Abstract
Background:
Maternal milk production requires the neuropeptide oxytocin. Individual variation in oxytocin function is a compelling target for understanding low milk production, a leading cause of breastfeeding attrition. Complicating the understanding of oxytocin pathways is that vasopressin may interact with oxytocin receptors, yet little is known about the role of vasopressin in lactation.
Research aims:
The aims of this study were (1) to describe maternal plasma oxytocin, vasopressin, and prolactin patterns during breastfeeding following low-risk spontaneous labor and birth in healthy first-time mothers and (2) to relate hormone patterns to maternal characteristics and breastfeeding measures.
Methods:
Eligible women were recruited before hospital discharge. Forty-six participants enrolled and 35 attended the study visit. Participants kept a journal of breastfeeding frequency, symptoms of lactogenesis, and infant weight. Plasma samples were obtained at breastfeeding onset on Day 4–5 postpartum, and repeated after 20 min. Hormones were measured with immunoassays. Infant weight change, milk transfer, and onset of lactogenesis were also measured.
Results:
Baseline oxytocin and vasopressin were inversely related to one another. Oxytocin and prolactin increased significantly across the 20-min sampling period while vasopressin decreased. Higher oxytocin was associated with higher maternal age, lower BMI, shorter active labor, physiologic labor progression, and less weight loss in the newborn. Higher vasopressin correlated with younger maternal age, higher BMI, and greater newborn weight loss.
Conclusions:
Oxytocin and vasopressin have contrasting relationships with maternal clinical characteristics and newborn weight gain in early breastfeeding infants. Further study is needed to understand how oxytocin and vasopressin influence lactation outcomes.
Keywords: breastfeeding, breastfeeding difficulties, hormones, lactogenesis, maternal physiology
Background
According to 2017 data, new mothers in the United States initiate breastfeeding at high rates (over 80%), yet exclusive breastfeeding falls to 47% by 3 months postpartum, which poses health risks to women and children (Centers for Disease and Control Prevention, 2018). A commonly cited reason for not providing only human milk is the mother’s own report of inadequate milk production (Kent, Gardner, & Geddes, 2016; Stuebe et al., 2014). Although there are many known barriers to optimal breastfeeding, factors that influence the physiology of milk production are important considerations. Mammalian milk production relies on many physiologic pathways. Oxytocin (OXT), a nonapeptide produced in the hypothalamus and released into circulation via the posterior pituitary, is the primary hormone responsible for the ejection of milk from the breast gland lumen (Crowley, 2015). Therefore, understanding variation in OXT function among women may be useful in identifying potential interventions for women at risk for suboptimal breastfeeding.
Oxytocin is an important trigger for prolactin (PRL) secretion from the anterior pituitary (Kennett & Mckee, 2012). PRL, the primary lactogogue, is also necessary for milk production (Crowley, 2015). PRL, as reported in classic human studies, appears to follow a pattern of secretion in labor; PRL falls during active and expulsive labor and rises after birth of the placenta and subsequent infant suckling (Rigg & Yen, 1977; Salamalekis, Pyrgiotis, Phoca, & Zourlas, 1991; Wladimiroff, Lo, de Meijer, Lamberts, & Schalekamp, 1983). During human childbirth, the influence of peripheral synthetic OXT administration on subsequent pituitary hormone secretion (e.g. OXT, PRL) has not been thoroughly examined. Some researchers have noted that synthetic OXT use during labor results in different patterns of PRL release during and after labor compared to those laboring without synthetic OXT, (Bremme & Eneroth, 1980; Haning et al., 1978; Jonas et al., 2009; Onur, Erçal, & Karslioglu, 1989; Rae, Hollebone, Chetty, Clausen, & McFarlane, 2007) although other researchers did confirm these observations (Haddad & Morris, 1983; Lao & Panesar, 1989). In only two studies have researchers examined patterns of OXT secretion post birth following exposure to synthetic OXT during the birth process (Gu et al., 2016; Jonas et al., 2009). Whether or not changes in the concentrations or patterns of release of PRL or OXT in the setting of synthetic OXT exposure leads to changes in lactation performance has not been examined. Although synthetic OXT administration has been linked to suboptimal breastfeeding outcomes, many researchers’ conclusions are limited by methodological issues and inadequate controls (Erickson & Emeis, 2017).
OXT and arginine vasopressin (AVP) are similar in structure, differing by only two amino acids, and both originate from adjacent collections of neurons in the hypothalamus. Their receptors also share some homology. AVP functions classically as a regulator of water loss and retention and vasoconstriction. Some scientists have noted that, given their similarities, OXT and AVP appear to demonstrate “cross-talk” between the two pathways (Arrowsmith & Wray, 2014)—with AVP activating OXT receptors and vice versa. That line of study has focused on implications for labor, but examination of maternal AVP in the setting of human lactation and breastfeeding outcomes has yet to be investigated.
Our first aim was to describe patterns of maternal plasma OXT, AVP, and PRL during the period of secretory activation (lactogenesis II) following low-risk spontaneous labor and birth in healthy primiparous women. In Aim 2 we relate hormone patterns to maternal characteristics and to breastfeeding outcomes. We hypothesized that levels of OXT, AVP, and PRL relate to markers of maternal milk production.
Methods
Design
The data for this analysis was derived from a pilot, prospective one-group, descriptive study with two data collection time points for hormone measurement. Given the delicate nature of the first few postpartum days with a first baby, we limited the scope of the hormone sampling to a single feeding and two blood samples to minimize participant burden. The local university Institutional Review Board approved this study as a minimal-risk protocol after expedited review.
Setting
The study took place at Oregon Health & Science University (OHSU), an academic tertiary care center in the Pacific Northwest region of the United States, where certified nurse-midwives and generalist and high risk obstetric/gynecologic physicians provide care to a patient population consisting of a mixture of the health service employees, patients from city public health clinics, and high risk patients referred from the surrounding region. The hospital provides both inpatient and out-patient lactation support to all women who desire the service. At OHSU, approximately 60% of families seeking perinatal care are privately insured and 40% access public insurance (Medicaid) for care. Culturally, breastfeeding initiation is desired amongst the majority of the patient population, and the hospital began the transition to becoming certified as Baby Friendly in 2017.
Sample
We used convenience sampling to screen inpatient postpartum electronic medical records for eligibility among the target population of newly delivered primiparous women who underwent spontaneous labor and birth. In the first 24–48 hr following birth, and prior to discharge from the hospital, authors (EE or CE) approached women who met study inclusion criteria. The inclusion criteria were as follows: (1) non-smoking; (2) English-speaking; (3) aged 18–43 years; and (4) intending to exclusively breastfeed. Women were excluded if they had had Cesarean or instrument assisted birth (forceps or vacuum), induced labor, a pre-pregnancy body mass index greater than 40, suspected hypoplastic breast tissue, breast surgery involving areola, serious obstetric conditions (intrauterine growth restriction, severe hypertension, gestational diabetes requiring medical management), or a personal history of uncontrolled or autoimmune thyroid disease or polycystic ovary syndrome. Women were also excluded if there was physical separation of neonate during the recovery period (e.g. observation or NICU admission), greater than 1,000 ml blood loss, blood transfusion, or iron infusion therapy. Women who had augmented labor with oxytocin were included if their exposure was less than or equal to 12 hr and no more than 20 mU/min maximum rate. We were powered to detect a correlation of at least r = .50 between two biomarkers with a sample size of 29 and an α of .05.
We presented the study protocol to 77 women; 31 (40%) declined to participate. The most common reason for declining was “feeling overwhelmed” by the baby’s needs or breastfeeding challenges (n = 12). Of the 46 participants who enrolled in the study and completed baseline data, 35 attended the follow-up visit on Day 4–5 postpartum.
Measurement
Medical record information and birth experience information was abstracted from the electronic record including total oxytocin dose (intrapartum and postpartum). Each day after enrollment, until the follow-up visit, participants completed a journal including frequency of feeding at the breast and breast pumping, level of breast fullness (timing of lactogenesis II measure), supplementation of breastfeeding, and infant weight if measured at an outpatient or home appointment. We assessed timing of onset of lactogenesis daily using maternal reports of breast fullness on a scale of 1–5 (1 = no change, 5 = uncomfortably full), which was validated to correspond to the presence of β-casein in maternal milk, signaling more mature milk production (Chen, Nommsen-Rivers, Dewey, Lonnerdal, & Lönnerdal, 1998; Dewey, Nommsen-Rivers, Heinig, & Cohen, 2003).
Weight measurements of the infants were obtained on a digital infant scale lined with a lightweight pad. In the study clinic, the weight of the blanket and a dry newborn diaper were subtracted from the newborn weight by zeroing the scale prior to placing the newborn. Milk transfer was assessed by subtracting each newborn’s post-feeding weight from a pre-feed weight after each breast and summing the total. Approximate milk transfer was determined as 1-gram weight change correlates to 1-ml volume. The percentage of the newborns’ weight (compared to birth weight) was also calculated. Newborn diapers stayed on during the weighing and were not changed until the end of the feeding session/final weight. The 12-point Infant Breastfeeding Behavior Tool (IBFAT), which has 91% inter-rater reliability (Matthews, 1988), was used to assess infant role in feeding.
Plasma PRL was assessed in a single determination using an electrochemiluminescence immunoassay on a Roche cobas e411 automated clinical platform. The assay range was 1–10,000 uIU/ml (0.0470–470 ng/ml). PRL samples were processed by the endocrine laboratory at the Oregon National Primate Research Center. In-house laboratory quality controls serve as assay standards. The laboratory intra-assay coefficient of variation (CV) was 4.6% and inter-assay CV was 7.1%. Plasma OXT and AVP assays were performed using enzyme immunoassay (EIA) methods (Carter et al., 2007; Kramer, Cushing, Carter, Wu, & Ottinger, 2004) from Arbor Assay (Ann Arbor, MI) and processed at the Kinsey Institute (Indiana University). Samples were run unextracted, in duplicate, with serial dilutions, and with standardized controls for reliability and validity (pg/ml). The intra-assay CV for the OXT assay was 9.27%. The CV was 8.91% on the AVP assay.
Data Collection
The study enrolled participants from July 2016 to October 2017. After reviewing the protocol, we obtained written informed consent from eligible participants. Personal medical data were entered into a password protected, secure database, and consent forms maintained in a locked cabinet to guard confidentiality. Participants were invited to an outpatient lactation clinic appointment for the Day 4–5 postpartum follow up or to the research clinic with the study staff if the participant declined the lactation consultation. During the follow-up visit, after getting settled comfortably in a padded phlebotomy chair that reclined as desired, participants breastfed when the baby exhibited feeding cues. Venous blood samples were collected at Time 1 (within 1–2 min of latching the baby) and again at Time 2 (20 min later), at which time OT and PRL typically peak (Hill, Chatterton, & Aldag, 1999). Blood was collected into a 6 ml EDTA tube at each draw, inverted several times and placed immediately into an ice bath. Samples were centrifuged following the 20-min study period at 1,600 g for 15 min at 4°C. Immediately after centrifugation, the plasma supernatant was pipetted into two aliquot tubes per blood draw and stored in a −80°C freezer. Plasma samples were successfully obtained in 32 and 31 participants at Time 1 and Time 2 respectively during the observed feeding. Participants received a gift card as remuneration for their time and effort.
Follow-up infant weight data were gathered from a combination of medical records, breastfeeding journal entries and in-person follow-up for a total of 38 of the 46 enrolled participants. Infants were weighed prior to feeding and following each breast to estimate milk-transfer volume. Participants were asked to feed their babies as desired, and the duration of feeding was recorded along with total infant weight change and the IBFAT score.
Data Analysis
To address Aim 1, we described patterns of maternal plasma OXT, AVP, and PRL during lactation using (a) a paired t-test to assess for change in hormone levels over the course of the feeding; (b) Spearman rank correlation between the plasma hormone concentrations (e.g., correlation between OXT and AVP); and (c) Spearman rank correlations with maternal characteristics (age, race, BMI, labor progress, and synthetic oxytocin administration). In addition, differences in mean levels of hormones were examined between white women and women of color, using an independent samples t-test. Spearman correlations were determined, given the nonnormal distributions in hormone concentrations.
To address Aim 2, we used Student’s t-test and Spearman rank correlations, where appropriate, to quantify associations between plasma hormones and breastfeeding outcomes (percentage of newborn weight loss/gain since birth, self-report of timing of breast fullness, number of breast feedings on days between discharge and follow-up, duration of study feed, milk transfer, and IBFAT score). We also examined the relationship between hormone levels and the clinical benchmark of a 5% loss of birth weight. As this was a pilot study, statistical significance was determined using a p-value < .10. The statistical analysis was carried out using StataSE 15 (College Station, TX: StataCorp LLC).
Results
Characteristics of the Sample
Characteristics of the sample are listed in Table 1. Most participants, 69% (n = 32) identified as white, 98% were married or partnered (n = 45), and 71.7% (n = 33) received care from a Certified Nurse-Midwife during labor and birth. Thirty-three participants (71.7%) received postpartum oxytocin, while only 11 participants had labor augmentation with oxytocin (23.9%). Male infants made up 57% (n = 26) of the sample. Immediately after birth, 87% of infants were put skin-to-skin with their mothers (n = 40).
Table 1.
Participants’ and Their Infants’ Characteristics (N = 46).
| Variable | M (SD) |
|---|---|
| Maternal Age | 30.9 (4.8) |
| Gestational Age (weeks) at Delivery | 39.9 (0.8) |
| Body Mass Index: End of Pregnancy | 28.5 (3.7) |
| Mean Weight Gained in Pregnancy (lbs) | 31.7 (10.1) |
| Latent Labor (hr) | 13.2 (11.5) |
| Active Labor (hr) | 5.4 (4.1) |
| Second Stage Labor (hr) | 1.9 (1.4) |
| Third Stage Labor (min) | 9.6 (5.4) |
| Blood Loss (ml) | 332 (120) |
| Epidural Duration (hr)a | 7.5 (2.7) |
| Total Intravenous Fluid Given Intrapartum (L) | 1.2 (1.5) |
| Dose Intrapartum of Synthetic Oxytocin (U)b | 1.3 (1.3) |
| Total Dose of Synthetic Oxytocin (U)c | 8.9 (8.3) |
| Infant Apgar Score at 5 min | 8.9 (0.25) |
| Birthweight of Infant (g) | 3394.3 (386.9) |
Note:
n = 18 received epidural,
n = 11 received augmentation,
n = 33 received synthetic oxytocin intrapartum and/or postpartum
Aim 1: Plasma Hormone Patterns
Mean and median plasma levels are shown in Table 2. OXT and PRL significantly increased over 20 min while AVP decreased by the 20-min mark. Overall, participants with higher baseline OXT had significantly lower baseline AVP (see Figure 1). The majority of participants (57%, n = 17) exhibited a rise in OXT and a decrease in AVP over the feeding; however, three participants’ plasma levels had the reverse pattern (drop in OXT and a rise in AVP).
Table 2.
Plasma Levels of OXT, PRL, and AVP in New Breastfeeding Healthy Women (Day 4–5).
| Time 1 (start of feeding) n = 32 | Time 2 (20 min later) n = 31 | Change (Time 2–Time 1) n = 30 | Paired t-test t | p | |
|---|---|---|---|---|---|
| OXT (pg/ml) | |||||
| M (SD) | 1641.5 (121.7) | 1713.6 (127.2) | 89.6 (120.0) | −4.1 | <.001 |
| Median (Min, Max) | 1634.4 (1381.2, 1867.8) | 1695.6 (1452.6, 1956.6) | 85.2 (−174.6, 429) | ||
| PRL (ng/ml) | |||||
| M (SD) | 311.81 (113.6) | 340.0 (106.2) | 26.2 (57.7) | −2.5 | .009 |
| Median (Min, Max) | 306.5 (77, 652) | 344.0 (166.0, 650.0) | 2 (−28, 228) | ||
| AVP (pg/ml) | |||||
| M (SD) | 1996.5 (294.6) | 1900.7 (302.1) | −112.36 (412.1) | 1.49 | .07 |
| Median (Min, Max) | 2018.1 (1548.6, 2772.0) | 1958.4 (1434.6, 2605.8) | −69.6 (−1147.9, 870.6) |
Note. PRL = prolactin; OXT = oxytocin; AVP = arginine vasopressin.
Figure 1.
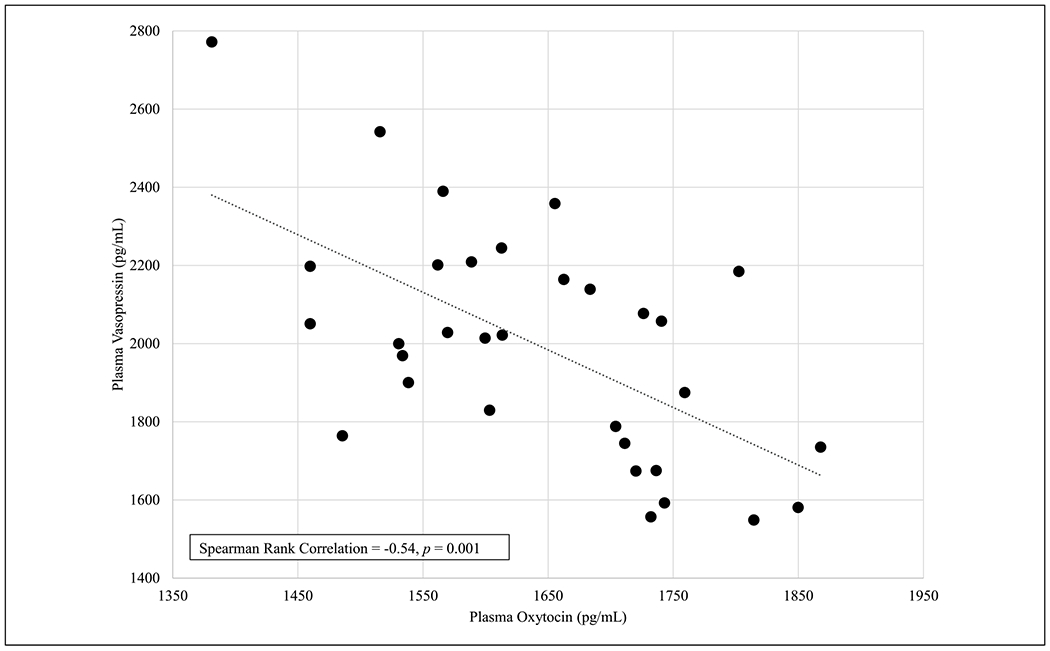
Relationship Between Oxytocin in Maternal Plasma During Early Lactation (N = 30).
Older participants had moderately higher OXT (0.36, p = .03) and lower AVP (−0.35, p = .05). Participants with higher BMIs at delivery demonstrated moderately lower OXT (−0.36, p = 0.04) and higher AVP (0.35, p = .05). On average, participants of color (n = 7) had mean (SD) PRL levels of 412.4 (50.8) ng/ml at baseline compared to 283.6 (18.3) ng/ml for participants who were white (n = 25; t = −2.96, two-tailed p-value = 0.006). In addition, participants of color had higher average AVP than white participants (2201.3 pg/ml versus 1939.1 pg/ml; t = −2.21, two-tailed p = .04). Non-parametric p-values for AVP and PRL, reported in Table 3, are a more conservative test of significance for this association. Relationships between baseline (Time 1) hormone expression and active labor duration, synthetic oxytocin, and epidural use during labor are noted in Table 3. Participants with the lowest duration of active labor (3 hr or less) had a mean OXT at baseline of 1720 pg/ml, compared to participants who had an active labor greater than 3 hr but less than 5 hr (1634.6 pg/ml) and those with the longest active phase, over 5 hr (1569.3 pg/ml). Notably, participants with labor augmentation with synthetic oxytocin also demonstrated an increase in AVP compared to women with spontaneous labor, who had a decrease in AVP (Figure 2) over the feeding.
Table 3.
Correlations Between Participants’ Plasma OXT, PRL, and AVP Values and Demographics and Clinical variables (N = 32).
| OXT | p | PRL | p | AVP | p | |
|---|---|---|---|---|---|---|
| Maternal Age | 0.36 | .03 | −0.30 | .10 | −0.35 | .05 |
| Participants of Color | −0.24 | .18 | 0.42 | .01 | 0.30 | .09 |
| BMI at Delivery | −0.36 | .04 | 0.04 | .84 | 0.35 | .05 |
| Gestational Age | 0.28 | .12 | −0.02 | .93 | −0.04 | .82 |
| Length of Active Labor | −0.50 | .003 | 0.04 | .81 | 0.20 | .27 |
| Augmentation of Labor | −0.30 | .08 | −0.13 | .47 | −0.15 | .39 |
| Synthetic Oxytocin Dose Used for Augmentation a | 0.72 | .03 | 0.63 | .06 | −0.43 | .24 |
| Duration Epidural Used b | −0.11 | .68 | −0.01 | .96 | 0.19 | .49 |
| Immediate Skin to Skin Newborn with Mother | 0.27 | .13 | 0.23 | .21 | −0.23 | .21 |
Note. PRL = prolactin; OXT = oxytocin; AVP = arginine vasopressin.
n = 9 received augmentation.
n = 15 received epidural.
Figure 2.
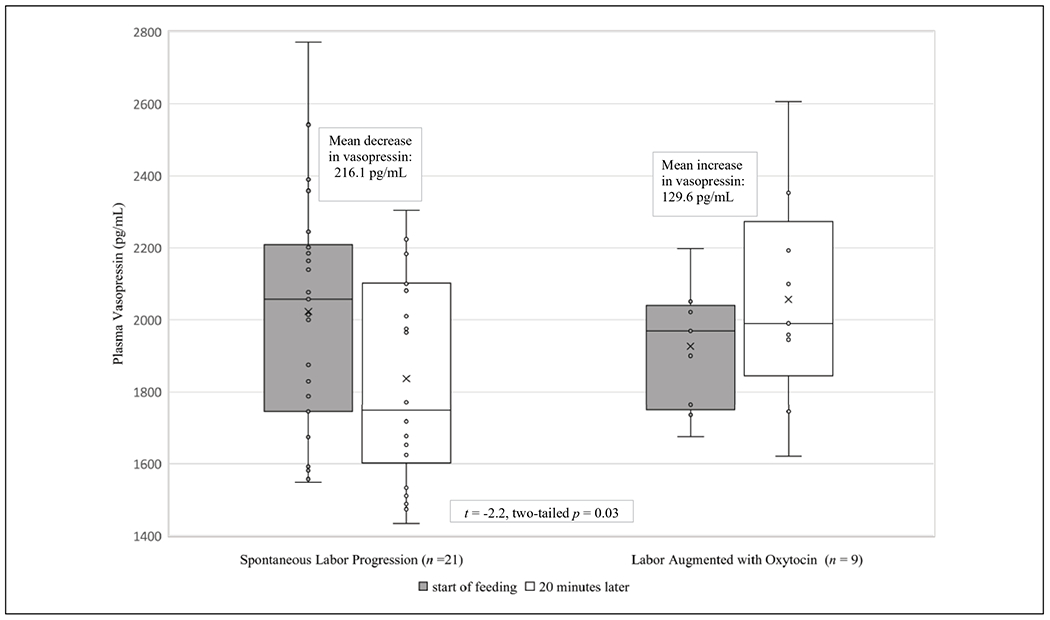
Maternal Plasma Vasopressin over 20 Min of Breastfeeding, Relationship to Labor Augmentation (N = 30).
Breastfeeding Outcomes
The percentage of newborn weight gained or lost after birth was related to OXT and AVP, with OXT and AVP having opposing relationships with this outcome. Mean OXT was significantly higher (1682.24 pg/ml vs. 1582.02 pg/ml, t = 2.5, one-tailed p = .009), and mean AVP was significantly lower (1917.2 pg/ml vs. 2112.32 pg/ml, t = −1.9, one-tailed p = .03) in participants whose babies had lost less than 5% of their birth weight by Day 4–5 postpartum. Furthermore, we examined the relationship between OXT and infant weight among the subset of participants who underwent a spontaneous labor progress. The correlation of maternal OXT levels with infant weight gain was stronger (Spearman rank 0.60, p = .002) when only examining the results from participants who did not receive synthetic oxytocin during labor (n = 23) see Figure 3.
Figure 3.
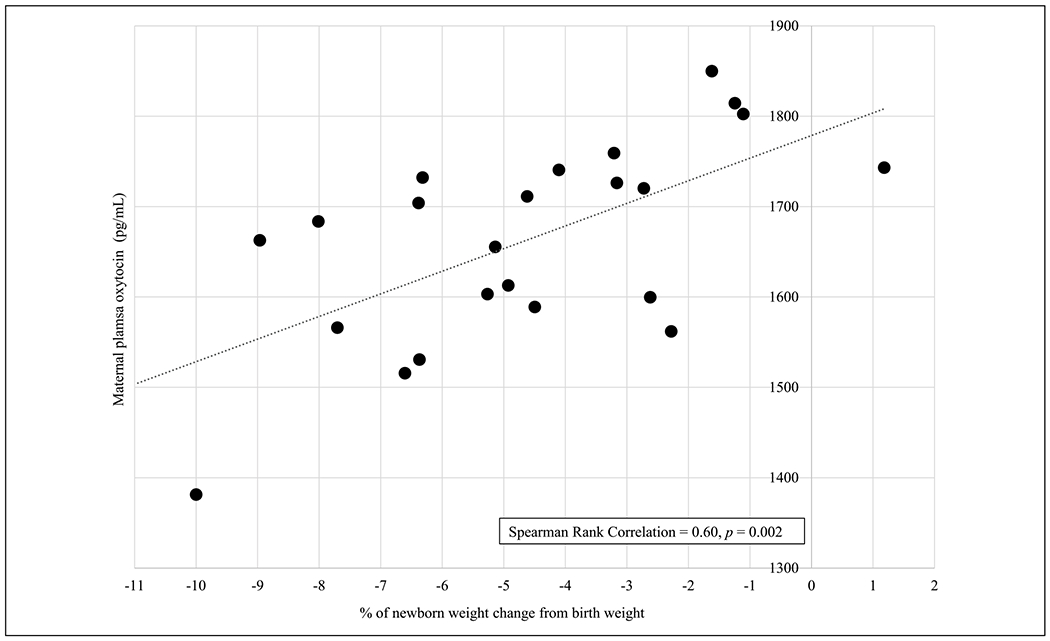
Plasma Oxytocin from Lactating Women who had Physiologic Labor Progress in Relation to Newborn Weight Change on Day 4–5 Postpartum (N = 23).
Self-report of breast-fullness/mature milk production varied within the sample. We considered onset as delayed if the participant reported breast fullness after 72 hr (Dewey et al., 2003). Eleven participants (30%) reported delayed onset of mature milk production with a mean (SD) of 84.1 (12.3) hr, which contrasts with the 25 participants who did not have delayed onset who reported an average of 52.9 (10.4) hr until notable breast fullness. Despite this variation, hours of onset of lactogenesis did not relate statistically to any of the hormones at baseline (data not shown). The IBFAT measure of newborn breastfeeding behavior was not related to maternal hormone concentrations. Finally, participants’ single milk transfer volume was related to PRL but not associated with OXT or AVP. Those participants who had more than 40 ml of milk transfer during the Day 4–5 postpartum visit (n = 12) had a mean PRL of 267.6 ng/ml compared to 338.4 ng/ml for participants with 40 ml or less (n = 12, t = 1.76, one-tailed p = .04). Interestingly post hoc Spearman correlation demonstrated that newborns with the highest percent of birth weight lost at follow up had moderately higher levels of milk transfer during the study feeding (−0.40, p = .02). Other breastfeeding outcomes included the length of feeding during the study visit and supplementation with formula during the first 4–5 days of life, and neither correlated to plasma values (see Table 4).
Table 4.
Correlations Between Participants’ Baseline Plasma OXT, PRL, and AVP Values and Breastfeeding on Day 4–5 Postpartum (N = 32).
| Hormone | Length of Feeding | p | Quantity Milk Transferred | p | Supplemented with Formula | p | % of Infant Birth Weight | p |
|---|---|---|---|---|---|---|---|---|
| OXT | 0.20 | .25 | −0.05 | .76 | −0.15 | .43 | 0.38 | .03 |
| PRL | 0.11 | .53 | −0.32 | .07 | 0.25 | .17 | −0.07 | .69 |
| AVP | −0.04 | .81 | 0.13 | .45 | 0.04 | .82 | −0.34 | .05 |
Note. PRL = prolactin; OXT = oxytocin; AVP = arginine vasopressin
Hours of onset perceived breast fullness, number of feedings/pumping on Day 2–3 were non-correlated with the hormones OXT, PRL, and AVP.
Discussion
Although our study did not include participants with prepregnancy obesity, our findings of lower OXT levels in participants with higher BMI are consistent with other research (Qian et al., 2014). According to recent research studies, women with higher BMI tend to need higher doses of synthetic OXT during labor induction and augmentation (Carlson, Corwin, & Lowe, 2017; Maeder et al., 2017), which may result from lower levels of endogenous production. In addition, overweight and obese women may have higher rates of undesired weaning due to breastfeeding difficulty (Stuebe et al., 2014). Early weaning could reflect lower OXT levels. Alternatively physical latching issues related to obesity may lead to lower OXT levels if breasts receive less stimulation. These findings point to a possibility of a shared mechanism for labor and lactation difficulties due to less robust OXT signaling in women with obesity. Additionally, obesity may modify OXT function. In contrast to findings reported by other researchers, we did not find a difference in PRL or PRL response according to body mass (Rasmussen & Kjolhede, 2004).
Few researchers have considered racial background in reporting PRL values in women; one team that examined this reported no significant differences (Pinheiro, Holmes, Pollak, Barbieri, & Hankinson, 2005). However, prolactin may be a marker of the stress response, particularly in the peripartum period (Torner, Toschi, Nava, Clapp, & Neumann, 2002). Examining PRL function in the context of stress may help understand how this may influence health disparities in lactation outcomes as well as the importance of stress on breastfeeding (Chen et al., 1998). Women of color also trended toward having higher AVP and lower OXT levels. This observation recapitulates existing literature which suggests that AVP function is linked to higher stress susceptibility, but OXT serves as a buffer to stress (Carter, 2017; Neumann & Landgraf, 2012). However, given the small proportion of participants of color in the sample, these results can only be considered hypothesis generating.
Higher AVP and lower OXT were noted among participants with babies who experienced greater levels of weight loss from birth. Newborn weight loss serves as an important proxy for milk production as it is often the clinical action point for starting supplementation with formula. One hypothesis for these opposing hormone actions in relation to milk production (and later newborn weight loss) could be due to higher levels of circulating levels of AVP triggering “cross-talk” or AVP binding to OXT receptors in the myoepithelium exerting a less specific or robust signal for milk ejection (Arrowsmith & Wray, 2014). As AVP expression may increase in a context of anxiety or fear (Carter, 2017), lactation under stress or stress caused by lactation difficulties may contribute further to the problem of milk production.
Another explanation for these findings is that OXT and AVP contained in maternal milk have direct influence on the neonate’s metabolism. This idea is supported by a recent study in mice showing that OXT found in maternal milk can be transported intact from dam to pups’ intestines (Higashida et al., 2017). A study of human neonates noted that higher AVP in the early neonatal plasma correlated to higher weight loss from birth, possibly as a compensatory mechanism against dehydration in the presence of higher weight loss (Marchini & Stock, 1997). Whether the OXT/AVP levels in infants correlate to that of maternal samples or to levels of these hormones within human milk is an area for further research. OXT and AVP did not correlate to the quantity of milk transferred during the study feeding, which could indicate that a single milk transfer measure is not a sufficient proxy for milk production. Overall, infant weight loss remains an important marker for intervention in lactation care compared to milk transfer measures.
Another finding of interest is the role of synthetic OXT used during the labor process on the plasma findings in our sample. Although the total number of women undergoing labor augmentation was small by study design, this relationship is supported by existing literature. Jonas et al. (2009) found that use of synthetic OXT for labor stimulation associated with lower levels in maternal plasma on Day 2 postpartum. Yet, Gu et al. (2016) found higher levels of plasma OXT at 2 months postpartum in women exposed to higher levels of intra-/postpartum synthetic OXT. We found women with longer active labor duration demonstrated lower OXT levels postpartum and were also more likely to have augmentation. A possible explanation for this outcome is that some women present to labor with less robust OXT function, which could influence both the labor and the postpartum periods. Whether the birth process itself, including medical interventions, could influence postpartum expression of these hormones remains to be explored (Bell, Erickson, & Carter, 2014). Future research should address whether OXT levels found postpartum indicate a cause or consequence of labor augmentation intervention by studying plasma levels before birth as well as postpartum in a larger sample undergoing labor augmentation.
If labor interventions, such as synthetic oxytocin administration, could have a lasting effect it could occur via a nervous system feedback or through direct binding of OXT receptors in the CNS. Until recently, it was thought that peripheral synthetic OXT could not pass the blood-brainbarrier. However, researchers conducting neuro-pharmacotherapuetic studies in primate models of high doses of peripherally administered synthetic OXT have shown increased OXT levels in the cerebral-spinal fluid (CSF) after intravenous administration (Freeman et al., 2016). In another non-human primate study, researchers intravenously administered labeled (d5-deuterated) OXT and later identified the labeled peptide within the CSF, indicating passage of peripherally administered OXT into the CNS (Lee et al., 2018). Even when large doses of synthetic OXT are administered intravenously, only small proportions appear to enter the CSF in non-human primates. Yet, OXT appears to last for at least 2 hr post administration, probably due to increased half-life in CSF. Ongoing pharmacologic study in the fields of psychiatry, obesity, and addiction will likely provide insights into how peripheral OXT administration influences the CNS (Viero et al., 2010). Many questions remain about whether obstetric use of synthetic oxytocin plays a longer-term role in maternal-child outcomes than previously understood.
Limitations
To our knowledge, we are the first investigators to report maternal OXT/AVP relationships during lactation with newborn weight loss outcomes. Nonetheless, we recognize the limitations in this analysis, including small sample size, attrition to the follow up visit, and measurement error due to the use of different scales for newborn weight (delivery room and in the follow up clinics). Self-report of maternal symptoms of breast fullness are subjective and may be another source of potential error. Our sample was also limited to healthy participants who were not obese prior to pregnancy. Finally, the women who agreed to participate in the study represent those who were possibly coping better with the initial breastfeeding relationship compared to those who declined participation.
Conclusion
In this study, we demonstrated that maternal plasma hormones after birth vary by personal and clinical characteristics and that an individual’s pattern of hormone secretion may influence milk production as indexed by early infant weight loss. Larger and more diverse samples are needed to fully examine the physiologic underpinnings of neurohormonal pathways underlying milk production and infant weight gain to support exclusive breastfeeding and long-term optimal outcomes.
Key Messages.
Few researchers have examined oxytocin function in relationship to breastfeeding outcomes, for example, infant weight gain.
Oxytocin and vasopressin were inversely correlated.
Higher levels of maternal vasopressin were associated with greater newborn weight loss on Day 4–5 postpartum.
This is the first study to characterize vasopressin in lactating women in relation to oxytocin function and breastfed newborn weight outcomes.
Acknowledgments
Special thanks the women and babies who contributed to this research during a sensitive time. Thanks also to Dr. Hossein Pournajafi-Nazarloo, Laura Lallande, CNM, IBCLC, Dr. Doria Thiele PhD, CNM, IBCLC, the Women’s Health Research Unit and the Endocrine Technologies Core at the Oregon National Primate Research Center (supported by NIH P51 OD011092).
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Support provided in part by the following: the Nursing Innovations Fund at the authors’ institution; the Dean’s Dissertation Fund at the authors’ institution; Jonas Veteran Healthcare Scholar Award; Kinsey Institute funding through Eunice Kennedy Shriver National Institute of Child Health and Human Development of the NIH under Award Number P01 075750. One author is currently supported as a Scholar of the Oregon Building Interdisciplinary Research Careers in Women’s Health K12 Program funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the NIH under Award Number K12HD043488.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Arrowsmith S, & Wray S (2014). Oxytocin: Its mechanism of action and receptor signalling in the myometrium. Journal of Neuroendocrinology, 26(6), 356–369. doi: 10.1in/jne.12154 [DOI] [PubMed] [Google Scholar]
- Bell AF, Erickson EN, & Carter CS (2014). Beyond labor: The role of natural and synthetic oxytocin in the transition to motherhood. Journal of Midwifery and Women’s Health, 59(1), 35–42. doi: 10.im/jmwh.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease and Control Prevention. (2018). Breastfeeding Report Card: United States. Retrieved from www.cdc.gov/breastfeeding
- Bremme K, & Eneroth P (1980). Changes in serum hormone levels during labor induced by oral pge2 or oxytocin infusion. Acta Obstetricia et Gynecologica Scandinavica, 59(92 S), 31–43. doi: 10.3109/00016348009156935 [DOI] [PubMed] [Google Scholar]
- Carlson NS, Corwin EJ, & Lowe NK (2017). Oxytocin augmentation in spontaneously laboring, nulliparous women: Multilevel assessment of maternal BMI and oxytocin dose. Biological Research for Nursing, 19(4), 382–392. doi: 10.1177/1099800417701831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS (2017). The oxytocin-vasopressin pathway in the context of love and fear. Frontiers in Endocrinology, 8(356), 1–12. doi: 10.3389/fendo.2017.00356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, & Schwertz D (2007). Oxytocin: Behavioral associations and potential as a salivary biomarker. Annals of the New York Academy of Sciences, 1098, 312–322. doi: 10.1196/annals.1384.006 [DOI] [PubMed] [Google Scholar]
- Chen DC, Nommsen-Rivers L, Dewey KG, Lonnerdal B, & Lönnerdal B (1998). Stress during labor and delivery and early lactation performance. American Journal of Clinical Nutrition, 68(2), 335–344. doi: 10.1093/ajcn/68.2.335 [DOI] [PubMed] [Google Scholar]
- Crowley WR (2015). Neuroendocrine regulation of lactation and milk production. Comprehensive Physiology, 5(1), 255–291. doi: 10.1002/cphy.c140029 [DOI] [PubMed] [Google Scholar]
- Dewey KG, Nommsen-Rivers LA, Heinig MJ, & Cohen RJ (2003). Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics, 112(3), 607–619. doi: 10.1542/peds.112.3.607 [DOI] [PubMed] [Google Scholar]
- Erickson EN, & Emeis CL (2017). Breastfeeding outcomes after oxytocin use during childbirth: An integrative review. Journal of Midwifery & Women’s Health, 62(4), 397–417. doi: 10.1111/jmwh.12601 [DOI] [PubMed] [Google Scholar]
- Freeman SM, Samineni S, Allen PC, Stockinger D, Bales KL, Hwa GGC, & Roberts JA (2016). Plasma and CSF oxytocin levels after intranasal and intravenous oxytocin in awake macaques. Psychoneuroendocrinology, 66, 185–194. doi: 10.1016/j.psyneuen.2016.01.014 [DOI] [PubMed] [Google Scholar]
- Gu V, Feeley N, Gold I, Hayton B, Robins S, Mackinnon A, … Zelkowitz P (2016). Intrapartum synthetic oxytocin and its effects on maternal well-being at 2 months postpartum. Birth, 43(1), 28–35. doi: 10.1111/birt.12198 [DOI] [PubMed] [Google Scholar]
- Haddad PF, & Morris NF (1983). Maternal serum prolactin levels during labour. Journal of Obstetrics and Gynaecology, 4(1), 42–46. doi: 10.3109/01443618309071229 [DOI] [Google Scholar]
- Haning RV, Barrett DA, Alberino SP, Lynskey MT, Donabedian R, & Speroff L (1978). Interrelationships between maternal and cord prolactin, progesterone, estradiol, 13,14-dihydro-15-keto-prostaglandin f2α, and cord cortisol at delivery with respect to initiation of parturition. American Journal of Obstetrics and Gynecology, 130(2), 204–210. doi: 10.1016/0002-9378(78)90367-8 [DOI] [PubMed] [Google Scholar]
- Higashida H, Furuhara K, Yamauchi A-M, Deguchi K, Harashima A, Munesue S, … Yamamoto Y (2017). Intestinal transepithelial permeability of oxytocin into the blood is dependent on the receptor for advanced glycation end products in mice. Nature Scientific Reports, 7(7883), 1–15. doi: 10.1038/s41598-017-07949-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill PD, Chatterton RT, & Aldag JC (1999). Serum prolactin in breastfeeding: State of the science. Biological Research for Nursing, 1(1), 65–75. [DOI] [PubMed] [Google Scholar]
- Jonas W, Johansson LM, Nissen E, Ejdebäck M, Ransjö-Arvidson AB, & Uvnäs-Moberg K (2009). Effects of intrapartum oxytocin administration and epidural analgesia on the concentration of plasma oxytocin and prolactin, in response to suckling during the second day postpartum. Breastfeeding Medicine, 4(2), 71–82. doi: 10.1089/bfm.2008.0002 [DOI] [PubMed] [Google Scholar]
- Kennett JE, & Mckee DT (2012). Oxytocin: An emerging regulator of prolactin secretion in the female rat. Journal of Neuroendocrinology, 24(3), 403–412. doi: 10.1111/j.1365-2826.2011.02263.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent JC, Gardner H, & Geddes DT (2016). Breastmilk production in the first 4 weeks after birth of term infants. Nutrients, 8(12), 1–6. doi: 10.3390/nu8120756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer KM, Cushing BS, Carter CS, Wu J, & Ottinger MA (2004). Sex and species differences in plasma oxytocin using an enzyme immunoassay. Canadian Journal of Zoology, 82(8), 1194–1200. doi: 10.1139/z04-098 [DOI] [Google Scholar]
- Lao TT, & Panesar NS (1989). The effect of labour on prolactin and cortisol concentrations in the mother and the fetus. European Journal of Obstetrics and Gynecology and Reproductive Biology, 30(3), 233–238. doi: 10.1016/0028-2243(89)90006- [DOI] [PubMed] [Google Scholar]
- Lee MR, Scheidweiler KB, Diao XX, Akhlaghi F, Cummins A, Huestis MA, … Averbeck BB (2018). Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: determination using a novel oxytocin assay. Molecular Psychiatry, 23(1), 115–122. doi: 10.1038/mp.2017.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeder AB, Vonderheid SC, Park CG, Bell AF, McFarlin BL, Vincent C, & Carter CS (2017). Titration of intravenous oxytocin infusion for postdates induction of labor across body mass index groups. JOGNN - Journal of Obstetric, Gynecologic, and Neonatal Nursing, 46(4), 494–507. doi: 10.1016/j.jogn.2017.02.006 [DOI] [PubMed] [Google Scholar]
- Marchini G, & Stock S (1997). Thirst and vasopressin secretion counteract dehydration in newborn infants. Journal of Pediatrics, 130(5), 736–739. doi: 10.1016/S0022-3476(97)80015-7 [DOI] [PubMed] [Google Scholar]
- Matthews MK (1988). Developing an instrument to assess infant breastfeeding behavior in the early neonatal period. Midwifery, 4, 154–165. doi: 10.1016/S0266-6138(88)80071-8 [DOI] [PubMed] [Google Scholar]
- Neumann ID, & Landgraf R (2012). Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends in Neurosciences, 35(11), 649–659. doi: 10.1016/j.tins.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Onur E, Erçal T, & Karslioglu I (1989). Prolactin and cortisol levels during spontaneous and oxytocin induced labour and the effect of meperidine. Archives of Gynecology and Obstetrics, 244(4), 227–232. doi: 10.1007/BF01560086 [DOI] [PubMed] [Google Scholar]
- Pinheiro SP, Holmes MD, Pollak MN, Barbieri RL, & Hankinson SE (2005). Racial differences in premenopausal endogenous hormones. Cancer Epidemiology Biomarkers and Prevention, 14(9), 2147–2153. doi: 10.1158/1055-9965.EPI-04-0944 [DOI] [PubMed] [Google Scholar]
- Qian W, Zhu T, Tang B, Yu S, Hu H, Sun W, … Yuan G (2014). Decreased circulating levels of oxytocin in obesity and newly diagnosed type 2 diabetic patients. Journal of Clinical Endocrinology and Metabolism, 99(12), 4683–4689. doi: 10.1210/jc.2014-2206 [DOI] [PubMed] [Google Scholar]
- Rae K, Hollebone K, Chetty V, Clausen D, & McFarlane J (2007). Follistatin serum concentrations during full-term labour in women—significant differences between spontaneous and induced labour. Reproduction, 134(5), 705–711. doi: 10.1530/REP-07-0208 [DOI] [PubMed] [Google Scholar]
- Rasmussen KM, & Kjolhede CL (2004). Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics, 113(5), e465–e471. doi: 10.1542/peds.113.5.e465 [DOI] [PubMed] [Google Scholar]
- Rigg LA, & Yen SSC (1977). Multiphasic prolactin secretion during parturition in human subjects. American Journal of Obstetrics and Gynecology, 128(2), 215–218. doi: 10.1016/0002-9378(77)90692-5 [DOI] [PubMed] [Google Scholar]
- Salamalekis E, Pyrgiotis E, Phoca I, & Zourlas P (1991). Maternal serum cortisol and prolactin variations during labor. Clinical and Experimental Obstetrics and Gynecology, 18(3), 199–202. [PubMed] [Google Scholar]
- Stuebe AM, Horton BJ, Chetwynd E, Watkins S, Grewen K, & Meltzer-Brody S (2014). Prevalence and risk factors for early, undesired weaning attributed to lactation dysfunction. Journal of Women’s Health, 23(5), 404–412. doi: 10.1089/jwh.2013.4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torner L, Toschi N, Nava G, Clapp C, & Neumann ID (2002). Increased hypothalamic expression of prolactin in lactation: Involvement in behavioural and neuroendocrine stress responses. European Journal of Neuroscience, 15(8), 1381–1389. doi: 10.1046/j.1460-9568.2002.01965.x [DOI] [PubMed] [Google Scholar]
- Viero C, Shibuya I, Kitamura N, Verkhratsky A, Fujihara H, Katoh A, … Dayanithi G (2010). Oxytocin: Crossing the bridge between basic science and pharmacotherapy. CNS Neuroscience and Therapeutics, 16(5), e138–e156. doi: 10.1111/j.1755-5949.2010.00185.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wladimiroff J, Lo R, de Meijer M, Lamberts S, & Schalekamp M (1983). Maternal prolactin, cortisol, growth hormone and noradrenalin profiles during labor and following delivery. European Journal of Obstetrics and Gynecology and Reproductive Biology, 14(6), 365–369. doi: 10.1016/0028-2243(83)90204-6 [DOI] [PubMed] [Google Scholar]


