Abstract
Introduction
Bipolar disorder (BD) is a chronic mental illness impacting 1–2% of the population worldwide and causing high rates of functional impairment. Patients with BD spend most of their time in depressive episodes and up to one-third of patients do not respond to adequate doses of medications. Although no consensus exists for definition of treatment-resistant bipolar depression (TRBD), failure of symptoms improvement despite an adequate trial of two therapeutic agents is a common theme of TRBD. In this paper, we review the evidence base of therapeutic interventions, challenges, and potential future directions for TRBD.
Methods
We conducted a literature search for randomized controlled trials on PubMed for the treatment of TRBD and ongoing trials for the treatment of TRBD/bipolar depression on clinicaltrials.gov.
Results
Several therapeutic agents have been investigated for TRBD. Adjunctive pramipexole and modafinil have data supporting short-term efficacy in TRBD, along with limited data for racemic intravenous ketamine. Celecoxib augmentation of escitalopram and treatment with metformin in patients with insulin resistance showed promising results. Right unilateral electroconvulsive therapy displayed statistically significant response rate and improvement, but not remission compared to pharmacotherapy. Trials for transcranial magnetic stimulation (TMS) have failed to show a significant difference from sham treatment in TRBD.
Future Trends
Pharmacological treatments with novel mechanisms of actions like brexpiprazole and vortioxetine are being investigated following successes in unipolar depression. Modified TMS protocols such as accelerated TMS are under investigation. Innovative approaches like psychedelic-assisted psychotherapy, interleukin-2, fecal microbiota transplantation and multipotent stromal cells are being studied.
Conclusion
Evidence on current treatment modalities for TRBD is limited with low efficacy. More research is needed for successful treatment of TRBD. Effective therapies and innovative approaches to treatment are being investigated and could show promise.
Keywords: TRBD, bipolar disorder, treatment-refractory depression, ketamine
Introduction
Bipolar disorder (BD) is a chronic mental illness that impacts 1–2% of the population worldwide.1 It causes high rates of functional impairment,2 poor quality of life, and has substantial economic effects.3 BD is characterized by episodes of depression and hypomania or mania, as well as subsyndromal symptoms that do not meet the full criteria for a mood episode, mostly of depressive nature and causing significant impairment.4 Likewise, the most time spent ill is in the depressive state rather than hypomania or mania in both type I and type II BD.5
It is not uncommon for patients to be refractory to treatments available for bipolar depression.6 Most mood stabilizers used for BD carry modest side-effect burden, such as metabolic syndrome and weight gain, while second-generation atypical antipsychotics (SGAs) have an additional risk of tardive dyskinesia. Unimodal antidepressants often prescribed in 40–50% of cases (but not FDA approved for BD) pose a substantial risk of treatment-emergent switch to mania/hypomania,7 further limiting the therapeutic options.8 A significant proportion (up to 33%) of patients are refractory to an adequate trial of therapeutic interventions. Even though treatment resistance is common in bipolar depression, there is relatively less research focused on treatment-resistant bipolar depression (TRBD). In part, this shortcoming could be attributed to the absence of a standardized definition of TRBD in the literature (Table 1).
Table 1.
Definitions of Treatment Resistance in Bipolar Depression
| Author / Study / Guideline | Definition/Criteria |
|---|---|
| Sachs, 19969 | Depression without remission despite two adequate trials of standard antidepressant agents (six weeks each), with or without augmentation strategies. |
| Yatham et al, 200310 | Non-response to a six-week trial with lithium at serum levels of ≥ 0.8 mmol ⁄L. |
| Nierenberg et al (STEP-BD study), 200611 | Bipolar disorder type I or II with a current DSM-IV major depressive episode of at least 8 weeks, and had not responded to treatment in first 12 weeks of treatment (according to the protocol) or had a well-documented failure to respond to at least two trials of antidepressants or an antidepressant and mood stabilizer. |
| Gitlin, 200612 | Criteria used for treatment-resistant unipolar depression would apply, with the proviso that failure to respond to mood stabilizers, as well as antidepressants. |
| Gajwani, 200913 | Stage I: Failed monotherapy trial of lithium, anticonvulsant, or atypical antipsychotic (quetiapine or olanzapine-fluoxetine combination) of adequate dose and for adequate duration. Stage II: Stage I plus failed trial of combination of two above mentioned medications. Stage III: Stage II plus failed trial of several different evidence-based adjunctive pharmacological compounds. Stage IV: Stage III plus neurostimulation (ie, ECT or VNS). |
| Pacchiarotti et al, 200914 | A depressive episode within bipolar disorder that fails to reach remission with adequately dosed lithium (0.8 mEq⁄l in the plasma) or to other adequate ongoing mood-stabilizing treatment, plus lamotrigine (50–200 mg⁄day) or with full dose (≥ 600 mg⁄day) of quetiapine as monotherapy (Additional definitions are provided for refractory, intractable, and involutional depression in bipolar disorder, indicating an increase in the degree of unresponsiveness.) |
| Lipsman et al, 201015 | Nonresponse to adequate trials of monotherapy with lithium or lamotrigine, as well as lithium or lamotrigine in combination with at least one anticonvulsant or antipsychotic. The addition of a third agent, if necessary, should be an antidepressant only. |
| Malhi et al, 201216 | Failure to reach remission despite two or three adequate trials of first-line medication, such as a mood stabilizer. |
| Poon et al, 201217 | Clinically unsatisfactory response following at least two, presumably adequate (by dose and duration), trials of dissimilar treatments within a specific phase of the illness (ie, depression), excluding patients who have responded, but are intolerant of the treatment regime. |
| Hidalgo-Mazzei et al, 201918 | Failure to reach sustained remission or tolerate at least two different adequate treatment trials, for at least 8 weeks at therapeutic doses with acceptable adherence, of monotherapy (quetiapine, lurasidone, lamotrigine, or olanzapine/ fluoxetine combination), or at least one of these as monotherapy and one of these in combination with lamotrigine, valproate, or lithium. Additional definition is provided for multi-therapy resistant bipolar depression. |
| Fountoulakis et al (CINP Guideline), 202019 | No significant reduction in MADRS/HDRS scores or significant increase in YMRS/MRS scores or YMRS and MRS scores exceed 5 and their recommended duration of treatment is 10–12 weeks. |
Abbreviations: CINP, International College of Neuropsychopharmacology; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders; fourth edition; ECT, Electroconvulsive therapy; HDRS, Hamilton Depression Rating Scale; MADRS, Montgomery–Asberg Depression Rating Scale; MRS, Mania Rating Scale; STEP-BD, Systematic Treatment Enhancement Program for Bipolar Disorder; VNS, Vagal nerve stimulation; YMRS, Young Mania Rating Scale.
Treatment resistance, or refractoriness, is frequently addressed in clinical practice guidelines on BD; however, the TRBD itself is not well defined.7,20–24 One exception is the most recent guideline of the International College of Neuropsychopharmacology (CINP) on treatment resistance in BD.19 Along with recommendations for interventions in treatment-resistant BD, the guideline defines acute TRBD, adapted from the International Society for Bipolar Disorders’ definition of treatment response and recovery (No significant reduction in Montgomery-Asberg Depression Rating Scale [MADRS] or Hamilton Depression Rating Scale [HDRS] scores or significant increase in Young Mania Rating Scale [YMRS] or Mania Rating Scale [MRS] scores or YMRS and MRS scores exceed 5, and the treatment duration is 10 to 12 weeks). They also emphasize that “nonresponse should be considered only after treatment according to the best evidence available.” Authors provided a treatment algorithm with varying degree of grading from level 1 to 4 for efficacy and recommendations.19
Another important milestone in TRBD research is the consensus definition study using a modified Delphi method by a representative panel of BD experts around the world.18 The panel established criteria for TRBD and multi-therapy-resistant bipolar depression in adults. Their established criteria for TRBD are failure to reach sustained remission or tolerate at least two different adequate treatment trials, for at least 8 weeks at therapeutic doses with acceptable adherence, of monotherapy (quetiapine, lurasidone, lamotrigine, or olanzapine/fluoxetine combination), or at least one of these as monotherapy and one of these in combination with lamotrigine, valproate or lithium, which was primarily based on the National Institute for Health and Care Excellence22 and the British Association for Psychopharmacology23 BD treatment guidelines. The panel also concluded that the same criteria should be used for both BD-I and BD-II, although the evidence base for treatment of BD-II is limited. The panel also established criteria for multi-therapy-resistant bipolar depression, where in addition to meeting TRBD criteria, it stipulates failure of at least one trial with an antidepressant for at least 8 weeks, cognitive behavioral therapy, and electroconvulsive therapy (ECT).
Considering that there have been only recent efforts to establish a consensus definition of TRBD, it is not surprising that most of the studies on TRBD use different criteria. As a result, it has been a challenge to compare the effectiveness of various interventions across studies. In this literature review, we aimed to summarize current evidence on available therapeutic interventions for TRBD and future directions in the field.
Methods
PubMed was queried with a combination of keywords used in the CINP study19 – “refractory” or “refractoriness” or “resistant” with “mania”, “manic”, “bipolar”, “manic-depressive”, or “manic depression”, on June 20, 2022. The keywords were chosen for their inclusivity and generalizability. No time frame had been set on the search parameters. When sufficiently available, only randomized clinical trials (RCTs) published in the English language literature were included. Additionally, systematic reviews were reviewed for studies not identified through initial search. We excluded review articles and comorbidities treatment studies. Initial review excluded studies based on the title. Remaining articles then underwent a review of the abstract. Identified articles were then read for content and included in the Tables 2–5. The discussion was then synthesized from the tables with additional background data added for clarification. Positive and negative studies that met the criteria were included. The following inclusion criteria were utilized:
Studies were in the English language.
Studies were reporting specifically on TRBD, not on affective disorders in general, BD or treatment-resistant depression (TRD). If the paper included different kinds of depression, there should be a stratification of the data regarding TRBD.
Studies were RCTs. Other relevant trials were mentioned for background information but not included in the tables or elaborated upon.
Studies were conducted on patients who continued to exhibit signs and symptoms of bipolar depression despite adequate trial of two appropriate medications for bipolar depression.
Post-hoc analyses were included.
Table 2.
Conventional Psychotropic Pharmacotherapy Studies for the Treatment of TRBD
| Author, Year | Study Design | Intervention | Sample Size (n) | Outcome measure | Key Outcome |
|---|---|---|---|---|---|
| Nierenberg et al, 200611 | 3-arm RCT | Lamotrigine | 66 | Rate of recovery | No significant difference between groups. |
| Inositol | Post-hoc analysis suggest lamotrigine may be superior. | ||||
| Risperidone | |||||
| Goldberg et al, 200432 | RCT | Placebo | 22 | Response (HDRS improvement >50%) | 67% of pramipexole responded vs 20% of placebo group. |
| Pramipexole (flexible dose) | CGI | Mean improvement in HDRS and CGI better in pramipexole group. | |||
| Frye et al, 200735 | RCT | Modafinil 177 mg/d | 85 | IDS | Significantly greater IDS change in treatment group compared with placebo. |
| Placebo | Response/remission | Response and remission were significant. | |||
| Murphy et al, 201433 | RCT | Naltrexone 50 mg/d | 30 | MADRS | No significant difference. |
| Placebo | HDRS |
Table 3.
Nonconventional Pharmacotherapy Studies for the Treatment of TRBD
| Author, Year | Study Design | Intervention | Sample Size (n) | Outcome Measure | Key Outcome |
|---|---|---|---|---|---|
| Unique RCTs Identified. | |||||
| Calkin et al, 202240 | RCT | Metformin 2000 mg/qd | 45 | MADRS | Significantly more IR-converters in metformin group. Converters experienced significant improvement in MADRS and GAF. |
| Placebo | GAF | ||||
| CGI-BP | |||||
| IR converters | |||||
| Halaris et al, 2020,39 Murata S et al, 202070 | RCT | Escitalopram (10–40 daily) + Celecoxib (200 mg twice daily) vs Escitalopram (10–40 daily) + placebo (twice daily) |
47 | HDRS HAM-A IL-1β |
Significantly more responders and remitters in Celecoxib group. Responders in the celecoxib group had a trend towards lower IL-1β. Significant decreases in HDRS and HAM-A scores at week 1. Celecoxib was well tolerated. |
| Post-hoc analyses of the above mentioned RCT (Halaris A et al 202039) | |||||
| Edberg et al, 202047 | Post-hoc analysis | Escitalopram (10–40 daily) + Celecoxib (200 mg twice daily) vs Escitalopram (10–40 daily) + placebo (twice daily) |
47 | Plasma MCP-1 levels | MCP-1 levels were not different in BDD vs HC subjects. |
| Castillo et al, 202045 | Post-hoc analysis | Escitalopram (10–40 daily) + Celecoxib (200 mg twice daily) vs Escitalopram (10–40 daily) + placebo (twice daily) 32 HC |
47 BD |
VEGF levels | VEGF was significantly higher at baseline in BD patients compared to HC. |
| No difference between BD groups after treatment. | |||||
| 32 HC | Baseline VEGF was a poor predictor of treatment response. | ||||
| Edberg et al, 201846 | Post-hoc analysis | Escitalopram (10–40 daily) + Celecoxib (200 mg twice daily) vs Escitalopram (10–40 daily) + placebo (twice daily) 32 HC |
47 BD | Plasma CRP | No significant difference in CRP levels between the groups (celecoxib and placebo) at baseline. Significant decrease in CRP levels among celecoxib group (versus placebo) by week 8. |
| 32 HC | |||||
Abbreviations: CGI, Clinical Global Impression Scale; CGI-BP, Clinical Global Impression Scale – Bipolar Version; CRP, C-reactive protein; GAF, Global Assessment of Functioning; HAM-A, Hamilton Rating Scale for Anxiety; HC, Healthy control; HDRS, Hamilton Depression Rating Scale; IDS, Inventory of Depressive Symptoms; IL-1β, Interleukin-1 beta; IR, Insulin Resistance; MADRS, Montgomery-Asberg Depression Rating Scale; MCP-1, Monocyte chemoattractant protein-1; RCT, Randomized controlled trial; TRBD, Treatment-resistant bipolar depression; VEGF, Vascular Endothelial Growth Factor.
Table 4.
Ketamine Studies for the Treatment of TRBD
| Author, Year | Study Design | Intervention | Sample Size (n) | Outcome measure | Key Outcome |
|---|---|---|---|---|---|
| Unique RCTs Identified | |||||
| Diazgranados et al, 201062 | RCT | Mood stabilizer+ Ketamine hydrochloride (0.5mg/kg) | 18 | MADRS | Significant improvement in depressive symptoms in ketamine group |
| Mood stabilizer +Placebo | Onset of action within 40 minutes. | ||||
| Zarate et al, 201263 | RCT | Mood stabilizer+ Ketamine hydrochloride (0.5mg/kg) | 15 | MADRS | Significant improvement in depressive symptoms in ketamine group |
| Mood stabilizer +Placebo | Onset in 40 min, effect lasted up to 3 days. | ||||
| Post-hoc analyses of the two previously mentioned RCTs | |||||
| Lally et al, 201465 | Post-hoc on 2 RCTs | Mood stabilizer + Ketamine hydrochloride (0.5mg/kg) | 36 | Level of anhedonia | Reduction of anhedonia independent of the reductions in general depressive symptoms |
| Mood stabilizer +Placebo | |||||
| Saligan et al, 201666 | Post-hoc on 2 RCTs | Mood stabilizer + Ketamine hydrochloride (0.5mg/kg) | 36 | Fatigue scores | Significantly lower fatigue scores in ketamine vs placebo at day 2 |
| Mood stabilizer +Placebo | |||||
| Xu et al, 201564 | Post-hoc on 2 RCTs | Lithium +Ketamine 0.5 mg/kg | 36 | MADRS | No statistically significant difference between mood stabilizer groups |
| Valproate + Ketamine 0.5 mg/kg | |||||
| Villasenor et al, 201467 | Post-hoc analysis | Ketamine | 22 | Metabolomic patterns | The metabolomic patterns were significantly different between the patients maintained on lithium and those maintained on valproate |
| Placebo | |||||
Table 5.
Randomized Controlled Trials of Electroconvulsive therapy
| Author, Year | Study Design | Intervention | Sample Size (n) | Outcome Measure | Key Outcomes |
|---|---|---|---|---|---|
| Unique RCTs Identified | |||||
| Sienaert et al, 200975 | RCT | Unilateral ECT 6 times threshold | 64 (13=Bipolar depression; 51=Unipolar depression) | HRSD | No difference between bipolar and unipolar depression in response or remission |
| Bifrontal ECT 1.5 times threshold | CGI | No difference in response to unilateral or bifrontal ECT | |||
| Patients with bipolar disorder showed more rapid response | |||||
| Bailine et al, 201076 | RCT | Right unilateral, bifrontal or bitemporal ECT | 220 (50=Bipolar depression; 170=Unipolar depression) | Remission/response rates | No difference between the groups |
| Schoeyen et al, 201572 | RCT | ECT vs algorithm-based pharmacological treatment | 73 | MADRS | ECT was more effective than algorithm-based pharmacotherapy. |
| Bjoerke-Bertheussen et al, 201874 | RCT follow-up study of Schoeyen et al72 | 6-months follow-up after: ECT vs Algorithm-based pharmacological treatment | 73 | MATRICS Consensus Cognitive Battery AMI-SF | MATRICS improved significantly in both groups No difference between groups |
| Post-hoc of the above mentioned RCT (Schoeyen H et al72) | |||||
| Kessler et al, 201473 | Post-hoc | ECT 3/week up to 6 weeks Algorithm-based pharmacological treatment | 73 | MATRICS Consensus Cognitive Battery AMI-SF | MATRICS improved significantly in both groups No difference between groups |
Abbreviations: AMI-SF, Autobiographical Memory Interview-Short Form; CGI, Clinical Global Impression Scale; ECT, Electroconvulsive therapy; HRSD, Hamilton Rating Scale for Depression; MADRS, Montgomery-Asberg Depression Rating Scale; MATRICS, Measurement and Treatment Research to Improve Cognition in Schizophrenia; RCT, Randomized controlled trial.
Results
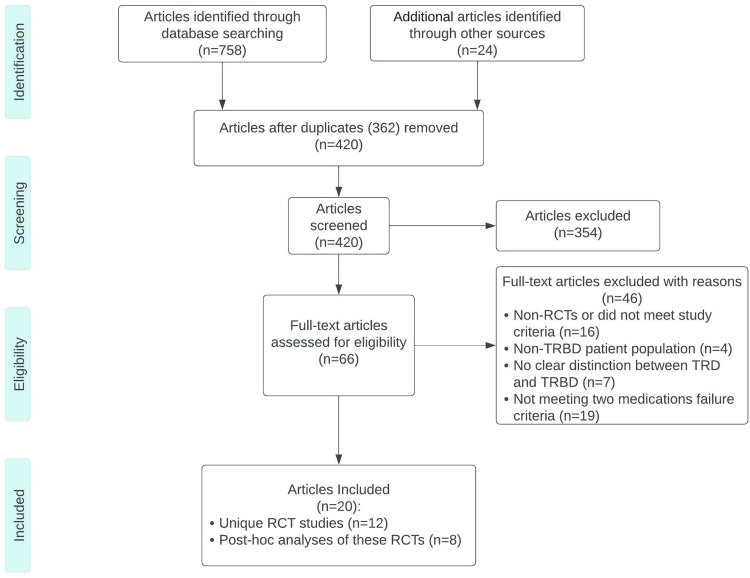
The results and studies included and extracted are summarized in the flowchart (Figure 1).
Figure 1.
The study flow chart showing the study identification and selection.
Abbreviations: RCT, randomized controlled trial; TRBD, treatment-resistant bipolar depression.
Conventional Psychotropic Medications (Table 2)
Studies that were conducted for the treatment of bipolar depression in patients who were already on a mood stabilizer support the addition of lamotrigine,25–27 L-sulpiride28 and lurasidone,29 but not imipramine30 or ziprasidone.31 These studies, however, were conducted on patients who are currently on a mood stabilizer but not necessarily failed a second medication, and thus do not meet our criteria for the diagnosis of TRBD (failure of two medications) and were thus excluded. Olanzapine-fluoxetine combination is FDA approved for treatment-resistant major depression but has not been investigated for TRBD.
In the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) equipoise study, 66 patients who were unresponsive to a mood stabilizer plus at least one antidepressant were randomized to receive lamotrigine (50 mg/d for 2 weeks followed by 50 mg twice daily for 2 weeks), inositol (2.5 to 5 g starting dose to a target of 10–15g/d), or risperidone (0.5 to 1 mg/d up to 6 mg as needed) for up to 16 weeks.11 While patients who were on lamotrigine had a 23.8% recovery rate compared to 17.4% and 4.6% in the inositol and risperidone groups, respectively, the differences did not reach statistical significance. Secondary outcomes on exit SUM-D (sum all depressive symptoms) scores were significantly lower in the lamotrigine group compared to inositol.
In a RCT comparing pramipexole to placebo in TRBD, pramipexole was significantly more effective than placebo in improving the HDRS score by ≥50% in 67% of the patients compared to 20% in placebo.32 Mean improvement in the Clinical Global Impression (CGI) and HDRS scores was also greater in the pramipexole group compared to placebo (48% vs 21%). 83% of the pramipexole group finished the study compared to 50% in the placebo group. This study was small (n = 22) and the high dropout rate should be taken into consideration.
Naltrexone displayed no significant difference when given in a 50mg/d dose compared to placebo in a post-hoc analysis on the MADRS and HDRS scales (p = 0.60 and p = 0.16 respectively).33
Adjunctive modafinil/armodafinil treatment has been investigated in several RCTs for bipolar depression.34 Adjunctive modafinil doses between 100 and 200 mg/day (mean dose of 177 mg/day) were shown to improve Inventory of Depressive Symptoms (IDS) scores significantly when compared with placebo in a 6-week trial among BD patients. Eighty-five patients on a mood stabilizer with or without antidepressant therapy were randomized to receive adjunctive modafinil or placebo.35 Percentage of participants who achieved a ≥50% improvement in IDS scores was greater in the modafinil group compared to placebo (43.9% vs 22.7%, p = 0.01). Remission rates were also higher (39% v 18%, p = 0.03). Notably, while the study did not stratify BD-I and BD-II patients, endpoint IDS scores controlling for baseline were significantly lower in BD-I compared with BD-II (p = 0.01). Three studies supported the efficacy of armodafinil, but because the patients were not required to have two failed medication trials, we did not include the evidence from those studies.36–38
Non-Conventional Psychotropic Medications (Table 3)
Because of the modest efficacy of conventional psychotropic medications, a shift in focus on research was geared towards medications that are not conventionally used for BD. Celecoxib39 and metformin40 have been studied as adjunctive medications for TRBD with varying degrees of success while pioglitazone41,42 had conflicting evidence in BD.
Dysregulation of the immune system was implicated as a factor contributing to the pathophysiology of BD.43 Additionally, studies using anti-inflammatories in major depressive disorder (MDD) suggest efficacy.44 Based on this premise, a group of researchers investigated the benefits of using celecoxib, a cyclooxygenase 2 (COX-2) inhibitor, in combination with escitalopram or placebo. Patients who had failed two or more medications (antidepressants and/or a mood stabilizer or SGA) for bipolar depression received escitalopram (10 mg twice daily) and randomized to receive celecoxib (200 mg twice daily) or placebo for 8-weeks.39 To minimize the risk of mania/hypomania, all the patients were prescribed a mood stabilizer (except lithium) and/or a SGA. Sixty-five participants were randomized, of which 55 completed the study and 47 had complete data sets to analyze. The odds ratio of response and remission in the celecoxib group compared to placebo were 4.13 (95% CI 1.03–18.48, p = 0.02) and 14.34 (95% CI 2.59–153.17, p < 0.0005), respectively. Two post-hoc analyses revealed that BD patients had significantly higher vascular endothelial growth factor levels at baseline that did not change with treatment, and C-reactive protein that decreased significantly more with treatment when compared to placebo.45,46 Monocyte chemoattractant protein-1 was negatively correlated in treatment non-responders and thus could predict response.47
Insulin resistance in type 2 diabetes mellitus is a risk factor of BD.48 Metformin (2000 mg/d) was therefore studied in 45 TRBD patients with insulin resistance in a quadruple-masked, parallel-group trial against placebo.40 MADRS scores showed significant improvement in insulin resistance converters (no longer met insulin resistance criteria) when compared to changes in non-converters. Transient gastrointestinal side effects occurred under both treatment conditions. Two studies that did not meet our criteria for TRBD have conflicting results on the use of pioglitazone in BD depression with one study supporting it41 and the other linking it with poor response.42
Thyroid hormone alterations have proven to play a role in mood regulation, and supplementation has shown to affect unipolar and bipolar depression;49 a retrospective chart review suggests treatment with triiodothyronine may be effective.50 However, there is a lack of RCTs investigating the efficacy of thyroid hormones in TRBD.
Ketamine (Table 4)
Several studies have reported that N-methyl-D-aspartate (NMDA)-receptor complexes are altered in patients with BD.51–53 Riluzole, a glutamatergic modulator, was shown to have antidepressant properties in BD while simultaneously found to enhance glutamatergic neurotransmission in cultured hippocampal mouse neurons.54,55 These lines of evidence seem to suggest that NMDA receptors play a role in BD.
Ketamine, an intravenous (IV) anesthetic, is a non-competitive NMDA receptor antagonist, has shown efficacy for TRD and TRBD.56–59 The S-enantiomer (esketamine) was recently FDA approved for TRD and MDD with suicidal ideation/self-injurious behaviors.60 Due to the considerable lag of onset of action in conventional bipolar depression therapies, the rapid action of ketamine, and the previous success of ketamine in TRD,61 sub-anesthetic doses of ketamine were investigated in two proof-of-concept placebo-controlled, randomized, double-blinded, crossover trials.62,63 In both studies, 18- to 65-year-old patients with TRBD without psychotic features who scored ≥20 on the MADRS scale at screening and baseline, and who had failed atleast 1 antidepressant trial and failed an open-trial of lithium or valproate (at least for 4 weeks) were selected. Patients were required to be on valproate or lithium and no other psychotropic medications throughout the study. Patients were randomized to receive either 0.5 mg/kg IV dose ketamine or placebo first. After two weeks, the groups underwent crossover to receive either ketamine or placebo for two more weeks. In the initial study,62 18 patients were randomized and 13 completed the study (72%). One dropped out during placebo phase and 4 during the ketamine phase. A linear mixed model indicated significant interaction between time and drug on the MADRS scale. Effect size was 0.52 (95% confidence interval [CI], 0.28–0.76) at 40 minutes with the largest effect shown two days after therapy. Post-hoc analysis showed a significant difference from minute 40 to 3 days after treatment (p < 0.001), but no difference at baseline, days 7, 10 or 14. Using valproate or lithium did not influence the result (p = 0.38). The replication study, conducted on 15 patients, had similar findings with post-hoc analysis indicating separation at 40 minutes through 3 days but not at 7, 10 or 14 days.63 79% of subjects responded to ketamine at some point during the study compared to 0% in the placebo group, whereas a considerable 64% of responders exhibited effect at 40 minutes. No serious adverse events were reported by either of the studies, with the most common adverse events in the ketamine group being dissociation, feeling strange, weird, or bizarre, dry mouth, tachycardia, and increased blood pressure. There was no statistically significant difference between the responses on the mood stabilizer groups (lithium vs valproate) (F1,28 = 2.51, P = 0.12, and d = 0.60) and no correlation between mood stabilizer level and ketamine antidepressant efficacy.64 These studies were limited by the small number of patients. None of the patients switched to mania while on ketamine, one patient switched in the placebo group. In a secondary analysis of the studies,64–66 ketamine significantly decreased levels of anhedonia independent of its depressive symptoms and caused significantly lower fatigue symptoms as compared to the placebo group. In a metabolomic analysis of 22 BD patients who received ketamine, blood samples were collected at 230 minutes post-administration and analyzed utilizing liquid chromatography; different metabolomic patterns between the lithium and valproate group were found.67 There were increases in lysophosphatidylethanolamines and lysophosphatidylcholines in responders compared to non-responders, suggesting alteration in mitochondrial beta-oxidation of fatty acids, although these differences were not due to ketamine. The current literature lacks evidence for serial infusions based on RCTs. Only two small, open-label studies using 6–8 serial infusions were conducted. The studies seem to suggest higher response rates than single infusion, but further exploration is needed.68,69,71
Tables 2–4 summarize the available published evidence based on RCTs of the pharmacotherapies for TRBD.
Neuromodulation
Electroconvulsive Therapy (Table-5)
Schoeyen et al72 conducted a RCT on TRBD comparing right unilateral ECT to algorithm-based pharmacological treatment. ECT comprised right unilateral placement of stimulus electrodes and brief pulse stimulation in 3 sessions per week for a period of 6 weeks. The right unilateral brief pulse ECT was significantly more effective than pharmacological treatment. The response rate in ECT vs pharmacotherapy (73.9% vs 35.0%, p=0.01) was significantly higher but there was little difference in the remission rate (34.8% vs 30.0%, p=0.74). The difference in mean scores for ECT and pharmacological treatment in MADRS scores was 6.6 points (SE = 2.05, 95% CI = 2.5–10.6), 9.4 points on the 30-item version of the Inventory of Depressive Symptomatology–Clinician-Rated (SE=2.49,95% CI = 4.6–14.3), and 0.7 points on the CGI (SE = 0.31, 95% CI = 0.13–1.36). The same clinical trial also studied the neurocognitive effects of ECT in bipolar depression, using Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) consensus cognitive battery.73 There were 39 patients that completed the neurocognitive assessments and there were no significant differences in the ECT group compared to pharmacological treatment group in terms of neurocognitive effects (no interaction effect; F1,37 = 1.52). Both groups had similar improvements in neurocognitive functions that correlated to improvement in depression scores post-treatment. However, there was a significant difference in autobiographical memory as measured by autobiographical memory interview-short form (AMI-SF). The scores were lower in ECT compared to the pharmacological group (72.9% vs 80.8%; p = 0.03). The findings fortify the use of ECT in the treatment of TRBD without compromising neurocognitive functions in patients. The same trial followed 26 patients up to 6 months and showed that MATRICS Consensus Cognitive Battery composite score improved by 4.1 points in both groups (p = 0.04) from baseline to 6 months (from 40.8 to 44.9 in pharmacological treatment and from 41.9 to 46.0 in the ECT group).74 They also reported a reduction in AMI-SF scores in both groups (72.3% pharmacological vs 64.3% ECT group; p = 0.09), thus, enhancing the evidence on no difference in the neurocognitive profile of patients between right unilateral ECT vs pharmacological treatment in bipolar depression.
A unique study compared the efficacy of ECT in bipolar depression to unipolar depression.75 The authors randomized 64 patients to bifrontal ECT and unilateral ECT, out of whom 13 patients had bipolar depression. Response rate was 84.62% in the bipolar group and 76.47% in the unipolar group, whereas remission was seen in 69% in bipolar and 65% in unipolar depression. There was no difference in response and remission, however, bipolar depression showed a more rapid rate of response to ECT compared to unipolar depression in the survival analysis. A similar multisite collaborative study was conducted to compare the relative efficacy of ECT in bipolar vs unipolar depression using three electrode placements: right unilateral, bifrontal, or bitemporal.76 22.7% of 220 patients had bipolar depression and the rest had unipolar depression. There was no difference in remission and response rates and number of ECT for both groups.
Table 5 summarizes the available evidence regarding ECT treatment for TRBD.
Transcranial Magnetic Stimulation
There have been three transcranial magnetic stimulation (TMS) studies so far and all failed to show a statistically significant difference compared to sham treatment for TRBD.77–79
Fitzgerald et al77 conducted a RCT on 49 patients using active sequential bilateral repetitive TMS (rTMS) for TRBD and reported no significant difference in mean reduction in depression rating scales or response rates at the end of 4 weeks between active and sham treatment.
Tavares et al78 investigated deep TMS (dTMS) in 50 TRBD patients who underwent 20 sessions of active or sham dTMS over the left dorsolateral prefrontal cortex. They reported active dTMS being superior to sham at 4 weeks (difference favoring dTMS = 4.88; 95% CI 0.43 to 9.32, p = 0.03), but no significant difference was seen at 8 weeks. A trend towards greater response rates was seen in the active (48%) vs sham (24%) groups (OR = 2.92; p = 0.08), but there was a nonsignificant difference in remission rates. The authors speculated dTMS was an effective and well-tolerated add-on treatment in TRBD patients on adequate pharmacotherapy.
Kito et al79 studied the effectiveness of conventional 37.5-minute vs 18.75-minute rTMS over the left prefrontal cortex in TRBD (n = 11) and treatment-resistant major depression (n = 19) patients. Treatment sessions were delivered for a total of 3000 pulses/day over 5 days a week, for 4‐6 weeks. 43.3% patients (13/30) showed remission at week 6. There were almost equal numbers of patients with TRBD (n = 5 in 37.5-min protocol, n = 6 in 18.75-min protocol) and treatment-resistant major depression (n = 10 in 37.5-min protocol, n = 9 in 18.75-min protocol) in both groups. There were no significant differences between the 37.5‐ and 18.75‐minute protocol groups in remission (46.7% vs 40.0%, p = 0.71) or response rates (60.0% vs 46.7%, p = 0.46) at week 6.
There was a single study on accelerated bilateral theta burst stimulation (TBS) among 300 TRD patients that included 36 patients with TRBD.80 It was a three-arm, single-blind RCT comparing accelerated bilateral TBS applied at 80–120% of the resting motor threshold, during which patients received 20 bilateral prefrontal TBS sessions over 10 days and left unilateral 10 Hz rTMS applied in 20 daily sessions to the left prefrontal cortex over 4 weeks. The overall treatment response rate was 43.7% and the remission rate was 28.2%; there were no significant differences across the three groups. The study reported no superior or rapid antidepressant effect with accelerated bilateral TBS compared to 10 Hz rTMS.
Vagus Nerve Stimulation (VNS)
Limited studies were available with VNS and most of them were conducted on mixed populations of TRD/TRBD and were open-label or prospective studies that did not meet our inclusion criteria. One RCT included a 10-week trial of VNS vs sham treatment in a group with a majority of TRD patients and few TRBD patients; however, this short-term study did not yield any short-term efficacy for adjunctive VNS treatment in TRD, but TRBD was not evaluated separately.81 This was followed by 2 years of an open treatment follow-up study82 to compare the response in TRBD patients (n = 25). The patients in VNS+treatment-as-usual (TAU) group had better initial response than patients in TAU group (63% vs 39%); also, the time to initial response was significantly quicker in VNS+TAU patients (p < 0.03). There was a significant reduction in mean suicidality score in the VNS+TAU group compared to the TAU group. Due to the nature of VNS, it was difficult to conduct the RCT. Thus, it is worth mentioning an open-label prospective study in TRBD over 5 years despite this not meeting our inclusion criteria.83 The authors reported a 63% response in VNS+TAU compared to only 39% in the TAU group, along with significant improvement in suicidality scores in the TAU+VNS group.
Deep Brain Stimulation
To our knowledge, there were no RCTs studying the effect of deep brain stimulation (DBS) in TRBD. An open-label pilot study supported the efficacy and long-term safety of DBS in TRD and TRBD (n = 7), with reported efficacy similar across both diagnoses.84 Further research is needed.
Future Trends and Directions
To gain a better understanding of the therapeutic interventions being investigated for bipolar depression, we also searched the ClinicalTrials.gov website for ongoing trials (Table 6).85–113 There are several studies examining the effectiveness of drugs that are already in use for other indications such as schizophrenia, MDD, or attention deficit and hyperactivity disorder in TRBD. Brexpiprazole is a promising agent that works as a partial agonist of dopamine D2 and D3 receptors, as well as the serotonin 5-HT1A receptor, and is used for schizophrenia and as an adjunct to antidepressants in MDD. There have been reports on its efficacy in bipolar depression114 and now it is being tested for treatment-resistant cases.88 A novel drug that has been recently found to be effective and safe in bipolar depression is lumateperone,115 which was initially approved for the treatment of schizophrenia. There is another ongoing trial for its efficacy in bipolar and unipolar depression with mixed features.85 Although it has not been investigated for treatment-resistant cases of bipolar depression, its unique mechanism of action (a serotonin 5-HT2A receptor antagonism, a dopamine D2 receptor presynaptic partial agonist and postsynaptic antagonist, a D1 receptor-dependent modulator of glutamate, and a serotonin reuptake inhibitor) makes it a good candidate for management of TRBD. Vortioxetine is a serotonin modulator and stimulator that is being studied for bipolar depression.86 It has been in use for MDD and has been found to be effective particularly in improving associated cognitive dysfunction.116 Another relatively new drug, an extended-release capsule of mixed salts of a single-entity amphetamine (Mydayis®) is approved for attention deficit and hyperactivity disorder treatment in adults. Its 16-hour extended duration of action makes it convenient for day-to-day use; it is being studied for its effectiveness and safety as an adjunct therapy in bipolar depression unresponsive to mood stabilizer treatment.87
Table 6.
Ongoing Bipolar Depression Trials Registered on ClinicalTrials.gov
| Bipolar Depression | Treatment Resistant Bipolar Depression | |
|---|---|---|
| Conventional drugs | ||
| Psychedelic drugs | ||
| Dietary supplement |
|
- |
| Neuromodulation | ||
| Other non-invasive interventions |
|
|
| Psychotherapy |
|
|
| Biological agents |
|
|
| Novel drugs |
Abbreviations: BD II, bipolar disorder type II; CBT, Cognitive Behavioral Therapy; IL-2, Interleukin-2; IV, intravenous.
Other than several ongoing ketamine studies that investigate its efficacy specifically in TRBD,89,91,117 there are several other substances with psychedelic effects being studied for bipolar depression: Xenon,97 an anesthetic drug and psilocybin,112,118 a naturally occurring alkaloid. There is preliminary evidence suggesting psilocybin-assisted psychotherapy’s efficacy in treatment-resistant major depression.119,120 The inclusion criteria for both ongoing psilocybin trials is BD-II depression, probably because it is a potent hallucinogen and to avoid possible induction of mania.121 While intranasal esketamine has been studied in treatment-resistant major/unipolar depression and showed promising results,122 there are currently no registered trials for its efficacy in TRBD on the ClinicalTrials.gov website.
Since ECT, TMS, and other neuromodulation therapies are extensively used in treatment-resistant mood disorders, it is not surprising that there are several studies evaluating the efficacy of emerging neuromodulation treatments like transcranial electric stimulation therapy96 or magnetic seizure therapy.105,123 Although the gold standard treatment remains ECT for TRBD, novel neuromodulation techniques have better tolerability. For example, one promising study is currently investigating intensive intermittent TBS, a patterned form of rTMS over a specific brain region, in TRBD.106 This has already been demonstrated to be as effective in treatment-resistant major/unipolar depression as standard forms of rTMS.94
Apart from those listed above, some other treatments that could be considered unconventional are being investigated for bipolar depression. These include low-dose interleukin-2 therapy,103 allogeneic fecal microbiota transplantation,108 and allogeneic bone marrow-derived multipotent mesenchymal stromal cell infusion,109 the latter being studied specifically for treatment-resistant cases.
Additionally, there are some drugs currently being studied specifically for bipolar depression, which might be considered for TRBD in the future. One is a non-racemic mixture consisting of 85% R-enantiomer and 15% S-enantiomer of amisulpride, potentially changing its receptor profile at the D2 and the 5-HT7.107
It would be reasonable to anticipate more clinical trials in bipolar depression for the drugs that have already been approved for the other indications, most notably schizophrenia and MDD. In the same way, other psychedelic substances and neuromodulation treatments are likely to be investigated for bipolar depression in the coming years, presumably following research on their efficacy and safety in MDD. However, studying psychedelics as monotherapy in the treatment of bipolar disorders is not encouraged currently because of their potential to trigger manic episodes.124 Thyroid-hormone augmentation for TRBD is another area which needs further investigation.125
Conclusion
Treatment-resistant bipolar depression is common and a challenging severe mental illness. Evidence on current treatment modalities for TRBD is limited and not uniform due to the lack of standardization of the definition and diagnosis. Most efforts at defining TRBD generally detail the failure of improvement in depression symptoms despite two presumably adequate trials of appropriate medications and are consistent with TRD definitions for major depression. Adjunctive pramipexole, modafinil, and racemic intravenous ketamine (limited data) support short-term efficacy in TRBD. Celecoxib augmentation of escitalopram and treatment with metformin in patients with insulin resistance showed promising results. Right unilateral ECT displayed a statistically significant response rate and improvement, but not remission, compared to pharmacotherapy.
Current TMS protocols are not as effective in TRBD; thus, further research is being conducted for modified TMS protocols. Other interventional modalities like VNS and DBS have not been adequately studied in TRBD and could be an exciting area of interest. Newer agents that have shown promise in MDD and TRD like brexpiprazole and vortioxetine can play a role and are currently being studied for the treatment of bipolar depression. Innovative approaches like psychedelic-assisted psychotherapy, interleukin-2, fecal microbiota transplantation, and multipotent stromal cells are being studied. There are currently no intranasal esketamine studies for TRBD; due to their efficacy in TRD, they should be considered for future TRBD trials. More research is needed for effective and innovative treatment approaches for TRBD.
Acknowledgments
We would like to thank Ms. Lori Solmonson for a thorough proofread of the manuscript.
Disclosure
B Singh has received research grant support from Mayo Clinic. Other authors have nothing to declare.
References
- 1.Clemente AS, Diniz BS, Nicolato R, et al. Bipolar disorder prevalence: a systematic review and meta-analysis of the literature. Braz J Psychiatry. 2015;37(2):155–161. doi: 10.1590/1516-4446-2012-1693 [DOI] [PubMed] [Google Scholar]
- 2.Burdick KE, Millett CE, Yocum AK, et al. Predictors of functional impairment in bipolar disorder: results from 13 cohorts from seven countries by the global bipolar cohort collaborative. Bipolar Disord. 2022;24(7):709–719. doi: 10.1111/bdi.13208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardner HH, Kleinman NL, Brook RA, Rajagopalan K, Brizee TJ, Smeeding JE. The economic impact of bipolar disorder in an employed population from an employer perspective. J Clin Psychiatry. 2006;67(8):1209–1218. doi: 10.4088/JCP.v67n0806 [DOI] [PubMed] [Google Scholar]
- 4.Altshuler LL, Post RM, Black DO, et al. Subsyndromal depressive symptoms are associated with functional impairment in patients with bipolar disorder: results of a large, multisite study. J Clin Psychiatry. 2006;67(10):1551–1560. doi: 10.4088/JCP.v67n1009 [DOI] [PubMed] [Google Scholar]
- 5.Kupka RW, Altshuler LL, Nolen WA, et al. Three times more days depressed than manic or hypomanic in both bipolar I and bipolar II disorder. Bipolar Disord. 2007;9(5):531–535. doi: 10.1111/j.1399-5618.2007.00467.x [DOI] [PubMed] [Google Scholar]
- 6.Mendlewicz J, Massat I, Linotte S, et al. Identification of clinical factors associated with resistance to antidepressants in bipolar depression: results from an European Multicentre Study. Int Clin Psychopharmacol. 2010;25(5):297–301. doi: 10.1097/YIC.0b013e32833c4ceb [DOI] [PubMed] [Google Scholar]
- 7.Yatham LN, Kennedy SH, Parikh SV, et al. Canadian network for mood and anxiety treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97–170. doi: 10.1111/bdi.12609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JH, Nunez NA, Gardea-Resendez M, et al. Short term second-generation antidepressant monotherapy in acute depressive episodes of bipolar II disorder: a systematic review and meta-analysis. Psychopharmacol Bull. 2022;52(2):45–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sachs GS. Treatment-resistant bipolar depression. Psychiatr Clin North Am. 1996;19(2):215–236. doi: 10.1016/S0193-953X(05)70285-9 [DOI] [PubMed] [Google Scholar]
- 10.Yatham LN, Calabrese JR, Kusumakar V. Bipolar depression: criteria for treatment selection, definition of refractoriness, and treatment options. Bipolar Disord. 2003;5(2):85–97. doi: 10.1034/j.1399-5618.2003.00019.x [DOI] [PubMed] [Google Scholar]
- 11.Nierenberg AA, Ostacher MJ, Calabrese JR, et al. Treatment-resistant bipolar depression: a STEP-BD equipoise randomized effectiveness trial of antidepressant augmentation with lamotrigine, inositol, or risperidone. Am J Psychiatry. 2006;163(2):210–216. doi: 10.1176/appi.ajp.163.2.210 [DOI] [PubMed] [Google Scholar]
- 12.Gitlin M. Treatment-resistant bipolar disorder. Mol Psychiatry. 2006;11(3):227–240. doi: 10.1038/sj.mp.4001793 [DOI] [PubMed] [Google Scholar]
- 13.Gajwani P. Treatment-refractory bipolar disorder: classification to aid in clinical management. Expert Opin Pharmacother. 2009;10(12):1907–1915. doi: 10.1517/14656560903064170 [DOI] [PubMed] [Google Scholar]
- 14.Pacchiarotti I, Mazzarini L, Colom F, et al. Treatment-resistant bipolar depression: towards a new definition. Acta Psychiatr Scand. 2009;120(6):429–440. doi: 10.1111/j.1600-0447.2009.01471.x [DOI] [PubMed] [Google Scholar]
- 15.Lipsman N, McIntyre RS, Giacobbe P, Torres C, Kennedy SH, Lozano AM. Neurosurgical treatment of bipolar depression: defining treatment resistance and identifying surgical targets. Bipolar Disord. 2010;12(7):691–701. doi: 10.1111/j.1399-5618.2010.00868.x [DOI] [PubMed] [Google Scholar]
- 16.Malhi GS, Bargh DM, Cashman E, Frye MA, Gitlin M. The clinical management of bipolar disorder complexity using a stratified model. Bipolar Disord. 2012;14(Suppl 2):66–89. doi: 10.1111/j.1399-5618.2012.00993.x [DOI] [PubMed] [Google Scholar]
- 17.Poon SH, Sim K, Sum MY, Kuswanto, CN, Baldessarini, RJ. Evidence-based options for treatment-resistant adult bipolar disorder patients. Bipolar Disord. 2012;14(6):573–584. doi: 10.1111/j.1399-5618.2012.01042.x. [DOI] [PubMed] [Google Scholar]
- 18.Hidalgo-Mazzei D, Berk M, Cipriani A, et al. Treatment-resistant and multi-therapy-resistant criteria for bipolar depression: consensus definition. Br J Psychiatry. 2019;214(1):27–35. doi: 10.1192/bjp.2018.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fountoulakis KN, Yatham LN, Grunze H, et al. The CINP guidelines on the definition and evidence-based interventions for treatment-resistant bipolar disorder. Int J Neuropsychopharmacol. 2020;23(4):230–256. doi: 10.1093/ijnp/pyz064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Practice Guideline for the Treatment of Patients with Bipolar Disorder (Revision). American Psychiatric Pub; 2002. [PubMed] [Google Scholar]
- 21.Grunze H, Vieta E, Goodwin GM, et al. The world federation of societies of biological psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2010 on the treatment of acute bipolar depression. World J Biol Psychiatry. 2010;11(2):81–109. doi: 10.3109/15622970903555881 [DOI] [PubMed] [Google Scholar]
- 22.National Institute for Health and Care Excellence (NICE). National institute for health and care excellence: guidelines. In: Bipolar Disorder: Assessment and Management. London: National Institute for Health and Care Excellence (NICE) Copyright © NICE 2019; 2018. [Google Scholar]
- 23.Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495–553. doi: 10.1177/0269881116636545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malhi GS, Bell E, Bassett D, et al. The 2020 royal Australian and New Zealand college of psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry. 2021;55(1):7–117. doi: 10.1177/0004867420979353 [DOI] [PubMed] [Google Scholar]
- 25.van der Loos ML, Mulder PG, Hartong EG, et al. Efficacy and safety of lamotrigine as add-on treatment to lithium in bipolar depression: a multicenter, double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70(2):223–231. doi: 10.4088/JCP.08m04152 [DOI] [PubMed] [Google Scholar]
- 26.Frye MA, Ketter TA, Kimbrell TA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;20(6):607–614. doi: 10.1097/00004714-200012000-00004 [DOI] [PubMed] [Google Scholar]
- 27.van der Loos ML, Mulder P, Hartong EG, et al. Efficacy and safety of two treatment algorithms in bipolar depression consisting of a combination of lithium, lamotrigine or placebo and paroxetine. Acta Psychiatr Scand. 2010;122(3):246–254. doi: 10.1111/j.1600-0447.2009.01537.x [DOI] [PubMed] [Google Scholar]
- 28.Bocchetta A, Bernardi F, Burrai C, Pedditzi M, Del Zompo M. A double-blind study of L-sulpiride versus amitriptyline in lithium-maintained bipolar depressives. Acta Psychiatr Scand. 1993;88(6):434–439. doi: 10.1111/j.1600-0447.1993.tb03487.x [DOI] [PubMed] [Google Scholar]
- 29.Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160–168. doi: 10.1176/appi.ajp.2013.13070984 [DOI] [PubMed] [Google Scholar]
- 30.Nemeroff CB, Evans DL, Gyulai L, et al. Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry. 2001;158(6):906–912. doi: 10.1176/appi.ajp.158.6.906 [DOI] [PubMed] [Google Scholar]
- 31.Sachs GS, Ice K, Chappell P. Efficacy and safety of adjunctive oral ziprasidone for acute treatment of depression in patients with bipolar I disorder. J Clin Psychiatry. 2011;72(10):1413–1422. doi: 10.4088/JCP.09m05934 [DOI] [PubMed] [Google Scholar]
- 32.Goldberg JF, Burdick KE, Endick CJ. Preliminary randomized, double-blind, placebo-controlled trial of pramipexole added to mood stabilizers for treatment-resistant bipolar depression. Am J Psychiatry. 2004;161(3):564–566. doi: 10.1176/appi.ajp.161.3.564 [DOI] [PubMed] [Google Scholar]
- 33.Murphy BL, Ravichandran C, Babb SM, Cohen BM. Naltrexone in bipolar disorder with depression: a double-blind, placebo-controlled study. J Clin Psychopharmacol. 2014;34(6):749–751. doi: 10.1097/JCP.0000000000000222 [DOI] [PubMed] [Google Scholar]
- 34.Nunez NA, Singh B, Romo-Nava F, et al. Efficacy and tolerability of adjunctive modafinil/armodafinil in bipolar depression: a meta-analysis of randomized controlled trials. Bipolar Disord. 2020;22(2):109–120. doi: 10.1111/bdi.12859 [DOI] [PubMed] [Google Scholar]
- 35.Frye MA, Grunze H, Suppes T, et al. A placebo-controlled evaluation of adjunctive modafinil in the treatment of bipolar depression. Am J Psychiatry. 2007;164(8):1242–1249. doi: 10.1176/appi.ajp.2007.06060981 [DOI] [PubMed] [Google Scholar]
- 36.Calabrese JR, Ketter TA, Youakim JM, Tiller JM, Yang R, Frye MA. Adjunctive armodafinil for major depressive episodes associated with bipolar I disorder: a randomized, multicenter, double-blind, placebo-controlled, proof-of-concept study. J Clin Psychiatry. 2010;71(10):1363–1370. doi: 10.4088/JCP.09m05900gry [DOI] [PubMed] [Google Scholar]
- 37.Calabrese JR, Frye MA, Yang R, Ketter TA. Efficacy and safety of adjunctive armodafinil in adults with major depressive episodes associated with bipolar I disorder: a randomized, double-blind, placebo-controlled, multicenter trial. J Clin Psychiatry. 2014;75(10):1054–1061. doi: 10.4088/JCP.13m08951 [DOI] [PubMed] [Google Scholar]
- 38.Ketter TA, Yang R, Frye MA. Adjunctive armodafinil for major depressive episodes associated with bipolar I disorder. J Affect Disord. 2015;181:87–91. doi: 10.1016/j.jad.2015.04.012 [DOI] [PubMed] [Google Scholar]
- 39.Halaris A, Cantos A, Johnson K, Hakimi M, Sinacore J. Modulation of the inflammatory response benefits treatment-resistant bipolar depression: a randomized clinical trial. J Affect Disord. 2020;261:145–152. doi: 10.1016/j.jad.2019.10.021 [DOI] [PubMed] [Google Scholar]
- 40.Calkin CV, Chengappa KNR, Cairns K, et al. Treating insulin resistance with metformin as a strategy to improve clinical outcomes in treatment-resistant bipolar depression (the TRIO-BD Study): a randomized, quadruple-masked, placebo-controlled clinical trial. J Clin Psychiatry. 2022;83:2. doi: 10.4088/JCP.21m14022 [DOI] [PubMed] [Google Scholar]
- 41.Zeinoddini A, Sorayani M, Hassanzadeh E, et al. Pioglitazone adjunctive therapy for depressive episode of bipolar disorder: a randomized, double-blind, placebo-controlled trial. Depress Anxiety. 2015;32(3):167–173. doi: 10.1002/da.22340 [DOI] [PubMed] [Google Scholar]
- 42.Aftab A, Kemp DE, Ganocy SJ, et al. Double-blind, placebo-controlled trial of pioglitazone for bipolar depression. J Affect Disord. 2019;245:957–964. doi: 10.1016/j.jad.2018.11.090 [DOI] [PubMed] [Google Scholar]
- 43.Fries GR, Walss-Bass C, Bauer ME, Teixeira AL. Revisiting inflammation in bipolar disorder. Pharmacol Biochem Behav. 2019;177:12–19. doi: 10.1016/j.pbb.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 44.Kohler-Forsberg O, Hjorthoj C, Nordentoft M, Mors O, Benros ME. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139(5):404–419. doi: 10.1111/acps.13016 [DOI] [PubMed] [Google Scholar]
- 45.Castillo MFR, Cohen A, Edberg D, et al. Vascular endothelial growth factor in bipolar depression: a potential biomarker for diagnosis and treatment outcome prediction. Psychiatry Res. 2020;284:112781. doi: 10.1016/j.psychres.2020.112781 [DOI] [PubMed] [Google Scholar]
- 46.Edberg D, Hoppensteadt D, Walborn A, Fareed J, Sinacore J, Halaris A. Plasma C-reactive protein levels in bipolar depression during cyclooxygenase-2 inhibitor combination treatment. J Psychiatr Res. 2018;102:1–7. doi: 10.1016/j.jpsychires.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 47.Edberg D, Hoppensteadt D, Walborn A, Fareed J, Sinacore J, Halaris A. Plasma MCP-1 levels in bipolar depression during cyclooxygenase-2 inhibitor combination treatment. J Psychiatr Res. 2020;129:189–197. doi: 10.1016/j.jpsychires.2020.06.010 [DOI] [PubMed] [Google Scholar]
- 48.Calkin CV, Ruzickova M, Uher R, et al. Insulin resistance and outcome in bipolar disorder. Br J Psychiatry. 2015;206(1):52–57. doi: 10.1192/bjp.bp.114.152850 [DOI] [PubMed] [Google Scholar]
- 49.Singh B, Sundaresh V. Thyroid hormone use in mood disorders: revisiting the evidence. J Clin Psychiatry. 2022;83:5. doi: 10.4088/JCP.22ac14590 [DOI] [PubMed] [Google Scholar]
- 50.Kelly T, Lieberman DZ. The use of triiodothyronine as an augmentation agent in treatment-resistant bipolar II and bipolar disorder NOS. J Affect Disord. 2009;116(3):222–226. doi: 10.1016/j.jad.2008.12.010 [DOI] [PubMed] [Google Scholar]
- 51.Law AJ, Deakin JF. Asymmetrical reductions of hippocampal NMDAR1 glutamate receptor mRNA in the psychoses. Neuroreport. 2001;12(13):2971–2974. doi: 10.1097/00001756-200109170-00043 [DOI] [PubMed] [Google Scholar]
- 52.McCullumsmith RE, Kristiansen LV, Beneyto M, Scarr E, Dean B, Meador-Woodruff JH. Decreased NR1, NR2A, and SAP102 transcript expression in the hippocampus in bipolar disorder. Brain Res. 2007;1127(1):108–118. doi: 10.1016/j.brainres.2006.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61(7):649–657. doi: 10.1001/archpsyc.61.7.649 [DOI] [PubMed] [Google Scholar]
- 54.Brennan BP, Hudson JI, Jensen JE, et al. Rapid enhancement of glutamatergic neurotransmission in bipolar depression following treatment with riluzole. Neuropsychopharmacology. 2010;35(3):834–846. doi: 10.1038/npp.2009.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zarate CA, Quiroz JA, Singh JB, et al. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry. 2005;57(4):430–432. doi: 10.1016/j.biopsych.2004.11.023 [DOI] [PubMed] [Google Scholar]
- 56.Sanacora G, Frye MA, McDonald, W. American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399–405. doi: 10.1001/jamapsychiatry.2017.0080 [DOI] [PubMed] [Google Scholar]
- 57.Gao M, Rejaei D, Liu H. Ketamine use in current clinical practice. Acta Pharmacol Sin. 2016;37(7):865–872. doi: 10.1038/aps.2016.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joseph B, Parsaik AK, Ahmed AT, Erwin PJ, Singh B. Systematic review on the efficacy of intravenous racemic ketamine for bipolar depression. J Clin Psychopharmacol. 2021. 41. 1:71–75. doi: 10.1097/JCP.0000000000001317 [DOI] [PubMed] [Google Scholar]
- 59.Singh B, Vande Voort JL, Frye MA, Kung S. Can ketamine be a safe option for treatment-resistant bipolar depression? Expert Opin Drug Saf. 2022;21(6):717–720. doi: 10.1080/14740338.2022.2045272 [DOI] [PubMed] [Google Scholar]
- 60.Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620–630. doi: 10.1176/appi.ajp.2018.17060720 [DOI] [PubMed] [Google Scholar]
- 61.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–354. doi: 10.1016/S0006-3223(99)00230-9 [DOI] [PubMed] [Google Scholar]
- 62.Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793–802. doi: 10.1001/archgenpsychiatry.2010.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zarate CA, Brutsche NE, Ibrahim L, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71(11):939–946. doi: 10.1016/j.biopsych.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu AJ, Niciu MJ, Lundin NB, et al. Lithium and valproate levels do not correlate with ketamine’s antidepressant efficacy in treatment-resistant bipolar depression. Neural Plast. 2015;2015:858251. doi: 10.1155/2015/858251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, Zarate CA. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saligan LN, Luckenbaugh DA, Slonena EE, Machado-Vieira R, Zarate CA. An assessment of the anti-fatigue effects of ketamine from a double-blind, placebo-controlled, crossover study in bipolar disorder. J Affect Disord. 2016;194:115–119. doi: 10.1016/j.jad.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Villasenor A, Ramamoorthy A, Silva Dos Santos M, et al. A pilot study of plasma metabolomic patterns from patients treated with ketamine for bipolar depression: evidence for a response-related difference in mitochondrial networks. Br J Pharmacol. 2014;171(8):2230–2242. doi: 10.1111/bph.12494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng W, Zhou YL, Wang CY, et al. Neurocognitive effects of six ketamine infusions and the association with antidepressant effects in treatment-resistant bipolar depression: a preliminary study. PeerJ. 2020;8:e10208. doi: 10.7717/peerj.10208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh B, Vande Voort JL, Kung S. Ketamine for treatment-resistant bipolar depression-need for more data! Bipolar Disord. 2021;23(7):728–729. doi: 10.1111/bdi.13129 [DOI] [PubMed] [Google Scholar]
- 70.Murata S, Murphy M, Hoppensteadt D, Fareed J, Welborn A, Halaris A. Effects of adjunctive inflammatory modulation on IL-1beta in treatment resistant bipolar depression. Brain Behav Immun. 2020;87:369–376. doi: 10.1016/j.bbi.2020.01.004 [DOI] [PubMed] [Google Scholar]
- 71.Wilkowska A, Wlodarczyk A, Galuszko-Wegielnik M, Wiglusz MS, Cubala WJ. Intravenous ketamine infusions in treatment-resistant bipolar depression: an open-label naturalistic observational study. Neuropsychiatr Dis Treat. 2021;17:2637–2646. doi: 10.2147/NDT.S325000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schoeyen HK, Kessler U, Andreassen OA, et al. Treatment-resistant bipolar depression: a randomized controlled trial of electroconvulsive therapy versus algorithm-based pharmacological treatment. Am J Psychiatry. 2015;172(1):41–51. doi: 10.1176/appi.ajp.2014.13111517 [DOI] [PubMed] [Google Scholar]
- 73.Kessler U, Schoeyen HK, Andreassen OA, et al. The effect of electroconvulsive therapy on neurocognitive function in treatment-resistant bipolar disorder depression. J Clin Psychiatry. 2014;75(11):e1306–e1313. doi: 10.4088/JCP.13m08960 [DOI] [PubMed] [Google Scholar]
- 74.Bjoerke-Bertheussen J, Schoeyen H, Andreassen OA, et al. Right unilateral electroconvulsive therapy does not cause more cognitive impairment than pharmacologic treatment in treatment-resistant bipolar depression: a 6-month randomized controlled trial follow-up study. Bipolar Disord. 2018;20(6):531–538. doi: 10.1111/bdi.12594 [DOI] [PubMed] [Google Scholar]
- 75.Sienaert P, Vansteelandt K, Demyttenaere K, Peuskens J. Ultra-brief pulse ECT in bipolar and unipolar depressive disorder: differences in speed of response. Bipolar Disord. 2009;11(4):418–424. doi: 10.1111/j.1399-5618.2009.00702.x [DOI] [PubMed] [Google Scholar]
- 76.Bailine S, Fink M, Knapp R, et al. Electroconvulsive therapy is equally effective in unipolar and bipolar depression. Acta Psychiatr Scand. 2010;121(6):431–436. doi: 10.1111/j.1600-0447.2009.01493.x [DOI] [PubMed] [Google Scholar]
- 77.Fitzgerald PB, Hoy KE, Elliot D, McQueen S, Wambeek LE, Daskalakis ZJ. A negative double-blind controlled trial of sequential bilateral rTMS in the treatment of bipolar depression. J Affect Disord. 2016;198:158–162. doi: 10.1016/j.jad.2016.03.052 [DOI] [PubMed] [Google Scholar]
- 78.Tavares DF, Myczkowski ML, Alberto RL, et al. Treatment of bipolar depression with deep TMS: results from a double-blind, randomized, parallel group, sham-controlled clinical trial. Neuropsychopharmacology. 2017;42(13):2593–2601. doi: 10.1038/npp.2017.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kito S, Miyazi M, Nakatani H, et al. Effectiveness of high-frequency left prefrontal repetitive transcranial magnetic stimulation in patients with treatment-resistant depression: a randomized clinical trial of 37.5-minute vs 18.75-minute protocol. Neuropsychopharmacol Rep. 2019;39(3):203–208. doi: 10.1002/npr2.12066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen L, Thomas EHX, Kaewpijit P, et al. Accelerated theta burst stimulation for the treatment of depression: a randomised controlled trial. Brain Stimul. 2021;14(5):1095–1105. doi: 10.1016/j.brs.2021.07.018 [DOI] [PubMed] [Google Scholar]
- 81.Rush AJ, Marangell LB, Sackeim HA, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005;58(5):347–354. doi: 10.1016/j.biopsych.2005.05.025 [DOI] [PubMed] [Google Scholar]
- 82.Nierenberg AA, Alpert JE, Gardner-Schuster EE, Seay S, Mischoulon D. Vagus nerve stimulation: 2-year outcomes for bipolar versus unipolar treatment-resistant depression. Biol Psychiatry. 2008;64(6):455–460. doi: 10.1016/j.biopsych.2008.04.036 [DOI] [PubMed] [Google Scholar]
- 83.McAllister-Williams RH, Sousa S, Kumar A, et al. The effects of vagus nerve stimulation on the course and outcomes of patients with bipolar disorder in a treatment-resistant depressive episode: a 5-year prospective registry. Int J Bipolar Disord. 2020;8(1):13. doi: 10.1186/s40345-020-0178-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holtzheimer PE, Kelley ME, Gross RE, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69(2):150–158. doi: 10.1001/archgenpsychiatry.2011.1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Intra-Cellular Therapies I. Clinical trial evaluating lumateperone monotherapy in the treatment of bipolar depression or major depressive disorder; 2020. Available from: https://ClinicalTrials.gov/show/NCT04285515. Accessed December 8, 2022.
- 86.University FAHoZ, University Z. Vortioxetine adjunctive treatment in bipolar depression; 2022. Available from: https://ClinicalTrials.gov/show/NCT05481957. Accessed December 8, 2022.
- 87.Clinic M, Hope L. 8 week multi-site study of MYDAYIS® for bipolar depression; 2020. Available from: https://ClinicalTrials.gov/show/NCT04235686. Accessed December 8, 2022.
- 88.Institute DMHU, University M, Hospital JG. Brexpiprazole treatment for bipolar I depression; 2021. Available from: https://ClinicalTrials.gov/show/NCT04569448. Accessed December 8, 2022.
- 89.University Health Network T. Maintenance ketamine infusions for treatment-resistant bipolar depression; 2022. Available from: https://ClinicalTrials.gov/show/NCT05339074. Accessed December 8, 2022.
- 90.Center SMH. Efficacy and safety of peropirone hydrochloride tablets in the treatment of adolescent bipolar disorder depression; 2020. Available from: https://ClinicalTrials.gov/show/NCT04826510. Accessed December 8, 2022.
- 91.Clinic M. Ketamine associated ACC GABA and glutamate change and depression remission; 2019. Available from: https://ClinicalTrials.gov/show/NCT03573349. Accessed December 8, 2022.
- 92.Consorcio Centro de Investigación Biomédica en Red MP, Institute SMR. Trehalose as add-on therapy in bipolar depression; 2016. Available from: https://ClinicalTrials.gov/show/NCT02800161. Accessed December 8, 2022.
- 93.Fe IdISL, Medicines SAo, Products H, Fe HUL. Efficacy of TBS in treatment resistant depression; 2021. Available from: https://ClinicalTrials.gov/show/NCT04998773. Accessed December 8, 2022.
- 94.Fitzgerald PB, Chen L, Richardson K, Daskalakis ZJ, Hoy KE. A pilot investigation of an intensive theta burst stimulation protocol for patients with treatment resistant depression. Brain Stimul. 2020;13(1):137–144. [DOI] [PubMed] [Google Scholar]
- 95.Hallahan DB, Institute SMR, National University of Ireland G, Ireland, HRB Clinical Research Facility Galway I, Galway UCH. Scopolamine in bipolar depression; 2021. Available form: https://ClinicalTrials.gov/show/NCT04211961. Accessed December 8, 2022.
- 96.Health NIoM, Center NIoHC. Transcranial Electric Stimulation Therapy (TEST) for Treatment Resistant Depression (TRD; 2022. Available from: https://ClinicalTrials.gov/show/NCT05172271. Accessed December 8, 2022.
- 97.Hospital MG. Xenon inhalation therapy for major depressive disorder and bipolar disorder; 2019. Available from: https://ClinicalTrials.gov/show/NCT03748446. Accessed December 8, 2022.
- 98.Hospital MG, Brain, Foundation BR, Willard J, Foundation ASM. BezafibrateTreatment for Bipolar Depression: a Proof of Concept Study; 2015. Available from: https://ClinicalTrials.gov/show/NCT02481245. Accessed December 8, 2022.
- 99.Janssen Pharmaceutica N.V. B. A Study of JNJ-55308942 in the treatment of bipolar depression; 2022. Available from: https://ClinicalTrials.gov/show/NCT05328297. Accessed December 8, 2022.
- 100.Kong CUoH. Adjunctive bright light therapy in patients with bipolar depression and eveningness; 2022. Available from: https://ClinicalTrials.gov/show/NCT05357313. Accessed December 8, 2022.
- 101.Korea Otsuka Pharmaceutical Co. A study of abilify® tablet (aripiprazole) as an adjunctive treatment in the bipolar depression; 2018. Available from: https://ClinicalTrials.gov/show/NCT03423680. Accessed December 8, 2022.
- 102.NeuroRx I, Prevail Infoworks I. NRX101 for bipolar depression and sub-acute suicidal ideation and behavior; 2022. Available from: https://ClinicalTrials.gov/show/NCT03395392. Accessed December 8, 2022.
- 103.Paris AP-Hd, Pharma I. Low dose IL-2 therapy in patients with a depressive episode in the course of a bipolar disorder; 2020. Available from: https://ClinicalTrials.gov/show/NCT04133233. Accessed December 8, 2022.
- 104.Paulo UoS. Creatine monohydrate as adjuvant therapy for bipolar depression; 2012. Available from: https://ClinicalTrials.gov/show/NCT01655030. Accessed December 8, 2022.
- 105.Paulo UoS, Paulo FdAàPdEdS. Electro-magnetic convulsive therapies for depression: a non-inferiority study; 2021. Available from: https://ClinicalTrials.gov/show/NCT05054699. Accessed December 8, 2022.
- 106.Pennsylvania Uo. Intensive TMS for bipolar depression; 2022. Available from: https://ClinicalTrials.gov/show/NCT05228457. Accessed December 8, 2022.
- 107.Sunovion. A clinical study of an investigational drug for the treatment of major depressive episode associated with bipolar I disorder; 2021. Available from: https://ClinicalTrials.gov/show/NCT05169710. Accessed December 8, 2022.
- 108.Taylor V, University Health Network T, Hospital WsC. Safety and efficacy of fecal microbiota transplantation in a population with bipolar disorder; 2018. Available from: https://ClinicalTrials.gov/show/NCT03279224. Accessed December 8, 2022.
- 109.The University of Texas Health Science Center H. Adjunctive allogeneic mesenchymal stem cells for treatment-resistant bipolar depression; 2021. Available from: https://ClinicalTrials.gov/show/NCT03522545. Accessed December 8, 2022.
- 110.University G, AB ACR. OSU6162 in bipolar depression (OBID); 2021. Available from: https://ClinicalTrials.gov/show/NCT05296356. Accessed December 8, 2022.
- 111.University JH, Fund BBR, Medical M, Austin UoTa. iTBS in bipolar I depression; 2022. Available from: https://ClinicalTrials.gov/show/NCT05375214. Accessed December 8, 2022.
- 112.University of California SF. Psilocybin therapy for depression in bipolar II disorder; 2022. Available from: https://ClinicalTrials.gov/show/NCT05065294. Accessed December 8, 2022.
- 113.University Qs, Clinic OP. Delivering electronic cognitive behavioural therapy to patients with bipolar disorder and residual depressive symptoms; 2020. Available from: https://ClinicalTrials.gov/show/NCT04664257. Accessed December 8, 2022.
- 114.Brown ES, Khaleghi N, Van Enkevort E, et al. A pilot study of brexpiprazole for bipolar depression. J Affect Disord. 2019;249:315–318. doi: 10.1016/j.jad.2019.02.056 [DOI] [PubMed] [Google Scholar]
- 115.Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry. 2021;178(12):1098–1106. doi: 10.1176/appi.ajp.2021.20091339 [DOI] [PubMed] [Google Scholar]
- 116.Frampton JE. Vortioxetine: a review in cognitive dysfunction in depression. Drugs. 2016;76(17):1675–1682. doi: 10.1007/s40265-016-0655-3 [DOI] [PubMed] [Google Scholar]
- 117.Gdansk MUo. A naturalistic study of ketamine for treatment resistant mood disorders; 2019. Available from: https://ClinicalTrials.gov/show/NCT04226963. Accessed December 8, 2022.
- 118.System SPH, Pathways C. The safety and efficacy of psilocybin in participants with type 2 bipolar disorder (BP-II) depression; 2021. Available from: https://ClinicalTrials.gov/show/NCT04433845. Accessed December 8, 2022.
- 119.Carhart-Harris RL, Bolstridge M, Rucker J, et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry. 2016;3(7):619–627. doi: 10.1016/S2215-0366(16)30065-7 [DOI] [PubMed] [Google Scholar]
- 120.Kisely S, Connor M, Somogyi AA, Siskind D. A systematic literature review and meta-analysis of the effect of psilocybin and methylenedioxymethamphetamine on mental, behavioural or developmental disorders. Aust N Z J Psychiatry. 2022;48674221083868. doi: 10.1177/00048674221083868 [DOI] [PubMed] [Google Scholar]
- 121.Hendin HM, Penn AD. An episode of mania following self-reported ingestion of psilocybin mushrooms in a woman previously not diagnosed with bipolar disorder: a case report. Bipolar Disord. 2021;23(7):733–735. doi: 10.1111/bdi.13095 [DOI] [PubMed] [Google Scholar]
- 122.McIntyre RS, Rosenblat JD, Nemeroff CB, et al. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am J Psychiatry. 2021;178(5):383–399. doi: 10.1176/appi.ajp.2020.20081251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.The University of Texas Health Science Center H. Magnetic seizure therapy in bipolar depression (MST-BpD); 2022. Available from: https://ClinicalTrials.gov/show/NCT04080778. Accessed December 8, 2022.
- 124.Bosch OG, Halm S, Seifritz E. Psychedelics in the treatment of unipolar and bipolar depression. Int J Bipolar Disord. 2022;10(1):18. doi: 10.1186/s40345-022-00265-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Seshadri A, Sundaresh V, Prokop LJ, Singh B. Thyroid hormone augmentation for bipolar disorder: a systematic review. Brain Sci. 2022;12(11):1540. doi: 10.3390/brainsci12111540 [DOI] [PMC free article] [PubMed] [Google Scholar]