Abstract
Lipopolysaccharide (LPS) pretreatment of mice resulted in a significantly enhanced survival after disseminated Cryptococcus neoformans infection. The survival was associated with reduced fungal burden in tissues. LPS-pretreated mice had lower levels of cytokines in blood, spleen, and lungs and higher levels in brain. Pentoxifylline abolished the beneficial effect of LPS pretreatment.
Whether the induction of endotoxin tolerance leads to an enhanced sensitivity to infection remains a challenging question. The data reported in the literature are rather controversial. Deleterious effects of endotoxin tolerance were suggested by a study in which pretreatment of mice with lipopolysaccharide (LPS) resulted in a lower titer of plasma interferon after challenge with Newcastle disease virus (26). Although this result may suggest a reduced ability of the animals to control viral infections, this question was not addressed in that study. Similarly, using a peritonitis model, it was reported that monophosphoryl lipid A pretreatment attenuated the levels of circulating cytokines and reduced the neutrophil margination in tissues (22). The efficiency of this pretreatment, however, was not addressed in terms of outcome. It was also reported that a few hours after LPS exposure, pretreated mice had an impaired capacity to clear Pseudomonas aeruginosa from their lungs (13). Injection of heat-killed gram-negative bacteria in baboons 12 h before an intravenous infusion of viable bacteria led to increased lung injury (25). In in vitro experiments it was observed that preexposure of macrophages to LPS resulted in reduced leishmanicidal activity (24). Furthermore, macrophages previously exposed to LPS are less responsive to various types of stimuli, including bacterial superantigens (16) and Staphylococcus aureus, Streptococcus pyogenes, or zymosan (2). In contrast, endotoxin tolerance was shown to be beneficial in preventing mortality after thermal injury (7), in protecting against hepatic and renal ischemia reperfusion (3, 8), and in reducing the size of myocardial infarction initiated by coronary occlusion (5).
No studies have yet addressed the consequences of endotoxin tolerance in terms of protection against fungal pathogens. Since disseminated Cryptococcus neoformans infection occurs in severely immunocompromised patients (15) who are likely to encounter other microorganisms, it was of interest to analyze the influence of endotoxin pretreatment on survival, fungal burden, and cytokine expression in blood and tissues after intravenous inoculation of C. neoformans.
Six- to 7-week old male BALB/c mice were made LPS tolerant as previously described (18) with intravenously injections of 2.5 μg of Escherichia coli LPS (0111:B4) (Sigma) in 0.1 ml of saline daily for 2 days and were intravenously inoculated on the third day with C. neoformans (2 × 106 live cells per mouse) (19). Control mice were injected with the same volume of saline. Blood samples were obtained by cardiac puncture, and lungs, spleens, and brains were excised. Determination of CFU, cytokine measurements, and analysis of nitrite and nitrate in blood and tissues were performed as previously described (19).
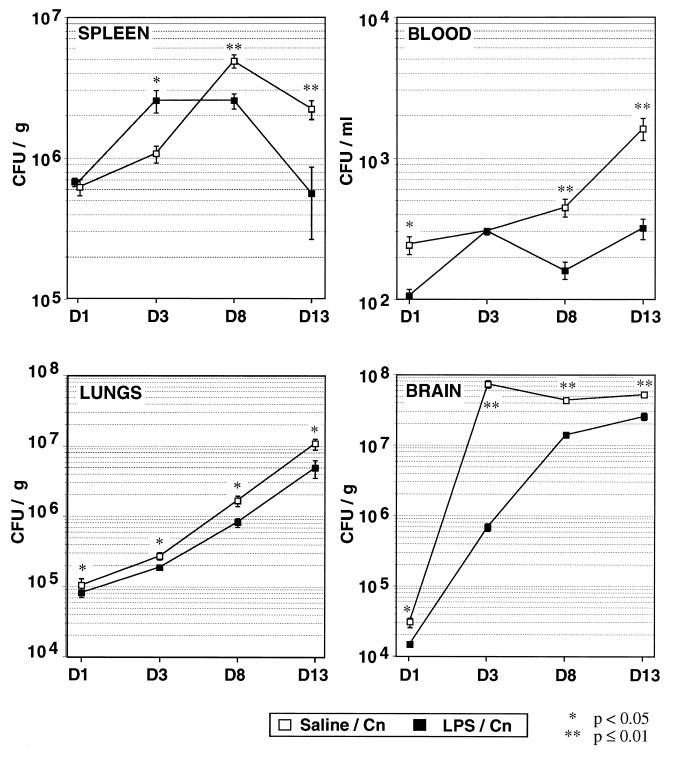
Pretreatment of mice with LPS was associated with a protective effect against a subsequent fungal challenge in terms of survival and fungal burden in blood and tissues (Fig. 1 and 2). The fungal burden was always higher in control mice than in the LPS-tolerant animals in the late days of the follow-up in all analyzed compartments and 1 day after infection in the lungs, the brain, and the blood. In contrast, a significantly and reproducibly higher number of CFU was observed on day 3 in the spleens of tolerant animals.
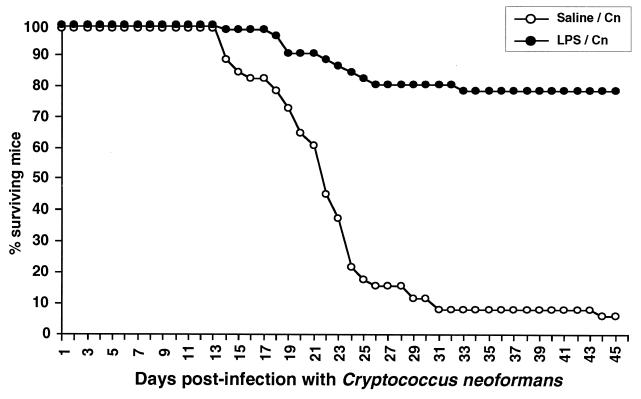
FIG. 1.
Survival curves for control and LPS-pretreated (2.5 μg for 2 days) mice following intravenous infection with 2 × 106 C. neoformans (Cn) cells. The data, expressed as percent surviving mice, are the cumulative results of four different experiments including a total of 51 mice in each group. Median survival times for control and LPS-tolerant mice were 22 and >49 days, respectively. The significance of the Kaplan-Meier survival curves was assessed by the Mantel-Cox log rank test (P < 0.0001).
FIG. 2.
Kinetics of CFU counts in spleen, lungs, brain, and blood after inoculation with C. neoformans (Cn) in normal and LPS-pretreated mice. The results are expressed as the means and standard errors of the means for seven mice in each group and are representative of three independent experiments. Significance was assessed by the Mann-Whitney U test. D, day.
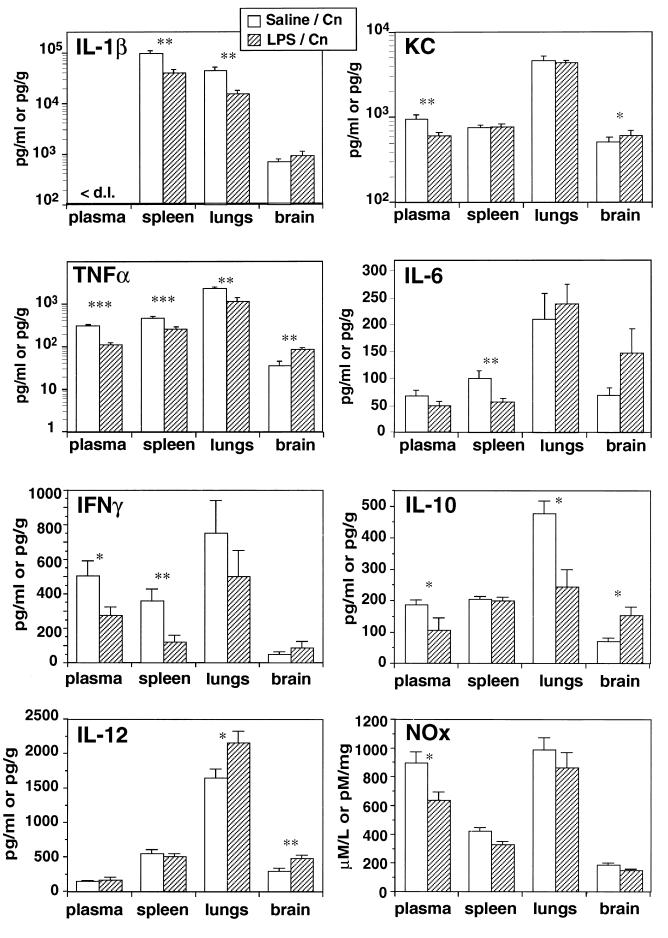
A survey of cytokine expression was performed over 13 days (data not shown). During the later course of infection, on day 13, lower levels of tumor necrosis factor alpha (TNF-α), KC chemokine, gamma interferon (IFN-γ), interleukin-10 (IL-10), and nitrite and nitrate in the blood; of TNF-α, IL-1β, IL-6, and IFN-γ in the spleen; and of TNF-α, IL-1β, and IL-10 in the lungs were observed in LPS-tolerant animals (Fig. 3). This observation most probably reflects the lower fungal burden in these tissues in tolerant mice. Interestingly, a reversed cytokine pattern was observed in the brains of tolerant mice, with higher levels of TNF-α, KC, IL-10, and IL-12. These results further illustrate that the brain behaves differently in terms of its cytokine network than the peripheral tissues (11) and that higher expression of certain cytokines in the brain was associated with protection (19).
FIG. 3.
Levels of IL-1β, KC, TNF-α, IL-6, IFN-γ, IL-10, IL-12, and nitrite and nitrate (NOx) in different compartments on day 13 postinfection in normal and LPS-pretreated mice. Cn, C. neoformans. The results are expressed as the means and standard errors of the means for 7 to 13 individual mice in each group. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
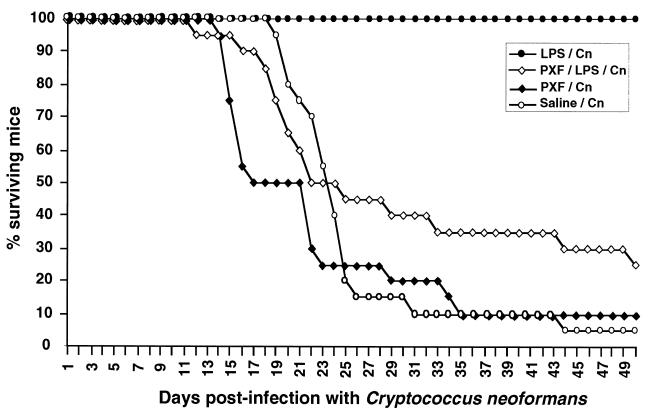
An early induction of cytokines, particularly TNF-α, during the LPS pretreatment may explain the observed protection. This was further suggested by experiments using pentoxifylline, an inhibitor of phosphodiesterases, which is known to prevent TNF production (6, 23). Pentoxifylline diminished the protective effect of the LPS pretreatment and by itself accelerated the occurrence of death in C. neoformans-infected mice, confirming the protective role of TNF in this fungal infection (1, 4, 9, 10, 19) (Fig. 4). To assess the role of IL-1, IL-1 receptor antagonist (200 μg/mouse) was injected in another group of animals 1 h before the LPS pretreatment. In our model IL-1ra failed to reverse the protective effect of the injection of endotoxin against C. neoformans infection (data not shown).
FIG. 4.
Survival curves for normal, LPS-pretreated, pentoxifylline (PXF)-pretreated, and pentoxifylline-plus LPS-pretreated mice after C. neoformans (Cn) fungal infection. The data are the cumulative results of two independent experiments including a total of 20 mice. PXF was injected 30 min before LPS or fungi. Control experiments revealed 8,151 ± 1,384 pg of TNF per ml in blood 90 min after LPS injection (100 μg/mouse), whereas only 707 ± 129 pg/ml was found in PXF-pretreated mice. For LPS-C. neoformans versus PXF-LPS-C. neoformans, P < 0.0001; for LPS-C. neoformans versus PXF-C. neoformans, P < 0.0001. For PXF-C. neoformans versus saline-C. neoformans, the median survival times were 17 and 23 days, respectively, and P = 0.05 when the comparison was performed between days 13 and 25.
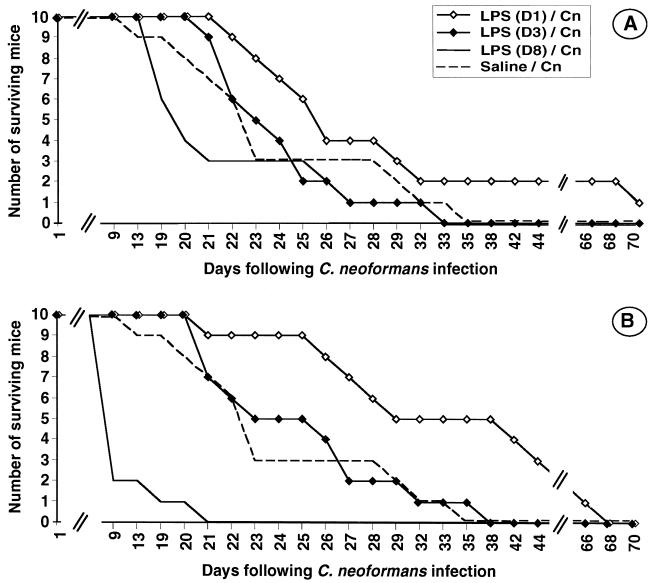
We also investigated whether delayed LPS injection could be beneficial. Thus, mice were injected 1, 3, and 8 days after the C. neoformans infection. As shown in Fig. 5A, no significant alteration of survival curves was observed when 2.5 μg of LPS was employed. In contrast, a protective effect was induced when a larger amount of LPS (25 μg) was injected 1 day after the fungal challenge (Fig. 5B). No protective effect was seen when the injection was performed 3 days later, and the injection even proved to be lethal when performed 8 days after infection, reminiscent of the increased toxicity of LPS when delivered with other microbial agents (17) or even with TNF (21).
FIG. 5.
Survival curves for mice infected with C. neoformans (Cn) and receiving either no LPS or 2.5 μg (A) or 25 μg (B) of LPS on day (D) 1, 3, or 8. The data are the results of experiments performed with 10 mice in each group. In panel B, for day 1 versus control P = 0.01; for day 8 versus control, P < 0.0001.
Our observations suggest that LPS pretreatment, which is known to be associated with the induction of a state of anergy, is also responsible for a priming effect after the production of cytokines that are known to be protective upon a further microbial challenge. Indeed, many authors have demonstrated that injection of IL-1 or TNF was protective against further microbial challenge, including fungal infection (10, 14). These protective effects may reflect the activation of microbicidal activity by mononuclear phagocytic cells (4, 20). It has also been shown that polymorphonuclear neutrophils (PMN) may be primed following LPS pretreatment (12). Since it has been reported that PMN are active in killing of C. neoformans, one can hypothesize that part of the efficient resistance to C. neoformans conferred by LPS pretreatment may reflect an enhanced microbicidal activity of PMN. Interestingly, the beneficial effect of LPS was associated with delivery either before or soon after the inoculation, further supporting the concept of a priming effect.
Although this model suggests that pretreatment with LPS may be of some advantage in preventing the development of an infection, one should be very careful before extrapolating these results for prophylactic purposes. Thus, on day 3 after inoculation, the pretreated animals had a reproducible higher CFU count in the spleen that was associated with a higher level of TNF, and late injection of LPS resulted in a shorter survival. In a model of viral infection with lymphocytic choriomeningitis virus, we failed to demonstrate any beneficial effect of LPS pretreatment (unpublished observation). Together our results suggest that LPS exposure can induce both a hyporeactive state, illustrated by the endotoxin tolerance phenomenon, and a priming effect which may favor antiinfectious responses.
Acknowledgments
O.L. was a recipient of a fellowship from SIDACTION and Assistance Publique-Hôpitaux de Paris. Part of the work was supported by a grant “Contrat Interne de Recherche Clinique” from the Pasteur Institute to Françoise Dromer.
We thank Jacques Callebert (Service de Biochimie, Hôpital Lariboisière) for the NOx measurements. We express our gratitude to Ernst Rietschel for his helpful comments and for reviewing the manuscript.
REFERENCES
- 1.Aguirre K, Havell E A, Gibson G W, Johnson L L. Role of tumor necrosis factor and gamma interferon in acquired resitance to Cryptococcus neoformans in the central nervous system of mice. Infect Immun. 1995;63:1725–1731. doi: 10.1128/iai.63.5.1725-1731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavaillon J M, Pitton C, Fitting C. Endotoxin tolerance is not a LPS-specific phenomenon: partial mimicry with IL-1, IL-10 and TGFβ. J Endotoxin Res. 1994;1:21–29. [Google Scholar]
- 3.Colletti L M, Remick D G, Campbell D A. LPS pretreatment protects from hepatic ischemia/reperfusion. J Surg Res. 1994;57:337–343. doi: 10.1006/jsre.1994.1152. [DOI] [PubMed] [Google Scholar]
- 4.Collins H L, Bancroft G J. Cytokine enhancement of complement-dependent phagocytosis by macrophages: synergy of tumor necrosis factor-alpha and granulocyte-macrophage colony-stimulating factor for phagocytosis of Cryptococcus neoformans. Eur J Immunol. 1992;22:1447–1454. doi: 10.1002/eji.1830220617. [DOI] [PubMed] [Google Scholar]
- 5.Eising G P, Mao L, Schmid-Schönbein G W, Engler R L, Ross J. Effects of induced tolerance to bacterial lipopolysaccharide on myocardial infarct size in rats. Cardiovasc Res. 1996;31:73–81. [PubMed] [Google Scholar]
- 6.Han J, Thompson P, Beutler B. Dexamethasone and pentoxifylline inhibit endotoxin-induced cachectin/tumor necrosis factor synthesis at separate points in the signaling patway. J Exp Med. 1990;172:391–394. doi: 10.1084/jem.172.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He W, Fong Y, Marano M A, Gershenwald J E, Yurt R W, Moldawer L L, Lowry S F. Tolerance to endotoxin prevents mortality in infected thermal injury: association with attenuated cytokine responses. J Infect Dis. 1992;165:859–864. doi: 10.1093/infdis/165.5.859. [DOI] [PubMed] [Google Scholar]
- 8.Heemann U, Szabo A, Hamar P, Müller V, Witzke O, Lutz J, Philipp T. Lipopolysaccharide pretreatment protects from renal ischemia/reperfusion injury. Possible connection to an interleukin-6 dependent pathway. Am J Pathol. 2000;156:287–293. doi: 10.1016/S0002-9440(10)64729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huffnagle G B, Toews G B, Burdick M D, Boyd M B, McAllister K S, McDonald R A, Kunkel S L, Strieter R M. Afferent phase production of TNF-alpha is required for the development of protective T cell immunity to Cryptococcus neoformans. J Immunol. 1996;157:4529–4536. [PubMed] [Google Scholar]
- 10.Kawakami K, Qifeng X, Tohyama M, Qureshi M H, Saito A. Contribution of tumour necrosis factor-alpha (TNF-alpha) in host defence mechanism against Cryptococcus neoformans. Clin Exp Immunol. 1996;106:468–474. doi: 10.1046/j.1365-2249.1996.d01-870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lortholary O, Improvisi L, Rayhane N, Gray F, Fitting C, Cavaillon J-M, Dromer F. Cytokines profiles are similar in AIDS patients and in mice with disseminated Cryptococcus neoformans infection. Infect Immun. 1999;67:6314–6320. doi: 10.1128/iai.67.12.6314-6320.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marie C, Muret J, Fitting C, Losser M-R, Payen D, Cavaillon J-M. Reduced ex vivo interleukin-8 production by neutrophils in septic and non-septic systemic inflammatory response syndrome. Blood. 1998;91:3439–3446. [PubMed] [Google Scholar]
- 13.Mason C M, Dobard E, Summer W R, Nelson S. Intraportal lipopolysaccharide suppresses pulmonary antibacterial defense mechanisms. J Infect Dis. 1997;176:1293–1302. doi: 10.1086/514125. [DOI] [PubMed] [Google Scholar]
- 14.Minami A, Fujimoto K, Ozaki Y, Nakamura S. Augmentation of host resistance to microbial infections by recombinant human interleukin-1 alpha. Infect Immun. 1988;56:3116–3120. doi: 10.1128/iai.56.12.3116-3120.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell T G, Perfect J R. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller-Alouf H, Alouf J E, Gerlach D, Fitting C, Cavaillon J M. Cytokine production by murine cells activated by erythrogenic toxin type A superantigen of Streptococcus pyogenes. Immunobiology. 1992;186:435–448. doi: 10.1016/S0171-2985(11)80396-7. [DOI] [PubMed] [Google Scholar]
- 17.Nansen A, Pravsgaard Christensen J, Marker O, Randrup Thomsen A. Sensitization to lipopolysaccharide in mice with asymptomatic viral infection: role of T-cell-dependent production of interferon-γ. J Infect Dis. 1997;176:151–157. doi: 10.1086/514017. [DOI] [PubMed] [Google Scholar]
- 18.Rayhane N, Fitting C, Cavaillon J-M. Dissociation of IFNγ from IL-12 and IL-18 during endotoxin tolerance. J Endotoxin Res. 1999;5:319–324. [Google Scholar]
- 19.Rayhane N, Lortholary O, Fitting C, Callebert J, Huerre M, Dromer F, Cavaillon J-M. Enhanced sensitivity of tumor necrosis factor/lymphotoxin-α deficient mice to Cryptococcus neoformans infection despite increased levels of nitrite/nitrate, gamma-interferon and interleukin-12. J Infect Dis. 1999;180:1637–1647. doi: 10.1086/315061. [DOI] [PubMed] [Google Scholar]
- 20.Roilides E, Lyman C A, Mertins S D, Cole D J, Venzon D, Pizzo P A, Chanock S J, Walsh T J. Ex vivo effects of macrophage colony-stimulating factor on human monocyte activity against fungal and bacterial pathogens. Cytokine. 1996;8:42–48. doi: 10.1006/cyto.1996.0006. [DOI] [PubMed] [Google Scholar]
- 21.Rothstein J L, Schreiber H. Synergy between tumor necrosis factor and bacterial products causes hemorrhagic necrosis and lethal shock in normal mice. Proc Natl Acad Sci USA. 1988;85:607–611. doi: 10.1073/pnas.85.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salkowski C A, Detore C, Franks A, Falk M C, Vogel S N. Pulmonary and hepatic gene expression following cecal ligation and puncture: monophosphoryl lipid A prophylaxis attenuates sepsis-induced cytokine and chemokine expression and neutrophil infiltration. Infect Immun. 1998;66:3569–3578. doi: 10.1128/iai.66.8.3569-3578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schade U F. Pentoxifylline increases survival in murine endotoxin shock and decreases formation of tumor necrosis factor. Circ Shock. 1990;31:171–181. [PubMed] [Google Scholar]
- 24.Severn A, Xu D, Doyle J, Leal L M, O'Donnell C A, Brett S J, Moss D W, Liew F Y. Pre-exposure of murine macrophages to lipopolysaccharide inhibits the induction of nitric oxide synthase and reduces leishmanicidal activity. Eur J Immunol. 1993;23:1711–1714. doi: 10.1002/eji.1830230747. [DOI] [PubMed] [Google Scholar]
- 25.Welty-Wolf K E, Carraway M S, Huang Y-C T, Simonson S G, Kantrow S P, Piantadosi C A. Bacterial priming increases lung injury in Gram negative sepsis. Am J Respir Crit Care Med. 1998;158:610–619. doi: 10.1164/ajrccm.158.2.9704064. [DOI] [PubMed] [Google Scholar]
- 26.Youngner J S, Stinebring W R. Interferon appearance stimulated by endotoxin bacteria or viruses in mice pre-treated with Escherichia coli endotoxin or infected with Mycobacterium tuberculosis. Nature. 1965;208:456–458. doi: 10.1038/208456a0. [DOI] [PubMed] [Google Scholar]