Abstract
The Monkeypox virus belongs to the Orthopoxvius genus, as does the specifically human smallpox virus. It is zoonotic and had never previously been considered as capable of human-to-human transmission over more than nine viral generation cycles. While relevant animal reservoirs have yet to be identified, non-human primates (NHP) are only accidental hosts. The potentially high number of current human shedders during the clinical phase (3 weeks maximum) raises the question of a risk in our countries of animals being contaminated by infected humans (reverse zoonosis). Cats as well as cows are susceptible to the Cowpox virus, another zoonotic Orthopoxvirus, which they transmit to humans. Dogs are much less susceptible to this virus and seem only receptive to Vaccinia virus (also belonging to the Orthopoxvirus genus). On the other hand, one study has demonstrated the pronounced susceptibility of the adult albino rabbit and of young animals of several rodent species to Monkeypox virus (MPXV). Given the susceptibility to MPXV of prairie dogs, which are American Sciuridae, the potential for infection of European squirrels cannot be ruled out.
Keywords: Monkeypox, Reverse zoonosis, Animal reservoir
Abbreviations
- CB
Congo Basin
- NHP
Non-human primates
- NP
New pets
- MPXV
Monkeypox virus
- WA
West Africa
The emergence of monkeypox outside of Africa in early May 2022, in Europe in particular and by human-to-human transmission alone, aroused considerable surprise and raised potential concern, especially insofar as the Monkeypox virus (MPXV) belongs to the Orthopoxvirus genus, as does the specifically human smallpox, which in 1980 was officially declared eradicated.
Contrary to the smallpox virus, the MPXV is zoonotic and had never previously been considered as capable of human-to-human transmission over more than nine viral generation cycles [1]. Moreover, the areas where it circulates in animal reservoirs correspond to the primary forests of central and western Africa, with two known lineages, namely CB (Congo basin), and WA (West Africa), the latter being at once less virulent and the only lineage to date to have made inroads outside the African continent [2].
While the animal reservoirs of MPXV have not been identified, it is now known that non-human primates (NPH) are only accidental hosts. On the other hand, different rodents and African squirrels are strongly suspected of being the main if not exclusive reservoirs of the virus [3], [4].
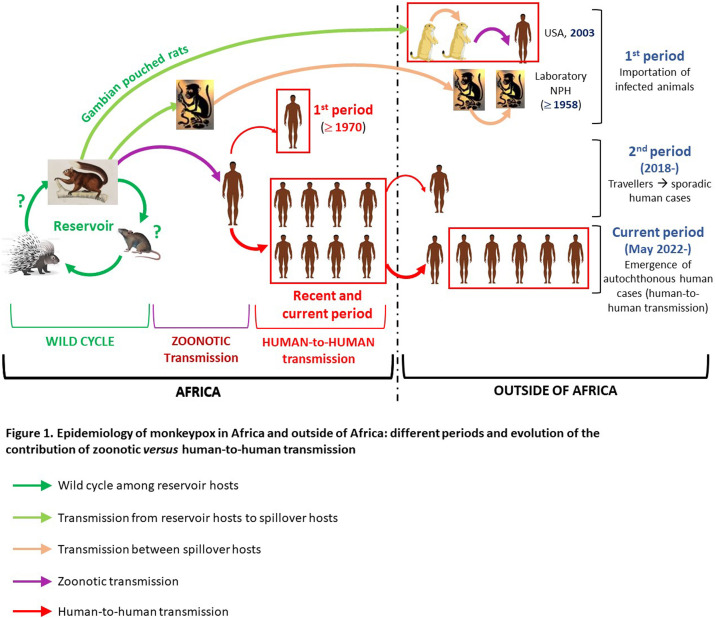
That said, MPXV (WA lineage) had previously, albeit rarely, managed to extract itself from its African “cradle”. In fact, it was in Denmark, in 1958, that the virus was discovered, following the importation of cynomolgus macaques for experimentations. Other imported cases were subsequently reported in laboratories in different parts of the world [5]. The most spectacular animal outbreak occurred in 2003 in the USA following the importation from Ghana of savanna cricetoma (Gambian pouched rats) as new pets (NP) [6]. They contaminated autochthonous pet prairie dogs, which were the source of 72 human zoonotic cases in six states (47 were confirmed). Interestingly, prairie dogs are Sciuridae, just like squirrels.
The first human cases outside of Africa (apart from the very particular outbreak of 2003 in the USA) were reported in 2018 but up until the unprecedented emergence in 2022, they remained sporadic (Fig. 1 ); only mild increases in numbers seem to have occurred. All of these cases were travellers coming or returning from Nigeria, plus one case of nosocomial transmission and one family cluster [7]. They were the secondary consequence of the abrupt reemergence of the WA lineage in Nigeria from 2017. While this increase in cases in historic foci, with increased human-to-human transmission are considered as resulting from the discontinuation of smallpox vaccination, other anthropic factors (deforestation, population growth…) have likewise strongly contributed [8], [9].
Figure 1.
Monkeypox epidemiology inside and outside of Africa: different periods in the evolution of zoonotic vs.human-to-human transmission.
The current large number of potential human shedders, occasioning during the clinical phase (3 weeks maximum) either direct contagiousness through skin and/or mucosal contact and emission in the air of infectious droplets, or indirect contagiousness through contamination of the patient's environment by secretions and scabs, raises the possibility of a risk of contamination not of humans by animals (as in Africa, or the USA in 2003), but rather of animals by humans (reverse zoonosis). This transmission could potentially concern pets, production animals and/or wild animals [10].
Not only are we largely unaware of their receptivity and susceptibility, but the animals contributing to the MPXV epidemiological cycle in Africa have yet to be formally identified. Only limited indications are presently available. What can we cautiously hypothesize, in this specific context, when we know that transmission from humans to animals presupposes:
-
•
virus emission by humans (demonstrated);
-
•
animal receptivity/susceptibility (unknown);
-
•
and a possibility of transmission of the virus (which probability will vary according to species categories)?
Since domestic animals evolve in close proximity to their owners, they would seem to be the most at risk of being contaminated by humans. While cats as well as cows are susceptible to the Cowpox virus, another zoonotic Orthopoxvirus, which can be transmitted by them to humans, not a single case of monkeypox attributable to a cat has been reported in Africa, and a serological survey failed to identify any seropositive cats (or domesticated ruminants). For dogs and ferrets, no data are available for MPXV to date. However, dogs have proven to be weakly susceptible to Cowpox virus and receptive to Vaccinia virus (also belonging to the Orthopoxvirus genus).
As regards new pets, the rabbit is the most frequent species, and a study has shown a high susceptibility of the adult albino rabbit through “natural” routes of infection. The rat and the mouse as pets belong to Rattus norvegicus and Mus musculus domesticus species respectively, neither of which is experimentally susceptible through “natural” routes of infection. However, under experimental conditions, the neonates of these species are highly susceptible to MPXV [11]. Regarding hamsters and guinea pigs, data are scarce. As for the Siberia chipmunk, since 2017 they cannot legally be kept as pets, at least in France.
Since synanthropic rats and mice belong to the same species as pet rats and mice, the same reasoning can apply. What is more, any contamination by infected humans could only occur indirectly, rendering even lower the likelihood of infection.
Lastly, as regards non-synanthropic wild species susceptible to infection, it is not sure that they exist, but were they to exist, they would most likely be rodents and squirrels. Given the susceptibility of prairie dogs (American Sciuridae), it cannot be ruled out that European squirrels could be infected. If the red squirrel has been found to be experimentally very susceptible, it does not approach humans, and there is no data for the gray squirrel, which can let humans feed it, but is apparently not present in France to date.
All in all, the risk of human-to-human transmission appears to be far greater than the risk of infection of animals by humans. That much said, a risk of transmission to domestic animals, particularly new pets (especially rabbits and neonates) cannot be ruled out. It also bears mentioning that while all Sciuridae potentially represent species at risk, they seldom approach humans.
Table 1 is an attempt at synthesis of the presumed levels of risk. The recommendations put forward are based on hypothetical levels of risk, and more generally on the precautionary principle, which should prevail when reasonable doubts appear. In any event, the best way to anticipate even a theoretical risk of transmission to animals would be to dry up this outbreak of human cases, by isolating them and vaccinating human contacts.
Table 1.
Available data on the receptivity and/or susceptibility of animal species to MPXV, presumed infection risk for certain species present in France and measures to recommend to prevent infection, be it theoretical, by a human case (according to [10], 2022).
| Order or Family | Data on their receptivity and/or susceptibility | Potential role as host? | Interactions with humans |
Theoretical risk in France of contamination of animals by infected humans | Measures to recommend by the precautionary principle to prevent infection of animals in a human case is confirmed in France par | |
|---|---|---|---|---|---|---|
| In Africa | Outside of Africa | |||||
| Sciuridae |
African squirrels: One clinical MPXV¨+ case . At least very receptive (Ab+ prevalence in infected area) i.e. Laboratorya: susceptible to very susceptible |
Potential reservoir hosts | Rare (hunting and bush meat) | Exceptional and not with live animals (contact with/eating bush meat ) | N.A. | N.A. |
|
Prairie dogs (USA) susceptible to very susceptible: (outbreak in the USA, 2003) i.e. Laboratory*: very susceptible |
Non-African hosts with reservoir potential? | N.A. | ++ (NP, essentially in the USA) | Exceptional (holding permit necessary) | NP: no contact with the human patient and his environment for 21 days after symptom onset (left in cage in a dedicated room)td: paraenter Prohibit their possession | |
| Red squirrel: No field data. Laboratorya: very susceptible at high dose | Almost nil (wild species) | Absent to exceptional | Presumably none, because red squirrels avoid humans | |||
| Gray squirrel: No data | Almost nil to possible (feeding) | Nil to almost nil today in France, but potentially present in the United Kingdom | Presumably none, because there are grey squirrels are not supposed to be present in France.td: paraenterIf they appear: don’t approach them, don’t feed them | |||
| Other small or medium-sized mammals, including rodents |
Wild African animals: Gambian pouched rat, African dormouse, jerboa, common rufous-nosed rat, African hedgehog, porcupine: MPXV+ and/or DNA and/or Ab. i.e. Laboratorya: Gambian pouched rat, African dormouse, Natal multimammate rat, cotton rat: very susceptible |
Potential reservoir hosts | Possible (hunting and bush meat) | Exceptional and not with live animals (contact with/eating bush meat) | N.A. | N.A. |
| Wild American mammals: common opossum, gray opossum, American woodchuck i.e. Laboratorya: thirteen-lined ground squirrel: very susceptible |
Non-African hosts with reservoir potential? | N.A. | Almost nil and only in America | |||
|
Rodents and lagomorphs present in Europe: no field data. i.e. Laboratorya: 1/ Rat, mice and rabbit: -Neonates: very susceptible. -Adults: not susceptible (except for rabbits, especially albino rabbits, and Asian house mice) 2/ Hamster and guinea pigs: not susceptible (neonates not tested) |
No role? Potential accidental hosts: rabbits or neonates (all species)? Potential spillover hosts? | N.A. | NP: very important (direct and indirect) | NP : potentially present | NP: no contact with the human patient and his environment for 21 days after symptom onset (left in cage in a dedicated room) | |
| Synanthropes: only indirect | Synanthropes: very limited and only indirect | Draconian rat control program (rat-extermination, food resource reduction…) | ||||
| Wild: absent or indirect | Wild: nil to practically nil and only indirect | Rodent control program | ||||
| Non-human primates |
African and Asian NHP: primary forests, zoos, experimental units: naturally susceptible to very susceptible i.e. Laboratorya: susceptible to very susceptible |
Accidental hosts | Possible (hunting and bush meat) | Nil except for zoos and, rarely, research laboratories | Limited by the fact that people shedding the virus are supposed to be on sick leave | N.A. |
| Domestic carnivores | Cat: no Abs in an infected African area, but only one study (67 cats) | No role? Accidental host? Potential spillover host? | Important (direct and indirect) | Very important (direct and indirect) | Nil to highly limited except if the cat is receptive and susceptible | Isolation of the owner if infected, disinfection of the environment |
| Dog and ferret: no data | No role? Accidental host? Potential spillover host? | Important for dogs, N.A. for ferret | Probably :very limited to nil, except if dogs and/or ferrets are receptive and/or susceptible | |||
| Ruminants |
Sheep and goats: no Ab in infected African area, but only one study (200 animals) Bovines : no data |
No role? Accidental hosts? | Important (direct and indirect) | Important | Probably minimal to nil | Isolation of the breeder if infected |
Ab: antibodies; +: positive; - : no data; N.A: not applicable : NP; new pets; NHP = non-human primates
Routes compatible with natural infection
Author's contribution
Nadia Haddad is the only author.
Disclosure of interest
The author declares that he has no competing interest.
References
- 1.Nolen L.D., Osadebe L., Katomba J., Likofata J., Mukadi D., et al. Extended human-to-human transmission during a monkeypox outbreak in the Democratic Republic of the Congo. Emerg Infect Dis. 2016;22:1014–1021. doi: 10.3201/eid2206.150579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunge E.M., Hoet B., Chen L., Lienert F., Weidenthaler H., et al. The changing epidemiology of human monkeypox-A potential threat?. A systematic review. PLoS Negl Trop Dis. 2022;16:e0010141. doi: 10.1098/rsos.171089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khodakevich L., Szczeniowski M., Nambu-ma-Disu, Jezek Z., Marennikova S., et al. Monkeypox virus in relation to the ecological features surrounding human settlements in Bumba zone, Zaire. Trop Geogr Med. 1987;39:56–63. [PubMed] [Google Scholar]
- 4.Tiee M.S., Harrigan R.J., Thomassen H.A., Smith T.B. Ghosts of infections past: using archival samples to understand a century of monkeypox virus prevalence among host communities across space and time. R Soc Open Sci. 2018;5:171089. doi: 10.1098/rsos.171089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arita I., Henderson D.A. Smallpox and monkeypox in non-human primates. Bull World Health Organ. 1968;39:277–283. [PMC free article] [PubMed] [Google Scholar]
- 6.Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 7.Mauldin M.R., McCollum A.M., Nakazawa Y.J., Mandra A., Whitehouse E.R., et al. Exportation of monkeypox virus from the african continent. J Infect Dis. 2022;225:1367–1376. doi: 10.3406/bavf.2022.70989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakazawa Y., Lash R.R., Carroll D.S., Damon I.K., Karem K.L., et al. Mapping monkeypox transmission risk through time and space in the Congo Basin. PLoS One. 2013;8:e74816. doi: 10.1371/journal.pone.0074816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen P.Y., Ajisegiri W.S., Costantino V., Chughtai A.A., MacIntyre C.R. Reemergence of human monkeypox and declining population immunity in the context of urbanization, Nigeria, 2017-2020. Emerg Infect Dis. 2021;27:1007–1014. doi: 10.3201/eid2704.203569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haddad N. Les animaux hors d’Afrique peuvent-ils être concernés par la flambée de monkeypox en cours, voire en devenir des acteurs importants ? Bull. Acad. Vét. France, https://academie-veterinaire-defrance.org/publications/bulletins-de-lavf, doi.org/10.3406/bavf.2022.70989.
- 11.Parker S., Buller R.M. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol. 2013;8:129–157. doi: 10.2217/fvl.12.130. [DOI] [PMC free article] [PubMed] [Google Scholar]