Abstract
A defect in the pksP gene of Aspergillus fumigatus is associated with the loss of conidial pigmentation, a profound change of the conidial surface structure, and reduced virulence. The structural change of the conidial surface structure was not observed in similar A. nidulans wA mutants. Our data indicate that the pigment of both species is important for scavenging reactive oxygen species and for protection of conidia against oxidative damage.
Aspergillus spp. are the predominant causative agents of invasive pulmonary aspergillosis (IPA), an often lethal infection of the immunocompromised host (4, 10, 13, 16). Since conidia are the infectious agent in IPA, recent studies focused on the elucidation of conidial factors contributing to pathogenicity (8, 17). Previously, we and others have shown that conidia lacking pigmentation due to the defective polyketide synthase gene pksP were less resistant to the attack by monocytes in vitro and showed reduced virulence in a murine animal model (8, 9, 17, 18). During these studies, it became apparent that coincubation of human phagocytes with pksP mutant conidia resulted in a marked increase in the release of reactive oxygen species (ROS) compared with wild-type (wt) conidia (8). Since a defective pksP gene not only impaired conidial pigmentation but concomitantly resulted in profound alterations of the conidial surface (8, 9), the question arose as to whether the large amounts of ROS detected after incubation of phagocytes with pksP mutant conidia were due to a change in the activation pattern of the cells or, alternatively, reflected the lack of ROS quenching capacity caused by the loss of conidial pigment. To address this question, conidia of wt strains of both Aspergillus fumigatus and the nonpathogenic fungus Aspergillus nidulans were compared with their respective pigmentless mutant strains.
The WA mutant of A. nidulans (strain WG370; wA3 bgaO biA1) lacking the conidial pigment due to a defective polyketide synthase gene (wA) was constructed by a sexual cross of appropriate parental strains (12) using standard genetic techniques (14). The wA gene product might have a function similar to that of the pksP gene product of A. fumigatus, although major differences between the pigment biosyntheses of the two Aspergillus species exist (2, 18; this study).
As previously reported, conidia of the A. fumigatus wt strain showed a rough surface; i.e., they had an ornamentation which was lacking in the pksP mutant strain (8, 9) (Fig. 1A and B). The A. nidulans wt conidia showed a similar ornamentation (Fig. 1C). In contrast to the pigmentless pksP mutant strain of A. fumigatus, however, similar pigmentless conidia of A. nidulans (wA) still exhibited the ornamentation characteristic of wild-type conidia (Fig. 1D). Taken together, the difference in surface structure between the A. fumigatus pksP mutant and the A. nidulans wA mutant further supports the assumption that different pathways exist for either conidial pigment biosynthesis or pigment deposition in the two species (1). As was noted previously with both cell types, i.e., human polymorphonuclear leukocytes (PMN) and monocytes, the amount of ROS detected in response to pigmentless A. fumigatus pksP mutant conidia was 10-fold higher than that of the respective wt conidia (8, 9) (Fig. 2A and B). To analyze the release of ROS upon incubation of the same cell types with A. nidulans conidia, ROS were measured on the basis of luminol-dependent chemiluminescence as was previously described (8). In brief, human PMN were prepared from freshly drawn heparinized blood and monocytes were isolated from the buffy coat. The cells were resuspended to give a final concentration of 2.5 × 106/ml in Hank's balanced salt solution–20 mM HEPES buffer (pH 7.3) containing 125 μM luminol (Sigma, Munich, Germany). Two hundred microliters of cell suspension was mixed with 20 μl of conidium suspensions in white flat-bottom microtiter plates (Greiner, Nürtingen, Germany) at an effector-to-target ratio of 1:10. Plates were placed in a microplate luminometer (Microlumat LB96p; EGG Berthold, Bad Wildbad, Germany) equipped with a temperature control device to keep plates at 37°C. Cells treated with 10 nM phorbol myristate acetate (PMA) (Sigma) served as positive controls, and cells incubated without conidia served as background controls. Wells were measured in 5 min intervals for 45 min. All assays were performed in duplicate.
FIG. 1.
Scanning electron micrographs of conidia. (A) A. fumigatus wild-type strain ATCC 46645. (B) A. fumigatus pksP mutant W (8, 9). (C) A. nidulans wild-type strain AXB4A (1). (D) A. nidulans wA mutant WG370 (this study).
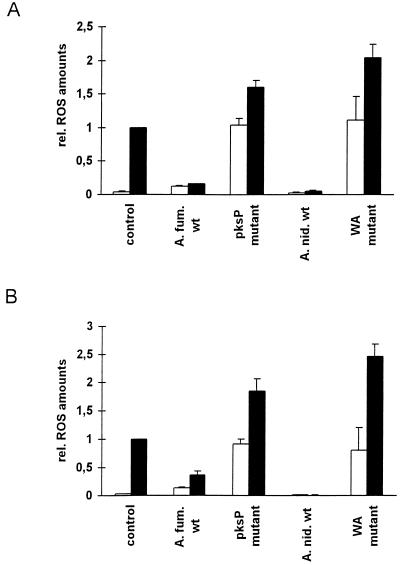
FIG. 2.
ROS release by phagocytes detected following their confrontation with conidia. Human monocytes (A) or human PMN (B) were incubated with conidia of the indicated strain (x axis) in the presence (■) or absence (□) of PMA. ROS release by untreated cells served as the background control. Data are given as relative ROS release. The release by PMA-stimulated cells was set to 1. The same strains described in the legend to Fig. 1 were used.
Results obtained with the A. nidulans conidia were similar to those obtained for A. fumigatus; i.e., the pigmentless wA mutant conidia led to a 10-fold increase in ROS release compared with wt conidia (Fig. 2A and B). The amounts of ROS released after coincubation of immune effector cells with pigmentless conidia were comparable to those observed after stimulation of the cells with PMA. Because the release of ROS was the same for both pigmentless A. fumigatus and A. nidulans conidia, the altered conidial surface of the A. fumigatus pigmentless mutant may not be responsible for the increased ROS release.
Some fungal pigments are known to quench ROS. This was shown, for example, in studies on oxidative damage of Wangiella dermatitidis and Cryptococcus neoformans and also on A. fumigatus conidia (3, 6–8). Therefore, it was conceivable that the apparent absence of oxidative burst by PMN in response to wt conidia of A. fumigatus or A. nidulans was due to a quenching effect of the wild-type pigments (11) (Fig. 2A and B). The ROS might well be released during the interaction of A. nidulans and A. fumigatus wt conidia with immune effector cells. However, their detection might be impaired due to immediate quenching of nascent ROS by the conidial pigment. To address this question, the ROS quenching ability of conidia was analyzed. For this purpose, the phagocytes were stimulated with 10 nM PMA in the presence or absence of A. nidulans and A. fumigatus wt as well as pigmentless mutant conidia (Fig. 2). When phagocytes were stimulated with PMA in the presence of wt conidia, the amounts of ROS detected decreased by 80 to 90% compared with the ROS release detected when PMA alone was applied (Fig. 2A and B). By contrast, stimulation of both PMN and monocytes by PMA in the presence of pksP or wA mutant conidia resulted in a 1.6- to 1.8-fold increase in detectable ROS compared with the stimulation observed with PMA alone, respectively, suggesting that the latter conidia cannot quench the ROS and, in addition, have an even additive stimulatory effect on ROS release (Fig. 2A and B). Taken together, these experiments strongly suggest that the pigments of both A. fumigatus and A. nidulans have ROS quenching ability.
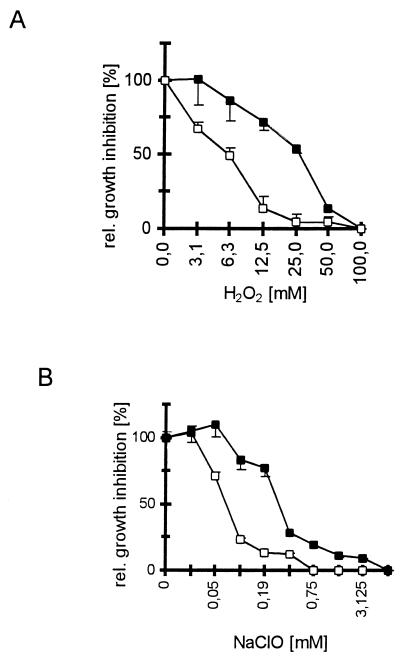
Further support for the hypothesis that Aspergillus conidial pigment functions as a ROS quencher was obtained from experiments on conidial damage by oxidizing agents. In a previous study, we had reported that pigmentless A. fumigatus pksP conidia were damaged significantly more by exposure to both H2O2 and NaOCl than were pigmented wt conidia (8). To test whether this is also true for A. nidulans, 1 × 106 conidia per microwell of both the pigmented wt and the pigmentless wA strain of A. nidulans were coincubated with either H2O2 or NaOCl at the concentrations indicated in Fig. 3. Subsequently, conidial growth was assessed by conversion of MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] (Serva, Heidelberg, Germany), and the relative growth inhibition was calculated. For comparison, oxidant concentrations leading to a 50% growth inhibition (IC50s) were determined. All procedures were carried out exactly as described previously (8). Exposure to H2O2 revealed an IC50 of 30 mM for A. nidulans wt conidia and 6 mM for wA conidia (Fig. 3A). The fivefold difference in IC50s observed here for A. nidulans pigmented and pigmentless conidia agreed well with previous reports on A. fumigatus conidia that demonstrated 10-fold-higher IC50s towards H2O2 or NaOCl of pigmented wt conidia compared with pigmentless pksP mutant conidia (8). When the susceptibility of A. nidulans conidia towards NaOCl was assessed, IC50s were found to be 0.2 mM for wt conidia and 0.07 mM for wA conidia (Fig. 3B). These results show that the loss of conidial pigment, in both A. nidulans and A. fumigatus, is linked with an increased susceptibility of conidia towards oxidizing agents. Taken together, these results strongly suggest that the release of ROS from human monocytes and PMN in response to pigmentless A. fumigatus conidia was not related to the change of the conidial surface, which is associated with the loss of the pigmentation only in A. fumigatus. Our findings indicate that the conidial pigments of both A. fumigatus and A. nidulans are potent quenching agents of ROS released from PMN and monocytes. Therefore, pigments might contribute to the relative resistance of conidia against the attack by neutrophils, as described for A. fumigatus (11). Furthermore, the lack of pigmentation resulted in an increased susceptibility towards oxidative attack, as seen in both A. nidulans and A. fumigatus (8). These findings underline the important role of fungal pigments, present in a variety of human mycopathogens, as protective agents against oxidant-based host defense mechanisms (5, 15, 19). However, they do not explain why A. fumigatus conidia can be pathogenic whereas this is rarely the case for A. nidulans conidia. Since the biosynthesis pathways of both conidia apparently differ, i.e., A. fumigatus apparently produces the conidial pigment via the 1,8-dihydroxynaphthalene (DHN)-melanin pathway, which seems to be lacking in A. nidulans (2, 17, 18), an attractive hypothesis is that during pigment biosynthesis A. fumigatus produces intermediates or shunt products interfering with an appropriate host response. Contrary to this hypothesis, Schnitzler et al. (15) recently reported that shunt products of DHN-melanin-deficient E. dermatitidis strains had no adverse effect on phagocytosis and intracellular killing by human PMN. However, A. fumigatus melanin biosynthesis apparently differs from the classical DHN-melanin pathway (18); hence, the possibility that during pigment biosynthesis in A. fumigatus compounds toxic to the immune effector cells contribute to the pathogenic potential of this human mycopathogen needs further elucidation. Alternatively, it is also conceivable that in addition to the DHN-melanin, the pksP gene product is also involved in the biosynthesis of other, yet unknown, polyketides which might be toxic to immune effector cells.
FIG. 3.
Susceptibility of pigmented and pigmentless A. nidulans conidia to damage by oxidants. The A. nidulans wt strain AXB4A (1) and the pigmentless wA strain WG370 (see the legend to Fig. 1) were used. First, 1 × 106 conidia per well of the wt (■) and wA (□) strains were incubated at 30°C for 14 h with H2O2 (A) or NaOCl (B), and then an MTT test (8) was performed on the wells. Wells incubated without oxidants served as the growth control, and wells containing only the oxidants served as background controls. The calculated damage, given as relative growth inhibition (y axis), was plotted against the respective oxidant concentration (x axis). All assays were run in duplicate (n = 3).
Acknowledgments
We gratefully acknowledge Sucharit Bhakdi for helpful discussions and Silvia Dobler for excellent technical assistance.
The work on A. fumigatus in the laboratories of A.A.B. and G.W. was supported by the Deutsche Forschungsgemeinschaft by grants Br-1130/5-3 and SFB 369, respectively.
REFERENCES
- 1.Brakhage A A, Browne P, Turner G. Regulation of Aspergillus nidulans penicillin biosynthesis and penicillin biosynthesis genes acvA and ipnA by glucose. J Bacteriol. 1992;174:3789–3799. doi: 10.1128/jb.174.11.3789-3799.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brakhage A A, Langfelder K, Wanner G, Schmidt A, Jahn B. Pigment biosynthesis and virulence. In: Brakhage A A, Jahn B, Schmidt A, editors. Aspergillus fumigatus: biology, clinical aspects and molecular approaches to pathogenicity. Basel, Switzerland: Karger AG; 1999. pp. 205–215. [Google Scholar]
- 3.Cooper C R, Jr, Szaniszlo P J. Melanin as a virulence factor in dematiaceous pathogenic fungi. In: Vanden Bossche H, Stevens D A, Odds F C, editors. Proceedings of the 5th Symposium on Topics in Mycology: Host-Fungus Interplay. Bethesda, Md: National Foundation for Infectious Diseases; 1997. pp. 81–93. [Google Scholar]
- 4.Denning D W. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–803. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 5.Dixon D M, Polak A, Szaniszlo P J. Pathogenicity and virulence of wild-type and melanin-deficient Wangiella dermatitidis. J Med Vet Mycol. 1987;25:97–106. doi: 10.1080/02681218780000141. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson E S, Hong J D. Redox buffering by melanin and Fe(II) in Cryptococcus neoformans. J Bacteriol. 1997;179:5340–5346. doi: 10.1128/jb.179.17.5340-5346.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson E S, Hove E, Emery H S. Antioxidant function of melanin in black fungi. Infect Immun. 1995;63:4944–4945. doi: 10.1128/iai.63.12.4944-4945.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jahn B, Koch A, Schmidt A, Wanner G, Gehringer H, Bhakdi S, Brakhage A A. Isolation and characterization of a pigmentless-conidium mutant of Aspergillus fumigatus with altered conidial surface and reduced virulence. Infect Immun. 1997;65:5110–5117. doi: 10.1128/iai.65.12.5110-5117.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langfelder K, Jahn B, Gehringer H, Schmidt A, Wanner G, Brakhage A A. Identification of a polyketide synthase gene (pksP) of Aspergillus fumigatus involved in conidial pigment biosynthesis and virulence. Med Microbiol Immunol. 1998;187:79–89. doi: 10.1007/s004300050077. [DOI] [PubMed] [Google Scholar]
- 10.Latgé J P. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levitz S M, Diamond R D. Mechanisms of resistance of Aspergillus fumigatus conidia to killing by neutrophils in vitro. J Infect Dis. 1985;152:33–42. doi: 10.1093/infdis/152.1.33. [DOI] [PubMed] [Google Scholar]
- 12.Mayorga M E, Timberlake W E. The developmentally regulated Aspergillus nidulans wA gene encodes a polypeptide homologous to polyketide and fatty acid synthases. Mol Gen Genet. 1992;235:205–212. doi: 10.1007/BF00279362. [DOI] [PubMed] [Google Scholar]
- 13.Ozsahin H, von Planta M, Muller I, Steinert H C, Nadal D, Lauener R, Tuchschmid P, Willi U V, Ozsahin M, Crompton N E, Seger R A. Successful treatment of invasive aspergillosis in chronic granulomatous disease by bone marrow transplantation, granulocyte colony-stimulating factor-mobilized granulocytes, and liposomal amphotericin-B. Blood. 1998;92:2719–2724. [PubMed] [Google Scholar]
- 14.Pontecorvo G, Roper J, Hemmons L, MacDonald K, Bufton A. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- 15.Schnitzler N, Peltroche-Llacsahuanga H, Bestier N, Zundorf J, Lutticken R, Haase G. Effect of melanin and carotenoids of Exophiala (Wangiella) dermatitidis on phagocytosis, oxidative burst, and killing by human neutrophils. Infect Immun. 1999;67:94–101. doi: 10.1128/iai.67.1.94-101.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segal B H, DeCarlo E S, Kwon-Chung K J, Malech H L, Gallin J I, Holland S M. Aspergillus nidulans infection in chronic granulomatous disease. Medicine (Baltimore) 1998;77:345–354. doi: 10.1097/00005792-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Tsai H F, Chang Y C, Washburn R G, Wheeler M H, Kwon-Chung K J. The developmentally regulated alb1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. J Bacteriol. 1998;180:3031–3038. doi: 10.1128/jb.180.12.3031-3038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai H F, Wheeler M H, Chang Y C, Kwon-Chung K J. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J Bacteriol. 1999;181:6469–6477. doi: 10.1128/jb.181.20.6469-6477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Aisen P, Casadevall A. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect Immun. 1995;63:3131–3136. doi: 10.1128/iai.63.8.3131-3136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]