Abstract
Background:
Preterm infants are at risk for severe infections due to their immature immune systems. Factors such as early life pain/stress experiences and feeding may influence immune activation and maturation of immune systems. However, the underlying mechanism remains unclear. Fecal calprotectin (FCP) is a noninvasive surrogate biomarker of mucosal inflammation in the gastrointestinal tract and has been used in detecting intestinal inflammation in specific pediatric gastrointestinal disorders.
Objective:
To describe the longitudinal trajectory of FCP levels in preterm infants and investigate the contributing factors that are associated with FCP levels.
Design:
A longitudinal study design was used.
Settings:
Preterm infants were recruited from 2 neonatal intensive care units (NICU) of a children’s medical center in the North-eastern US.
Methods:
Preterm infants were followed during their first 4 weeks of NICU hospitalization. Stool samples were collected twice per week to quantify the FCP levels. Cumulative pain/stress experiences and feeding types were measured daily. A linear mixed-effect model was used to examine the associations between FCP levels and demographic and clinical characteristics, cumulative pain/stress, and feeding over time.
Results:
Forty-nine preterm infants were included in the study. Infants’ FCP levels varied largely with a mean of 268.7±261.3 µg/g and increased over time. Preterm infants experienced an average of 7.5±5.0 acute painful procedures and 15.3±20.8 hours of chronic painful procedures per day during their NICU stay. The mean percentage of mother’s own milk increased from the first week (57.1±36.5%) to the fourth week (60.7±38.9%) after birth. Elevated FCP concentration was associated with acute and cumulative (chronic) pain/stress levels, mother’s own milk, non-White race, and higher severity of illness score.
Conclusions:
FCP levels were elevated in preterm infants with wide interindividual and intraindividual variations. Cumulative pain/stress during the NICU hospitalization, feeding, race, and health status may influence FCP concentrations in early life that may be associated with inflammatory gut processes.
Keywords: Fecal calprotectin, Gut inflammation, Preterm infant, Pain, Stress
Introduction
Preterm infants are at a greater risk of morbidity and mortality related to severe infections (ie, necrotizing enterocolitis) due to their developmental immaturity of the gut, characterized by immature (leaky) mucosal and epithelial barriers and immature immune systems1,2. Factors influencing the maturation of gut immune systems are multifactorial, which may include gestational age, feeding and nutrition, infection, antibiotic use, and gut microbiome colonization. Moreover, preterm infants are often exposed to numerous stressors, both physically and emotionally in the neonatal intensive care unit (NICU)3. Physical stress such as painful procedures in NICU were associated with elevated cortisol levels in early childhood4 and the microbial colonization in the developing gut5, which suggests early life stress may influence the programming of the immune system in preterm infants. However, there is a paucity of research exploring the effects of early life experience, especially cumulative stress and feeding on gut inflammation in preterm infants.
Fecal calprotectin (FCP) is a noninvasive surrogate biomarker of mucosal inflammation in the gastrointestinal tract6–9. Calprotectin accounts for about 60% of the cytosolic proteins in neutrophils10 and is involved in the process of neutrophil defense and immunoglobulin production11. Elevated FCP is related to the migration of neutrophil granulocytes into the luminal aspect of the intestinal mucosa, which usually occurs during intestinal inflammation12,13. As an extremely stable protein, FCP has become a useful assessment tool for detecting intestinal inflammation in specific pediatric gastrointestinal disorders14. Despite increased research on calprotectin in recent years, there is inadequate data available on FCP in preterm infants. Several studies have suggested that elevated FCP excretion may result from the maturation of the gut immune system rather than inflammation in neonates15,16. Therefore, it is important to understand the dynamic patterns of FCP and the factors that are involved in regulating FCP. Although previous studies have reported that FCP is associated with the birth delivery mode17, postnatal age18,19, feeding and nutrition8,15,19–21, and antibiotic treatment22,23, the results are somewhat inconsistent. Furthermore, the relationships between procedural stress and calprotectin levels in the neonatal period were not explored.
The objectives of this study were: (1) to examine the trend of FCP levels, cumulative pain/stress experience, and feeding types in preterm infants during the first four weeks of life in NICU; (2) to identify early life factors that are associated with FCP excretion in preterm infants.
Methods
Study design
A longitudinal cohort study design was used to investigate the trend of FCP levels and associated contributing factors in preterm infants. The present study was an extension of a clinical study to investigate the mechanisms of the gut-brain axis and the microbiome in the regulation of early-life pain/stress in preterm infants (K23NR014674). The original study used a prospective longitudinal design to examine preterm infants’ gut microbiome patterns over the first 4 weeks of NICU stay24. The study was approved by the Institutional Review Board at the research institute and the medical center where infants were recruited. Informed consent were obtained from the parent(s) or legal guardian(s) of infants before data collection.
Setting and participants
From 2013 to 2017, a total of 93 stable preterm infants born between 26 and 34 weeks of gestational age were recruited and followed for 3–4 weeks in the NICUs of a children’s medical center located in the Northeastern US. Infants from the original study who met the eligibility criteria were selected for this analysis. The inclusion criteria are: Infants who were born at 26–34 weeks gestational age, aged 0–3 days old, and cared for in an incubator. The infant’s mother or father had to be 18 years old and above to consent. Infants with the following conditions were excluded from the study: (1) known congenital anomalies; (2) severe periventricular/intraventricular hemorrhage (≥Grade III); (3) undergone minor or major surgery or procedures such as inguinal hernia repair, laparotomy, thoracotomy, diaphragmatic hernia repair, or intestinal resection; and (4) history of illicit drug exposure during the current pregnancy. Forty-nine infants from the original study who had complete daily pain/stressor data and had at least 1 stool sample per week for the first 4 weeks were selected for the present study. The effect size in detecting FCP as the gut inflammatory marker is 3.6 in colic infants25, and 0.96 in necrotizing enterocolitis infants26.
Outcome measures and data collection
Demographic data and health characteristics
Demographic information including gestational age at birth, birth weight, sex, mode of delivery, and rupture of membranes were extracted from the medical records. The severity of illness soon after birth was measured using the Score for Neonatal Acute Physiology–Perinatal Extension II (SNAPEII)27. Generic names and duration of antibiotics use (standard doses per day) postnatally were also retrieved from the medical database.
FCP assay
Daily stool samples from preterm infants were collected by research nurses in the original study using sterile, disposable spatulas during diaper changes and then placed into a sterile specimen container28. Upon collection, samples were immediately frozen at −80 °C and then transported to the University lab in a cooler containing dry ice and stored at −80 °C until processing. The infant’s stool samples on the third and seventh day of each week during the first 4 weeks of NICU stay were selected for assay and analysis. If the samples were unavailable on the above days, samples collected within 3 days before or after the targeted day were used for the analysis. FCP ELISA assay was performed using PhiCal kits (Calprest, Eurospital S.p.A, Trieste, Italy) on each select sample according to the manufacturer’s instructions. All the samples and standard controls from the kits were tested in duplicate and inter-assay and intra-assay coefficients of variation were calculated for quality control. The lowest detection limit of the kit is 6.25 ng/mL. The reported sensitivity and specificity of the PhiCal test in the pediatric population were 93.2% and 86.6%, respectively29.
Cumulative pain/stress
The Neonatal Infant Stressor Scale (NISS) is a checklist-based instrument to quantify cumulative procedural stressors preterm infants experience during their NICU stay30. The instrument was originally developed to measure NICU pain/stressors in Australia. We modified the NISS to include daily cumulative painful/stressful procedures that are common in NICU practices in the United States3,30. The modified NISS instrument consists of 70 acute painful/stressful procedures (eg, heel stick, and chest tube insertion) and chronic painful/stressful events (eg, nasogastric tube in situ and indwelling chest tube) in the categories of daily care, feeding, imaging, blood draw, peripheral venous access, central venous access, respiratory care, surgeries and major procedures, and infection. Each procedure/event is assigned a pain severity level from 1 to 5 (1=not painful/stressful; 2=a little; 3=moderate; 4=very; and 5=extremely painful/stressful). Acute pain/stress (weighted) scores were compiled by summing up the weighted frequency of each acute event, whereas chronic stress scores were calculated by summing up the weighted duration of each chronic event. Cumulative acute and chronic pain/stress were calculated, respectively, by summing up daily pain/stressor data for preterm infants’ first 4 weeks of life. The instrument was validated clinically with salivary cortisol31. The frequency of acute painful/stressful procedures and the hours of chronic painful/stressful events were collected prospectively by the infant’s bedside nurse on paper-based forms at each shift and were entered into REDCap by the research staff.
Feeding type
Infant daily feeding information, including the frequency of the infant fed by mother’s own milk (MOM), human donor’s milk (HDM), or formula over the first 4 weeks of life were also extracted from the medical record. The frequency and percentage of each feeding type (MOM, HDM, or formula) for each infant were calculated daily and over each week24.
Statistical analysis
The preterm infants’ demographic and clinical characteristics were evaluated by descriptive statistics. Visualization techniques conducted by R package ggplot2 were generated to present the trend of FCP levels of stool samples, acute and chronic pain/stress levels, and feeding types over the 4 weeks after infant birth. The relationships of acute and chronic pain/stress, feeding types, and antibiotic use with FCP levels were assessed by the linear mixed model (LMM) using the lme4 package in R32. The random intercept and random slope of preterm infants were considered in the LMM. Statistical inference was performed by the t test with Satterthwaite’s approximated degree of freedom using the lmerTest package in R33, which has a better type-I error control than other hypothesis testing methods for LMM34. All analyses were conducted in R 3.6.3.
Results
Infant characteristics
The demographic characteristics ( Table 1 ) of the 49 infants were female (51.0%), White (71.4%), and non-Hispanic (81.6%). The majority of infants were delivered by c-section (71.4%) at 31 (SD=1.7) gestational weeks with an average birth weight of 1477.4 (SD=417.9) g. Approximately 78% received resuscitation at delivery. The average SNAPEII score within 24 hours of birth was 6.4 (SD=8.9).
Table 1.
Demographic and clinical characteristics of preterm infants (N=49).
| Demographic | n (%) | |
|---|---|---|
| Sex | ||
| Male | 24 (49.0) | |
| Female | 25 (51.0) | |
| Race | ||
| White | 35 (71.4) | |
| African American | 11 (22.4) | |
| Multiple race | 2 (4.1) | |
| Not known | 1 (2.0) | |
| Ethnicity | ||
| Hispanic | 9 (18.4) | |
| Non-Hispanic | 40 (81.6) | |
| Delivery type | ||
| Vaginal | 14 (28.6) | |
| Cesarean section | 35 (71.4) | |
| PROM | ||
| Yes | 17 (34.7) | |
| No | 31 (63.3) | |
| Birth | ||
| Multiple birth | 21 (42.9) | |
| Single birth | 28 (57.1) | |
| Resuscitation at birth | ||
| Yes | 38 (77.6) | |
| No | 11 (22.4) | |
| Antibiotics use | ||
| Ampicillin | 32 (65.3) | |
| Gentamycin | 30 (61.2) | |
| Cefotaxime | 3 (6.1) | |
| Other* | 1 (2.0) | |
| Mean (SD) | Range | |
| Gestational age (wks) | 31.0 (1.7) | 26 1/7–33 3/7 |
| Birth weight (g) | 1477.4 (417.9) | 703–2640 |
| Birth length (cm) | 40.5 (3.2) | 32.5–47.0 |
| Birth head circumference (cm) | 28.2 (2.1) | 24.0–34.5 |
| SNAPEII | 6.4 (8.9) | 0–44 |
| Mother age (y) | 32.5 (5.7) | 19–46 |
Other: ceftazidime and sulfamethoxazole-trimethoprim.
PROM indicates premature rupture of membranes; SNAPEII, Score for Neonatal Acute Physiology–Perinatal Extension II.
Temporal trend in FCP concentration
A total of 296 stool samples collected during the 4 weeks of NICU stay were used for the FCP quantification. Of these samples, 48 were collected in week 1, 99 in week 2, 94 in week 3, and 55 in week 4. The earliest stool sample was collected on postnatal day 3. The overall mean FCP concentration over the first 4 weeks was 268.7±261.3 µg/g Figure 1 A illustrates the temporal trend of FCP levels over time on both daily and weekly scales. The mean FCP levels were high in week 1 (352.2±305.3 mg/g), then decreased in week 2 (164.0±193.6 mg/g), and gradually increased in week 3 (297.0±260.2 mg/g) and week 4 (336.1±274.1 mg/g).
Figure 1.
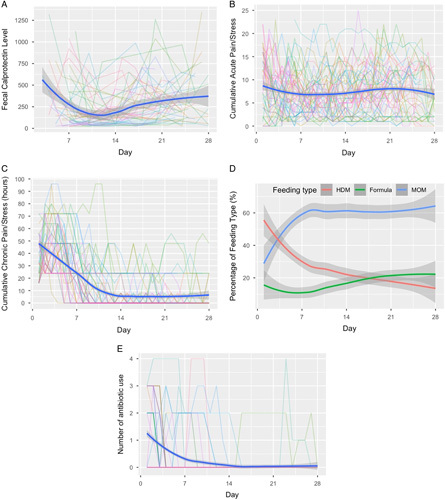
Trends of fecal calprotectin (FCP), pain/stress experience (NISS scores), feeding types and antibiotic use over the first four weeks (28 postnatal days) of NICU stay. (A) FCP levels. (B) Cumulative acute pain/stress. (C) Cumulative chronic pain/stress. (D) Percentage of feeding types in daily feeding; HDM = Human donor’s milk, MOM = Mother’s own milk. (E) Number of daily antibiotic use. Each colored line represents the outcome trajectory of each preterm infant over the first 28 postnatal days; The blue curve represents the longitudinal mean outcome values across all infants; the gray band represents the confidence interval of the mean outcome values.
Temporal trends in modified NISS scores
Trends of acute and chronic pain/stress experience (NISS scores) over time were depicted in ( Figs. 1 B, C ), respectively. Nine babies were discharged before the fourth week of follow-up, which reduced the sample size to 40 in week 4.
Cumulative acute pain/stress
The daily averaged frequencies of acute pain/stress activities experienced by the preterm infants were 7.7±4.1 in week 1, 6.9±3.6 in week 2, 7.7±4.2 in week 3, and 7.5±4.6 in week 4 ( Fig. 1 B ). The weighted acute pain/stress scores are presented in Supplemental Table 1 (Supplemental Digital Content 1, http://links.lww.com/NR9/A2). In the 4 weeks of NICU stay, preterm infants experienced an average of 7.5±5.0 acute pain/stress procedures per day, and the corresponding daily averaged acute pain/stress (weighted) score was 18.5±12.3.
Cumulative chronic pain/stress
During the first week after birth, infants experienced relatively high levels of chronic pain/stress (38.0±14.2 cumulative hours) and then the chronic pain/stress levels decreased in week 2 (10.1±13.5 cumulative hours). In weeks 3 and 4, 20 (40.8%) infants did not have documented chronic pain/stress and the daily averaged chronic pain/stress cumulative hours was 5.4±9.2 and 5.4±11.0 hours, respectively. The weighted chronic pain/stress scores are presented in Supplemental Table 1 (Supplemental Digital Content 1, http://links.lww.com/NR9/A2). On average, the preterm infants experienced 15.3±20.8 cumulative hours of chronic painful/stressful events per day during the first 4 weeks of NICU stay ( Fig. 1 C ).
Temporal trends in feeding types
The mean percentage of MOM feeding increased over the first week (57.1±36.5%) and then remained stable in week 2 (59.7±36.2%), week 3 (60.1±40.5%), and week 4 (60.7±38.9%) ( Fig. 1 D ). From week 1 to week 4 the percentage of HDM gradually decreased during the NICU stay and there was a slight increase in the percentage of daily formula milk intake over time (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/NR9/A2).
Temporal trends in antibiotic use
The trends of daily systemic antibiotic use were depicted in Figure 1 E and Supplemental Table 1 (Supplemental Digital Content 1, http://links.lww.com/NR9/A2). A majority of infants (n=27, 55.1%) received systemic antibiotics in the first 48 hours after birth. The number of infants who were given antibiotics decreased over time and only 3 infants (6.1%) received antibiotics in the last 2 weeks.
LMM and statistical inference
Both acute pain/stress and chronic pain/stress scores were significantly associated with FCP levels using a linear mixed-effect model ( Table 2 ). The likelihood ratio test was then applied and confirmed the statistically significant association between FCP and acute pain/stress (P=0.008) and between FCP and chronic pain/stress (P<0.001). Table 2 also presents the hypothesis testing results based on the t test with Satterthwaite’s approximated degree of freedom, showing a statistically significant interaction term by time for both acute pain (P=0.008) and chronic pain (P=0.004). The associations between pain and FCP level are also illustrated in Supplemental Figure 1 (Supplemental Digital Content 1, http://links.lww.com/NR9/A2). Notably, the negative effects of acute pain/stress on FCP in week 1 increased over time and converted to a positive effect in week 2, which continually increased for the remaining weeks (Supplemental Fig. 1A, Supplemental Digital Content 1, http://links.lww.com/NR9/A2). A positive association between chronic pain/stress and FCP was observed in weeks 1 and 2, which decreased to negative values in week 3 and continually decreased in week 4 (Supplemental Fig. 1B, Supplemental Digital Content 1, http://links.lww.com/NR9/A2).
Table 2.
Contributing factors to the FCP levels: results of the fixed effect in the linear mixed model.
| Fixed effect | Estimate | t | P |
|---|---|---|---|
| Week | 0.25 | 2.95 | 0.004** |
| Standardized acute pain/stress | −0.26 | −1.79 | 0.074 |
| Standardized chronic pain/stress | 0.50 | 4.46 | <0.001*** |
| Week* standardized acute pain/stress | 0.16 | 2.69 | 0.008** |
| Week* standardized chronic pain/stress | −0.20 | −2.98 | 0.004** |
| Feeding type in the last 3 d: mixed with formula milk | −0.33 | −2.21 | 0.028* |
| If use antibiotic in the last 7 d | 0.04 | 0.24 | 0.809 |
| Sex: female | −0.06 | −0.31 | 0.755 |
| Race: non-White | 0.50 | 2.13 | 0.039* |
| Ethnicity: non-Hispanic | 0.04 | 0.14 | 0.888 |
| Gestational age (wk) | 0.10 | 1.21 | 0.231 |
| Birth weight (kg) | −0.05 | −0.14 | 0.889 |
| Delivery: cesarean section | −0.33 | −1.29 | 0.204 |
| PROM: no | 0.15 | 0.69 | 0.493 |
| SNAPEII | 0.03 | 2.48 | 0.017* |
PROM indicates premature rupture of membranes; SNAPEII, Score for Neonatal Acute Physiology–Perinatal Extension II. *<0.05, **<0.01, ***<0.001.
Mean FCP levels were significantly higher in non-White infants compared with White infants (b=0.49, P=0.039) and for infants fed with MOM exclusively compared with those who were fed with a mix of MOM and formula (b=−0.33, P=0.028). In addition, we observed a significant positive association between the FCP level and SNAPEII (b=0.03, P=0.017). However, no statistically significant associations were found between FCP and antibiotic use, sex, birth gestational age, and birth weight.
Discussion
This study is one of the first to investigate infant gut immune development using the FCP measurement and explore the relationship between early life pain/stress and feeding with FCP in preterm infants. Our study showed that FCP levels changed over time and were dynamically associated with infant NICU experiences including postnatal acute and chronic painful/stressful events, and feeding types.
FCP, a cytoplasmic protein in neutrophil granulocytes, originates primarily from neutrophils as they migrate to the intestinal mucosa. Because calprotectin is not present in human milk35, a high FCP level likely reflects gut inflammation in preterm infants. The current study showed that FCP levels of preterm infants were elevated compared with healthy full-term infants, which is consistent with previous research22,36. Preterm infants with immature intestinal mucosal and enhanced intestinal permeability28,37 may result in increased transepithelial migration of neutrophils through intercellular junctions, and the release of calprotectin into the gut lumen38,39.
High interindividual and intraindividual variability of FCP was observed in our study and was confirmed not related to the technical aspects of the FCP assay. Indeed, all samples and standard controls were tested in duplicate with low inter-assay and intra-assay coefficient of variation; both were lower than 10% in the present study. In addition, our sample collection procedure strictly followed the institutional standard protocol, where samples were immediately collected at diaper change and transferred to a −80 °C freezer until analysis. Considering the stable nature of the FCP, it is unlikely that there were degradations of the FCP during the data collection procedure14. Our findings are consistent with previous studies showing high FCP levels and large interindividuals and intraindividual variation in preterm infants19,22,40.
In this cohort of preterm infants, FCP levels decreased from the first to the second week and then subsequently increased until the fourth week of life. This finding is congruent with previous studies18,40,41. FCP is extremely resistant to degradation both in vitro and in vivo. Therefore the elevated FCP level in meconium may not reflect the gut inflammation during the first week of life but rather be related to the cumulative effect of FCP in meconium during pregnancy. No correlation was found between infant gestational age and FCP levels. It is possible that the FCP level in infants before 34 weeks of gestational age remains constantly high and does not vary significantly within the window of time our measurements were made. Our findings add more specificity to the previous studies on FCP level and gestational age19,21,40,42,43.
We found that both daily acute and cumulative chronic pain/stress levels were significantly correlated with FCP levels in preterm infants during the NICU stay. Even though this is the first study conducted to examine the relationship between cumulative pain/stress experience and FCP levels in preterm infants, the findings supported our hypothesis that stress may be associated with the activation of inflammatory processes in the gut. Notably, the NISS scores were calculated daily based on the number of medical procedures the infants experienced in the NICU. Therefore, FCP may also reflect the inflammation directly caused by these medical procedures as well as the infants’ medical conditions. It is unclear why correlation patterns with FCP levels differed between acute and chronic pain/stress. In animal models, stress activates hypothalamic-pituitary-adrenal axis, results in neutrophil infiltration, increased epithelial permeability and abnormal expression of the proinflammatory cytokines and chemokines44. An earlier study found that salivary cortisol was positively correlated with acute pain/stress but not chronic pain/stress in preterm infants31. Taken together with our findings, it suggests acute and chronic pain/stress may regulate the immune system through different mechanistic pathways.
Some of the variations in FCP levels were also explained by the feeding regimen in our study. Infants who received exclusive MOM tended to have an increased FCP level compared with those fed with mixed type, which is in line with Groer et al’s8 report on very low birth weight infants during the first 4 weeks of life. Li et al21 and Asgarshirazi et al20 also reported that the FCP level was higher in breastmilk-fed infants than in nonbreastfed cohorts. The increased FCP level in MOM-fed preterm infants may suggest a protective effect of human milk in the immature gut and the effect may vary as the gut immune system matures. MOM contains microbiota and oligosaccharides, which are essential in establishing commensal bacteria and promoting the maturation of preterm infants’ guts45. Our previous study reported that MOM promotes the diversity of the gut microbiome and early transition to adult-like microbial patterns in preterm infants compared with HDM and formula24,45. Another study confirmed the effect of enteral bacteria in promoting FCP production, which may explain the postnatal changes in FCP levels in our population22. In addition, MOM contains more than 109 leukocytes per liter for the first several months of lactation as well as other bioactive substances such as pro-inflammatory cytokines, antibodies and other immune-stimulating factors20,46,47. Thus, elevated FCP may also be a response to these immune factors present in MOM.
This study also observed that the mean FCP level of the non-White infants was significantly higher than the White group, which is consistent with a recent study that reported higher FCP levels in African Americans than in White infants48. African American mothers have been found with elevated levels of inflammatory markers such as IL-4 and IL-6 during the second trimester of pregnancy which may contribute to the high preterm birth rate and also higher FCP level of their infants49. Our findings suggest that race and other social determinant health factors may be associated with biomarkers of gut inflammation including FCP that we need to address in infant health care.
No differences in FCP levels were found between infants with or without postnatal antibiotics use which may be due to the homogeneity of the antibiotics used, which is consistent with Groer et al’s study50. Other studies reported lower FCP levels in infants with antibiotic use18,22,40. Antibiotics have been known to influence gut bacterial colonization and disrupt microbial communities. In our study, infants were primarily administered ampicillin and gentamicin during the first 48 hours for prophylaxis from sepsis. Further investigation of the gut microbiome in association with FCP will provide greater insight into how antibiotics influence infants’ immune development in early life.
There are several limitations in this study. First, the mother’s medical information was not collected for this project. Mother’s antenatal conditions such as intrauterine inflammation and antibiotic use may influence the newborn’s FCP level. Secondly, we did not include measures of other inflammatory markers such as cytokines or c-reactive protein to map the sources and possible pathways of the inflammation. Previous evidence of FCP as an early biomarker in predicting intestinal distress and enteropathy is still somewhat inconsistent23,51. Thirdly, gut microbiome data were not included in this analysis to examine the correlations between the microbiome, FCP and stress. A previous study showed that FCP was correlated with the relative abundance of Klebsiella in preterm infants52, indicating that the gut microbiome may be an essential pathway in modulating the gut inflammatory process. Further studies are needed to explore the mediation effect of gut microbiome patterns on external stimuli—in particular stress—on gut inflammation.
Conclusion
This study demonstrates that preterm infants have high FCP concentrations with wide interindividual and intraindividual variation during the first 4 weeks of life. The elevated FCP levels are positively associated with higher MOM intakes, being non-White, and higher SNAPEII scores at birth. The relationship between FCP and pain/stress during NICUs staying varies over time. Further research including more comprehensive gut inflammatory measures is needed to clarify the role of pain/stress and gut health in preterm infants.
Author contribution
W.X.: conceptualization, writing (original draft preparation), funding acquisition. Y.Z.: formal analysis. W.Z.: investigation. J.C.: data curation and validation. K.M.: resources. N.H.: conceptualization and writing (review and editing). W.A.H.: methodology, funding acquisition. X.C.: conceptualization, writing (review and editing), funding acquisition.
Conflict of interest disclosures
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Sources of funding
This publication was supported by the American Nurses Foundation Academy of Neonatal Nurses, (PI: W.X.), National Association of Neonatal Nurses Small Grant, (PI: W.X.) National Institute of Nursing Research of the National Institutes of Health (NIH-NINR) under Award Number K23NR014674 (PI: X.C.) and NR016928 (PI: X.C.) and Affinity Research Collaboratives award through University of Connecticut Institute for Systems Genomics (PI: X.C.). Additional support from the Division of Intramural Research, NINR-NIH, 1ZIANR000018 (PI: W.A.H.).
Supplementary Material
Footnotes
W.X. and X.C. are co-corresponding authors.
Data Availability Statements: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Published online 23 November 2022
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.InterdisciplinaryNursingResearch.com.
Contributor Information
Wanli Xu, Email: wanli.xu@uconn.edu.
Yiming Zhang, Email: yiming.3.zhang@uconn.edu.
Wenxiao Zhao, Email: zhaowx912@163.com.
Jie Chen, Email: jie.2.chen@uconn.edu.
Naveed Hussain, Email: Hussain@uchc.edu.
Wendy A. Henderson, Email: wendy.henderson@uconn.edu.
Xiaomei Cong, Email: xiaomei.cong@yale.edu.
References
- 1. Adams M, Bassler D, Bucher HU, et al. Variability of very low birth weight infant outcome and practice in Swiss and US Neonatal Units. Pediatrics 2018;141(5):e20173436. [DOI] [PubMed] [Google Scholar]
- 2. Sharma AA, Jen R, Butler A, et al. The developing human preterm neonatal immune system: a case for more research in this area. Clin Immunol 2012;145(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cong X, Wu J, Vittner D, et al. The impact of cumulative pain/stress on neurobehavioral development of preterm infants in the NICU. Early Hum Dev 2017;108:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grunau RE, Cepeda IL, Chau CM, et al. Neonatal pain-related stress and NFKBIA genotype are associated with altered cortisol levels in preterm boys at school age. PLoS One 2013;8(9):e73926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun Z, Xu W, Cong X, et al. Log-contrast regression with functional compositional predictors: linking preterm infant’s gut microbiome trajectories to neurobehavioral outcome. Ann Appl Stat 2020;14(3):1535–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walsham NE, Sherwood RA. Fecal calprotectin in inflammatory bowel disease. Clin Exp Gastroenterol 2016;9:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ 2010;341:c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Groer M, Ashmeade T, Louis-Jacques A, et al. Relationships of feeding and mother’s own milk with fecal calprotectin levels in preterm infants. Breastfeed Med 2016;11:207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ho TT, Groer MW, Luciano AA, et al. Red blood cell transfusions increase fecal calprotectin levels in premature infants. J Perinatol 2015;35(10):837–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dale I, Brandtzaeg P, Fagerhol MK, et al. Distribution of a new myelomonocytic antigen (L1) in human peripheral blood leukocytes. Immunofluorescence and immunoperoxidase staining features in comparison with lysozyme and lactoferrin. Am J Clin Pathol 1985;84(1):24–34. [DOI] [PubMed] [Google Scholar]
- 11. Roseth AG, Fagerhol MK, Aadland E, et al. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol 1992;27(9):793–8. [DOI] [PubMed] [Google Scholar]
- 12. Roseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol 1999;34(1):50–54. [DOI] [PubMed] [Google Scholar]
- 13. Bjerke K, Halstensen TS, Jahnsen F, et al. Distribution of macrophages and granulocytes expressing L1 protein (calprotectin) in human Peyer’s patches compared with normal ileal lamina propria and mesenteric lymph nodes. Gut 1993;34(10):1357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koninckx CR, Donat E, Benninga MA, et al. The use of fecal calprotectin testing in paediatric disorders: a position paper of the European Society for Paediatric Gastroenterology and Nutrition Gastroenterology Committee. J Pediatr Gastroenterol Nutr 2021;72(4):617–40. [DOI] [PubMed] [Google Scholar]
- 15. Costa S, Patti ML, Perri A, et al. Effect of different milk diet on the level of fecal calprotectin in very preterm infants. Front Pediatr 2020;8:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Savino F, Castagno E, Calabrese R, et al. High faecal calprotectin levels in healthy, exclusively breast-fed infants. Neonatology 2010;97(4):299–304. [DOI] [PubMed] [Google Scholar]
- 17. Lee YM, Min CY, Choi YJ, et al. Delivery and feeding mode affects fecal calprotectin levels in infants <7months old. Early Hum Dev 2017;108:45–48. [DOI] [PubMed] [Google Scholar]
- 18. Park JS, Cho JY, Chung C, et al. Dynamic changes of fecal calprotectin and related clinical factors in neonates. Front Pediatr 2020;8:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moussa R, Khashana A, Kamel N, et al. Fecal calprotectin levels in preterm infants with and without feeding intolerance. J Pediatr (Rio J) 2016;92(5):486–92. [DOI] [PubMed] [Google Scholar]
- 20. Asgarshirazi M, Shariat M, Nayeri F, et al. Comparison of fecal calprotectin in exclusively breastfed and formula or mixed fed infants in the first six months of life. Acta Med Iran 2017;55(1):53–58. [PubMed] [Google Scholar]
- 21. Li F, Ma J, Geng S, et al. Comparison of the different kinds of feeding on the level of fecal calprotectin. Early Hum Dev 2014;90(9):471–5. [DOI] [PubMed] [Google Scholar]
- 22. Rouge C, Butel MJ, Piloquet H, et al. Fecal calprotectin excretion in preterm infants during the neonatal period. PLoS One 2010;5(6):e11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Campeotto F, Elie C, Rousseau C, et al. Faecal calprotectin and gut microbiota do not predict enteropathy in very preterm infants. Acta Paediatr 2021;110(1):109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cong X, Judge M, Xu W, et al. Influence of feeding type on gut microbiome development in hospitalized preterm infants. Nurs Res 2017;66(2):123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rhoads JM, Fatheree NY, Norori J, et al. Altered fecal microflora and increased fecal calprotectin in infants with colic. J Pediatr 2009;155(6):823–8.e821. [DOI] [PubMed] [Google Scholar]
- 26. Yoon JM, Park JY, Ko KO, et al. Fecal calprotectin concentration in neonatal necrotizing enterocolitis. Kor J Pediatr 2014;57(8):351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Richardson DK, Corcoran JD, Escobar GJ, et al. SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J Pediatr 2001;138(1):92–100. [DOI] [PubMed] [Google Scholar]
- 28. Cong X, Xu W, Romisher R, et al. Gut microbiome and infant health: brain-gut-microbiota axis and host genetic factors. Yale J Biol Med 2016;89(3):299–308. [PMC free article] [PubMed] [Google Scholar]
- 29. Radillo O, Pascolo L, Martelossi S, et al. Fecal calprotectin: diagnostic accuracy of the immunochromatographic CalFast assay in a pediatric population. J Clin Lab Anal 2016;30(5):500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Newnham CA, Inder TE, Milgrom J. Measuring preterm cumulative stressors within the NICU: the Neonatal Infant Stressor Scale. Early Hum Dev 2009;85(9):549–55. [DOI] [PubMed] [Google Scholar]
- 31. Pourkaviani S, Zhang X, Spear EA, et al. Clinical validation of the Neonatal Infant Stressor Scale with preterm infant salivary cortisol. Pediatr Res 2020;87(7):1237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bates D, Mächler M, Bolker B, et al. Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:14065823. 2014.
- 33. Kuznetsova A, Brockhoff PB, Christensen RH. lmerTest package: tests in linear mixed effects models. J Stat Softw 2017;82(1):1–26. [Google Scholar]
- 34. Luke SG. Evaluating significance in linear mixed-effects models in R. Behav Res Methods 2017;49(4):1494–502. [DOI] [PubMed] [Google Scholar]
- 35. Olafsdottir E, Aksnes L, Fluge G, et al. Faecal calprotectin levels in infants with infantile colic, healthy infants, children with inflammatory bowel disease, children with recurrent abdominal pain and healthy children. Acta Paediatr 2002;91(1):45–50. [DOI] [PubMed] [Google Scholar]
- 36. Kapel N, Campeotto F, Kalach N, et al. Faecal calprotectin in term and preterm neonates. J Pediatr Gastroenterol Nutr 2010;51(5):542–7. [DOI] [PubMed] [Google Scholar]
- 37. Weaver LT, Laker MF, Nelson R. Intestinal permeability in the newborn. Arch Dis Child 1984;59(3):236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parkos CA, Colgan SP, Delp C, et al. Neutrophil migration across a cultured epithelial monolayer elicits a biphasic resistance response representing sequential effects on transcellular and paracellular pathways. J Cell Biol 1992;117(4):757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Berstad A, Arslan G, Folvik G. Relationship between intestinal permeability and calprotectin concentration in gut lavage fluid. Scand J Gastroenterol 2000;35(1):64–69. [DOI] [PubMed] [Google Scholar]
- 40. Josefsson S, Bunn SK, Domellof M. Fecal calprotectin in very low birth weight infants. J Pediatr Gastroenterol Nutr 2007;44(4):407–13. [DOI] [PubMed] [Google Scholar]
- 41. Nakayuenyongsuk W, Christofferson M, Stevenson DK, et al. Point-of-care fecal calprotectin monitoring in preterm infants at risk for necrotizing enterocolitis. J Pediatr 2018;196:98–103. e101. [DOI] [PubMed] [Google Scholar]
- 42. Yang Q, Smith PB, Goldberg RN, et al. Dynamic change of fecal calprotectin in very low birth weight infants during the first month of life. Neonatology 2008;94(4):267–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Campeotto F, Kalach N, Lapillonne A, et al. Time course of faecal calprotectin in preterm newborns during the first month of life. Acta Paediatr 2007;96(10):1531–3. [DOI] [PubMed] [Google Scholar]
- 44. Deng Q, Chen H, Liu Y, et al. Psychological stress promotes neutrophil infiltration in colon tissue through adrenergic signaling in DSS-induced colitis model. Brain Behav Immun 2016;57:243–54. [DOI] [PubMed] [Google Scholar]
- 45. Xu W, Judge MP, Maas K, et al. Systematic review of the effect of enteral feeding on gut microbiota in preterm infants. J Obstet Gynecol Neonatal Nurs 2018;47(3):451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wallace JM, Ferguson SJ, Loane P, et al. Cytokines in human breast milk. Br J Biomed Sci 1997;54(2):85–87. [PubMed] [Google Scholar]
- 47. Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res 2007;61(1):2–8. [DOI] [PubMed] [Google Scholar]
- 48. Desorcy-Scherer K, Weaver M, Parker LA. Exploring social and demographic factors as determinants of intestinal inflammation in very low birth-weight infants. Adv Neonatal Care 2021;21(6):443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Giurgescu C, Engeland CG, Templin TN, et al. Racial discrimination predicts greater systemic inflammation in pregnant African American women. Appl Nurs Res 2016;32:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Groer MW, Gregory KE, Louis-Jacques A, et al. The very low birth weight infant microbiome and childhood health. Birth Defects Res C Embryo Today 2015;105(4):252–64. [DOI] [PubMed] [Google Scholar]
- 51. Jung JH, Park SH. Correlation between fecal calprotectin levels in meconium and vitamin D levels in cord blood: association with intestinal distress. J Clin Med 2020;9(12):4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ho TTB, Groer MW, Kane B, et al. Enteric dysbiosis and fecal calprotectin expression in premature infants. Pediatr Res 2019;85(3):361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


