Abstract
The emergence of the SARS-CoV-2 has affected several production services including the water production and delivery processes. This study considered sachet water quality during the advent of the SARS-CoV-2 pandemic using multivariate statistics and Water Quality Index, Water Pollution Index and, hygienic and sanitation practices of sixty-two (62) sachet water vendors using a panel assessment approach. The findings showed that vendors did not adhere to proper hygienic practices as ninety-four (94%) of them did not have health clearance, ninety (90%) did not frequently wash their receptacles for selling daily, and most of them stored and sold in unhygienic environments. Majority of the producers violated Food and Drugs Authority Regulations. The Empirical Orthogonal Function analysis showed that total iron, Total Heterotrophic Bacteria, Salmonella, Cl−, E. coli, and fecal and total coliforms were the controlling elements in the water. All the brands were below threshold limits based on the physical water assessment. However, enteric bacteria were observed in all the brands. Water Quality and Water Pollution Indices (WQI and WPI) described all the sachet water brands (vendors and production sites) as excellent for drinking. The WQI computations for samples from the production and vending sites respectively ranged from 0.12 to 0.36 and 0.27–0.42 whereas WPI presented 0.22–0.31 and 0.23–0.32. Comparatively, samples from vendors had elevated elemental concentrations and loads. This suggests that besides sachet water contamination during production and transportation, vendors significantly impacted the quality of sachet water. Sensitization on proper hygienic practices for sachet water production and vending and routine assessment of the quality of sachet water produced or sold is recommended.
Keywords: Physical, Enteric bacteria, Physicochemical, Sachet water quality, Production and vending sites
1. Introduction
The emergence and prevalence of the SARS-CoV-2 have had several impacts on the economic-production sectors including water production and delivery processes. Even before the advent of the pandemic, many developing countries lagged in the provision of piped water supply to their citizens (Abanyie et al., 2019). Though access to safe drinking water is imperative to promoting health, Dupas et al. (2020) revealed that, globally, about 1.9 billion people lack safe drinking water. Abanyie et al. (2019) described this inadequacy as a driving force to the public resorting to unsafe sources of water, especially for drinking purposes. Filling this service gap, the past decade has seen an astronomic increase in the production, supply, and consumption of bottled and sachet water (Obiri-Danso et al., 2003). There has been an astronomic production of packaged-sachet water which has become an important source of drinking water. It has emerged as a common source of drinking water in Ghana due to its low cost, availability, and provision of safe-instant drinking water which ultimately drives towards achieving the water target of the Sustainable Development Goal 6.
Sachet water production in Ghana faces several challenges that tend to impact the quality of water (Stoler et al., 2012). Due to the affordability and portability of sachet water, its quality and desirability is a share of problems (Manjaya et al., 2019). Several studies including Obiri-Danso et al. (2003), MacArthur and Darkwa (2013), and Emenike et al. (2017) have described sachet water as possible vehicles for the transmission of enteric pathogens, and heavy metals. Similarly, Omalu et al. (2011) mentioned that though hygiene, taste, and physical appearance are paramount reasons for public and personal acceptance of sachet water, it may not be entirely free of pathogens since the source of water, the treatment and packaging processes, transportation and storage can impact its quality. Water vendors could also contaminate sachet water through unhygienic practices (Manjaya et al., 2019). For instance, Dzotsi et al. (2016) attributed more than 80% of diarrhea cases in Accra to the consumption of sachet water. Nguyen et al. (2014) indicated that drinking any type of water sold is a significant risk factor for cholera infection. Similarly, MacArthur and Darkwa (2013) showed that several sachet water brands do not meet the recommended standards for drinking water. Addo et al. (2009) attributed this to the limited standard industrialized model for delivering safe drinking water due to their inability to afford efficient but expensive technologies. In this regard, Olaoye and Onilude (2009) suggest the need to examine and ascertain the quality of sachet water to safeguard the health of consumers.
Over two decades, Damongo, the capital of the Savanna Region of Ghana has lacked piped water supply due to the siltation of the Damongo Agric Institute (College) dam which supplied the township with potable water. Though mechanized boreholes have been drilled, these are inadequate and sparsely sited with some broken-down. Groundwater potentials are also limited due to the underlying Voltaian Geological Formation of the area (Ghana Statistical Service (GSS), 2014). GSS (2014) further showed that just 2.9% and 2.8% of the population in the entire municipality respectively have access to pipe-borne water in and out of their dwellings. In lieu of pipe water, low-cost sachet water is sold. Consequently, some sachet-package drinking water companies within and outside Damongo as far as Kintampo (126 miles), Techiman (163.2 miles), Tamale (77.4 miles) and, Bole (61.5 miles) have taken advantage of this to provide the people of Damongo with safe-drinkable water whiles ensuring profit. Considering the distances covered in the quest to do business, the quality of water provided may be compromised since irregular monitoring of sachet water quality is one of the factors capitalized by sachet water producers (Oyelude and Ahenkorah, 2012).
Coupled with these factors, hygiene, production practices, and transportation could also impact the quality of sachet water sold in the area. Although similar studies have been conducted in other parts of Ghana, most of them focused on the mineral and/or microbiological quality, making corrective measures more reactive than preventive. It is suspected that following the emergence of the COVID-19 pandemic which has caused economic and social disruptions, sachet water production companies may flout standards. Also, there is no categorical study on sachet water quality that has been conducted in the entire Savanna Region. In addition, the area houses the Mole National Park which suggests that the health of tourists is also at risk. This study, therefore, seeks to examine the physical, microbiological, and physicochemical qualities of sachet water from production sites and on the market, and sanitation and hygiene practices of sachet water vendors that could compromise the quality in Damongo, northern Ghana. Key areas considered are (1) a comparative water quality assessment between production and vending sites, (2) evaluating the implications of vendors hygiene and sanitation practices on sachet water quality, (3) the physical characteristics of the brands considered in the study, (4) compliance to regulatory policies on water production by sachet water producers and (5) employing the use of the novel Water Pollution Index approach to determine the quality of the water samples. This will serve as a conceptual framework for regulatory bodies to enhance sachet water quality monitoring and environmental health, and sanitation assessment of sachet water vendors and vending sites to protect and promote public health and wellbeing.
2. Materials and methods
2.1. Overview of the study area
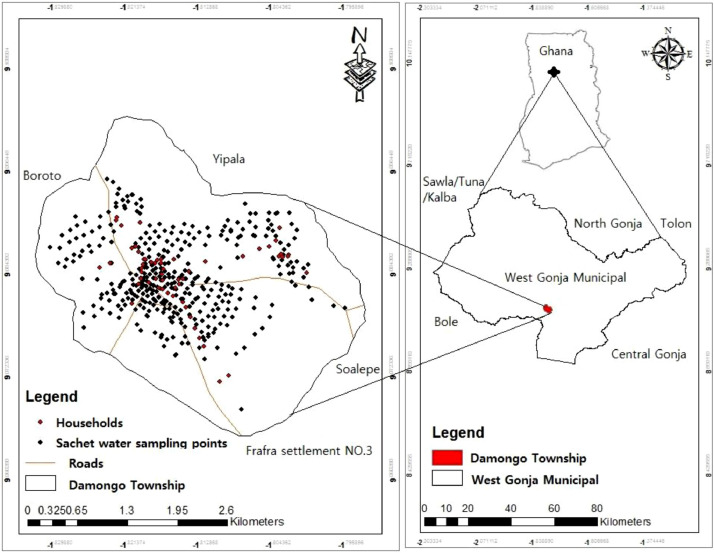
This study was conducted in Damongo, the regional capital of the Savannah Region of Ghana. It also serves as the administrative capital of the West Gonja Municipality (WGM) which occupies an area of 4715 km2. It lies within latitudes 8° 32′ and 10° 2′ North and longitudes 1° 5′ and 2° 58′ West (Fig. 1 ). The area shares boundaries to the north with Yipala, southwards with Frafra settlement N0.3, to the east and west with Soalepe and Boroto respectively. The area houses the Mole National Park (GSS, 2014). It is located within the Guinea Savanna and has an adulating topography with altitudes between 150 and 200 m above sea level. Damongo is home to 20,735 people and an estimated 3275 households. Temperatures are generally high with a mean annual temperature of 27 °C (Mahama, 2019). The study further indicated that the maximum temperature occurs in the dry season (March-April) and the lowest occurs between December and January. Humidity is very low with erratic rainfall. The geology of the area is characterized mainly by the Voltaian rock units with mudstones and sandstones in the Alluvial Damongo Formation, and the extreme western part of the area is composed of granitic materials. Groundwater potential is limited due to the geological formation of the area (GSS, 2014).
Fig. 1.
Map of the area and sampling points.
2.2. Research design
The study was designed to examine the quality of sachet waters and vendors' sanitation and hygiene practices in the Damongo township. The study employed both qualitative and quantitative approaches which included the determination of the physical characteristics, microbial, and physicochemical qualities of various sachet water brands, and the hygiene and sanitation practices of sachet water vendors. Using the panel size determination approach, ten (10) assessors were deployed to assess the level of hygiene and sanitation of the vendors, the receptacles used for selling sachet water, and the hygienic conditions of the areas where selling was done using a checklist. The assessors were to examine the vendors using a five-point Likert scale of strongly agree, agree, neutral, disagree, and strongly disagree. The assessors comprised environmental science, health, and sanitation personnel. The obtained results were presented in percentages. They also determined other physical and physicochemical parameters such as recommended labels on packaging bag/sachets, the odor, taste, and appearance of the branded water samples. Both tables and figures were used to present and visualize statistically derived data.
2.3. Determination of vendors sample size and the identification of sachet water brands
The sample size of vendors was estimated using the formula proposed by Cochran (1963) which is presented as:
| (1) |
Using an error margin of 0.05, a confidence level of 95%, and a standard deviation of 0.2%.
| (2) |
In Eqs. (1) and 2, γ = sample size, α = z value of confidence level, e = desired level of precision, and σ = product of the estimated proportion of an attribute that is present in the population and 1-p.
Based on the computed sample size, 62 sachet water vendors (stores = 31; and roadside sellers = 31) were involved in this study. The water vendors were interviewed to identify the brands of sachet water sold in the township.
2.4. Panel size (X) determination for sanitation and hygiene conditions of the vendors
The number of persons required to assess the hygienic and sanitation conditions of the sachet water vendors was computed using the permutation formula suggested by Cochran and Cox (1957). This is shown as:
| (3) |
This study considered 62 vendors. Hence, this is presented as:
| (4) |
In applying this formula, Tetteh et al. (2004) indicated that the sample size should either be a factor or multiple of the result (X). A panel of 10 which is a factor of and representative sample size was considered adequate for this study. The panel used a checklist to assess the vendors, sanitation of selling points, and the taste, appearance, and odor of the sachet water samples.
2.5. Sachet water sampling
Sachet water samples were collected in triplicates. Using ArcGIS 9.3©, the area was subdivided into six grids. Except for the business area where 17 sachet water samples were collected (due to the relatively large number of vendors), 9 samples were collected from vendors in each of the other grids. The samples were prepared for analysis following the American Public Health Association Standards for the examination of water and wastewater (American Public Health Association, 1995; 2017).
2.6. Analytical and experimental methods
2.6.1. Sampling and preparation of sachet water samples
A total of 120 sachet water samples were purchased from sachet water vendors (10 samples each of the 8 brands) and the production sites (5 samples each of the 8 brands) for this study. Techniques and methods followed for the collection, preservation, and analysis of the water samples followed the recommended standards by APHA (1995; 2017).
2.6.2. Physical assessment of sachets
The physical appearance and external examination of the brands were examined for regulatory compliance which included registration (Food and Drugs Authority (FDA)), batch numbers, location, manufacturing and expiry dates, brand name, producers’ details, sources of water used for packaging, additives, the volume of water, and nutritional constituents of the various sachet waters.
2.6.3. Microbiological quality of sachet water
The filter membrane technique using a cellulose membrane filter of 0.45 µm pore size was adopted except for Total Heterotrophic Bacteria (THB) count which was determined using the pour plate method. The enteric bacterial considered in this study were total coliform (TC), Fecal coliform (FC), Escherichia coliform (E. coli), THB, and Salmonella (SS). Total and fecal coliforms were respectively enumerated using M-Endo and M-FC media whereas E. coli and Salmonella spp., and THB were determined using MacConkey Sorbitol and MI agars and the pour plate analytical procedures detailed in the APHA (1998; 2017) methods for the examination of water and wastewater.
2.6.4. Physical characteristics of water samples
This study involved the measurement of turbidity, pH, Electrical conductivity (EC) (µs/c), color, Total Dissolved Solids (TDS), appearance, taste, and odor. Based on the analytical techniques stipulated by APHA (1998), Turbidity and TDS were determined using the electronic colorimeter model DR./890 whereas pH was determined using a pH meter (model WTW 323). Meanwhile, electrical conductivity was measured using a conductivity meter (HACH 2100) and a Lovibond visual color comparator (M- 2000).
2.6.5. Chemical properties of sachet water samples
Following the APHA (1998; 2017) standards, the strong acid titration and the ultraviolet spectrophotometer methods were respectively used in determining the levels of alkalinity and Sulfate (SO4 −) in the water samples. In measuring chloride (Cl−), the Ethylenediaminetetraacetic acid (EDTA) titration method was adopted, whereas calcium (Ca2+) and magnesium (Mg2+) were determined using the fast-sequential Atomic Absorption Spectrometer (AAS) model AA240FS. Meanwhile, the SPADNS method was used in measuring the concentration of fluoride (F −) in the sachet water samples.
2.7. Statistical and mathematical analysis of data
The obtained data were summarized into descriptive statistical parameters using the R software and Microsoft Excel (2016 version). The expected values of the data are presented in Table 4, Table 5, Table 6. Water GraphPad Prism 5 was adapted to present descriptive statistics and correlation (covariance-variance) analysis whereas Empirical Orthogonal Function (EOF) was done on the obtained data and the cumulative proportions were used in the interpretation of the results. The analyses were conducted using the first moment/expected values which were computed using the expression:
| (5) |
The idea of indexing water quality with a numerical value was developed in the 1960s by Horton (1965). The Water Quality Index (WQI) of the water samples was determined following Vasanthavigar et al. (2010). This is presented as:
| (6) |
| (7) |
| (8) |
| (9) |
Where Rwi = relative weight, Awi = sum of the assigned weight of each parameter, n = the number of parameters, qi = quality rating for each parameter, Ci = the concentration of each chemical parameter in each water sample in mg/L, Si = the standard of each chemical parameter in mg/L and SIi = the sub-index of the ith parameter.
Table 4.
Statistical summary of the centered enteric bacteria loads of sachet water from the production sites and vendors.
| Parameter | Collection point | Centered values of Enteric Bacteria Load (CFU/100 ml) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PL | YG | NN | AK | WD | AJ | ED | AC | Min | Max | Mean | SD | Median | Skew | Kurt | ||
| Fecal coliform | Production | 0.3 | 0.7 | 0.0 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.00 | 0.70 | 0.3 | 0.2 | 1.0 | 0.8 | 3.8 |
| Vendor | 1.0 | 1.0 | 0.7 | 0.7 | 1.3 | 1.0 | 0.7 | 1.3 | 0.7 | 1.3 | 1.0 | 0.3 | 1.0 | 0.3 | −1.4 | |
| Total coliform | Production | 6.3 | 5.3 | 8.3 | 8.0 | 4.7 | 4.0 | 8.7 | 6.8 | 4.0 | 8.7 | 6.5 | 1.8 | 6.6 | −0.2 | −1.6 |
| Vendor | 6.7 | 5.7 | 9.1 | 10.2 | 6.9 | 7.2 | 9.1 | 7.3 | 5.7 | 10.2 | 7.8 | 0.5 | 7.3 | 0.4 | −1.0 | |
| Salmonella | Production | 0.7 | 0.0 | 1.0 | 0.7 | 0.3 | 0.7 | 0.0 | 0.3 | 0.0 | 1.0 | 0.5 | 0.4 | 1.0 | −0.1 | −1.4 |
| Vendor | 0.7 | 0.7 | 0.7 | 1.0 | 1.3 | 1.3 | 0.3 | 1.0 | 0.3 | 1.3 | 0.9 | 0.3 | 0.9 | −0.2 | −0.4 | |
| E. coli | Production | 0.7 | 0.7 | 0.7 | 0.3 | 0.7 | 1.0 | 0.7 | 0.3 | 0.3 | 1.0 | 0.6 | 0.2 | 1.0 | −0.4 | 0.2 |
| Vendor | 0.7 | 1.7 | 1.0 | 0.7 | 1.7 | 1.0 | 1.3 | 1.0 | 0.7 | 1.7 | 1.1 | 0.4 | 1.0 | 0.6 | −1.1 | |
| THB | Production | 18.3 | 18.3 | 32.0 | 18.7 | 13.7 | 21.3 | 18.7 | 13.7 | 13.7 | 32.0 | 19.3 | 5.7 | 65.7 | 1.7 | 3.9 |
| Vendor | 73.7 | 63.7 | 77.3 | 65.3 | 70.6 | 74.0 | 65.7 | 55.3 | 55.3 | 77.3 | 68.2 | 7.1 | 68.2 | −0.6 | 0.1 | |
Table 5.
Statistical summary of the centered Physical characteristics of sachet water.
| Parameter | Collection point | Expected values of Physical Parameters |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PL | YG | NN | AK | WD | AJ | ED | AC | Min | Max | Mean | SD | Med. | Skew | Kurt | WHO (2011) | ||
| Turbidity (NTU) | Vendor | 0.63 | 0.71 | 0.45 | 0.67 | 0.81 | 0.42 | 0.65 | 0.79 | 0.42 | 0.81 | 0.64 | 0.14 | 0.66 | −0.62 | −0.68 | 5 |
| Production | 0.61 | 0.69 | 0.43 | 0.67 | 0.79 | 0.41 | 0.65 | 0.77 | 0.41 | 0.79 | 0.63 | 0.13 | 0.67 | −0.71 | −0.67 | ||
| pH | Vendor | 8.11 | 7.25 | 7.56 | 7.23 | 6.69 | 7.14 | 6.89 | 6.65 | 6.65 | 8.11 | 7.19 | 0.48 | 7.19 | 0.88 | 0.78 | 6.5–8.5 |
| Production | 7.97 | 7.13 | 7.47 | 7.21 | 6.62 | 7.10 | 6.83 | 6.60 | 6.60 | 7.97 | 7.11 | 0.46 | 7.14 | 0.77 | 0.52 | ||
| EC (µs/cm) | Vendor | 10.0 | 13.0 | 9.0 | 16.0 | 12.0 | 14.0 | 9.0 | 11.0 | 9.0 | 16.0 | 11.75 | 2.49 | 11.50 | 0.53 | −0.65 | 1500 |
| Production | 10.0 | 9.0 | 7.0 | 14.0 | 11.0 | 13.0 | 8.0 | 11.0 | 7.0 | 14.0 | 10.40 | 2.39 | 12.0 | 0.16 | −0.83 | ||
| TDS (mg/l) | Vendor | 61.0 | 57.0 | 43.0 | 67.0 | 84.0 | 39.0 | 47.0 | 64.0 | 39.0 | 84.0 | 57.75 | 14.68 | 59.0 | 0.49 | 0.03 | 1000 |
| Production | 56.0 | 55.0 | 41.0 | 65.0 | 76.0 | 35.0 | 43.0 | 61.0 | 35.0 | 76.0 | 54.38 | 13.94 | 57.0 | 0.08 | −0.10 | ||
| Appearance | Vendor | C | C | C | C | C | C | C | C | – | – | – | – | – | – | – | C |
| Production | C | C | C | C | C | C | C | C | – | – | – | – | – | – | – | ||
| Taste | Vendor | U | U | U | U | U | U | U | U | – | – | – | – | – | – | – | U |
| Production | U | U | U | U | U | U | U | U | – | – | – | – | – | – | – | ||
| Color (Hz) | Vendor | C | C | C | C | C | C | C | C | – | – | – | – | – | – | – | C |
| Production | C | C | C | C | C | C | C | C | – | – | – | – | – | – | – | ||
| Odor | Vendor | U | U | U | U | U | U | U | U | – | – | – | – | – | – | – | U |
| Production | U | U | U | U | U | U | U | U | – | – | – | – | – | – | – | ||
C = Clear and U = Unobjectionable.
Table 6.
Statistical summary of the centered Chemical quality of sachet water.
| Parameter | Collection point | Mean values of Chemical Parameters |
(WHO, 2011) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PL | YG | NN | AK | WD | AJ | ED | AC | Min | Max | Mean | SD | Median | Skew | Kurt | |||
| Total iron (mg/l) | Vendor | 0.26 | 0.23 | 0.25 | 0.22 | 0.28 | 0.31 | 0.17 | 0.22 | 0.17 | 0.31 | 0.24 | 0.04 | 0.24 | −0.1 | 0.37 | 0.3 |
| Production | 0.25 | 0.21 | 0.25 | 0.20 | 0.26 | 0.26 | 0.15 | 0.19 | 0.15 | 0.26 | 0.22 | 0.04 | 0.23 | −0.70 | −0.57 | ||
| Fluoride (mg/l) | Vendor | 0.27 | 0.73 | 0.78 | 0.33 | 0.16 | 0.84 | 0.37 | 0.28 | 0.16 | 0.84 | 0.47 | 0.27 | 0.35 | 0.49 | −1.90 | 1.5 |
| Production | 0.23 | 0.71 | 0.77 | 0.29 | 0.13 | 0.77 | 0.35 | 0.27 | 0.13 | 0.77 | 0.44 | 0.27 | 0.37 | 0.45 | −2.0 | ||
| Calcium (mg/l) | Vendor | 16.12 | 16.65 | 13.48 | 16.87 | 17.34 | 23.17 | 13.45 | 15.71 | 13.45 | 23.17 | 16.60 | 3.03 | 16.40 | 1.51 | 3.42 | 75 |
| Production | 15.91 | 16.46 | 13.24 | 16.65 | 17.02 | 22.83 | 13.12 | 15.70 | 13.12 | 22.83 | 16.37 | 3.01 | 16.65 | 1.44 | 3.30 | ||
| Chloride (mg/l) | Vendor | 15.75 | 9.14 | 9.61 | 10.27 | 9.36 | 11.79 | 8.65 | 7.92 | 7.92 | 15.75 | 10.31 | 2.48 | 9.50 | 1.80 | 3.48 | 250 |
| Production | 15.57 | 9.19 | 9.36 | 10.03 | 9.17 | 11.49 | 8.46 | 7.90 | 1.03 | 15.57 | 9.02 | 4.05 | 9.36 | −0.62 | 2.72 | ||
| Magnesium (mg/l) | Vendor | 3.58 | 5.54 | 5.81 | 8.17 | 4.69 | 7.95 | 6.71 | 6.39 | 3.58 | 8.17 | 6.11 | 1.55 | 6.10 | −0.19 | −0.51 | 30 |
| Production | 3.51 | 5.27 | 5.72 | 8.16 | 4.49 | 7.50 | 6.57 | 6.18 | 3.51 | 8.16 | 5.93 | 1.53 | 6.39 | −0.11 | −0.50 | ||
| Sulfate (mg/l) | Vendor | 9.12 | 12.27 | 14.23 | 13.28 | 15.67 | 6.45 | 5.62 | 3.89 | 3.89 | 15.67 | 10.07 | 4.41 | 10.70 | −0.18 | −1.78 | 250 |
| Production | 9.10 | 12.24 | 14.20 | 13.27 | 15.65 | 6.44 | 5.62 | 3.89 | 3.89 | 15.65 | 10.05 | 4.39 | 12.27 | −0.17 | −1.77 | ||
Following Hossain and Patra (2020) Water Pollution Index was computed using the formula:
| (10) |
| (11) |
In Eqn. (10), PL = Pollution Load, Ic = observed concentration of the ith parameter whereas Sd = standard or the highest permissible limit for the various parameter considered. WPI = Water Pollution Index and n = n number of parameters (Eqn. (11)).
3. Results and discussion
3.1. Identification of sachet water brands
A reconnaissance survey was done within the Damongo township to identify the various brands of sachet water sold. The study identified eight different sachet water brands at the time of the study. These were identified and presented as PL, YG, NN, AK, WD, AT, ED, and AC. These brands were either produced within the setting or produced and transported from other areas.
3.2. Hygiene and sanitation practices of sachet water vendors
Stoler (2012) revealed that several studies have shown a chain of water contamination from the source point to consumption. Ashbolt (2004) attributed contamination of sachet drinking water to poor personal hygiene of handlers and general environmental hygiene. Though sachet water is airtight, leakages observed during production and disruptions resulting from packaging, loading, transporting, and offloading bagged sachet water could cause breakages that expose them to external routes of contamination. Therefore, inasmuch as production conditions are essentials, the conditions in which water is sold are essential since the quality of water provided to consumers could be compromised. The results obtained showed that 52% of the vendors used ice-chest for selling sachet water, whereas 6 (9%) and 24 (39%) vendors respectively used metal and plastic receptacles. About 21 (66%) of the receptacles used did not have lids, and 26 (81%) did not wash their ice-chests daily making them unhygienic for rendering such public service. This could expose sachet water sold on the market to contamination.
Considering the sanitation and hygiene conditions of the vendors, strongly disagree had the highest average score of 36 whereas neutral and disagree were 21 and 15 respectively (Table 2). Meanwhile, agree and strongly agree respectively recorded average scores of 10 and 8 as shown in Table 2. This showed that most of the sachet water vendors did not adhere to proper sanitation and hygiene practices. External factors emanating from the practices of the vendors could impact the quality of the water sold since vendors may transfer pathogens and other contaminants to the sachet water which therefore makes the protection of public health concern. In this regard, the United Nations 2019 report on Water, Sanitation, and Hygiene indicates that unsafe hygiene practices are widespread, resulting in debilitating impacts on public health (United Nations, 2019).
Table 2.
Frequency results of Sanitation and Hygiene conditions of the vendors (N = 10).
| Vendors’ hygiene and sanitation (%) | |||||
|---|---|---|---|---|---|
| Conditions | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| The vendor appear clean | 0 | 0 | 50 | 20 | 30 |
| Knew the importance of ensuring cleanness | 50 | 20 | 30 | 0 | 0 |
| Clean dress | 10 | 30 | 30 | 30 | 0 |
| Vendor washed hands frequently | 0 | 0 | 0 | 0 | 100 |
| Vendor had hand sanitizer/soap | 0 | 0 | 0 | 0 | 100 |
| Looked physically healthy | 20 | 40 | 10 | 30 | 0 |
| Washed before re-refrigerating unsold sachet water | 0 | 0 | 0 | 0 | 100 |
| The storage area was clean | 0 | 10 | 40 | 30 | 20 |
| Vendor's overall sanitation and hygiene | 0 | 0 | 50 | 40 | 10 |
| Average | 8 | 10 | 21 | 15 | 36 |
| Environmental conditions of vending sites (%) | |||||
| Animals/insects (flies) around the area of selling | 60 | 30 | 10 | 0 | 0 |
| Garbage around the selling area | 40 | 20 | 20 | 10 | 10 |
| Clean premises | 50 | 30 | 10 | 10 | 0 |
| Average | 17 | 9 | 3 | 0 | 0 |
| Receptacle used for selling (%) | |||||
| Receptacle was clean | 10 | 10 | 50 | 20 | 10 |
| People placed their hands to pick sachet water was hygienic | 60 | 30 | 10 | 0 | 0 |
| Average | 7 | 4 | 6 | 2 | 10 |
| Yes | No | ||||
| Vendor had an active health permit for selling | 4 (6%) | 58 (94%) | |||
| Vendor handled sachet water with bare hands | 62 (100%) | 0 | |||
| Receptacles with lids | 23 (37%) | 39 (63%) | |||
| Did the Vendor wash ice blocks before adding to cool the water? | 59 (95%) | 3 (5%) | |||
| Vendor handled money with bare hands | 62 (100%) | 0 | |||
| Vendor washed receptacle daily | 6 (10%) | 56 (90%) | |||
| People placed their hands to remove sachet water when purchasing from vendors | 34 (55%) | 28 (45%) | |||
| Vendor recognized the importance of ensuring proper sanitation and hygiene | 59 (95%) | 3 (5%) | |||
This study revealed that most (60%) of the areas where sachet waters were sold had animals and insects (flies) around. Khalil et al. (1994) described flies as bacterial enteropathogens, which makes public health a concern based on the environmental and sanitation conditions prevailing at the vending points. Similarly, 40% of the vendors had a high score of having refuse around their selling areas, which translates into 50% of highly unclean areas. A neutral score (50%) was recorded for the cleanliness of the receptacles used for selling the sachet water. Most of the vendors (60%) allowed purchasers to pick sachet waters personally. These could serve as pathogenic vectors and physical mechanisms to contaminating the water. This explains the variations in pathogenic, chemical, and physical attributes shown in Table 3, Table 4, Table 5, Table 6.
Table 3.
Physical Examination of Sachet water samples.
| Factor | PL | YG | NN | AK | WD | AT | ED | AC |
|---|---|---|---|---|---|---|---|---|
| Batch number | – | * | – | – | – | – | – | – |
| Manufacturing and expiry dates | + | – | + | – | + | + | + | + |
| Location | + | + | + | + | + | + | + | + |
| Conditions for proper storage | + | – | – | + | + | + | – | – |
| Brand name | + | + | + | + | + | + | + | + |
| Producer's name | – | – | + | + | + | + | + | + |
| Contact address | + | + | – | + | – | + | + | + |
| Source of water | – | – | – | – | – | – | – | – |
| Additives | – | – | – | – | – | – | – | – |
| Nutritional constituents | – | – | – | – | – | – | – | – |
| Directives for proper disposal | + | – | + | – | * | + | + | * |
| Volume of water | + | – | + | + | + | + | + | + |
| FDA Registration | – | + | – | – | + | – | + | + |
| Faded sachet water package | + | + | + | – | + | – | – | + |
| Leaking after storing for a week | – | – | + | + | + | – | + | – |
+ = present; * = present but faded; and - = absent.
The research further showed that 58% of the vendors did not have health clearance that declared them healthy for rending this public-economic service. However, all the vendors handled money and sachet water with bare hands, and neither of them had hand sanitizers to disinfect their hands in the course of selling to protect personal and public health. External contaminants/pathogens could be transferred unto the surfaces of the sachet water, and eventually into the sachets through the broken/leaking areas. It is therefore prudent that vendors are considered medically healthy to vend water to reduce waterborne diseases such as cholera, hepatitis, and typhoid.
About 63% of the vendors used receptacles without lids. Alike with Manjaya et al. (2019), ice-cubes were placed into receptacles to keep the sachet water cold for public consumption. Nevertheless, the study indicated that 95% of the vendors did not wash the ice cubes before doing this. These practices could be possible routes for contaminating sachet waters. Considering the leakages observed in some brands (Table 3), pathogens could enter the sachet waters through these openings. Relating to Recio and Gomez (2013) on the importance of ensuring sanitation and hygiene at vending areas, a majority (95%) of the vendors recognized the need for ensuring proper sanitation and hygiene in selling water to the public. The findings affirm the assertion of Chinenye and Amos (2017) that unhygienic practices by vendors and filthy environments for selling sachet water impact the quality of sachet water sold on the market.
3.3. Physical assessment of sachets
Table 3 presents the results of the physical examination of the sampled sachets. The study revealed that only brand YG representing 13% had a batch number. However, this was faded and illegible. Also, considering details of the dates of manufacturing and expiry, all the brands indicated the span (months after manufacture) of the water produced. However, the date of manufacture was not shown, making it difficult to determine the stipulated span. All the samples showed the locations of the production sites and brand names. Nevertheless, brands WD and ED and, AC did not give explicit locations as they stated “opposite total filling station”, and “Kintampo” respectively. Brands NN, YG, ED, and AC did not indicate the conditions for proper storage of sachet water whereas brands PL and YG did not indicate the producers’ names. These respectively represented 50% and 25% of the total brands assessed in this study. The study showed that two brands; NN and WD did not show the contact addresses of the manufacturers. Meanwhile, all the brands did not indicate the sources of the water used for packaging, the additives, and the nutritional constituents of the packaged water (Table 3).
Except for brands TG and AK, the remaining brands diagrammatically indicated disposal of used sachet water into dustbins. This could influence sachet water users to dispose used sachet bags properly. However, this indication was faded and illegible on brands WD and AC. The study showed that only brand TG did not indicate the volume of the water whereas brands PL, NN, AK, and AT, representing 50% of the brands did not have registration numbers from the FDA, Ghana. Meanwhile, the inscriptions on samples PL, YG, NN, WD, and AC sachets were faded and unreadable, whereas brands YG, NN, WD, and ED leaked after being stored for a week. Olaoye and Onilude (2009) and Dzodzomenyo et al. (2018) in Nigeria and Ghana that most sachet brands either do not have or have fake addresses, forged standard seals, and incorrect information about the producers. Similarly, some sachet brands lacked details including manufacture and expiry dates (brands YG and AK), producers' or company name, and registration numbers. These violations make it difficult for regulatory authorities to conduct routine assessments, ensure compliance with water quality standards, and prosecute violators. Also, all the brands did not indicate the sources of water used, the additives, and the concentrations of the nutritional constituents of the bagged water. This could affect public health since these could either be above or below threshold limits and could impede toxicological studies.
3.4. Microbiological quality of packaged water
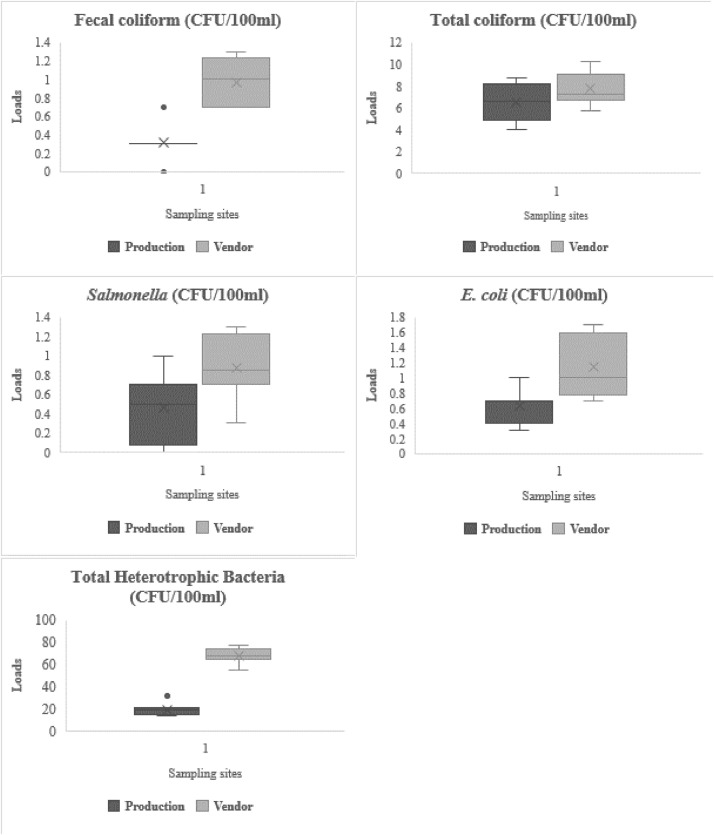
The centered results for the bacterial loads of the sachet water are presented in Table 4 and Fig. 2. The study showed that mean fecal coliform count ranged from 0 to 1.3 CFU/100 ml, and 0.7–1.3 CFU/100 ml for samples collected from the production sites and vendors respectively (Fig. 2). Total coliform and Salmonella loads were respectively between 4.0–8.7 CFU/100 ml, and 0–1.0 CFU/100 ml for samples from the manufacturers and 5.7–10.2 CFU/100 ml, and 0.3–1.3 CFU/100 ml for samples from the vendors. E. coli counts were 0.3–1.0 CFU/100 ml (production sites) and 0.7–1.3 CFU/100 ml (vendors). THB loads fell between 13.7–21.3 CFU/100 ml (production sites) and 55.3–77.3 CFU/100 ml (vendors) (Table 4). Packaged water, no matter their sources, are susceptible to microbial, toxic organics, and inorganic contamination (Anyamene and Ojiagu, 2014). The presence of coliforms in potable water indicates water contamination and is used to assess the cleanliness and integrity of water distribution systems (Opara and Nnodim, 2014). Water is considered to pose no risk to human health when fecal and total coliforms, E. coli, and Salmonella counts/100 ml are zero (World Health Organization, 2011) and THB below 500 CFU/100 ml by the Ghana Standards Authority (GSA) as stated by Akrong et al. (2019).
Fig. 2.
Enteric Bacteria Loads of Sachet water samples from Production sites and Vendors.
The enteric bacteria load of the sachet water obtained from the production sites suggest poor quality control and sanitation measures considered in the production of the waters whereas the further increase in loads in samples from the vendors also indicated poor adherence to sanitation and hygienic conditions which significantly increased loads of bacteria pathogens of sachet water on the market and further suggest the possible presence other organisms of health concerns including norovirus, Giardia spp., Shigella spp., and Cryptosporidium spp. (World Health Organization, 1996).
MacArthur and Darkwa (2013) in the Central Region of Ghana attributed the unavailability of handwashing facilities at sachet water production sites, poor handwashing by staff before and during production, the lack of hygienic clothes and protective materials (gloves and cups), talking during production as unhygienic practices that contribute significantly to the microbial quality of packaged water intended for public use. This shows that health implications associated with enteric bacteria such as cholera, abdominal pain, gastroenteritis, bacillary dysentery, typhoid, diarrhea, urinary infections, and hepatitis stated by Anyamene and Ojiagu (2014) emanating from sachet water consumption are expected in the areas. The wide load difference between the THB loads and the other enteric bacteria suggests the presence of other opportunistic pathogenic microbes including Acinetobacter, Aeromonas, Flavobacterium, Klebsiella, Moraxella, Serratia, and Xanthomonas as indicated by WHO (2017). The close deviations, skewness, lopsidedness, and tailedness presented in Table SC indicate a very close relationship between the enteric bacteria loads of samples from vendors and producers.
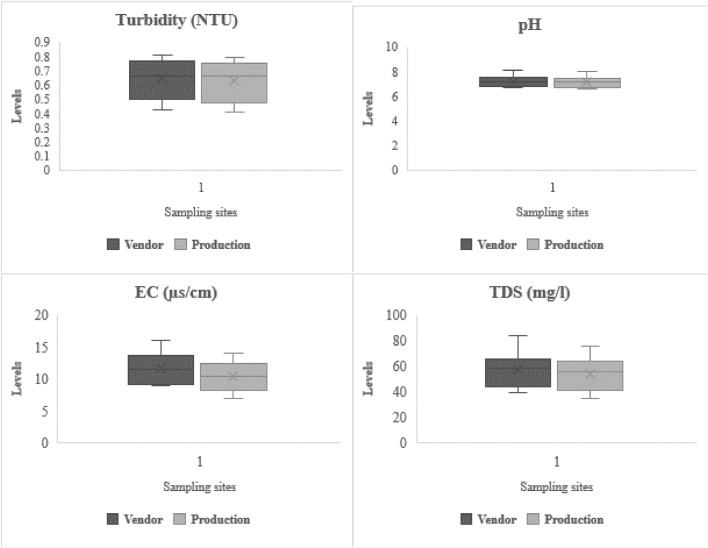
3.5. Physical properties of sachet water
Table 5 and Fig. 3 show the averaged results of the physical properties of the sampled sachet water. The results indicate an increase in turbidity and TDS from the production sites to vending points as the sachet water samples from vendors recorded TDS (39.0–84.0 mg/l) and turbidity (0.46–0.81 NTU) whiles those collected from the production sites ranged from 35.0 to 76.0 mg/l and 0.41 and 0.77 NTU for TDS and turbidity (Table 5). The EC results of samples from the vendors and the producers recorded first moment values of 11.75 µs/cm and 10.4 µs/cm respectively. Also, the expected values of 7.19 and 7.11 were obtained for pH for samples from the vendors and production sites respectively. These were within the permissible limits of 6.5–8.5 by WHO (2011). Inferentially, the brands can be described as neutral water. This suggests that based on the recorded pH, the sachet water brands may pose no health threats such as acidosis or alkalosis (Asamoah and Amorin, 2011). The pH results were dissimilar to the findings of MacArthur and Darkwa (2013) in a study conducted in the Central Region of Ghana where the pH of some packaged water was acidic, ranging from 5.3 to 6.5, and 5.4 to 7.6 by Ngmekpele (2015) in Obuasi. However, these were similar to Oyelude and Ahenkorah (2012) and Toma et al. (2013) where pH ranged between 6.80–8.15, and 6.9–7.9 in Bolgatanga, Ghana, and Erbil city, Iraq, respectively. All the samples; from vendors and production sites were clear in color and appearance and unobjectionable in taste and odor.
Fig. 3.
Results of physical parameters.
Turbidity presents the measure of suspended particles in water. Following the recommended guideline of 5 NTU by WHO (2011), the obtained results were within safe limits as the results ranged between 0.41–0.79 NTU, and 0.42–0.81 NTU for samples from the production sites and vendors respectively (Fig. 3). In northeastern Ghana, Iraq, and Nigeria, Oyelude and Ahenkorah (2012), Toma et al. (2013), and Adefemi and Azeez (2019) respectively obtained similar results; 0.45–1.72 NTU, 0.17–2.20 NTU, and 0.01–1.22 NTU. This study was disparate from Duwiejuah et al. (2013) where turbidity ranged from 1.0 to 7.0 NTU in Northern Ghana. Based on this, no health implications are expected from consuming any of the brands. However, the slightly elevated turbid nature of samples obtained from the vendors over those from the production sites indicates contamination sources between the production and vending stages.
The TDS results (43–84 mg/l) (vendors) and 41–76 mg/l (production sites) were well below the permissible limits of 1000 mg/l stipulated by WHO (2011). These results were alike with 4–204 mg/l by Oyelude and Ahenkorah (2012) in Bolgatanga, 11–54 mg/l by Ngmekpele (2015) in Obuasi, and 14.9–114.3 mg/l by Duwiejuah et al. (2013) in Tamale. The results suggest no health implications associated with TDS. Electrical conductivity values ranged from 8.0 to 14.0 µs/cm in samples obtained from the production sites and 9.0–16.0 µs/cm in samples from vendors. These were within the 1500 µs/cm recommended by WHO (2011). Similar to Oyelude and Ahenkorah (2012), the results of color were clear for samples taken from vendors and directly from the producers whereas Toma et al. (2013) and Ngmekpele (2015) showed higher results.
Color, taste, and appearance of the sachet water samples may not pose any adverse health effects on public health or discourage people from consuming such drinking water sources as the water samples were generally colorless and possessed unobjectionable odor and taste, alike with Oyelude and Ahenkorah (2012), and Adefemi and Azeez (2019). The deviations, lopsidedness, and tailedness presented in Table 5 and Fig. 3 indicate a very close relationship between the physical properties of samples obtained from vendors and those collected directly from the producers.
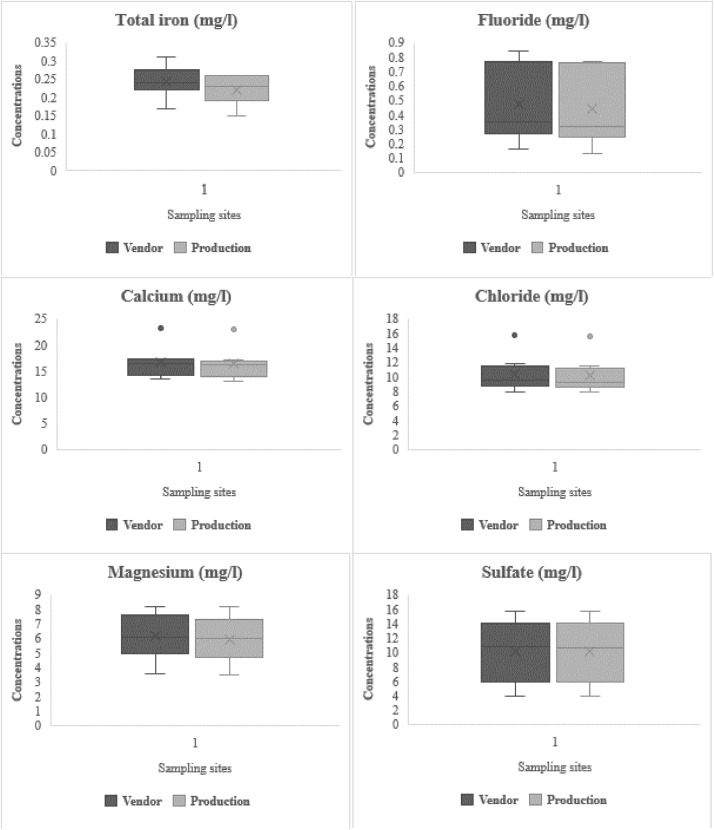
3.6. Chemical quality of water samples
Summaries of the centered values of the chemical quality of packed water examined are presented in Table 6 and Fig. 4. The measured concentration of total iron in mg/l had a minimum amount of 0.17 and 0.15 and maximum values of 0.31 and 0.26 for samples taken from vendors and producers respectively. APHA (1995) discussed that in water, F − may either be naturally occurring or purposively added. The expected values of fluoride (F −) in the samples from the production sites and vendors were 0.44 mg/l and 0.47 mg/l. These were below the WHO (2011) of 1.5 mg/l for human consumption. This also suggests that sufficient amounts of F −are available to avert tooth decay and skeletal and dental fluorosis. Following Nordqvist (2018), the obtained results indicate that thyroid problems including hyperparathyroidism, osteoarthritis, high blood pressure, heart failure, myocardial damage, and low fertility were not expected as health implications associated with the consumption of sachet water within the locality.
Fig. 4.
Chemical quality of sachet water samples.
The samples recorded central values of Ca2+; 16.60 mg/l and 16.37 mg/l for samples from the production sites and vendors. Meanwhile, the level of Cl−were summarized as 10.31 mg/l and 9.02 mg/l in the same order. The centered values of magnesium (Mg2+) for samples from the production sites and vendors were 5.93 mg/l and 6.11 mg/l correspondingly whiles the concentration of sulfate (SO4 2−) in water samples from vendors were 3.89–15.67 mg/l, whereas that of the production sites were 3.89–15.65 mg/l. The amount of calcium (Ca2+) gives an indication of the level of hardness of water which is predominantly influenced by geological factors and the seepage of industrial waste into water sources. Magnesium is also described as an essential mineral in water. Fig. 4 shows outliers for results obtained for Ca2+. Alike with the findings of Oyelude and Ahenkorah (2012), in all the samples, Ca2+concentration was higher than Mg2+. However, both variables were within the thresholds of 75 mg/l for Ca2+and 30 mg/l for Mg2+ (WHO, 2011). These are consistent with Duwiejuah et al. (2013) where the obtained Ca2+ranged from 6.4 to 15.2 mg/l in Tamale, Northern Ghana, and Asamoah and Amorin (2011) (2.0–16.8 mg/l) in Tarkwa-Nsuaem, Southern Ghana.
Chloride is a non-cumulative toxin. The Cl−levels shown in Table SE reveal within-threshold results against the WHO (2011) suggestion of 250 mg/l. The below threshold results are beneficial since a high amount of Cl− can contribute to saltiness and laxative effects in water (Singh et al., 2008). Fig. 4 shows outliers for results obtained for Cl−. The results were dissimilar with Asamoah and Amorin (2011) where elevated concentrations of Cl−up to 270 mg/l were recorded in analyzed samples from Tarkwa-Nsuaem. However, there were comparable with Oyelude and Ahenkorah (2012) (6.03–24.80 mg/l) and Ngmekpele (2015) (3.99–10.24 mg/l) conducted in Bolgatanga and Obuasi, Ghana.
Obiri-Danso et al. (2003) mentioned that iron is one of the most predominant sources of consumer complaints in the water industry, especially in developing countries. The obtained results of total iron presented in Table SE and Fig. 4 showed that the concentrations of total iron in both spectrums of the study were below the recommended standard of 0.30 mg/l stipulated by WHO (2011). The study related with Obiri-Danso et al. (2003), Asamoah and Amorin (2011), and Oyelude and Ahenkorah (2012) in studies conducted in Kumasi Metropolis, the Tarkwa-Nsuaem and Bolgatanga Municipalities respectively, which presented maximum iron concentrations of 0.1 mg/l, 0.01 mg/l, and 0.28 mg/l. The results suggest that impacts such as unpleasant odor and taste related to high iron concentration revealed by Smedley et al. (1995) are not expected.
The obtained results of sulfate (3.89–15.67 mg/l) for samples collected from vendors and 3.89–15.65 mg/l for those directly taken from the production sites fell within the “no effect” range of 250 mg/l (WHO, 2011). These relate to Singla et al. (2014) in a study conducted in India where centered SO4 2- values of 8.91 mg/l and 20.88 mg/l were respectively obtained in bottled and sachet water samples. These results further correlated with that of Asamoah and Amorin (2011), and Oyelude and Ahenkorah (2012) as they respectively ranged from 0.01 mg/l to 4.0 mg/l and 3.83 mg/l to 23.63 mg/l, indicating no expected health implications such as respiratory problems, laxative and cathartic effects related to SO4 2− (Sajil Kumar et al., 2020).
3.7. Covariance-Variance analysis of sachet water
Results for the Pearson correlation (r) analysis are presented in Table 7. The variance-covariance analysis is a means for determining the level of closeness of dissimilar variables (Mugheri et al., 2019). The study showed a direct relationship between FC, and TC (r = 0.59), E. coli (r = 0.64) and THB (r = 0.76). The presence of FC suggests the occurrence of these enteric bacteria too as FC is described as an indicator organism of fecal contamination in water (Jeon et al., 2019). Though TC established an inverse relation with Mg2+ (r=−0.51), it showed a positive correlation with THB (r = 0.69) and total iron (TI) (r = 0.52). Dissimilar to Abua et al. (2012) in Nigeria, where a negative association (r=−0.28) was identified between Cl− and pH, a positive relation (r = 0.57) was established in this study. However, the Cl−-pH relationship correlated with that of Emenike et al. (2017) in Ado-Odo Ota, Southwestern Nigeria where an r=−0.28 was established in the first week of assessment. The correlation analysis did not only present a directly significant association of THB with FC and TC but with Salmonella (SS) r=(0.58) and E. coli (r = 0.62). These firm the results presented in Table SB, indicating poor sanitation and hygienic practices during production and vending. Salmonella was further related to total iron positively (r = 0.71). Turbidity did not only show a direct relationship with TDS (r = 0.86) but negatively with pH (r=−0.50) and F − (r=−0.76). F −also established an inverse correlation with TDS (r=−0.77) whereas Cl−was indirectly related to Mg2+ (r=−0.55) and directly with pH (r = 0.57). Meanwhile, Ca2+showed a positive association with EC (r = 0.67) and total iron (r = 0.62). Unlike the findings of Emenike et al. (2017) where pH-TDS associations (r = 0.267, r = 0.203, r = 0.057, and r = 0.183) were shown in weeks 1, 2, 3, and 4 respectively, this study presented r=−0.29. This suggests that the level of pH was influenced by the level of dissolved solids in the water. However, like with the results of EC-Mg2+in week 1 by Emenike et al. (2017) (r = 0.59), this present study showed r = 0.52. This could be attributed to the fact that Mg2+is described as a good conductor of electrical charges (Freund et al., 1993).
Table 7.
Variance-Covariance Matrix of Sachet water samples.
| FC | TC | SS | E. coli | THB | Turb | pH | EC | TDS | TI | F− | Ca2+ | Cl− | Mg2+ | SO42− | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FC | 1.00 | ||||||||||||||
| TC | 0.59 | 1.00 | |||||||||||||
| SS | 0.45 | 0.26 | 1.00 | ||||||||||||
| E. coli | 0.64 | 0.40 | 0.36 | 1.00 | |||||||||||
| THB | 0.76 | 0.69 | 0.58 | 0.62 | 1.00 | ||||||||||
| Turb | 0.32 | 0.00 | −0.24 | 0.06 | −0.13 | 1.00 | |||||||||
| pH | −0.09 | 0.40 | 0.17 | −0.14 | 0.21 | −0.50 | 1.00 | ||||||||
| EC | 0.29 | −0.03 | 0.47 | 0.09 | 0.21 | 0.11 | −0.13 | 1.00 | |||||||
| TDS | 0.33 | 0.18 | 0.12 | 0.04 | 0.01 | 0.86 | −0.29 | 0.31 | 1.00 | ||||||
| TI | 0.29 | 0.52 | 0.71 | 0.29 | 0.36 | −0.36 | 0.30 | 0.29 | 0.05 | 1.00 | |||||
| F− | −0.05 | −0.02 | 0.12 | 0.21 | 0.18 | −0.76 | 0.23 | −0.06 | −0.77 | 0.26 | 1.00 | ||||
| Ca2+ | 0.19 | 0.01 | 0.40 | 0.13 | 0.04 | −0.28 | −0.07 | 0.67 | −0.10 | 0.62 | 0.28 | 1.00 | |||
| Cl− | 0.15 | 0.48 | 0.16 | 0.17 | 0.26 | −0.29 | 0.57 | −0.15 | −0.19 | 0.48 | 0.08 | 0.20 | 1.00 | ||
| Mg2+ | −0.09 | −0.51 | 0.16 | −0.14 | 0.04 | −0.28 | −0.36 | 0.52 | −0.33 | −0.23 | 0.31 | 0.32 | −0.55 | 1.00 | |
| SO42− | −0.05 | 0.25 | 0.20 | 0.14 | 0.09 | 0.05 | 0.20 | 0.08 | 0.39 | 0.35 | 0.02 | −0.13 | −0.13 | −0.25 | 1.00 |
FC = Fecal coliform, TC = Total coliform, SS = Salmonella, THB = Total Heterotrophic Bacteria, Turb. = Turbidity, EC = Electrical conductivity, TDS = Total Dissolved Solids, and TI = Total iron.
3.8. Empirical orthogonal function (EOF)
The EOF was performed on the dataset to identify the main controls on the quality of the sachet water, and Tables 8 and 9 present the outputs. EOF does not only identify the significant components and factors that aid in the interpretation of large data, but it also visualizes the correlation between the variables and limits the number of variables (Loh et al., 2020). The variables were transformed using normal score transformation before the Empirical Orthogonal Function (EOF) was done whereas the North et al. (1982) method was used in selecting the components with the highest signals. The EOF results presented in Tables 8 and 9 showed five (5) components. The components explained 85% of the total variance. EOF 1 eigenvectors which explained 28% of the total variance were attributed to a weighted sum of total iron, THB, Salmonella, total coliform, Cl−, E. coli, and fecal coliform. This indicates that the sachet water samples were highly contaminated with enteric bacteria, relating to poor sanitation practices during production, transporting, or vending. Though the results of total iron directly showed no potential effects, the EOF study revealed hidden impacts emanating from the concentration of total iron. Based on this, factors such as unpleasant odor and taste related to iron could be experienced in the sachet water when stored (Smedley et al., 1995). Inferentially, these contained the highest signals of the data and predominantly influenced the quality of the sachet water. The data further shows that TDS, turbidity, F −, E. coli, and fecal coliforms were the weighted sum of EOF 2 and described 22% of the water quality. Meanwhile, EOF 3 and 4 eigenvectors were governed by a weighted sum of Mg2+, EC, pH, Cl−, and Ca2+, and total iron, E. coli, and SO4 2−. They respectively determined 17% and 11% of the water quality. EOF 5 had a weighted sum of SO4 2− and Cl− contributing 7% to the water quality.
Table 8.
Total Variance of Sachet water quality.
| Total Variance Explained | ||||||
|---|---|---|---|---|---|---|
| Component | Initial Eigenvalues |
Extraction Sums of Squared Loadings |
||||
| Total | % of Variance | Cumulative% | Total | % of Variance | Cumulative% | |
| 1 | 4.19 | 27.95 | 27.95 | 4.19 | 27.95 | 27.95 |
| 2 | 3.27 | 21.79 | 49.74 | 3.27 | 21.79 | 49.74 |
| 3 | 2.58 | 17.17 | 66.91 | 2.58 | 17.17 | 66.91 |
| 4 | 1.63 | 10.87 | 77.78 | 1.63 | 10.87 | 77.78 |
| 5 | 1.15 | 7.66 | 85.43 | 1.15 | 7.66 | 85.43 |
| 6 | 0.86 | 5.71 | 91.14 | |||
| 7 | 0.47 | 3.10 | 94.24 | |||
| 8 | 0.38 | 2.53 | 96.77 | |||
| 9 | 0.24 | 1.58 | 98.35 | |||
| 10 | 0.14 | 0.95 | 99.30 | |||
| 11 | 0.07 | 0.44 | 99.74 | |||
| 12 | 0.03 | 0.19 | 99.94 | |||
| 13 | 0.01 | 0.05 | 99.98 | |||
| 14 | 0.00 | 0.01 | 100.00 | |||
| 15 | 0.00 | 0.00 | 100.00 | |||
Table 9.
Summary of EOF results.
| Variables | Components |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Total iron | .828 | −0.092 | .031 | .500 | −0.040 |
| THB | .782 | .192 | .063 | −0.457 | .130 |
| Salmonella | .746 | .004 | .386 | .180 | .043 |
| Total coliform | .717 | .361 | −0.395 | −0.106 | .031 |
| Fecal coliform | .631 | .511 | .215 | −0.428 | −0.139 |
| E. coli | .592 | .259 | .109 | −0.500 | .157 |
| TDS | −0.036 | .907 | .182 | .362 | .019 |
| Turbidity | −0.339 | .901 | .136 | −0.005 | −0.122 |
| Fluoride | .333 | −0.763 | .028 | −0.247 | .300 |
| Magnesium | −0.163 | −0.446 | .788 | −0.212 | .097 |
| EC | .303 | .087 | .771 | .287 | −0.132 |
| pH | .409 | −0.298 | −0.601 | .248 | .054 |
| Chloride | .543 | −0.112 | −0.566 | .114 | −0.502 |
| Calcium | .462 | −0.422 | .500 | .375 | −0.313 |
| Sulfate | .235 | .251 | −0.082 | .500 | .774 |
3.9. Water quality and water pollution indices
Water Quality Index and Water Pollution Index are described as effective water quality assessment methods (Vasistha and Ganguly, 2020; Hossain and Patra, 2020). Tables 10 and 11 respectively present the computations and results of the WQI and WPI. The study showed that all sachet water brands sampled from production and vending sites were “excellent” water sources as described by Ramakrishnaiah et al. (2009) and Hossain and Patra (2020) in Table 1 . The levels of contamination in all the brands were negligible. However, samples taken from the vendors were slightly higher than those from the production sites. This indicates that vending practices influenced the quality of sachet water.
Table 10.
Standards, weight, and relative weight for WQI computation.
| Variables | Guidelines | Weight (wi) | Relative weight (wi) |
|---|---|---|---|
| pH | 7.5 | 5 | 0.14 |
| EC | 1000 | 4 | 0.11 |
| TDS | 1500 | 5 | 0.14 |
| Total iron | 0.3 | 3 | 0.08 |
| Fluoride | 1.5 | 5 | 0.14 |
| Calcium | 75 | 3 | 0.08 |
| Chloride | 250 | 3 | 0.08 |
| Magnesium | 30 | 3 | 0.08 |
| Sulfate | 250 | 5 | 0.14 |
Table 11.
WQI and WPI results of sachet water.
| Collection point | PL | YG | NN | AK | WD | AJ | ED | AC |
|---|---|---|---|---|---|---|---|---|
| Water Quality Index | ||||||||
| Production | 0.24 | 0.19 | 0.20 | 0.17 | 0.15 | 0.36 | 0.13 | 0.12 |
| Vendors | 0.42 | 0.32 | 0.35 | 0.30 | 0.28 | 0.38 | 0.27 | 0.27 |
| Water Pollution Index | ||||||||
| Production | 0.26 | 0.28 | 0.29 | 0.26 | 0.25 | 0.31 | 0.22 | 0.24 |
| Vendors | 0.26 | 0.28 | 0.28 | 0.26 | 0.25 | 0.32 | 0.23 | 0.24 |
Table 1.
WQI and WPI classifications by Ramakrishnaiah et al. (2009) and Hossain and Patra (2020).
| WQI value | Description of water for drinking | WPI value | Description of water for drinking |
|---|---|---|---|
| < 50 | Excellent water | < 0.5 | Excellent water |
| 50–100 | Good | 0.5 – 0.75 | Good water |
| 100–200 | Poor | 0.75 – 1 | Moderately polluted water |
| 200–300 | Very poor | > 1 | Highly polluted water |
| > 300 | Unsuitable |
4. Policy plan for prevention of contamination of sachet water
The high level of patronage of sachet water in developing countries such as Ghana makes it a particularly critical matter of public health concern. The point of contamination of the product includes the source of the water, the production and delivery processes, and the handling of the product with the vendors. To avoid contamination, there must therefore be strict and adequate supervision at each stage of the processes indicated. In Ghana; the control, distribution, and sale of these products lie squarely in the purview of the Ghana Standards Authority (G.S.A), the Food and Drugs Authority (F.D.A) as well as the Environmental departments of the Local Government Machinery. As it is the situation in most of the developed world, the appropriate structures remain in place to ensure the suitable control and oversight of this sector, however, the issue lies with implementation and enforcement. These authors argue that due to the duplication of oversight responsibility for the sachet water industry, there is a lack of assertive and coordinated supervision of the sector players. It may therefore be suitable to form a joint task force drawn from the G.S.A, F.D.A and the Environmental of Local Government Authority will be charged with the strict enforcement of the relevant standards in terms of sources of water, treatment processes, packaging, and delivery processes.
Also, a significant proportion of the quality issues with sachet water are eminently treatable with some cost-effective methods such as chelation and reverse osmosis which are capable of removing inorganic substances and all forms of wastes. The purification processes also include Nano-filtration which is capable of removing inorganic chemicals, some large organic compounds, and pre-filtration which are known to be useful in treating these different sources of groundwater for human consumption as well as other treatment processes. The authors propose that the relevant authorities could team up with the National Association of Sachet and Packaged Water Producers to offer training in these processes which may be applied where suitable. Modes of hygienic handling of the product can be printed on pictorial charts or flyers which will be presented to the vendors by the delivery persons. This will provide a cost-effective means of sensitizing the sellers/vendors on how to properly handle the product with minimal contamination.
In summary, the authors propose;
-
•
The formation of a joint task force comprised all the regulatory oversight bodies in charge of the sachet water industry to fill the gaps left due to the duplication of functions.
-
•
To organize periodic training in collaboration with the National Association of Sachet and Packaged Water Producers in water treatment processes which may be applied where suitable.
-
•
Sensitization of the sellers/vendors on how to properly handle the product with minimal contamination via pictorial charts of flyers.
5. Conclusion
Unhygienic production and vending practices are factors that significantly impact the quality of sachet water. A physical examination of the identified brands showed that majority of brands did not have batch numbers. Furthermore, all the brands did not indicate the sources of water used for packaging, additives, and nutritional constituents, and nearly half of the brands did not indicate their Ghana FDA Registration numbers. An assessment of the quality of packaged water sampled from Damongo did not indicate physicochemical contamination but enteric bacteria contamination was observed in all the studied brands. The detection of pollution and fecal indicator organism, total and fecal coliforms, E. coli, and THB in samples obtained from both production and vending points. However, samples obtained from vendors had elevated contamination than those directly from the production sites. Though the WQI (0.12–0.42) and WPI (0.23–0.32) computation described all the sachet water brands as safe for drinking, the presence of enteric bacteria loads could pose deleterious public health implications. This was studied to have emanated from unhygienic practices in the production and selling lines. Poor storage and sanitation conditions were observed amongst vendors, the use of unclean receptors for selling and unwashed/unsterile hands to pick water were poor vending practices observed. Regulatory bodies mandated to handle issues related to consumables should ensure that persons and institutions involved in the water packaging business adhere to regulations to protect public health whiles doing business. Routine assessment on the quality of sachet water produced or sold should be enhanced. The WGM Assembly should intensify efforts to improve sanitation conditions in the Damongo township.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
Acknowledgement
Special thanks to all who advised us on how to modify this research.
Funding
This research did not receive any grant from any funding agency, commercial or profit sectors.
Compliance with ethical standards
References
- Abanyie S.K., Ampadu B., Saeed Z.M., Amuah E.E.Y., Douti N.B., Owusu G. The roles of community-based water and sanitation management teams (WSMTs) for sustainable development: an example of the Bawku West District, Ghana. AJEST. 2019;13(11):439–449. [Google Scholar]
- Abua M.A., Iwara A.I., Ibor U.W., Deekor T.D., Ewa E.E., Lasisi C.J. A critical assessment of quality status of selected sachet water in Calabar Municipality, Nigeria. Int. J. Biosci. 2012;2:19–26. [Google Scholar]
- Addo K.K., Mensah G.I., Bekoi M., Bonsu C., Akyeh M.L. Bacteriological quality of sachet water produced and sold in Teshie-Nungua suburbs in Accra. Adfand Online. 2009:4. [Google Scholar]
- Adefemi O.S., Azeez M.A. Chemical assessment of sachet water in Ado-Ekiti Metropolis, Nigeria. Chem. Sci. Int. J. 2019:1–6. [Google Scholar]
- Akrong M.O., Amu-Mensah F.K., Amu-Mensah M.A., Darko H., Addico G.N.D., Ampofo J.A. Seasonal analysis of bacteriological quality of drinking water sources in communities surrounding Lake Bosomtwe in the Ashanti Region of Ghana. Appl. Water Sci. 2019;9(4):1–6. [Google Scholar]
- Anyamene N.C., Ojiagu D.K. Bacteriological Analysis of sachet water sold in Akwa Metropolis, Nigeria. IJAB. 2014;3:120–122. [Google Scholar]
- American Public Health Association. 20th Ed. Washington; American: 1995. Standard Method For the Examination of Water and Waste Water; pp. 67–69. [Google Scholar]
- APHA. 20th Ed. Washington; D.C: 1998. Standard Methods for the Examination of Water and Wastewater. pp. 4-145 to 4-146. [Google Scholar]
- APHA. American Public Health Association, American Water Works Association, and Water Env; Washington: 2017. APHA 2017 Standard Methods For Examination of Water and Wastewater. Federation ISBN. [Google Scholar]
- Asamoah D.N., Amorin R. Assessment of the quality of bottled/sachet water in the Tarkwa-Nsuaem municipality (TM) of Ghana. Res. J. Appl. Sci. Eng. Technol. 2011;3(5):377–385. [Google Scholar]
- Ashbolt N.J. Microbial contamination of drinking water and disease outcome in developing regions. Toxicology. 2004;198:229–238. doi: 10.1016/j.tox.2004.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinenye I.J., Amos O.O. Effect of storage and exposure to sunlight on the quality of sachet water sold in Ibadan metropolis. SJPH. 2017;5(4):321. [Google Scholar]
- Cochran P., Cox A. Longman Group Ltd.; New York: 1957. Principle of Statistics; pp. 120–130. [Google Scholar]
- Cochran W.G. 2nd Ed. John Wiley and Sons, Inc; New York: 1963. Sampling Techniques. [Google Scholar]
- Dupas P., Nhlema B., Wagner Z., Wolf A., Wroe E. Expanding access to clean water for the rural poor: experimental evidence from Malawi. Nat. Bureau Econ. Res. 2020 http://www.nber.org/papers/w27570 Available at. [Google Scholar]
- Duwiejuah A.B., Cobbina S.J., Akrong M.O. Effect of storage on the quality of sachet-vended water in the Tamale Metropolis, Ghana. J. Environ. Prot. 2013;4(06):629–637. [Google Scholar]
- Dzodzomenyo M., Fink G., Dotse-Gborgbortsi W., Wardrop N., Aryeetey G., Coleman N., Hill A., Wright J. Sachet water quality and product registration: a cross-sectional study in Accra, Ghana. J. Water Health. 2018;16(4):646–656. doi: 10.2166/wh.2018.055. [DOI] [PubMed] [Google Scholar]
- Dzotsi E., Odoom J.K., Opare J.K., Davies-Teye B.B. Outbreak of cholera, greater accra region Ghana 2014. J. Sci. Res. Rep. 2016;9(3):1–12. [Google Scholar]
- Emenike P.C., Tenebe T.I., Omeje M., Osinubi D.S. Health risk assessment of heavy metal variability in sachet water sold in Ado-Odo Ota, South-Western Nigeria. Environ. Monit. Assess. 2017;189(9):480. doi: 10.1007/s10661-017-6180-3. [DOI] [PubMed] [Google Scholar]
- Freund F., Freund M.M., Batllo F. Critical review of electrical conductivity measurements and charge distribution analysis of magnesium oxide. J. Geophys. Res. 1993;98(B12):22209–22229. [Google Scholar]
- Ghana Statistical Service (GSS) West Gonja District Analytical Report. 2014. 2010 Population and Housing Census.http://www2.statsghana.gov.gh/docfiles/2010_District_Report/Northern/West_Gonja.pdf Available at. [Google Scholar]
- Horton R.K. An index number system for rating water quality. J. Water Pollut. Control Fed. 1965;37(3):300–306. [Google Scholar]
- Hossain M., Patra P.K. Water pollution index–A new integrated approach to rank water quality. Ecol Indic. 2020;117 [Google Scholar]
- Jeon D.J., Ligaray M., Kim M., Kim G., Lee G., Pachepsky Y.A., Cha D.H., Cho K.H. Evaluating the influence of climate change on the fate and transport of fecal coliform bacteria using the modified SWAT model. Sci. Total Environ. 2019;658:753–762. doi: 10.1016/j.scitotenv.2018.12.213. [DOI] [PubMed] [Google Scholar]
- Khalil K., Lindblom G.B., Mazhar K., Kaijser B. Flies and water as reservoirs for bacterial enteropathogens in urban and rural areas in and around Lahore, Pakistan. Epidemiol. Infect. 1994;113(3):435–444. doi: 10.1017/s0950268800068448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh Y.S.A., Akurugu B.A., Manu E., Aliou A.S. Assessment of groundwater quality and the main controls on its hydrochemistry in some Voltaian and basement aquifers, northern Ghana. Groundw. Sustain. Dev. 2020;10 [Google Scholar]
- MacArthur R.L., Darkwa S. Production and Vendors practices that compromise the quality of “sachet” water in the central region, Ghana. Int. J. Sci. Technol. Soc. 2013;1:64–70. [Google Scholar]
- Mahama R.A. 2019. Nature and Causes of Solid Waste Crime at Damongo in the Northern Region of Ghana. (Doctoral dissertation) [Google Scholar]
- Manjaya D., Tilley E., Marks S.J. Informally Vended Sachet Water: handling Practices and Microbial Water Quality. Water (Basel) 2019;11(4):800. [Google Scholar]
- Mugheri M.H., Pathan A.M., Sayed M.A., Maira M., Soomro D.B., Ali amur S., Soomro N.A. Assessment of drinking water quality district jamshoro sindh pakistan: a case study. Int. J. Curr. Res. 2019;11(03):1812–1816. [Google Scholar]
- Ngmekpele B.S. Sachet water quality in Obuasi, Ashanti Region, Ghana. J Biol Agric Healthc. 2015:37–42. [Google Scholar]
- Nguyen V.D., Sreenivasan N., Lam E., Ayers T., Kargbo D., Dafae F., Jambai A., Alemu W., Kamara A., Islam M.S., Stroika S. Cholera epidemic associated with consumption of unsafe drinking water and street-vended water—Eastern Freetown, Sierra Leone, 2012. Am. J. Trop. Med.Hyg. 2014;90(3):518–523. doi: 10.4269/ajtmh.13-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordqvist C. Why Do We Have Fluoride in Our Water? Medical News Today; 2018. Fluoride: risk, uses, and side effects.https://www.medicalnewstoday.com/articles/154164.php Available at. [Google Scholar]
- North G.R., Bell T.L., Cahalan R.F., Moeng F.J. Sampling errors in the estimation of empirical orthogonal functions. Monthly Weather Rev. 1982;110(7):699–706. [Google Scholar]
- Obiri-Danso K., Okore-Hanson A., Jones K. The microbiological quality of drinking water sold on the streets in Kumasi, Ghana. Lett. Appl. Microbiol. 2003;37(4):334–339. doi: 10.1046/j.1472-765x.2003.01403.x. [DOI] [PubMed] [Google Scholar]
- Olaoye O.A., Onilude A.A. Assessment of microbiological quality of sachet-packaged drinking water in Western Nigeria and its public health significance. Public Health. 2009;123(11):729–734. doi: 10.1016/j.puhe.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Opara A.U., Nnodim J. Prevalence of Bacteria in bottled and sachet water sold in Owerri metropolis. IJSID. 2014;4:117–122. [Google Scholar]
- Oyelude E.O., Ahenkorah S. Quality of sachet water and bottled water in Bolgatanga municipality of Ghana. Res J Appl Sci Eng Technol. 2012;4(9):1094–1098. [Google Scholar]
- Ramakrishnaiah C.R., Sadashivaiah C., Ranganna G. Assessment of water quality index for the groundwater in Tumkur Taluk, Karnataka State, India. E-J. Chem. 2009;6(2):523–530. [Google Scholar]
- Recio R.B., Gomez J.E.A., Jr Street vendors, their contested spaces, and the policy environment: a view from Caloocan, Metro Manila. Environ. Urbaniz. Asia. 2013;4(1):173–190. [Google Scholar]
- Sajil Kumar P.J., Mohanan A.A., Ekanthalu V.S. Hydrogeochemical analysis of Groundwater in Thanjavur district, Tamil Nadu; influences of geological settings and land use pattern. Geol., Ecol. Landscapes. 2020;4(4):306–317. [Google Scholar]
- Singh A.K., Mondal G.C., Kumar S., Singh T.B., Tewary B.K., Sinha A. Major ion chemistry, weathering processes and water quality assessment in upper catchment of Damodar River basin, India. Environ. Geol. 2008;54(4):745–758. [Google Scholar]
- Singla A., Hansa, Kundu B.P., Singh S., Singh K., Jain S. Physico-chemical and bacterial evaluation of packaged drinking water marketed in Delhi-potential public health implications. J. Clin. Diagn. Res. 2014;8(3):246. doi: 10.7860/JCDR/2014/7845.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedley P.L., Edmunds W.M., West J.M., Gardner S.J., Pelig-Ba K.B. 1995. British Geological Survey Technical Report WC/95/43; p. 122. [Google Scholar]
- Stoler J. Improved but unsustainable: accounting for sachet water in post-2015 goals for global safe water. Trop. Med. Internat. Health. 2012;17(12):1506–1508. doi: 10.1111/j.1365-3156.2012.03099.x. [DOI] [PubMed] [Google Scholar]
- Stoler J., Weeks J.R., Fink G. Sachet drinking water in Ghana's Accra-Tema metropolitan area: past, present, and future. J. Water Sanit. Hyg. Dev. 2012;2(4):223–240. doi: 10.2166/washdev.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetteh I.K., Awuah E., Frempong E. Development of a weighting system for use in environmental health impact assessment associated with water impoundment projects in Ghana and its application I. J. Ghana Sci. Ass. 2004;6(2):2004. [Google Scholar]
- Toma J.J., Ahmed R.S., Abdulla Z.K. Application of water quality index for assessment water quality in some bottled water Erbil City, Kurdistan Region, Iraq. J. Adv. Lab. Res. Biol. 2013;4(4):128–134. [Google Scholar]
- United Nations. 2019. Water, Sanitation and Hygiene.https://www.unwater.org/water-facts/water-sanitation-and-hygiene/Accessed Available at. December 13, 2020. [Google Scholar]
- Vasanthavigar M., Srinivasamoorthy K., Vijayaragavan K., Ganthi R.R., Chidambaram S., Anandhan P., Manivannan R., Vasudevan S. Application of water quality index for groundwater quality assessment: thirumanimuttar sub-basin, Tamilnadu, India. Environ. Monit. Assess. 2010;171(1–4):595–609. doi: 10.1007/s10661-009-1302-1. [DOI] [PubMed] [Google Scholar]
- Vasistha P., Ganguly R. Assessment of spatio-temporal variations in lake water body using indexing method. Environ. Sci. Pollut. Res. 2020;27(33):41856–41875. doi: 10.1007/s11356-020-10109-3. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) 2nd ed. 1996. Guidelines For Drinking-Water Quality; pp. 940–949.http://www.who.int/water_sanitation_health/GDWQ/Summary_tables/Sumtab.html Vol. 2 Health criteria and other supporting informationand WHO, 1998. Addendum to Vol. 2. p. 281-283. Geneva, World Health Organization. Available at. [Google Scholar]
- WHO Guidelines for drinking-water quality. WHO Chron. 2011;38(4):104–108. [PubMed] [Google Scholar]
- WHO . 2017. Guidelines For Drinking-Water Quality. first addendum to the fourth edition. [Google Scholar]