Abstract
Introduction:
Cognitive impairment is common in the course of Parkinson’s disease (PD) and displays a continuum from subjective cognitive impairment to dementia. Illuminating the pathophysiological processes associated with the continuum may help create follow-up and new treatment approaches. In this context, large-scale intrinsic connectivity networks are widely investigated to elucidate the neural processes underlying PD and are promising as non-invasive biomarkers. This systematic review aims to examine the alterations in large-scale intrinsic connectivity networks in the continuum of PD-associated cognitive impairment.
Method:
ScienceDirect, Web of Science, and PubMed databases were searched with the specified keywords. The studies obtained as a result of this review were investigated by the PRISMA criteria, which were taken as a basis for the systematic review and writing of meta-analyses.
Results:
A total of 974 studies were obtained from three databases. Twenty studies were included in the systematic review based on predetermined eligibility criteria. Among the large-scale connectivity networks examined in these studies, it was found that sensory-motor networks decreased their connectivity in the continuum of PD-associated cognitive impairment, and there were conflicting results in terms of cognitive networks.
Conclusion:
Well-designed longitudinal studies are needed to clarify the alterations in the intrinsic connectivity networks in the PD cognitive impairment continuum. In these studies, it is necessary to define the cognitive disorder groups well, to control the connectivity changes that may occur due to dopaminergic treatment, and to evaluate Parkinson’s patients with subjective cognitive impairment and dementia within the continuum.
Keywords: Functional magnetic resonance imaging, intrinsic connectivity networks, Parkinson’s disease, Parkinson’s disease dementia, Parkinson’s disease with mild cognitive impairment, resting-state networks
INTRODUCTION
The current opinion on brain functions is based on representing various information domains in segregated neural communities and integrating pieces of information through dynamic interactions between circuits at multiple spatial scales of the central nervous system. This view derives from a wide variety of studies based on measurement techniques applied at various spatial scales of the organization of the nervous system organization, ranging from submicroscopic scale of molecular events and the microscopic scale of single-cell recordings to the mesoscopic scale of interaction within local-scale neuronal ensembles and the macroscopic scale of large-scale brain networks. The common point of all previous studies conducted based on the spatial scales mentioned above is that they are either carried out to inspect the development and change of the brain in healthy people or to elucidate the pathogenetic processes of various neuropsychiatric diseases. Regarding neuropsychiatric diseases, one of the most studied subjects is cognitive disorders. Recent studies on cognition have increasingly focused on the network paradigm (1). This paradigm postulates that cognitive functions arise from the collaborative work of distributed and interconnected brain systems organized into large-scale networks (2).
In this context, functional magnetic resonance imaging (fMRI) has been the most widely used method for examining large-scale brain networks (3). fMRI is the recording of brain activity based on changes in the level of cerebral blood oxygenation. There are two main areas where information about how the brain works can be obtained by examining brain activity. The first is localization, which tries to associate certain brain areas with specific functions. Many researchers use behavioral tasks that participants perform in fMRI to localize functionally specialized regions of the brain that are activated in response to a particular aspect of behavior, and task-related activation is localized by measuring blood oxygenation level-dependent (BOLD) signal across different conditions (4).
Another approach used to study brain activity is connectivity imaging, which explores how brain regions communicate and how information is transferred from one brain area to another. Connectivity neuroimaging is categorized as structural imaging and functional imaging. In structural connectivity neuroimaging, diffusion tensor imaging (DTI) and methods derived from it display axonal connectivity pathways. In contrast, functional connectivity neuroimaging displays low-frequency BOLD signal fluctuations (0.01-0.1 Hz) that show a high degree of synchronization at rest in brain regions that are spatially distant from each other. These fluctuations are components of functional intrinsic connectivity networks (ICNs) (5–7).
Highlights
Cognitive impairment (CI) is common and continuous in Parkinson’s disease (PD).
fMRI is widely used in PD-associated CI research.
The connectivity of sensory-motor networks is decreased in PD-associated CI.
There are conflicting results in the connectivity of congitive networks in the PD-associated CI.
Longitudinal stuides are required to use intrinsic connectivity networks (ICNs) as biomarkers in the PD-associated CI.
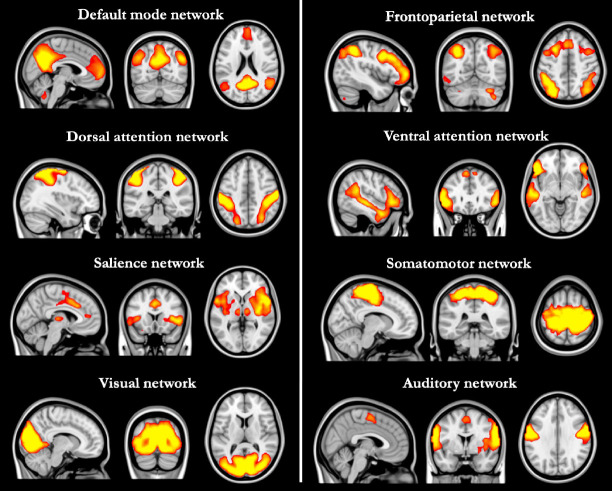
These fluctuations can be analyzed as hypothesis-driven or data-driven fluctuations (8). The hypothesis-driven approach investigates changes in the connectivity of a pre-selected brain region or regions, called the seed, with other brain regions. These examinations can be in the form of investigating the connectivity of the selected seed with other anatomically or functionally bound seeds (seed-to-seed) or determining the connectivity of the voxels in the whole brain (seed-to-voxel). Data-driven approaches attempt to identify consistent spatial fluctuation patterns in the BOLD signal. In this context, the most used data-driven analysis method is independent component analysis (ICA). ICA splits the BOLD signal into independent components represented by three-dimensional spatial maps and a corresponding time series. While these components may have a neural basis, head movement or physiological noises may also appear as other components (9). Neural components, namely ICNs, show a spatial pattern consistent with brain regions activated during cognitive tasks. These spatial similarities were considered when naming these networks (Figure 1). Because ICNs are highly consistent and reproducible both within and between participants, they have the potential as a non-invasive biomarker by revealing the neural processes underlying various neuropsychiatric diseases (1,7)
Figure 1.
Large-scale intrinsic connectivity networks
Parkinson’s Disease-Associated Cognitive Impairment
In Parkinson’s disease (PD), which is diagnosed with conventional motor findings, the counterpart of these findings is neurodegeneration, represented by the loss of dopaminergic neurons in the substantia nigra pars compacta (10–12). Nigrostriatal dopaminergic deafferentation leads to motor symptoms of the disease. However, considering the caudo-rostral progression of the disease from the lower brain stem, the motor symptoms elicited by the involvement of nigral dopaminergic neurons correspond to the middle stage of the disease. Although the diagnostic criteria consist of motor findings, it is understood that non-motor symptoms such as chronic constipation, depression, anxiety, parasomnia, and hyposmia can be seen years before motor symptoms appear with the involvement of the lower brain stem and the olfactory bulb (13). One essential non-motor manifestation of PD is cognitive impairment, which is seen up to 6 times more frequently in the course of PD than in normal population (14).
Although the spectrum of cognitive impairment associated with PD is often defined as a continuum extending from PD with mild cognitive impairment (PD-MCI) to PD dementia (PD-D), it is known that approximately 39% of newly diagnosed PD patients also have subjective cognitive impairment (PD-SCI) (15). SCI represents an individually perceived decline in cognitive ability even though the patient normally performs on standardized cognitive tests and is not associated with an acute event (16).
In contrast, PD-MCI refers to the non-functional decline in cognitive ability reported by the patient or patient’s relatives or observed by the clinician. For the diagnosis of PD-MCI, the Movement Disorder Society (MDS) has proposed a two-stage operational scheme. According to this scheme, in the Stage I criteria, impairment in a proposed global cognitive screening test or impairment in at least two neuropsychological tests when a limited number of tests are used to measure cognition leads to diagnosis of PD-MCI (17,18). According to Stage II criteria, PD-MCI is diagnosed, when 1-2 standard deviations below the norm in one of the five cognitive domains (attention and working memory, executive functions, language, memory, and visuospatial functions) evaluated with at least two neuropsychological tests or in one test in two different cognitive domains (17). MDS criteria may introduce some variability due to lack of specificity regarding cutoff points for impairment in neuropsychological testing. Within this framework, the most recent studies use a threshold value of 1.5 for standard deviations below the normative means of tests (19). PD-MCI can be classified into single or multiple domains, depending on the number of cognitive domains affected. If there is deterioration in two or more tests in one cognitive domain, the PD-MCI is classified as a single domain, while if there is impairment in at least one test in each of the two or more cognitive domains, it is classified as multiple domain (17).
Two phenotypes of PD-MCI, “benign” for an executive disorder and “malign” for a visuospatial disorder, were also defined, and called the “dual syndrome hypothesis,” according to which, dysfunction in the frontostriatal dopaminergic circuitry leads to executive dysfunction, and patients with this phenotype do not always progress to PD-D. The posterior cortical/visual-spatial phenotype, called malign due to cholinergic transmission changes, is a risk factor for PD-D (20–23).
The main feature of the PD-D criteria of MDS is an insidious decline in more than one cognitive area that is severe enough to impair daily living functions and lasts for at least six months (24). Notably, various neuropsychiatric manifestations such as apathy, depression, visual hallucinations, and psychosis may accompany PD-D. As in the diagnosis of PD-MCI, a two-stage system has been developed for the diagnosis of PD-D. According to Stage I PD-D criteria, PD develops before the onset of dementia after excluding major depression, delirium, and other abnormalities that may complicate the diagnosis, scoring below 26 on the Mini-Mental State Examination (MMSE), motor symptoms independently have cognitive deficits severe enough to affect daily life and have impairment in at least two of the four cognitive domains (months reversed or seven backward, lexical fluency or clock drawing, pentagon drawing, and three-word recall) on the MMSE required (24–26). Stage II evaluation, on the other hand, evaluates four areas: global cognitive efficiency, subcortico-frontal features of PD-D, instrumental (cortical-mediated) functions, and neuropsychiatric features (For detailed information, see. Dubois et al. 2007). Also, atrophy on cranial MRI is associated with PD-D; however, the pattern of regional atrophy is variable and can appear in any brain region (27–29).
Uncovering the neural mechanisms underlying the persistence of PD-associated cognitive impairment may be beneficial for the follow-up of the disease and for developing new treatment approaches. In this context, studies investigating resting-state functional connectivity in the framework of the network approach are increasing rapidly, and it is thought that these studies will also be vital in terms of the clinical effect of the disease. This systematic review aims to examine studies investigating changes in large-scale intrinsic connectivity networks in the continuum of PD-associated cognitive impairment ranging from normal cognition to dementia.
METHOD
This systematic review was conducted in line with PRISMA criteria, which were used to write systematic reviews and meta-analyses (30). First, a literature search was conducted in ScienceDirect, Web of Science, and PubMed databases, using search terms suitable for the systematic review (Table 1), covering the entire text (Last search date 18 April 2022). As a result, a total of 974 studies were accessed, of which 643 were in ScienceDirect, 224 were in Web of Science, and 107 in PubMed. Then, the titles and abstracts of these studies were reviewed, and the scanning phase was completed after the studies were removed because the same study appeared in all three databases (n=394), meeting abstracts (n=40), and the letter to the editor (n=5). The full texts of 535 articles were reviewed to examine the eligibility criteria. Eligibility criteria defined within the scope of this study: 1) The study should be a research or meta-analysis article; 2) Include at least one of the PD-MCI or PD-D groups that address the PD-associated cognitive impairment continuum (studies comparing healthy controls and the cognitively normal PD (PD-CN) group were not considered appropriate because they could not be evaluated in the cognitive impairment continuum); 3) Diagnosis of PD-associated cognitive disorder according to current criteria; and 4) Investigation of large-scale intrinsic connectivity networks based on the fMRI. As a result of the application of these criteria, 515 studies were excluded for different causes (Figure 2), and 20 studies were included in the systematic review.
Table 1.
Distribution of publications obtained as a result of database search with search terms
| Search term | ScienceDirect | Web of Science | PubMed | Total |
|---|---|---|---|---|
| “Parkinson’s Disease” AND (“cognitive impairment” OR “dementia”) AND “Default Mode Network” | 171 | 147 | 60 | 378 |
| “Parkinson’s Disease” AND (“cognitive impairment” OR “dementia”) AND “Dorsal Attention Network” | 58 | 10 | 8 | 76 |
| “Parkinson’s Disease” AND (“cognitive impairment” OR “dementia”) AND “Ventral Attention Network” | 33 | 3 | 4 | 40 |
| “Parkinson’s Disease” AND (“cognitive impairment” OR “dementia”) AND “Visual Network” | 46 | 7 | 4 | 57 |
| “Parkinson’s Disease” AND (“cognitive impairment” OR “dementia”) AND (“Motor Network” OR “Somatomotor Network” OR “Sensorymotor Network” OR “Sensory-motor Network”) | 161 | 19 | 7 | 187 |
| “Parkinson’s Disease” AND (“cognitive impairment” OR “dementia”) AND (“Frontoparietal Network” OR “Fronto-parietal Network” OR “Central Executive Network” OR “Central-executive Network” OR “Executive Control Network”) | 110 | 20 | 14 | 144 |
| “Parkinson’s Disease” AND (“cognitive impairment” OR “dementia”) AND (“Salience Network” OR “Cingulo-opercular Network”) | 52 | 17 | 10 | 79 |
| “Parkinson’s Disease” AND (“cognitive impairment” OR “dementia”) AND “Auditory Network” | 12 | 1 | 0 | 13 |
| Total | 643 | 224 | 107 | 974 |
Figure 2.
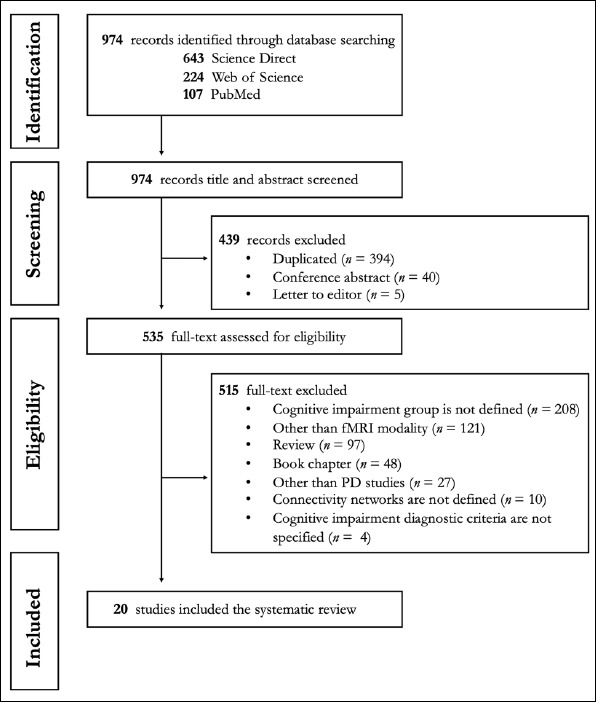
PRISMA flowchart for identification and eligibility of articles. n = number of articles
RESULTS
The designs, samples, diagnostic criteria, analysis methods, and findings of the 20 studies by the eligibility criteria are summarized in Table 2. In this context, alterations in large-scale networks in the PD-associated cognitive impairment continuum will be discussed under separate headings.
Table 2.
The designs, samples, diagnostic criteria, and summary findings of studies reviewed
| Study (reference) | Design | Sample | Diagnostic criteria | Method | Results |
|---|---|---|---|---|---|
| Amboni et al. 2015 (33) | Cross-sectional | 20 HC – 21 PD-CN – 21 PD-MCI | MDS Stage I | ICA | -Decreased DMN connectivity in PD-CN and PD-MCI than in HC -Decreased FPN connectivity in PD-MCI than PD-CN and PD-CDN than HC -Visuospatial functions, memory, and executive functions/attention scores of the PD-CN and PD-MCI groups were positively correlated with FPN. |
| Baggio et al. 2015 (45) | Cross-sectional | 36 HC – 43 PD-CN – 22 PD-MCI | MDS Stage I | ICA | -DMN-DAN and DMN-left FPN connectivity increased in PD-MCI compared to PD-CN and HC. This increased connectivity was negatively correlated with visuospatial scores. -In PD-MCI, intra-DAN and DAN-FPN connectivity decreased compared to PD-CN and HC. Decreased connectivity was positively associated with attention/executive function scores. |
| Gorges et al. 2015 (34) | Cross-sectional | 22 HC – 17 PD-CN – 14 PD-MCI | MDS Stage I | ICA | -Decreased DMN, DAN, and SMN connectivity in PD-MCI than in both PD-CN and HC -Decreased VAN connectivity in PD-MCI than in PD-CN |
| Hou et al. 2016 (35) | Cross-sectional | 22 HC – 18 PD-CN – 14 PD-MCI | MDS Stage II | SBA | -Decreased DMN and DAN-FPN connectivity in PD-MCI than in PD-CN -In all PD groups, DMN and DAN connectivity was positively correlated with attention/working memory score, and intra-DMN connectivity was positively correlated with memory score. |
| Pereza et al. 2017 (48) | Cross-sectional | 30 HC – 62 PD-CN – 37 PD-MCI | MDS Stage II | ICA | -In PD-CN than HC, decreased DAN, SMN, and right FPN connectivity, increased left FPN connectivity -Decreased VN and DAN connectivity in PD-MCI than HC -Decreased FPN and VAN connectivity in PD-MCI than PD-CN |
| Bezdicek et al. 2018 (55) | Meta-analysis | 30 HC – 15 PD-CN – 16 PD-MCI | MDS Stage II | MACM | -Altered connectivity between PD-MCI and PD-CN, especially in DAN and FPN |
| Diez-Cirarda et al. 2018 (36) | Cross-sectional | 26 HC – 12 PD-CN – 23 PD-MCI | MDS Stage II | ICA, SBA | -Decreased DMN and thalamus connectivity in PD-MCI than HC -Decreased connectivity between FPN-SMN, FPN-VN SMN-VN, and SMN-AN in PD-MCI than HC |
| Kawabata et al. 2018 (38) | Cross-sectional | 24 HC – 28 PD-CN – 20 PD-aMCI – 24 PD-naMCI | MDS Stage I | ICA | -The intra-DMN connectivity of PD-aMCI is lower than that of HC, PD-CN, and PD-naMCI -Connectivity between DMN and CBN correctly classified PD-aMCI and PD-naMCI with 86.4% |
| Aracil-Bolanos et al. 2019 (58) | Cross-sectional | 34 PD-CN – 19 PD-MCI | MDS Stage I | SBA | -Intra-SN network coherence was higher in PD-CN than PD-MCI -Increased connectivity between DMN-SN in PD-MCI than PD-CN -SN node degree was positively correlated with global cognition and visuospatial scores; however negatively correlated with the TMT-B score |
| Chung et al. 2019 (39) | Cross-sectional | 30 HC – 50 PD-aMCI – 50 PD-naMCI | MDS Stage II | SBA | -Decreased connectivity of DMN, FPN, and DAN in PD-MCI than HC, however, no connectivity difference between the two MCI groups. -Increased SN connectivity in PD-aMCI compared to both PD-naMCI and HC |
| Klobusiakova et al. 2019 (49) | Cross-sectional and longitudinal | 51 HC – 17 PD-CN – 22 PD-MCI | MDS Stage II | SBA | -Decreased FPN-DMN, FPN-VN, and FPN-DAN connectivity in PD-CN compared to HC -Decreased FPN-VN connectivity in PD-CN compared to PD-MCI -Increased FPN-DMN and FPN-VN connectivity in the whole PD group after 1-year follow-up |
| Wolters et al. 2019 (42) | Meta-analysis | 353 HC – 289 PD-CN – 222 PD-MCI – 68 PD-D | MDS Stage I and II | AES-SDM | -Decreased DMN, AN, and FPN connectivity in PD-MCI compared to HC -Decreased DMN connectivity in PD-MCI compared to PD-CN |
| Arslan et al. 2020 (43) | Cross-sectional | 15 HC – 26 PD-CN – 27 PD-MCI | MDS Stage I | ASL | -Hypoperfusion in FPN, in HC compared to PD-CN -Hypoperfusion in DMN, FPN, SMN, DAN and VN in PD-MCI compared to HC -SMN hypoperfusion in PD-MCI compared to PD-CN |
| Chen et al. 2020 (40) | Cross-sectional | 18 HC – 45 PD-CN – 22 PD-MCI | MDS Stage I | SBA | -Decreased node centrality in SMN, increased node centrality in DMN, VN, and FPN in PD-MCI compared to PD-CN |
| Hou et al. 2020 (41) | Cross-sectional | 28 HC – 19 PD-CN – 22 PD-MCI | MDS Stage II | SBA | -Decreased node centrality in DMN, FPN, and SMN, increased node centrality in VN and FPN in PD-MCI compared to HC |
| Azamat et al. 2021 (51) | Cross-sectional | 17 PD-CN – 18 PD-MCI – 16 PD-D | MDS Stage I, MDS Task Force | ASL | -In PD-D; FPN and VN hypoperfusion compared to PD-MCI, PD-CN, and the merging of these two groups |
| Cascone et al. 2021 (50) | Cross-sectional | 21 HC – 22 PD-CN – 37 PD-MCI | MDS Stage I | SBA | -The topological brain-network resilience of FPN is decreased in PD-MCI compared to PD-CN and HC |
| Hou et al. 2021 (37) | Cross-sectional | 28 HC – 19 PD-CN – 28 PD-MCI | MDS Stage II | ICA | -Decreased connectivity in DMN, SMN, and VN in PD-MCI compared to HC -DMN connectivity was positively correlated with memory score, and VN connectivity was positively correlated with visuospatial functions score in the PD-MCI group -SMN and UPDRS-III scores are negatively correlated in all PD -Decreased connectivity between SMN-LN and between VAN-VN compared to HC in both PD-CN and PD-MCI |
| Ruppert et al. 2021 (46) | Cross-sectional | 16 HC – 36 PD-CN – 12 PD-MCI | MDS Stage II | ICA, SBA | -Increased metabolic DMN connectivity in PD-CN and PD-MCI compared to HC -Both increased and decreased metabolic DMN connectivity between PD-CN and PD-MCI -Decreased DMN connectivity in PD-CN compared to HC whereas increased DMN connectivity in PD-MCI compared to both HC and PD-CN -General cognitive, visuospatial functions and attention scores and intra-DMN connectivity were negatively correlated, while executive functions score and intra-DMN connectivity were positively correlated |
| Schindlbeck et al. 2021 (44) | Cross-sectional | 49 HC – 98 PD-CN – 45 PS-sMCI – 36 PD-mMCI – 19 PD-D | MDS Stage I, PD-D diagnosis: Dementia Rating Scale | ICA, SBA | -The expression scores of the DMN component, which includes the posterior cingulate cortex, cingulum, and thalamus, are decreased in PD-MCI compared to PD-CN -Ventral DMN expression scores are positively correlated with verbal recall scores |
AES-SDM: Anisotropic effect size Seed-based d Mapping; aMCI: Amnestic mild cognitive impairment; AN: Auditory network; ASL: Arterial spin labeling; CBN: Cerebellar-brain stem network; DAN: Dorsal attention network; DMN: Default mode network; FPN: Frontoparietal network; HC: Healthy control; ICA: Independent component analysis; LN: Limbic network; MACM: Meta-analytic co-activation map; MCI: Mild cognitive impairment; MDS: Movement Disorders Society; mMCI: Multi-domain mild cognitive impairment; naMCI: Non-amnestic mild cognitive impairment; PD: Parkinson’s disease; PD-CN: Cognitively normal PD; PD-D: PD with dementia; PD-MCI: PD with mild cognitive impairment; SBA: Seed-based analyses; sMCI: Single domain mild cognitive impairment; SMN: Somatomotor network; SN: Salience network; TMT-B: Trail Making Test-Part B; UPDRS-III: The Unified Parkinson’s Disease Rating Scale-III (Motor section); VAN: Ventral attention network; VN: Visual network.
Default Mode Network
The default mode network (DMN) consists of bilateral and symmetrical cortical regions, including the medial and lateral parietal cortex, medial prefrontal cortex, medial and lateral temporal cortices, and limbic components, covering the hippocampal formation and the amygdala (31). DMN is closely related to cognitive processes and plays an active role in functions such as emotional processing, self-referential mental activity, and recall of previous experiences (31, 32). DMN has been the most researched ICN in PD and other neuropsychiatric disorders (1, 29). Amboni et al. (2015) found that connectivity of DMN structures, including the inferior parietal cortex, posterior cingulate cortex, and medial temporal lobe, did not differ between the PD-CN and PD-MCI groups (33). In contrast, PD-CN and PD-MCI groups had lower DMN connectivity than healthy controls (HCs). In another study, DMN connectivity in the PD-MCI group was lower than in PD-CN and HC (34).
Similarly, in a seed-based study conducted by Hou et al. (2016) in drug-naive, early-stage PD patients, it was found that PDs with cognitive impairment decreased the functional connectivity of region of interests (ROIs) of DMN with dorsal attention network (DAN) and frontoparietal network (FPN) compared to PD-CNs (35). They also showed that connectivity between the DMN (anterior temporal cortex) and the DAN (middle temporal gyrus) was positively correlated with attention and working memory scores. The connectivity within the DMN itself (between the hippocampus and the inferior temporal gyrus) was positively correlated with memory scores. While two studies (36, 37) suggested that intra-DMN connectivity decreased in the PD-MCI group compared to HC, however, without any difference between the PD-CN and PD-MCI groups; Kawabata et al. (2018) stated that the intra-DMN connectivity of PD patients with amnestic MCI (PD-aMCI) was found to decrease compared to both the non-amnestic PD-MCI (PD-naMCI) group and the PD-CN group. They also suggested that connectivity between the DMN and the cerebellar-brainstem network could correctly distinguish PD-aMCI and PD-naMCI at 86.4% with the support vector machine classification (38).
On the other hand, in the study of Chung et al. (2019), including PD-aMCI and PD-naMCI groups and HCs, there was no difference in terms of DMN connectivity between the two MCI groups, but when they compared the PD-MCI group as a single group and compared it with healthy controls, found that DMN showed bilaterally decreased connectivity of the entire cortex (39). In two studies using graph theory, Chen et al. (2020) showed increased nodal centrality in DMN in PD-MCI compared to PD-CN (40), and Hou et al. (2020) found decreased nodal centrality in newly diagnosed and drug-naive PD-MCI patients compared to HC (41). A meta-analysis of functional connectivity studies by Wolters et al. (2019), which included many of the studies mentioned above, revealed that the PD-MCI (n=222) group had decreased DMN connectivity compared to both the HC (n=353) and the PD-CN (n=289) groups and suggested that quantifying the connectivity of DMN as a biomarker for PD-associated cognitive impairment would be necessary for understanding the pathophysiology of the disease (42).
In the studies summarized, ICNs have been defined based on the characteristic of the BOLD signal. However, it is possible to perform studies on connectivity networks using different methods apart from analyzing the BOLD signal. Arslan et al. (2020), with the arterial spin labelling (ASL) method, which allows the measurement of cerebral blood flow, showed that there was hypoperfusion in the precuneus component of DMN in the PD-MCI group compared to the HC group. In addition, they classified the PD-MCI group in terms of H1/H1 and H1/H2 haplotypes associated with the MAPT gene polymorphism. They found that the H1/H1 haplotype of the PD-MCI group showed hypoperfusion in regions including the precuneus and anterior cerebellum components of DMN (43). Also, in an FDG-PET study, which allows the measurement of metabolic activity in the brain, it was found that the expression scores of the DMN component, including the precuneus, cingulum, and thalamus, were decreased in PD-MCI compared to PD-CN, and the expression scores of the ventral DMN are positively correlated with verbal recall scores (44).
The general conclusion that can be drawn from studies conducted on the continuum of PD-associated cognitive impairment is that DMN connectivity decreases as the severity of cognitive impairment increases. On the other hand, in two different studies conducted on the PD-associated cognitive impairment continuum, findings of increased DMN connectivity were also reported. In the first of these, it was found that the midline and frontal/temporal components of the DMN increased functional connectivity of the posterior DAN and FPN components in the PD-MCI group compared to both the PD-CN and HC groups, and this hyperconnectivity was found to be negatively related to the visuospatial function scores (45). A recent FDG-PET study showed increased metabolic DMN connectivity in the PD-CN and PD-MCI groups compared to the HC group, and both increased and decreased intra-DMN connectivity in various regions in the PD groups with and without cognitive impairment (46). In the same study, as a result of the analysis of DMN by ICA, it was found that DMN connectivity was decreased in PD-CN compared to HC but increased in PD-MCI compared to both HC and PD-CN. Besides, general cognition, visuospatial and attention scores were negatively correlated with intra-DMN connectivity and positively correlated with executive functions scores.
Frontoparietal Network
The frontoparietal network (FPN) consists of bilateral dorsolateral prefrontal cortex and posterior parietal cortex components, which are associated with higher-level cognitive functions such as actively holding and processing information in working memory, problem-solving based on rules, and making decisions in the context of goal-directed behaviour (47). After DMN, FPN has been the most investigated ICN in the PD-associated continuum of cognitive impairment. Studies seem to agree that Parkinson’s patients without cognitive impairment reveal lower FPN connectivity than HCs (33, 42, 48-50). In functional connectivity studies comparing PD-MCI and HC groups, findings were also reported that FPN connectivity was lower than that in HCs (36, 39, 42). However, a study investigating cerebral blood flow demonstrated hypoperfusion in areas involving FPN in PD-CN and PD-MCI compared to HC (43). In another study examining the relationship between brain-network topology and cognition in Parkinson’s disease, it was found that HC and PD-CN groups were significantly more resistant to network disruption in FPN than in the PD-MCI group (50).
There are contradictory findings in studies comparing Parkinson’s patients with and without cognitive impairment in terms of FPN. While Amboni et al. (2015) suggested that the FPN connectivity of the PD-MCI group was lower than that of the PD-CN group and that this low connectivity was positively associated with visuospatial functions, memory and attention scores (33), Baggio et al. (2015) suggested that the connectivity between FPN and DMN increased and increased connectivity was positively correlated with visuospatial scores (45). On the other hand, Pereza et al. (2017) reported decreased FPN connectivity in PD-MCI compared to PD-CN (48), and in parallel, Azamat et al. (2021) in Parkinson’s disease dementia, reported hypoperfusion in regions involving FPN compared to PD-CN, PD-MCI, and non-demented Parkinson’s patients by way of merging the two groups (51).
Dorsal and Ventral Attention Networks
Corbetta and Shulmann (2002) depicted two anatomically and functionally different attention systems in the human brain; the dorsal attention network (DAN) and the ventral attention network (VAN) (52). While DAN and VAN are considered two anatomically separated cortical systems with functionally specialized nodes that support specific processes for controlling attention, it has been shown that DAN includes the intraparietal sulcus and the frontal eye field, whereas VAN consists of the temporoparietal junction and ventral inferior frontal gyrus (IFG-pars orbitalis) components (53). While DAN is related to top-down processes in the goal-directed voluntary control of visuospatial attention, VAN plays a role in detecting unexpected bottom-up stimuli and triggering attention shifts (54). In initial studies performed (34, 45, 48), it was found that the connectivity of both within-DAN and DAN to other networks was lower in those with PD-MCI compared to both the PD-CN and HC groups, and this decreased connectivity was positively associated with executive function scores (45). It was also suggested that VAN connectivity was decreased in PD-MCI compared to PD-CN; however, there was no connectivity change in VAN in the comparison of HC and PD-CN (34, 48). In line with this finding, Arslan et al. (2020) revealed hypoperfusion in the regions involving DAN compared to HC in PD-MCI, and there was no change in DAN or VAN perfusion in the comparison of PD-MCI and PD-CN groups (43). A recent study comparing HCs with the PD-aMCI and PD-naMCI groups showed that DAN connectivity in the PD-MCI groups decreased compared to the HC group; however, there was no difference between the two PD-MCI groups (39). In addition, a PD-associated cognitive impairment continuum meta-analysis highlighted the importance of altered connectivity about cognition in PD, particularly in DAN and FPN (55).
Salience Network
The salience network (SN) emerges as an ICN containing cortical nodes covering the anterior cingulate cortex and ventral anterior insular cortices and subcortical components consisting of the amygdala, hypothalamus, ventral striatum, and the specific brainstem nuclei (56). One of the least explored networks in the PD-associated cognitive impairment continuum, SN is associated with responsiveness to homeostatically relevant stimuli and whether their valence is negative (punitive) or positive (reinforcing) (57). Findings related to SN were reported in only two of the studies covered in this systematic review. In the first of these studies (58), regions of interest of SN, DMN, and FPN were selected within the framework of a seed-based approach, and it was found that the intra-SN network coherence was higher in PD-CN compared to PD-MCI. However, they also suggested that SN-DMN connectivity was increased in the PD-MCI group compared to the PD-CN group. In addition, analyses based on graph theory displayed that the node degree of the SN and the scores of global cognition and visuospatial functions were positively correlated with the Trail Making Test Form B (TMT-B) score. In the second study, amnestic and non-amnestic PD-MCI groups were compared with the seed-based analysis method and revealed that the SN connectivity of the amnestic PD-MCI group was higher than both the non-amnestic PD-MCI and HC groups (39). In the same study, the patients were followed for 1- 4 years, and it was reported that the risk of conversion to PD-associated dementia (PD-D) in patients with amnestic-type cognitive impairment was 2.3 times higher than in patients with PD without amnestic-type. In summary, it can be stated that as the severity of cognitive impairment increases in PD, the SN connectivity increases, but further studies are required.
Somatomotor Network
The foundation for the ICN studies was laid when Biswal et al. (1995) published the first study revealing that the bilateral motor cortices are not quiet at rest and that there is a high correlation between the BOLD time series of these regions suggesting ongoing information processing and functional connectivity (59). Within this framework, the somatomotor network (SMN) was the first ICN to be discovered. All of the studies covered in this systematic review have a common finding: SMN connectivity decreases in the continuum of PD-associated cognitive impairment. While Gorges et al. (2015) showed that patients with PD-MCI had decreased SMN connectivity compared to both PD-CN and HC (34), Pereza et al. (2017) showed that patients with PD-CN had decreased SMN connectivity compared to HC (37), Hou et al. (2021) revealed a decrease in connectivity in SMN compared to HC in newly diagnosed and drug-naive patients with PD-MCI. In addition, Hou et al. (2021) showed that SMN connectivity in a group of all Parkinson’s patients was negatively correlated with UPDRS-III (Unified Parkinson’s Disease Rating Scale – Motor Evaluation) scores (37). In parallel with functional connectivity studies, Arslan et al. (2020) reported SMN hypoperfusion in patients with PD-MCI according to both PD-CN and HC groups (43). Two studies on graph theory determined that patients with PD-MCI exhibited lower nodal centrality in SMN compared to patients with PD-CN (40) and HCs (41).
Visual Network
Studies on defining large-scale intrinsic connectivity networks revealed that the visual network (VN) consisted of two different components: the medial visual network (primary visual network) and the lateral visual network (extrastriate visual network). Anatomically, the medial visual network includes the primary visual cortices in the bilateral calcarine fissure (BA17), lingual gyrus, lateral geniculate nuclei, inferior precuneus, and the lateral visual network includes the visual association cortices (BA18, 19, and 37) (5). A longitudinal study of the PD-associated cognitive impairment continuum revealed that patients with PD-CN had decreased VN-FPN connectivity compared to patients with PD-MCI but increased VN-FPN connectivity in all Parkinson’s patients after a one-year of follow-up (49). However, in various studies, it has been found that patients with PD-CN and patients with PD-MCI show lower VN connectivity compared to healthy controls (36, 37, 48), and there is an expected positive correlation between VN connectivity and visuospatial functions scores (37). In two studies for the detection of cerebral blood flow, hypoperfusion was detected in the VN-containing regions in the PD-MCI group compared to HCs (43), and in the VN regions in the PD-D group compared to both the PD-MCI and PD-CN groups (51). Two studies with the graph theory approach revealed increased node centrality between VN nodes in PD-MCI compared to PD-CN (40) and HC (41). Based on these findings, it can be argued that as the severity of cognitive impairment increases in PD, there is a decrease in functional connectivity and perfusion of the VN regions, whereas the increase in the node centrality of the VN.
Auditory Network
The auditory network (AN) is a sensory network covering the primary (BA41 and 42) and secondary auditory cortices (BA22), including the Heschl gyrus, planum polare, planum temporale, lateral superior temporal gyrus, and posterior insular cortex (5). Findings related to AN were found in only two of the articles reviewed within the scope of this study. The first one showed that connectivity between AN and SMN decreased in patients with PD-MCI compared to HCs (36), and the other, the second study, which was a meta-analysis, indicated a decrease in AN connectivity in patients with PD-MCI compared to HCs (42).
DISCUSSION
This systematic review inspected twenty studies on ICNs in the Parkinson’s disease-associated cognitive impairment continuum. In this framework, the focus is on the studies based on the default mode network (DMN), frontoparietal network (FPN), dorsal and ventral attention networks (DAN and VAN), salience network (SN), somatomotor network (SMN), visual network (VN) and auditory network (AN), which are frequently studied in the fMRI literature. It is possible to classify these connectivity networks as cognitive (DMN, FPN, DAN, VAN, SN) and sensory-motor networks (SMN, VN, AN) (7). It can be said that the results related to sensory-motor networks in the studies investigated within the scope of this review have internal consistency. The general opinion to draw from these studies is that the connectivity of SMN, VN, and AN decreases in the PD-associated cognitive impairment continuum. Nevertheless, consistent and inconsistent findings have been reported in studies on cognitive networks. Therefore, some points should be considered when interpreting the results from studies reviewed.
First, the criteria used in the diagnosis of PD-MCI may differ. Although the research conducted in line with the established criteria accepted by the Movement Disorders Society for diagnosing PD-MCI is reviewed within the scope of this study, there are two variations of MDS criteria, Stage I and Stage II, as mentioned in the introduction. Stage II criteria include a comprehensive neuropsychological assessment, while Stage I criteria can be considered an abbreviated assessment. In a study that compared these two criteria, Parkinson’s patients without dementia were evaluated according to Stage I criteria with two different screening tests (Montreal Cognitive Assessment Scale, MoCA, and Parkinson’s Disease-Cognitive Rating Scale, PD-CRS) and Stage II with a detailed neuropsychological evaluation, and the obtained groups were examined in terms of structural (voxel-based morphometry and cortical thickness), and functional (functional connectivity and graph theory) changes. In these assessments, Stage II criteria were set as the gold standard (60). In groups diagnosed with PD-MCI by PD-CRS and in the one diagnosed with PD-MCI according to Stage II criteria, a decrease in grey matter concentration was detected in the precuneus and posterior cingulate cortex, which are structures belonging to DMN, as well as a reduction of cortical thickness in the precuneus. However, no overlapping results were obtained in groups diagnosed according to Stage II criteria and MoCA. Nonetheless, in the seed-based analysis of ICNs, no functional connectivity difference was observed between PD-CN and PD-MCI in groups diagnosed with PD-MCI according to Stage II criteria and MoCA, but in the group diagnosed with PD-CRS, connectivity within the SN was found to be higher in the PD-CN group than in the PD-MCI group. However, it was reported that connectivity between DMN-SN and DMN-FPN was higher in the PD-MCI group compared to PD-CN. As a result of the analyses carried out within the framework of graph theory, it was found that the node degree of a node in two nodes of the SN and the centrality of a node of the DMN were higher than PD-MCI in PD-CN in all three diagnostic groups. The node degree of one node in the SN was higher in the PD-MCI group than in the PD-CN group in groups diagnosed with MoCA according to Stage II. On the other hand, it was reported that the centrality of a different node in the SN was higher than PD-MCI in PD-CN according to the diagnosis with MoCA, while it was higher than PD-CN in PD-MCI according to the Stage II diagnosis (60). There were also outcomes in only one PD-CRS, MoCA, and Stage II diagnostic group. PD-MCI is a risk factor for PD-D (61, 62); studies have shown that both Stage I and Stage II diagnostic criteria have prognostic validity for PD-D development. However, the sensitivity and specificity of Stage I criteria are considered lower than Stage II (19).
Another issue is the fluctuations between PD-MCI and PD-CN in the PD-associated cognitive impairment continuum. In longitudinal studies, it has been determined that approximately 20-30% of patients with a diagnosis of PD-MCI revert to normal cognition (63 - 66). However, it has also been reported that patients who revert to normal cognition can return to the MCI stage and that the risk of dementia increases in the long term (60, 65). A meta-analysis study showed that the rate of return to normal cognition in PD-MCI according to Stage I criteria is approximately two times higher than in patients diagnosed according to Stage II criteria (67). In a study carried out in Türkiye, it was determined that 20.8% of patients diagnosed with PD-MCI revert to normal cognition within an average of 1.5 years of follow-up, and education was an important variable in reverting to normal cognition. In addition, a decrease in grey matter concentration was found in the regions including FPN and VAN in patients with stable PD-MCI compared to patients reverting to normal cognition (68). However, no study based on ICNs included the PD group reverting to normal cognition in the PD-associated cognitive impairment continuum. In this context, longitudinal studies are required to understand better the relationships between cognitive profile fluctuations and large-scale connectivity networks. However, benign PD-MCI, which rarely progresses to dementia and is associated with frontostriatal dopaminergic deficiency, and malign PD-MCI, a serious risk factor for dementia and associated with posterior cortical deficits, have not been investigated based on ICNs. In particular, a longitudinal investigation of FPN connectivity in benign PD-MCI and VN connectivity in malign PD-MCI is required.
One reason for the inconsistencies between the results presented on cognitive connectivity networks in the studies reviewed in this systematic review may be that the PD groups in all studies consisted of patients under treatment except in three studies (35, 37, 41). It is known that newly diagnosed Parkinson’s patients also meet the diagnosis of PD-MCI at rates varying between 20% and 55% (69-71). Since dopaminergic therapy used in Parkinson’s disease affects cognition and ICNs (72-76), comparing newly diagnosed and drug-naive PD-MCI and PD-CN patients may be of significant importance in elucidating the neural basis of cognitive impairment in PD.
It was observed that none of the twenty studies reviewed had a PD group with subjective cognitive impairment, and only two included PD-D. A longitudinal study revealed that approximately 30% of Parkinson’s patients with subjective cognitive complaints developed cognitive impairment within two years (77). In this context, patients with subjective cognitive complaints should also be included in longitudinal neuroimaging studies, and the relationships between connectivity networks and the risk of developing cognitive impairment should be clarified. Wolters et al. (2019) included patients from the PD-D group in their meta-analysis. However, they combined the PD-MCI and PD-D groups in statistical analyses to obtain a PD group with a single cognitive impairment (42). In this context, there are restricted results about alterations in large-scale connectivity networks in Parkinson’s disease-associated dementia. Long-term follow-up of Parkinson’s patients on a continuum from subjective cognitive impairment to dementia may help identify various risk factors for the development of both mild cognitive impairment and dementia.
CONCLUSION
Throughout the Parkinson’s disease-associated cognitive impairment continuum, there seems to be varying degrees of alterations in ICNs. It can be stated that these changes manifest themselves as a decrease in connectivity in sensory-motor networks. It is considered that well-controlled longitudinal studies are required to reveal the changes in cognitive networks. In future studies, it is crucial to define the cognitive disorder groups well and control the changes in connectivity networks due to dopaminergic treatment. Conducting longitudinal studies, especially in patients with subjective cognitive impairments and extending to dementia with extended follow-up periods may reveal the pathophysiological mechanisms underlying PD-associated cognitive impairment and may benefit the use of ICNs as a biomarker.
Acknowledgments:
Ulaş Ay acknowledges the support of Turkish Council of Higher Education for 100/2000 CoHE doctoral scholarship.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – UA; Design – UA, HG; Supervision – HG; Resource – (-); Material - (-); Data Collection and/or Processing - (-); Analysis and/or Interpretation - UA, HG; Literature Search – UA, HG; Writing – UA, HG; Critical Appraisal – HG.
Conflict of Interest: The authors declared that there is no conflict of interest.
Financial Disclosure: This study was supported by the Istanbul University Research Projects Unit (IU-BAP). Project No: BAP-2019K12-149071.
REFERENCES
- 1.Baggio HC, Junqué C. Functional MRI in Parkinson's disease cognitive impairment. Int Rev Neurobiol. 2019;144:29–58. doi: 10.1016/bs.irn.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Bressler SL, Menon V. Large-scale brain networks in cognition:emerging methods and principles. Trends Cogn Sci. 2010;14(6):277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Bandettini PA. Twenty years of functional MRI. The science and the stories. Neuroimage. 2012;62(2):575–588. doi: 10.1016/j.neuroimage.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 4.Matthews P, Jezaard P. Functional magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2004;75:6–12. https: //jnnp.bmj.com/content/jnnp/75/1/6.full.pdf . [PMC free article] [PubMed] [Google Scholar]
- 5.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc B Biol Sci. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glover GH. Overview of functional magnetic resonance imaging. Neurosurg Clin N Am. 2011;22(2):133–139. doi: 10.1016/j.nec.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bijsterbosch J, Smith S, Beckmann C. Introduction to resting state fMRI functional connectivity. First ed. New York, USA: Oxford University Press; 2017. [Google Scholar]
- 8.Harı E, Ay U, Neşe H, Bayram A, Demiralp T. Manyetik rezonans görüntüleme temelli fonksiyonel bağlantısallık yöntemleri. İstanbul Tıp Fakültesi Derg. 2020;83(1):71–80. [Google Scholar]
- 9.Griffanti L, Douaud G, Bijsterbosch J, Evangelisti S, Alfaro-Almagro F, Glasser MF, et al. Hand classification of fMRI ICA noise components. Neuroimage. 2017;154:188–205. doi: 10.1016/j.neuroimage.2016.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rüb U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson's disease (preclinical and clinical stages) J Neurol. 2002;249(Suppl 3):III/1–5. doi: 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- 11.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318(1):121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 13.Schapira AH, Tolosa E. Molecular and clinical prodrome of Parkinson disease:implications for treatment. Nat Rev Neurol. 2010;6(6):309–317. doi: 10.1038/nrneurol.2010.52. [DOI] [PubMed] [Google Scholar]
- 14.Arsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sørensen Risk of dementia in Parkinson's disease:a community-based, prospective study. Neurology. 2001;56(6):730–736. doi: 10.1212/wnl.56.6.730. [DOI] [PubMed] [Google Scholar]
- 15.Chua CY, Koh MR, Chia NS, Ng SY, Saffari SE, Wen M-C, et al. Subjective cognitive Complaints in early Parkinson's disease patients with normal cognition are associated with affective symptoms. Park Relat Disord. 2021;82:24–28. doi: 10.1016/j.parkreldis.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease:Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27(3):349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skorvanek M, Goldman JG, Jahanshahi M, Marras C, Rektorova I, Schmand B, et al. Global scales for cognitive screening in Parkinson's disease:critique and recommendations. Mov Disord. 2018;33(2):208–218. doi: 10.1002/mds.27233. [DOI] [PubMed] [Google Scholar]
- 19.Aarsland D, Batzu L, Halliday GM, Geurtsen GJ, Ballard C, Chaudhuri KR, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Prim. 2021;7(1):1–21. doi: 10.1038/s41572-021-00280-3. [DOI] [PubMed] [Google Scholar]
- 20.Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain. 2007;130(Pt 7):1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 21.Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, et al. The distinct cognitive syndromes of Parkinson's disease:5 year follow-up of the CamPaIGN cohort. Brain. 2009;132(Pt 11):2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 22.Klein JC, Eggers C, Kalbe E, Weisenbach S, Hohmann C, Vollmar S, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology. 2010;74(11):885–892. doi: 10.1212/WNL.0b013e3181d55f61. [DOI] [PubMed] [Google Scholar]
- 23.Martínez-Horta S, Kulisevsky J. Is all cognitive impairment in Parkinson's disease “mild cognitive impairment”? J Neural Transm. 2011;118(8):1185–1190. doi: 10.1007/s00702-011-0675-9. [DOI] [PubMed] [Google Scholar]
- 24.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 25.Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, et al. Diagnostic procedures for Parkinson's disease dementia:recommendations from the Movement Disorder Society Task Force. Mov Disord. 2007;22(16):2314–2324. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- 26.Goetz CG, Emre M, Dubois B. Parkinson's disease dementia:definitions, guidelines, and research perspectives in diagnosis. Ann Neurol. 2009;64(Suppl 2):S81–S92. doi: 10.1002/ana.21455. [DOI] [PubMed] [Google Scholar]
- 27.Burton EJ, McKeith IG, Burn DJ, Williams ED, O'Brien JT. Cerebral atrophy in Parkinson's disease with and without dementia:a comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain. 2004;127(4):791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- 28.Hwang KS, Beyer MK, Green AE, Chung C, Thompson PM, Janvin C, et al. Mapping cortical atrophy in Parkinson's disease patients with dementia. J Parkinsons Dis. 2013;3(1):69–76. doi: 10.3233/JPD-120151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanskey JH, McColgan P, Schrag AE, Acosta-Cabronero J, Rees G, Morris HR, et al. Can neuroimaging predict dementia in Parkinson's disease? Brain. 2018;141(9):2545–2560. doi: 10.1093/brain/awy211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions:explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Raichle ME. The Brain's Default Mode Network. Annu Rev Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 32.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amboni M, Tessitore A, Esposito F, Santangelo G, Picillo M, Vitale C, et al. Resting-state functional connectivity associated with mild cognitive impairment in Parkinson's disease. J Neurol. 2015;262(2):425–434. doi: 10.1007/s00415-014-7591-5. [DOI] [PubMed] [Google Scholar]
- 34.Gorges M, Müller HP, Lulé D, Pinkhardt EH, Ludolph AC, Kassubek J. To rise and to fall:functional connectivity in cognitively normal and cognitively impaired patients with Parkinson's disease. Neurobiol Aging. 2015;36(4):1727–1735. doi: 10.1016/j.neurobiolaging.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 35.Hou Y, Yang J, Luo C, Song W, Ou R, Liu W, et al. Dysfunction of the default mode network in drug-naïve parkinson's disease with mild cognitive impairments:a resting-state fMRI study. Front Aging Neurosci. 2016;8:247. doi: 10.3389/fnagi.2016.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Díez-Cirarda M, Strafella AP, Kim J, Peña J, Ojeda N, Cabrera-Zubizarreta A, et al. Dynamic functional connectivity in Parkinson's disease patients with mild cognitive impairment and normal cognition. Neuroimage Clin. 2018;17:847–855. doi: 10.1016/j.nicl.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou Y, Wei Q, Ou R, Zhang L, Yuan X, Gong Q, et al. Different resting-state network disruptions in newly diagnosed drug-naïve Parkinson's disease patients with mild cognitive impairment. BMC Neurol. 2021;21(1):327. doi: 10.1186/s12883-021-02360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawabata K, Watanabe H, Hara K, Bagarinao E, Yoneyama N, Ogura A, et al. Distinct manifestation of cognitive deficits associate with different resting-state network disruptions in non-demented patients with Parkinson's disease. J Neurol. 2018;265(3):688–700. doi: 10.1007/s00415-018-8755-5. [DOI] [PubMed] [Google Scholar]
- 39.Chung SJ, Park YH, Yun HJ, Kwon H, Yoo HS, Sohn YH, et al. Clinical relevance of amnestic versus non-amnestic mild cognitive impairment subtyping in Parkinson's disease. Eur J Neurol. 2019;26(5):766–773. doi: 10.1111/ene.13886. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Liu M, Wu Z, Cheng H. Topological abnormalities of functional brain network in early-stage Parkinson's disease patients with mild cognitive impairment. Front Neurosci. 2020;14:616872. doi: 10.3389/fnins.2020.616872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hou Y, Wei Q, Ou R, Yang J, Gong Q, Shang H. Impaired topographic organization in Parkinson's disease with mild cognitive impairment. J Neurol Sci. 2020;414:116861. doi: 10.1016/j.jns.2020.116861. [DOI] [PubMed] [Google Scholar]
- 42.Wolters AF, van de Weijer SC, Leentjens AF, Duits AA, Jacobs HI, Kuijf ML. Resting-state fMRI in Parkinson's disease patients with cognitive impairment:A meta-analysis. Parkinsonism Relat Disord. 2019;62:16–27. doi: 10.1016/j.parkreldis.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 43.Arslan DB, Gurvit H, Genc O, Kicik A, Eryurek K, Cengiz S, et al. The cerebral blood flow deficits in Parkinson's disease with mild cognitive impairment using arterial spin labeling MRI. J Neural Transm (Vienna) 2020;127(9):1285–1294. doi: 10.1007/s00702-020-02227-6. [DOI] [PubMed] [Google Scholar]
- 44.Schindlbeck KA, Vo A, Mattis PJ, Villringer K, Marzinzik F, Fiebach JB, et al. Cognition-related functional topographies in Parkinson's disease:localized loss of the ventral default mode network. Cereb Cortex. 2021;31(11):5139–5150. doi: 10.1093/cercor/bhab148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baggio H-C, Segura B, Sala-Llonch R, Marti M-J, Valldeoriola F, Compta Y, et al. Cognitive impairment and resting-state network connectivity in Parkinson's disease. Hum Brain Mapp. 2015;36(1):199–212. doi: 10.1002/hbm.22622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruppert MC, Greuel A, Freigang J, Tahmasian M, Maier F, Hammes J, et al. The default mode network and cognition in Parkinson's disease:a multimodal resting-state network approach. Hum Brain Mapp. 2021;42(8):2623–2641. doi: 10.1002/hbm.25393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menon V. Large-scale brain networks and psychopathology:a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Peraza LR, Nesbitt D, Lawson RA, Duncan GW, Yarnall AJ, Khoo TK, et al. Intra- and inter-network functional alterations in Parkinson's disease with mild cognitive impairment. Hum Brain Mapp. 2017;38(3):1702–1715. doi: 10.1002/hbm.23499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klobušiakova P, Mareček R, Fousek J, Výtvarova E, Rektorova I. Connectivity between brain networks dynamically reflects cognitive status of Parkinson's disease:a longitudinal study. J Alzheimers Dis. 2019;67(3):971–984. doi: 10.3233/JAD-180834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cascone AD, Langella S, Sklerov M, Dayan E. Frontoparietal network resilience is associated with protection against cognitive decline in Parkinson's disease. Commun Biol. 2021;4(1):1021. doi: 10.1038/s42003-021-02478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azamat S, Arslan DB, Erdogdu E, Kicik A, Cengiz S, Eryürek K, et al. Detection of visual and frontoparietal network perfusion deficits in Parkinson's disease dementia. Eur J Radiol. 2021;144:109985. doi: 10.1016/j.ejrad.2021.109985. [DOI] [PubMed] [Google Scholar]
- 52.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–125. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 53.Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems:distinct neural circuits but collaborative roles. Neuroscientist. 2014;20(2):150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. J Neurosci. 2005;25(18):4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bezdicek O, Ballarini T, Růžička F, Roth J, Mueller K, Jech R, et al. Mild cognitive impairment disrupts attention network connectivity in Parkinson's disease:a combined multimodal MRI and meta-analytical study. Neuropsychologia. 2018;112:105–115. doi: 10.1016/j.neuropsychologia.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 56.Seeley WW. The salience network:a neural system for perceiving and responding to homeostatic demands. J Neurosci. 2019;39(50):9878–9882. doi: 10.1523/JNEUROSCI.1138-17.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bartra O, McGuire JT, Kable JW. The valuation system:A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aracil-Bolaños I, Sampedro F, Marín-Lahoz J, Horta-Barba A, Martínez-Horta S, Botí M, et al. A divergent breakdown of neurocognitive networks in Parkinson's disease mild cognitive impairment. Hum Brain Mapp. 2019;40(11):3233–3242. doi: 10.1002/hbm.24593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 60.Aracil-Bolaños I, Sampedro F, Marín-Lahoz J, Horta-Barba A, Martínez-Horta S, Gónzalez-de-Echávarri JM, et al. Tipping the scales:how clinical assessment shapes the neural correlates of Parkinson's disease mild cognitive impairment. Brain Imaging Behav. 2022;16(2):761–772. doi: 10.1007/s11682-021-00543-3. [DOI] [PubMed] [Google Scholar]
- 61.Hoogland J, Boel JA, de Bie RM, Schmand BA, Geskus RB, Dalrymple-Alford JC, et al. Risk of Parkinson's disease dementia related to level I MDS PD-MCI. Mov Disord. 2019;34(3):430–435. doi: 10.1002/mds.27617. [DOI] [PubMed] [Google Scholar]
- 62.Hoogland J, Boel JA, de Bie RM, Geskus RB, Schmand BA, Dalrymple-Alford JC, et al. Mild cognitive impairment as a risk factor for Parkinson's disease dementia. Mov Disord. 2017;32(7):1056–1065. doi: 10.1002/mds.27002. [DOI] [PubMed] [Google Scholar]
- 63.Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D. Dementia and survival in parkinson disease:a 12-year population study. Neurology. 2008;70(13):1017–1022. doi: 10.1212/01.wnl.0000306632.43729.24. [DOI] [PubMed] [Google Scholar]
- 64.Santangelo G, Vitale C, Picillo M, Moccia M, Cuoco S, Longo K, et al. Mild cognitive impairment in newly diagnosed Parkinson's disease:A longitudinal prospective study. Parkinsonism Relat Disord. 2015;21(10):1219–1226. doi: 10.1016/j.parkreldis.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 65.Pedersen KF, Larsen JP, Tysnes O-B, Alves G. Natural course of mild cognitive impairment in Parkinson disease. Neurology. 2017;88(8):767–774. doi: 10.1212/WNL.0000000000003634. [DOI] [PubMed] [Google Scholar]
- 66.Domellöf ME, Ekman U, Forsgren L, Elgh E. Cognitive function in the early phase of Parkinson's disease, a five-year follow-up. Acta Neurol Scand. 2015;132(2):79–88. doi: 10.1111/ane.12375. [DOI] [PubMed] [Google Scholar]
- 67.Saredakis D, Collins-Praino LE, Gutteridge DS, Stephan BC, Keage HA. Conversion to MCI and dementia in Parkinson's disease:a systematic review and meta-analysis. Park Relat Disord. 2019;65:20–31. doi: 10.1016/j.parkreldis.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 68.Ay U, Kiçik A, Erdoğdu E, Kurt E, Bilgiç B, Gürvit İH, et al. Hafif kognitif bozuklukla seyreden Parkinson hastalığında kognisyonun değişimine etki eden faktörlerin incelenmesi:1,5 yıllık izlem çalışması. 57 Ulusal Nöroloji Kongresi;2021 27 Kasım - 4 Aralık. Antalya, Türkiye. pp. 21–22. https: //avesis.istanbul.edu.tr/yayin/14974c4a-23ed-4813-bf62-3fbccbb74895/hafif-kognitif-bozuklukla-seyreden-parkinson-hastaliginda-kognisyonun-degisimine-etki-eden-faktorlerin-incelenmesi-1-5-yillik-izlem-calismasi .
- 69.Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, et al. MDS task force on mild cognitive impairment in Parkinson's disease:critical review of PD-MCI. Mov Disord. 2011;26(10):1814–1824. doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Janvin C, Aarsland D, Larsen JP, Hugdahl K. Neuropsychological profile of patients with Parkinson's disease without dementia. Dement Geriatr Cogn Disord. 2003;15(3):126–131. doi: 10.1159/000068483. [DOI] [PubMed] [Google Scholar]
- 71.Mattay VS, Tessitore A, Callicott JH, Bertolino A, Goldberg TE, Chase TN, et al. Dopaminergic modulation of cortical function in patients with Parkinson's disease. Ann Neurol. 2002;51(2):156–164. doi: 10.1002/ana.10078. [DOI] [PubMed] [Google Scholar]
- 72.Dang LC, O'Neil JP, Jagust WJ. Dopamine supports coupling of attention-related networks. J Neurosci. 2012;32(28):9582–9587. doi: 10.1523/JNEUROSCI.0909-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berman BD, Smucny J, Wylie KP, Shelton E, Kronberg E, Leehey M, et al. Levodopa modulates small-world architecture of functional brain networks in Parkinson's disease. Mov Disord. 2016;31(11):1676–1684. doi: 10.1002/mds.26713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhong J, Guan X, Zhong X, Cao F, Gu Q, Guo T, et al. Levodopa imparts a normalizing effect on default-mode network connectivity in non-demented Parkinson's disease. Neurosci Lett. 2019;705:159–166. doi: 10.1016/j.neulet.2019.04.042. [DOI] [PubMed] [Google Scholar]
- 75.Aracil-Bolaños I, Sampedro F, Pujol J, Soriano-Mas C, Gónzalez-de-Echávarri JM, Kulisevsky J, et al. The impact of dopaminergic treatment over cognitive networks in Parkinson's disease:stemming the tide? Hum Brain Mapp. 2021;42(17):5736–5746. doi: 10.1002/hbm.25650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Erro R, Santangelo G, Barone P, Picillo M, Amboni M, Longo K, et al. Do subjective memory complaints herald the onset of mild cognitive impairment in Parkinson disease? J Geriatr Psychiatry Neurol. 2014;27(4):276–281. doi: 10.1177/0891988714532015. [DOI] [PubMed] [Google Scholar]