, Artacho A, Abdollahi-Roodsaz S, Öckinger J, Kullberg S, et al. The lung microbiota in early rheumatoid arthritis and autoimmunity. Microbiome. 2016;4(1):60. doi: 10.1186/s40168-016-0206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sparks JA, He X, Huang J, Fletcher EA, Zaccardelli A, Friedlander HM, et al. Rheumatoid Arthritis Disease Activity Predicting Incident Clinically Apparent Rheumatoid Arthritis-Associated Interstitial Lung Disease: A Prospective Cohort Study. Arthritis Rheumatol. 2019;71(9):1472–82. doi: 10.1002/art.40904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Liu R, Zhang Y, Zhou J, Li Y, Xu Y, et al. A retrospective study on the predictive implications of clinical characteristics and therapeutic management in patients with rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol. 2020;39(5):1457–70. doi: 10.1007/s10067-019-04846-1. [DOI] [PubMed] [Google Scholar]
- 19.Ledingham J, Gullick N, Irving K, Gorodkin R, Aris M, Burke J, et al. BSR and BHPR guideline for the prescription and monitoring of non-biologic disease-modifying anti-rheumatic drugs. Rheumatology (Oxford) 2017;56(6):865–8. doi: 10.1093/rheumatology/kew479. [DOI] [PubMed] [Google Scholar]
- 20.Fraenkel L, Bathon JM, England BR, St Clair EW, Arayssi T, Carandang K, et al. American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2021;73(7):1108–23. doi: 10.1002/art.41752. [DOI] [PubMed] [Google Scholar]
- 21.Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR. Predictors of progression of HRCT diagnosed fibrosing alveolitis in patients with rheumatoid arthritis. Ann Rheum Dis. 2002;61(6):517–21. doi: 10.1136/ard.61.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkins SR, Turesson C, Myers JL, Tazelaar HD, Ryu JH, Matteson EL, et al. Morphologic and quantitative assessment of CD20+ B cell infiltrates in rheumatoid arthritis-associated nonspecific interstitial pneumonia and usual interstitial pneumonia. Arthritis Rheum. 2006;54(2):635–41. doi: 10.1002/art.21758. [DOI] [PubMed] [Google Scholar]
- 23.Duarte AC, Cordeiro A, Fernandes BM, Bernardes M, Martins P, Cordeiro I, et al. Rituximab in connective tissue disease-associated interstitial lung disease. Clin Rheumatol. 2019;38(7):2001–9. doi: 10.1007/s10067-019-04557-7. [DOI] [PubMed] [Google Scholar]
- 24.Robles-Perez A, Dorca J, Castellví I, Nolla JM, Molina-Molina M, Narváez J. Rituximab effect in severe progressive connective tissue disease-related lung disease: preliminary data. Rheumatol Int. 2020;40(5):719–26. doi: 10.1007/s00296-020-04545-0. [DOI] [PubMed] [Google Scholar]
- 25.Druce KL, Iqbal K, Watson KD, Symmons DPM, Hyrich KL, Kelly C. Mortality in patients with interstitial lung disease treated with rituximab or TNFi as a first biologic. RMD Open. 2017;3(1):e000473. doi: 10.1136/rmdopen-2017-000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Md Yusof MY, Iqbal K, Darby M, Lettieri G, Vital EM, Beirne P, et al. Effect of rituximab or tumour necrosis factor inhibitors on lung infection and survival in rheumatoid arthritis-associated bronchiectasis. Rheumatology. 2020;59(10):2838–46. doi: 10.1093/rheumatology/kez676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Characteristics of 20 patients whose pre- and post-RTX data were available
| Male sex, n (%) | 12 (60.0) |
| Age at first RTX infusion, mean (SD), years | 61.5 (5.8) |
| RA disease duration at first RTX, median (Q1-Q3), years | 12.2 (4.4-19.8) |
| ILD disease duration at first RTX, median (Q1-Q3), years | 2.6 (0.1-5.2) |
| Age at RA diagnosis, mean (SD), years | 48.6 (13.2) |
| Age at ILD diagnosis, mean (SD), years | 58.1 (7.0) |
| RF positivity, n (%) | 19 (95.0) |
| ACPA positivity*, n (%) | 15 (88.2) |
| ANA positivity†, n (%) | 9 (47.4) |
| Higher CRP (> 5 mg/L), n (%) | 14 (70.0) |
| CRP levels,mg/L, mean (SD) | 40.3 (58.9) |
| Higher ESR (>20 mm/hour), n (%) | 16 (80.0) |
| ESR levels, mean (SD) | 37.7 (18.2) |
| Smoking status, n (%) | |
| Never | 6 (30.0) |
| Ex-smoker | 12 (60.0) |
| Current | 2 (10.0) |
| Secondary Sjögren’s syndrome, n (%) | 1 (5.0) |
| Prior anti-TNF treatment, n (%) | 4 (20.0) |
| Concomitant DMARDs | |
| Methothrexate | 1 (5.0) |
| Azathioprine | 1 (5.0) |
| Leflunomide | 9 (45.0) |
| Mycophenolate mofetil | 2 (10.0) |
| Steroids | 18 (90.0) |
| DAS28 at first RTX infusion, mean (SD) | 5.1 (1.5) |
| Radiographic pattern, n (%) | |
| Usual interstitial pneumonia | 10 (50.0) |
| Non-specific interstitial pneumonia | 7 (35.0) |
| Organizing pneumonia | 2 (10.0) |
| Indeterminate | 1 (5.0) |
| Median pre-RTX DLCO ¥ , %, (Q1-Q3) | 71 (54-79) |
| Median pre-RTX FVC § , %, (Q1-Q3) | 79 (64-100) |
| Follow-up duration, median (Q1-Q3), years | 2.7 (2.1-3.8) |
RA= rheumatoid arthritis, ILD= interstitial lung disease, RTX= rituximab, RF= romatoid factor, ACPA= anti-citrullinated protein antibody, CRP= C reactive protein, ESR= erythrocyte sedimentation rate, anti-TNF= anti tumor necrosis factor, DMARDs= disease-modifying antirheumatic drugs, DAS28= disease activity score 28, DLCO= diffusion capacity of carbon monoxide, FVC= forced vital capacity
*Data of 17 patients were available, †Data of 19 patients were available, ¥Data of 15 patients were available, §Data of 13 patients were available


