Abstract
In July 2018, pediatric type 1 diabetes (T1D) care at Stanford suffered many of the problems that plague U.S. health care. Patient outcomes lagged behind those of peer European nations, care was delivered primarily on a fixed cadence rather than as needed, continuous glucose monitors (CGMs) were largely unavailable for individuals with public insurance, and providers’ primary access to CGM data was through long printouts. Stanford developed a new technology-enabled, telemedicine-based care model for patients with newly diagnosed T1D. They developed and deployed Timely Interventions for Diabetes Excellence (TIDE) to facilitate as-needed patient contact with the partially automated analysis of CGM data and used philanthropic funding to facilitate full access to CGM technology for publicly insured patients, for whom CGM is not readily available in California. A study of the use of CGM for patients with new-onset T1D (pilot Teamwork, Targets, and Technology for Tight Control [4T] study), which incorporated the use of TIDE, was associated with a 0.5%-point reduction in hemoglobin A1c compared with historical controls and an 86% reduction in screen time for providers reviewing patient data. Based on this initial success, Stanford expanded the use of TIDE to a total of 300 patients, including many outside the pilot 4T study, and made TIDE freely available as open-source software. Next steps include expanding the use of TIDE to support the care of approximately 1,000 patients, improving TIDE and the associated workflows to scale their use to more patients, incorporating data from additional sensors, and partnering with other institutions to facilitate their deployment of this care model.
The Challenge
In July 2018, pediatric type 1 diabetes (T1D) care at Stanford suffered many of the problems that plague U.S. health care. Patient outcomes lagged those of peer European nations, care was delivered primarily on a fixed cadence rather than as needed, continuous glucose monitors (CGMs) were largely unavailable for individuals with public insurance, and provider access to CGM data was inefficient and frustrating. Traditional T1D care revolved around a patient performing fingerstick blood glucose readings multiple times per day and visiting a care provider’s clinic four times per year to have their hemoglobin A1c (HbA1c) measured and receive feedback on glucose management. There were few opportunities to identify deteriorating glucose management between scheduled visits (Figure 1).
FIGURE 1. Glucose Management with Fixed-Cadence Visits (Hypothetical).
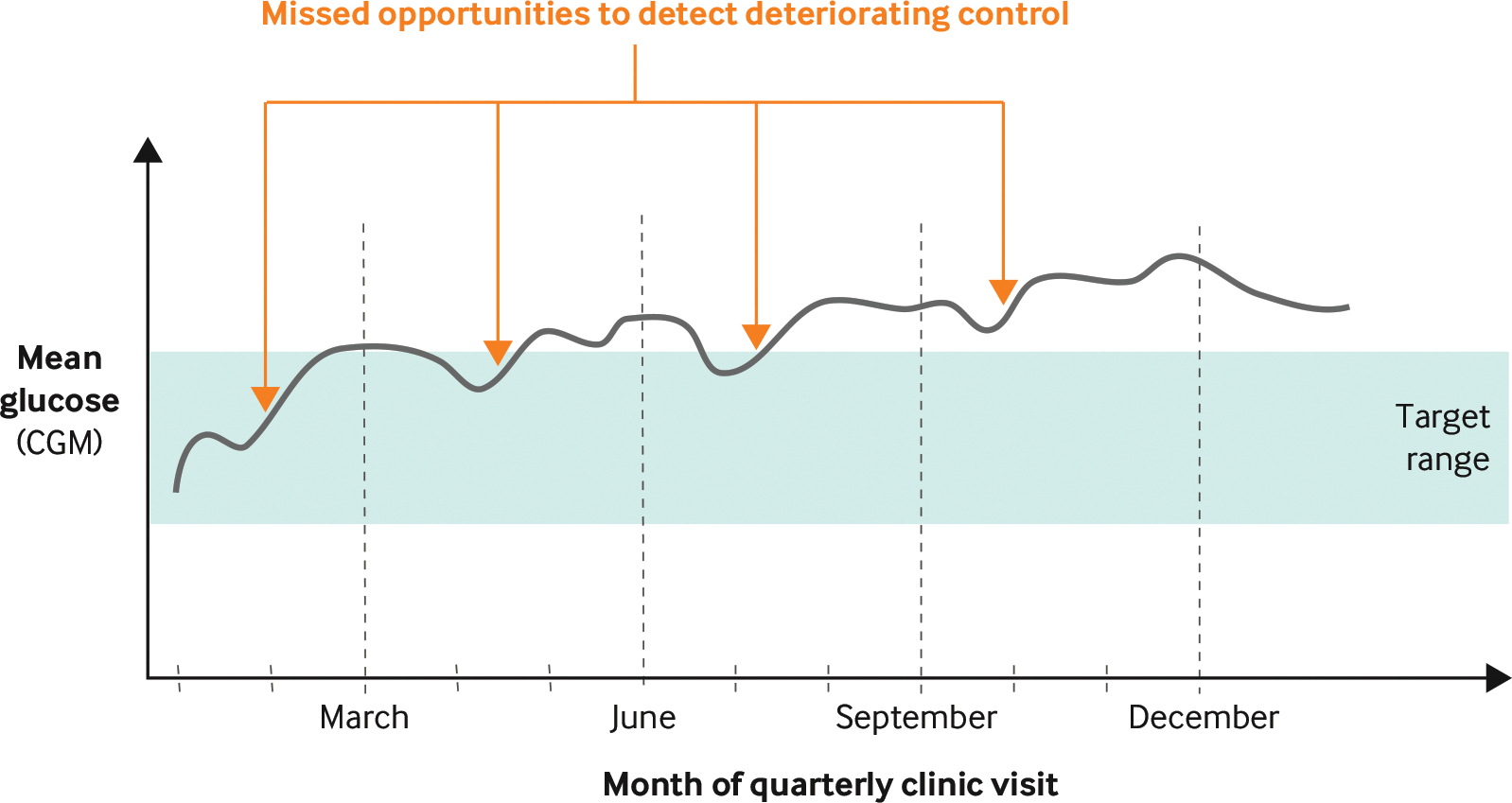
Glucose management improves after fixed-cadence visits, but not enough to make up for episodes of deterioration occurring between visits. The trend illustrated is consistent with an observed decline in postdiagnosis glucose management.
CGM = continuous glucose monitor.
Source: Lucile Packard Children’s Hospital Stanford
NEJM Catalyst (catalyst.nejm.org) © Massachusetts Medical Society
Even for those patients who use a CGM, providers would often review CGM trace data only at quarterly visits. At Stanford, as across much of the rest of the United States, certain factors were associated with worse access to care and worse outcomes, including being publicly insured, being Latinx or Black, having lower household income, or living in a rural county.1 We at Stanford, as in the United States generally, were failing to achieve HbA1c targets, especially in children, adolescents, and young adults who have significantly higher HbA1c than those in peer European countries.2
The Goal
Our goals were to improve glucose management, as measured by HbA1c and consensus CGM-derived metrics, for patients with T1D cared for by the pediatric endocrinology clinic at Lucile Packard Children’s Hospital Stanford (LPCH). LPCH is a women’s and children’s academic medical center, and the pediatric endocrinology clinic is a multispecialty group of approximately 25 physicians and 40 nonphysician care providers, including nurse practitioners, psychologists, certified diabetes care and education specialists (CDCESs), registered dieticians, endocrine nurses, and social workers providing care for approximately 1,200 youth with T1D.
The landmark Diabetes Control and Complications Trial established in 1993 that intensive management of T1D resulted in lower HbA1c and reduced vascular complications.3 Our hypothesis was that leveraging continuously collected data to identify early signs of deteriorating glucose management would facilitate as-needed patient contact and better glucose management (Figure 2).
FIGURE 2. Glucose Management As-Needed Visits Based on Remote Monitoring via Continuous Glucose Monitors (Hypothetical).
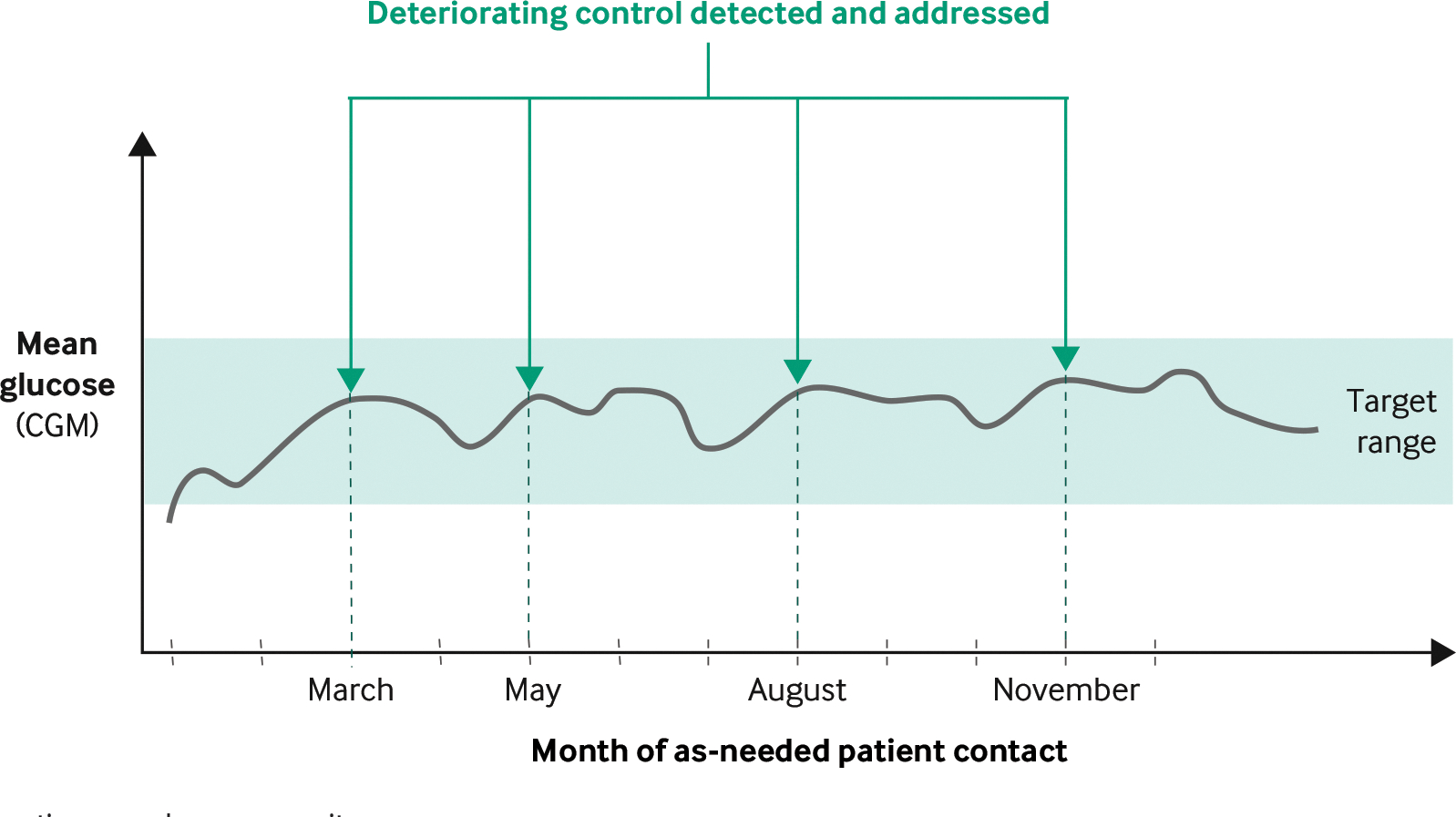
Deterioration in glucose management is detected, and patients are contacted with guidance on how to improve management. The trend illustrated is consistent with a slower observed decline in postdiagnosis glucose management associated with the use of Timely Interventions for Diabetes Excellence (TIDE).
CGM = continuous glucose monitor.
Source: Lucile Packard Children’s Hospital Stanford
NEJM Catalyst (catalyst.nejm.org) © Massachusetts Medical Society
The Execution
We developed the Teamwork, Targets, and Technology for Tight Control (4T) program.4,5 We focused on the early introduction of CGMs with asynchronous remote monitoring of glucose data by CDCESs. To facilitate the review of CGM data (up to 9,000 glucose data points per patient per month), we designed the Timely Interventions for Diabetes Excellence (TIDE) platform to analyze patient-level data for the entire population and identify those patients whose glucose management was deteriorating. TIDE is compatible with data generated by any make and model of CGM and runs on a server that allows providers convenient, secure access to personalized patient data for the entire population. In December 2018, we deployed an initial version of TIDE for use for patient care by one pediatric endocrinologist and one CDCES. The tool immediately reduced provider screen time by aggregating data for all patients, eliminating the need for providers to log into and review each patient’s CGM data one at a time. Over the subsequent 3 years, we gathered user feedback and iteratively updated the visual interface, the algorithms that rank the patients, the metrics used, and how TIDE is hosted. We have expanded the use of TIDE beyond the original study. TIDE is now used by 3 CDCESs in the clinic as a routine part of patient care for almost 300 patients. We are on track to expand the use of TIDE to all CDCESs and to our entire clinic population of approximately 1,000 pediatric patients with T1D. The present article presents the execution, challenges, and lessons learned from designing and deploying the technology and workflows of the program.
Patient Populations
TIDE is currently in use for youth in three institutional review board–approved studies: 4T pilot, 4T phase 1, and CGM Time in Range Program (CGM TIPs). In the 4T pilot program, started in 2018, youth were offered a CGM in the month after diagnosis to complement standard of care. Starting in 2019, youth in the 4T pilot study were offered a CGM with remote patient monitoring (RPM) consisting of weekly CGM data review by CDCESs. The 4T phase 1 study started in 2020 with all patients offered a CGM and weekly RPM in the month after diagnosis. After the successful use of TIDE for patients with new-onset T1D, TIDE was deployed with modified settings to support the CGM TIPs for youth with established T1D on public insurance who were unable to receive insurance approval for a CGM or who had frequent gaps in coverage. In CGM TIPs, patients were offered monthly RPM.
Among the three studies, TIDE is now used to support the care of 296 youth with T1D (Table 1). All of those enrolled gave informed consent for the care team to review the data collected by their CGM every week and to send them a message with suggestions for glucose management, when appropriate. Patients were not billed for RPM; the costs of these studies were covered with research funding, and we are currently studying financial viability models of RPM. We have previously published details of the study populations and data showing that the use of TIDE has been associated with sustained CGM use, improved glucose management, and reduced provider screen time.4–11
Table 1.
Characteristics of Patient Populations Whose Care Is Supported with the Use of TIDE
| Study | |||
|---|---|---|---|
| Characteristic | Pilot 4T with remote monitoring | 4T phase 1 | GCM TIPs |
| No. of patients | 89 | 124 | 83 |
| Age, mean (SD) | 11.91 (4.13) | 11.19 (4.53) | 15.40 (4.05) |
| Sex | |||
| Female | 54 (46) | 48 (60) | 52 (43) |
| Male | 46 (43) | 52 (64) | 49 (40) |
| Self-identified race | |||
| White | 35 (31) | 38 (47) | 24 (20) |
| Asian/Pacific Islander | 15 (13) | 13 (16) | 2 (2) |
| Black | 0 (0) | 1 (1) | 6 (5) |
| Other | 5 (5) | 30 (37) | 55 (46) |
| Not stated | 26 (23) | 22 (27) | 12 (10) |
| Ethnicity | |||
| Non-Hispanic | 48 (43) | 52 (64) | 30 (25) |
| Hispanic | 19 (17) | 28 (35) | 47 (39) |
| Not stated | 26 (23) | 20 (25) | 23 (19) |
| Insurance type | |||
| Private | 75 (67) | 65 (80) | 0 (0) |
| Public | 25 (22) | 34 (42) | 100 (83) |
| Both | 0 (0) | 2 (2) | 0 (0) |
| Years since diabetes diagnosis at enrollment, mean (SD) | Enrolled within 1 mo of onset | Enrolled within 1 mo of onset | 4.36 (3.155) |
Data are presented as percent (n) unless otherwise indicated. TIDE = Timely Interventions for Diabetes Excellence, 4T = Teamwork, Targets, and Technology for Tight Control, GCM TIPs = Time in Range Program. Source: Lucile Packard Children’s Hospital Stanford
Clinical Workflow
Each CDCES uses TIDE on a scheduled cadence (e.g., weekly for some patient populations and monthly for others) to identify the patients most likely to benefit from provider contact. TIDE displays population-level data with one row per patient, metrics exceeding predefined thresholds in red, and patients ranked by the likelihood of requiring contact (Figure 3). TIDE uses American Diabetes Association consensus metrics to identify patients for contact: the number of days the CGM was active and collected more than a minimum percentage of valid readings (ACT), mean glucose, the percentage of time in range (TIR) defined as readings 70–180 mg/dL, the change in the TIR from the previous review period (percent change in TIR), the percentage of time extremely hypoglycemic defined as readings lower than 54 mg/dL, and the percentage of time hypoglycemic defined as readings lower than 70 mg/dL as well as a variety of more refined metrics, such as the percentage of time very high, defined as readings higher than 250 mg/dL. Based on these metrics, TIDE ranks patient likelihood of requiring contact and identifies those above a predefined threshold. TIDE is designed to balance the number of patients identified with the capacity of the clinic. The algorithms used to do so have been refined based on provider feedback and the analysis of historical data gathered at our clinic.8
FIGURE 3. Timely Interventions for Diabetes Excellence Population-Level View.
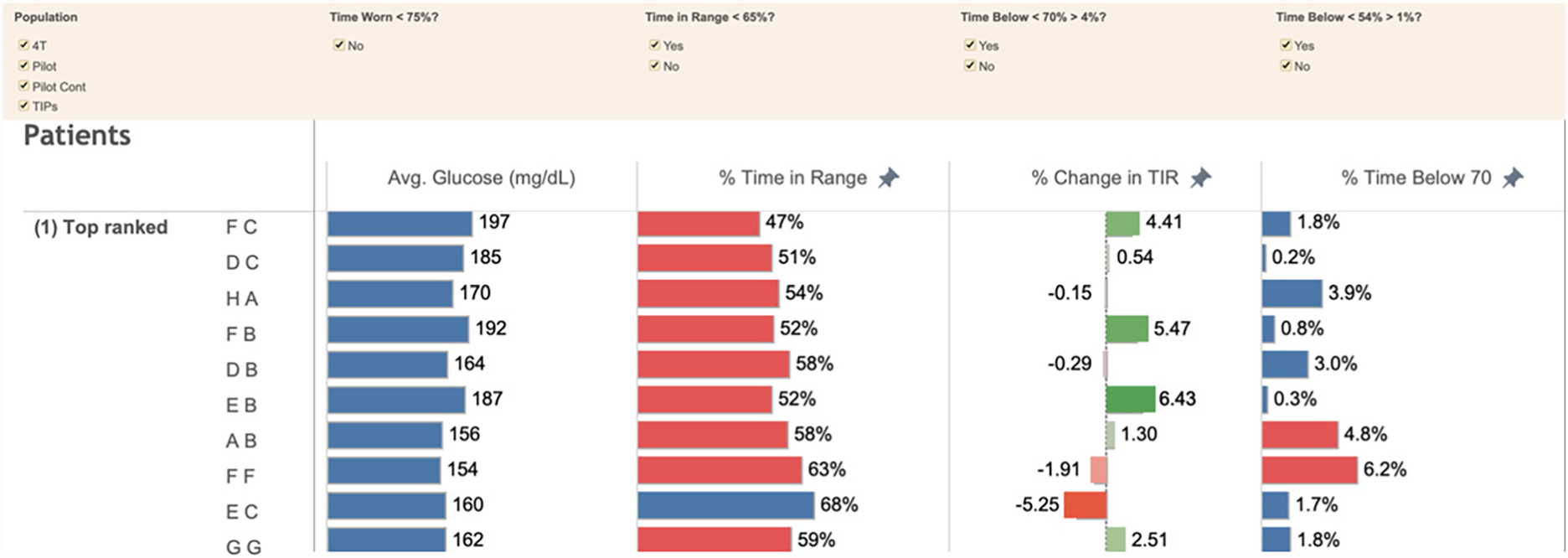
Rows correspond to individual patient data, are ranked by the likelihood of the patient requiring contact, and display metrics exceeding predefined thresholds in red.
Avg. = average, Cont = continued, 4T = Teamwork, Targets, and Technology for Tight Control, TIPs = Time in Range Program, TIR = time in range.
Source: Lucile Packard Children’s Hospital Stanford
NEJM Catalyst (catalyst.nejm.org) © Massachusetts Medical Society
When CDCESs select a patient, TIDE provides the detailed patient-level data necessary to make decisions such as insulin dose adjustments (Figure 4). For those participants who they believe would benefit, CDCESs use the electronic medical record (EMR) to send secure messages to provide insulin dose adjustments, education, or encouragement. CDCESs make insulin dose adjustments and provide clinical guidance within institutional parameters, with the support of a physician available for consult. Those patients who do not participate in portal messaging are contacted via telephone call. CDCESs also contact patients with significant gaps in CGM data to discuss CGM usage, but this is relatively rare, with one of our studies reporting 90.8% median percent time with CGM data over 2-week intervals.6
FIGURE 4. Timely Interventions for Diabetes Excellence Individual-Level View.
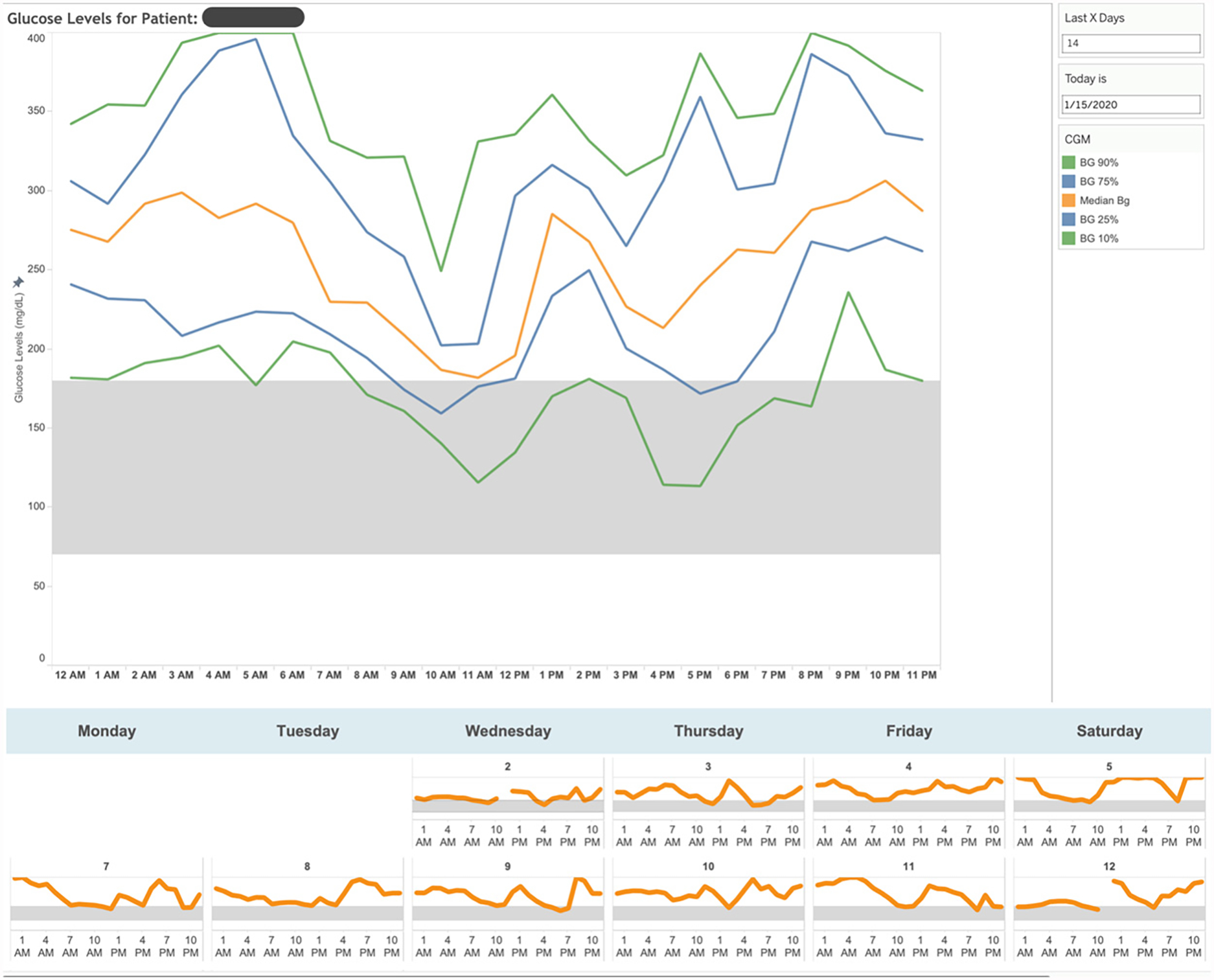
For the patient selected, detailed time series data reveal opportunities to improve glucose management - postprandial management for the patient shown.
BG = blood glucose, CGM = continuous glucose monitor.
Source: Lucile Packard Children’s Hospital Stanford
NEJM Catalyst (catalyst.nejm.org) © Massachusetts Medical Society
Hurdles
To date, relatively few analytics-based models for health care have been implemented and shown to improve patient care. Even large, multicenter studies of decision-support tools based on well-accepted evidence have failed to produce desired improvements.12,13 There is a disconnect at the center of this missed opportunity. Team-based population-level care delivery requires coordination and judgment across a multiskilled provider team, but most models based on artificial intelligence (AI) are designed for use by a single provider to make a single decision for a single patient based on a black box recommendation. Overcoming this disconnect requires a “human-centered” approach to AI integration in health care, in which providers play a leading role in the development, implementation, and interpretability of the technology intended to assist them.
Decision-Support Tools Should Be Developed Iteratively, in Close Partnership with Those Who Will Ultimately Use Them
We sought to develop tools to transform the deluge of data generated by CGMs into actionable decision support. An important goal was to avoid contributing to provider frustration associated with a poorly designed user experience.14 Initially, we made the all-too-common mistake of pursuing the most sophisticated individual-level models we could build. We developed several models, including one that improved over the current gold-standard approach to estimating HbA1c based on CGM data.11 While this did make possible more accurate estimates when the Covid-19 pandemic reduced the frequency of HbA1c testing, none of these models provided actionable information for improved workflows or clinical decision-making.
We switched to an agile approach.15 We rapidly generated and tested numerous, relatively simple ideas to improve caregiver decision-making. After several rapid rounds of provider-centered iteration, we settled on a tool to summarize a few useful glucose-management metrics. This version of TIDE presented the care team with a convenient synopsis of patient glucose management, saving the time that would otherwise be required to review each patient’s data. In the years since, the care team has continued to generate feedback based on which the visual interface, glucose metrics used, and algorithms continue to be revised.15
Decision Support Should Be Designed for the Workflow of the Entire Care Team and Focus on the Entire Population
We sought to prioritize patients who would most benefit from provider contact without inadvertently neglecting those who were managing their glucose relatively well while also ensuring that no patient would go too long without their data being reviewed. We analyzed historical patient data to rank the patients displayed in the population-level part of TIDE and analyzed provider workflows and time constraints to limit the total number of patients displayed.
After several iterations of testing and feedback from care providers, we made two improvements to TIDE to reduce the time required per patient and, thus, increase the number of patients for whom the CDCES could provide RPM. First, we reduced the number of data reviews per patient by 36% (Figure 5, from period 1 to period 2). We did so by developing an algorithm that reduced patients being identified inappropriately as likely to benefit from provider contact; average positive predictive power increased from 65% to 76%, while average sensitivity did not change significantly (91% to 90%).8
FIGURE 5. Time Savings Associated with the Use of Timely Interventions for Diabetes Excellence.
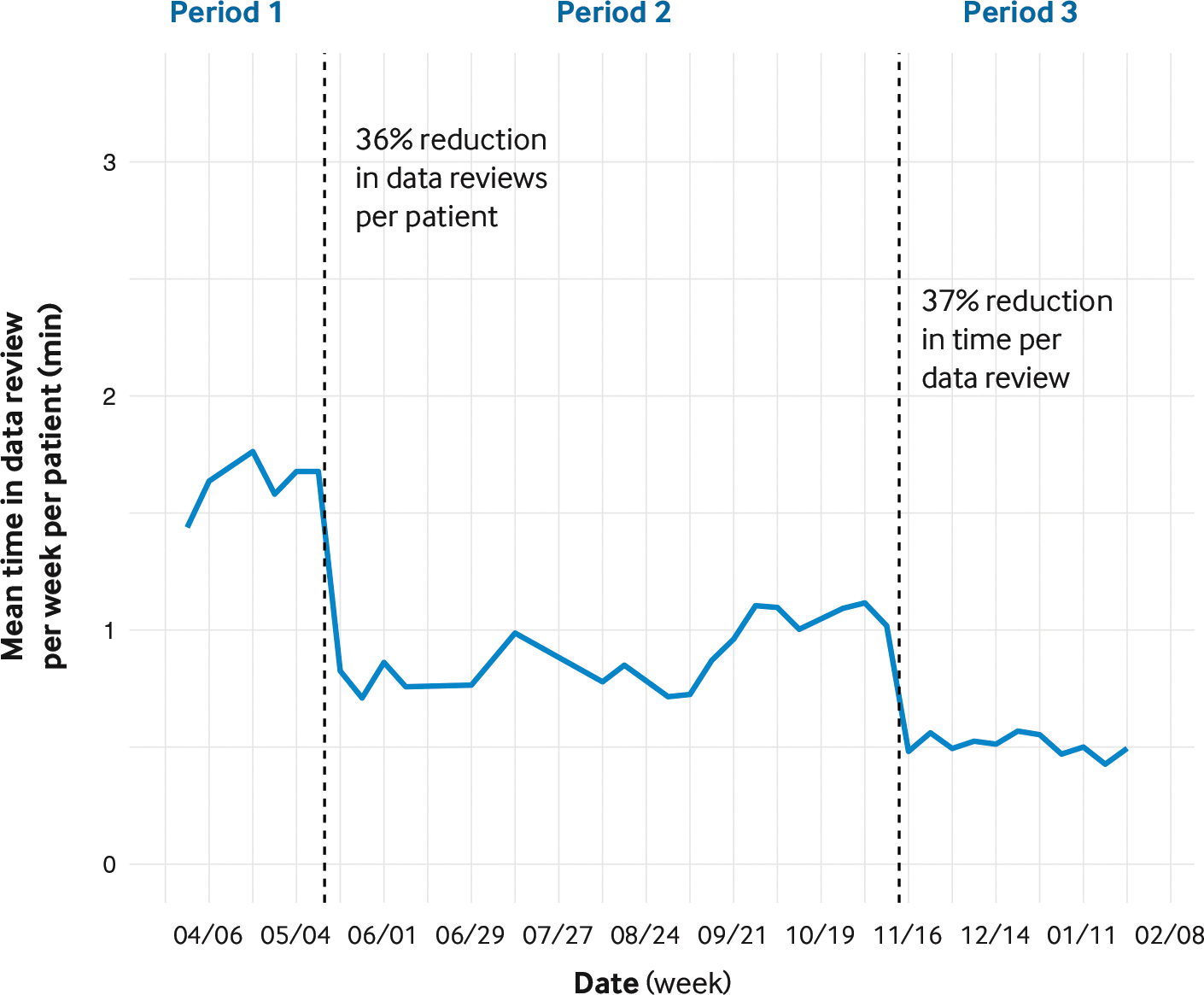
Period 1 is the baseline. Before period 2, improved algorithms reduced the average number of data reviews per patient. Before period 3, additional data displays were included in Timely Interventions for Diabetes Excellence (TIDE) to reduce the time required for data review.
Source: Adapted from Ferstad JO, Vallon JJ, Jun D, et al. Population-level management of type 1 diabetes via continuous glucose monitoring and algorithm-enabled patient prioritization: precision health meets population health. Pediatr Diabetes 2021;22:982–91 with the permission of the authors
NEJM Catalyst (catalyst.nejm.org) © Massachusetts Medical Society
Second, we reduced the time required to review each patient’s data by 37% (Figure 5, from period 2 to period 3).8 We did so by displaying additional metrics, access to which allowed the CDCES to complete data review entirely within TIDE without the need to access a CGM data portal. Providers welcomed the reduced number of unnecessary reviews and reduced screen time. As a result, the same CDCES had the capacity to offer RPM to more patients and expand program enrollment without working additional hours. We are partnering with other institutions interested in deploying TIDE to tune the parameter settings for their deployment to maximize the number of patients they identify for contact while limiting the workload to within their clinic capacity.
Institutional Support or Philanthropic Funding May Be Required, at Least Initially, to Ensure Equitable Access for Underserved Populations
The 4T program initially provided the first month of CGM supplies but relied on insurance coverage to cover the ongoing costs of the CGM, which can exceed $5,000 per year without insurance coverage (e.g., Dexcom G6 transmitters cost $1,200 each and must be replaced every 3 months). We quickly found that our patients with public insurance were not receiving ongoing coverage for the CGM and would have to return to fingerstick glucose readings after 1 month of CGM use. Limiting access to CGMs created disparities in access to our program and its benefits on glycemic outcomes and quality of life.
To promote equitable access, we obtained philanthropic funding from the Association of Auxiliaries Endowment for Children at LPCH to provide a CGM for patients with public insurance and funding for mobile devices such as an iPod touch (required for data connectivity) so that all youth could participate in remote monitoring. We found that these youth benefited from uninterrupted access to a CGM, and we are actively disseminating these findings in the hopes of informing policy.9
The Team
TIDE was initially developed by faculty with appointments in the Stanford Schools of Engineering and Medicine, a large interdisciplinary National Institutes of Health–funded research group, and several CDCESs. Executive leaders from operations and from information services at LPCH were essential for scaling the program and improving its stability and reliability.
Metrics
Glucose management was evaluated using HbA1c, TIR (70–180 mg/dL), and clinically significant hypoglycemia (lower than 54 mg/dL). Compared with 272 historical control patients, for the 135 patients in the 4T phase 1 study, the HbA1c was 0.54%, 0.52%, and 0.58% points lower at months 6, 9, and 12 postdiagnosis (Figure 6).6
FIGURE 6. Hemoglobin A1c for Patients in the Historical Control Group and the Teamwork, Targets, and Technology for Tight Control in Newly Diagnosed Type 1 Diabetes Phase 1 Study.
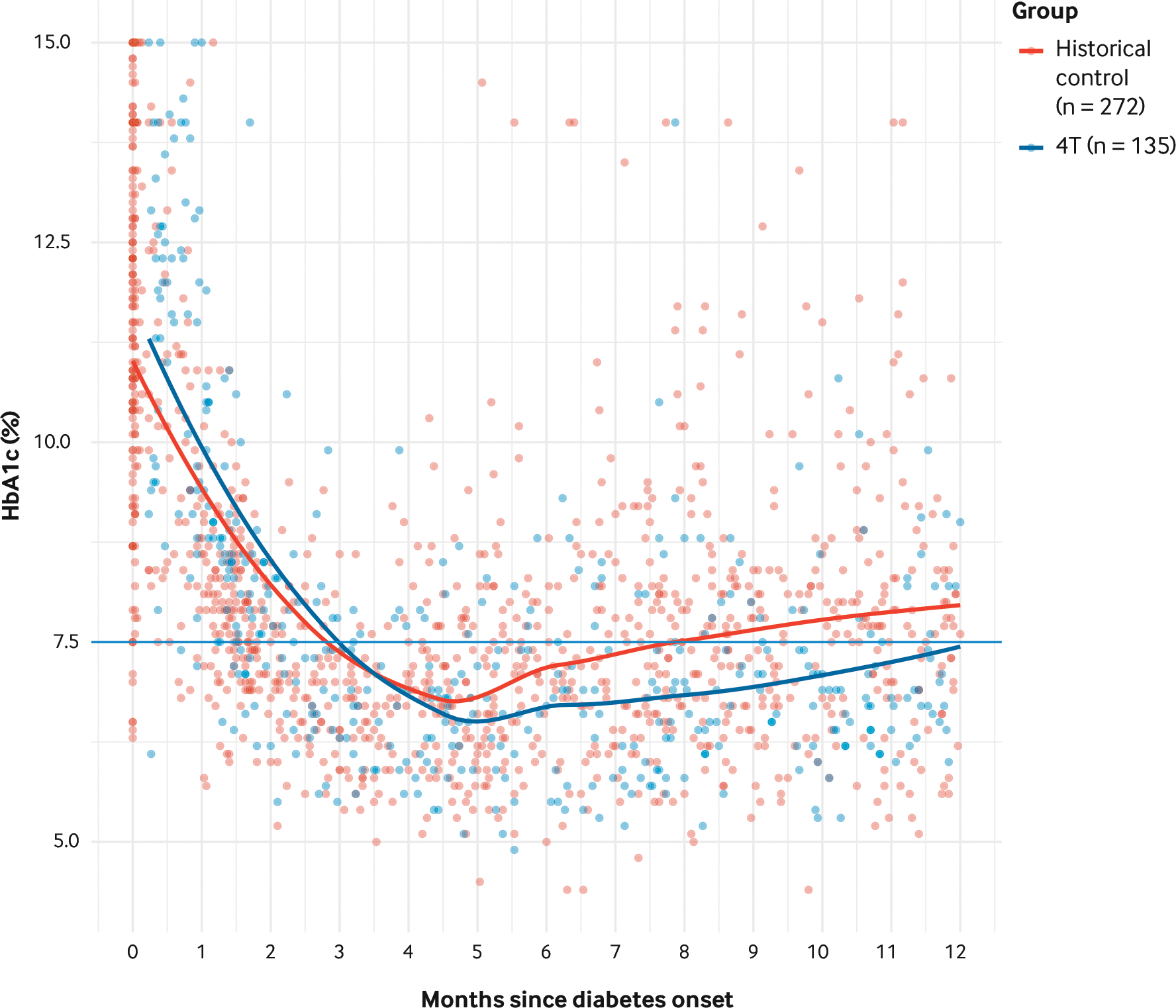
Compared with 272 historical control patients, for the 135 patients in the Teamwork, Targets, and Technology for Tight Control (4T) phase 1 study, hemoglobin A1c (HbA1c) was 0.54%, 0.52%, and 0.58% points lower at months 6, 9, and 12 postdiagnosis.
Source: Adapted from Prahalad P, Ding VY, Zaharieva DP, et al. Teamwork, targets, technology, and tight control in newly diagnosed type 1 diabetes: pilot 4T study. J Clin Endocrinol Metab. 2021;107:998–1008. https://academic.oup.com/jcem/advance-article-abstract/doi/10.1210/clinem/dgab859/6445182. https://doi.org/10.1210/clinem/dgab859 with the permission of the authors
NEJM Catalyst (catalyst.nejm.org) © Massachusetts Medical Society
Within the 4T phase 1 cohort, patients monitored with TIDE had better glucose management than those not monitored with TIDE. The 89 participants monitored with TIDE had an HbA1c that was 0.14%, 0.18%, and 0.14% lower at 6, 9, and 12 months postdiagnosis than the 46 participants who enrolled prior to the initiation of TIDE. For participants enrolled in the pilot 4T study monitored with the use of TIDE, the postonset declines in glucose management and TIR were less severe, and their TIR was 8.8% points higher at 12 months than participants monitored without the use of TIDE (Figure 7).8 For the 80 youth for whom sufficient data were available at the time of analysis, clinically significant hypoglycemia was infrequent and not associated with TIR.10
FIGURE 7. Time in Range Patients in the Pilot Teamwork, Targets, and Technology for Tight Control Study Using and Not Using Timely Interventions for Diabetes Excellence.
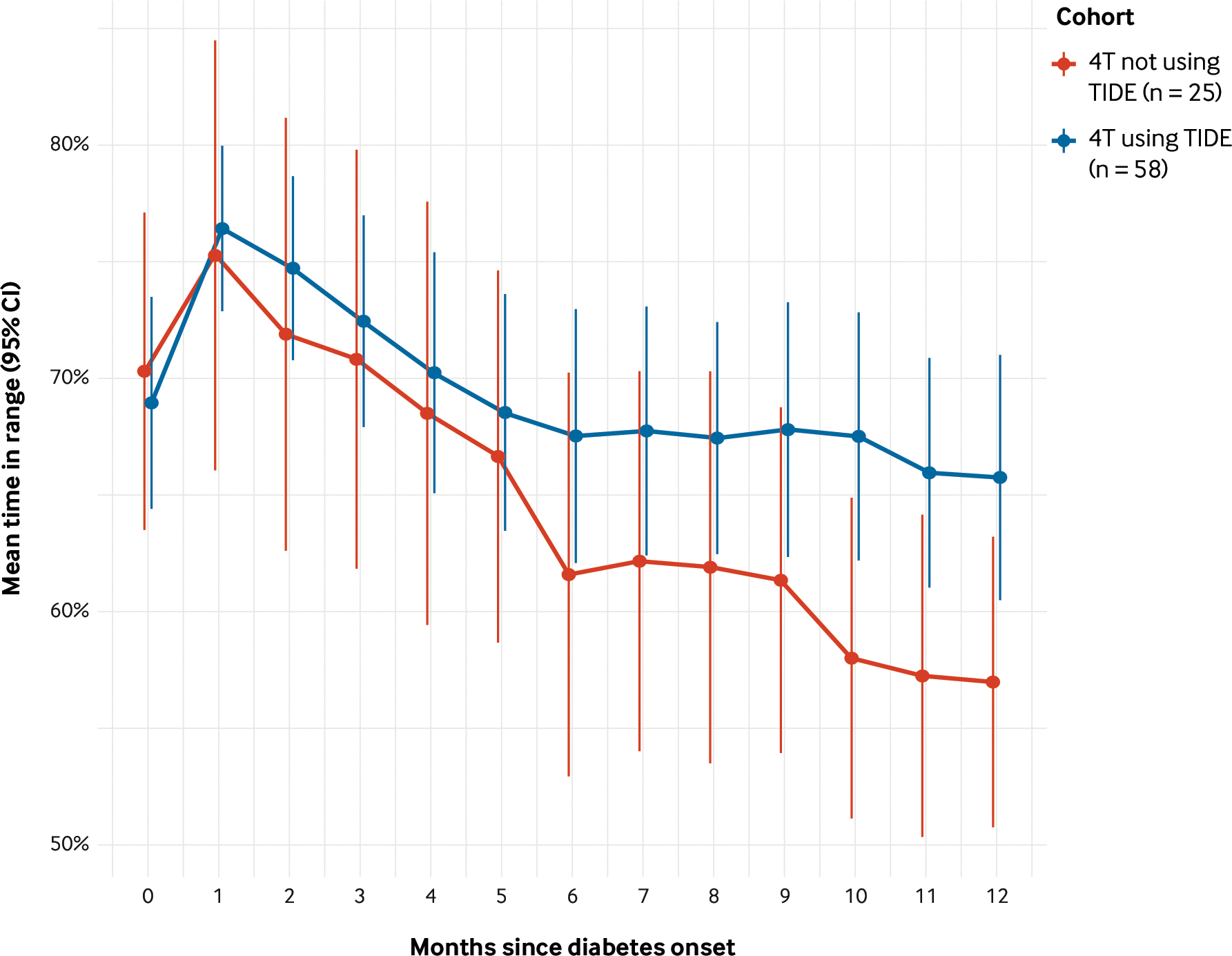
For participants enrolled in the pilot Teamwork, Targets, and Technology for Tight Control (4T) study monitored with the use of Timely Interventions for Diabetes Excellence (TIDE), the postonset declines in glucose management and time in range (TIR) were less severe, and their TIR was 8.8% points higher at 12 months than participants monitored without the use of TIDE.
CI = confidence interval.
Source: Adapted from Ferstad JO, Vallon JJ, Jun D, et al. Population-level management of type 1 diabetes via continuous glucose monitoring and algorithm-enabled patient prioritization: precision health meets population health. Pediatr Diabetes 2021;22:982–91 with the permission of the authors
NEJM Catalyst (catalyst.nejm.org) © Massachusetts Medical Society
Next Steps
Three lines of work are in progress to increase the value that patients receive with care based on TIDE. First, we continue to improve the metrics and algorithms used to identify the patients most likely to benefit from provider contact. We are using the data collected by TIDE and the variation in when patients are or are not contacted to develop statistical methods to better estimate the causal effects of contact by a care provider for a patient flagged by TIDE. Developing causal estimates will facilitate the use of algorithms to identify the patients most likely to benefit from intervention as well as the interventions most likely to be effective. Second, we are working to expand the population of patients whose care is supported with TIDE. Internally, we are recruiting patients in our clinic, both patients with new-onset and established T1D, for remote monitoring with a goal of enrolling 1,000 patients over the next 3 years. Externally, we are partnering with a large U.S. hospital and a large health care system in Australia for them to deploy the use of TIDE for their populations. To support both efforts, we have developed simulation models to support the operational planning of TIDE and to evaluate the financial feasibility of its use based on the cost of provider time and reimbursement rates for telemedicine-based care. Finally, we are integrating data from insulin pumps and exercise trackers into TIDE to generate a much richer picture of patient glucose management and improvement opportunities.
An important next step for these efforts is to partner with an analytics vendor to extract data from Epic to TIDE, push data from TIDE to the patient chart in Epic, and contact patients directly through TIDE. We developed TIDE entirely outside of our Epic EMR, using an iterative process based on user feedback. Deploying an initial version of TIDE in this manner took less than a month, and small changes could be made in hours or days. We took this approach because our previous projects to develop relatively simple analytics within Epic and its Cognitive Computing module required over a year for initial deployment and weeks or months for minor modifications. However, working outside of Epic limits the patient data available to TIDE and requires users to switch from TIDE to Epic to send messages or update the patient chart.
We are living through a sea change in the technology that supports patient care. The digital transformation of diabetes care includes not only novel sensors, such as CGMs, activity trackers, and heart rate monitors, but also the rapid expansion of telehealth (including video and telephone visits, emails and text messaging, Web and mobile apps, etc.), accelerated by Covid-19. These changes promise to enable a future in which measurement, monitoring, and care of patients can happen at a much faster cadence than just four times per year. Beyond T1D, many of the methods may apply to the management of type 2 diabetes, a disease that impacts over 30 million people in the United States. Beyond diabetes, much of the management of chronic diseases, such as heart disease, may benefit from as-needed patient contact based on the partially automated analysis of data available from devices such as smart scales and wearable devices such as activity and heart rate monitors.
Where to Start
We were able to leverage a diversity of talent across Stanford University: the medical school, pediatric hospital, engineering school, and philanthropic foundations. Other institutions hoping to leverage TIDE to improve T1D management and reduce provider screen time can access a freely available version of TIDE on Amazon Web Services (for which our team will provide documentation and instructions). The technical barriers to deploying TIDE are minimal, it can be hosted on a wide variety of platforms, and it does not require integration with the EMR.
The present work offers several best practices to maximize the likelihood of the successful use of TIDE or any other tool for the automated analysis of CGM data. First, we recommend that the configuration of TIDE (the metrics displayed and the thresholds used) be determined iteratively, starting with metrics most similar to those already in use at the institution and updated based on user feedback.
Second, the criteria for patient contact and the cadence of the use of TIDE should be carefully planned to fit the workflow and population of the clinic, again starting with a few initial users targeting a few patients and iterating based on their experience. Clinics should customize the algorithms based on which TIDE identifies patients for contact in order to identify those who would benefit from contact without exceeding the capacity of the clinic.
Finally, institutions serving patients without the resources to access CGM technology should find funding to enroll a few such patients in a trial in order to determine the resources the clinic needs to make this care equitably accessible. When designed around the care team and the patient population, technology-enabled, telemedicine-based clinical care has the potential to improve quality, equity, and provider satisfaction.
KEY TAKEAWAYS.
Wearable sensors and the use of telemedicine may facilitate detecting deteriorating health and providing as-needed patient care for individuals with T1D as well as other types of chronic disease.
Decision-support tools should be developed iteratively, in close partnership with those who will ultimately use them.
Decision-support tools should be designed for the workflow of the entire care team and focus on the entire population.
Institutional support or philanthropic funding may be required, at least initially, to ensure equitable access for underserved populations.
Acknowledgments
We thank the 4T study team, including Ananta Addala, Dessi Zaharieva, Nora Arrizon-Ruiz, Franziska Bishop, Annette Chmielewski, Barry Conrad, Ana Cortes, Manisha Desai, Victoria Ding, Rebecca Gardner, Simrat Ghuman, Carolyn Herrera, Korey Hood, Julie Hooper, Brianna Leverenz, Jeannine Leverenz, Erica Pang, Natalie Pageler, Piper Sagan, Julie Senaldi, Anjoli Martinez-Singh, and Michelle Wiedemann. We thank the numerous engineering students and physicians who contributed to the technical design of TIDE, the design of the operational model, and its iterative improvement: Johannes O. Ferstad, Jacqueline J. Vallon, Oseas Ayerdi, Daniel Jun, Angela Gu, Anastasiya Vitko, Dianelys P. Morales, Ming Yeh Lee, Christos Vasilakis, Andrew Shin, and Esli Osmanlliu. Finally, we thank the members of LPCH Information Services that helped deploy a server-based version of TIDE and improve its functionality: Glen Loving, Conner Brown, Austin Powell, and Brendan Watkins. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R18DK12242), the Helmsley Charitable Trust, an International Society for Pediatric and Adolescent Diabetes-JDRF Fellowship, Stanford Diabetes Research Center, and Lucile Packard Children’s Hospital Stanford Association of Auxiliaries Endowment for Children.
Footnotes
Disclosures: David Scheinker, Priya Prahalad, Ramesh Johari, David M. Maahs, and Rick Majzun have nothing to disclose.
Contributor Information
David Scheinker, Associate Professor, Pediatrics, Stanford University, Stanford, California, USA; Executive Director, Lucile Packard Children’s Hospital Stanford, Palo Alto, California, USA; Faculty, Clinical Excellence Research Center, Stanford University, California, USA.
Priya Prahalad, Associate Professor, Pediatrics, Stanford University, Stanford, California, USA.
Ramesh Johari, Professor, Management Science and Engineering, Stanford University, Stanford, California, USA.
David M. Maahs, Professor, Pediatrics, Stanford University, Stanford, California, USA.
Rick Majzun, Chief Operating Officer, Lucile Packard Children’s Hospital Stanford, Palo Alto, California, USA.
References
- 1.Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care 2020;44:258–79 10.2337/dci20-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charalampopoulos D, Hermann JM, Svensson J, et al. Exploring variation in glycemic control across and within eight high-income countries: a cross-sectional analysis of 64,666 children and adolescents with type 1 diabetes. Diabetes Care 2018;41:1180–7 10.2337/dc17-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan DM; DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014;37:9–16 10.2337/dc13-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prahalad P, Addala A, Scheinker D, Hood KK, Maahs DM. CGM initiation soon after type 1 diabetes diagnosis results in sustained CGM use and wear time. Diabetes Care 2020;43:e3–4 10.2337/dc19-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prahalad P, Zaharieva DP, Addala A, et al. Improving clinical outcomes in newly diagnosed pediatric type 1 diabetes: Teamwork, Targets, Technology, and Tight Control—the 4T study. Front Endocrinol (Lausanne) 2020;11:360 10.3389/fendo.2020.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prahalad P, Ding VY, Zaharieva DP, et al. Teamwork, targets, technology, and tight control in newly diagnosed type 1 diabetes: pilot 4T study. J Clin Endocrinol Metab 10.1210/clinem/dgab859/6445182 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheinker D, Gu A, Grossman J, et al. Algorithm-enabled, personalized glucose management for type 1 diabetes at the population scale: a prospective evaluation in clinical practice. January 20, 2021. (https://www.semanticscholar.org/paper/Algorithm-Enabled%2C-Personalized-Glucose-Management-Scheinker-Gu/51bd3294aaa5b1a8f5e3671588a6f18660a16f21). preprint. [DOI] [PMC free article] [PubMed]
- 8.Ferstad JO, Vallon JJ, Jun D, et al. Population-level management of type 1 diabetes via continuous glucose monitoring and algorithm-enabled patient prioritization: precision health meets population health. Pediatr Diabetes 2021;22:982–91 10.1111/pedi.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Addala A, Maahs DM, Scheinker D, Chertow S, Leverenz B, Prahalad P. Uninterrupted continuous glucose monitoring access is associated with a decrease in HbA1c in youth with type 1 diabetes and public insurance. Pediatr Diabetes 2020;21:1301–9 10.1111/pedi.13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Addala A, Zaharieva DP, Gu AJ, et al. Clinically serious hypoglycemia is rare and not associated with time-in-range in youth with new-onset type 1 diabetes. J Clin Endocrinol Metab 2021;106:3239–47 10.1210/clinem/dgab522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grossman J, Ward A, Crandell JL, Prahalad P, Maahs DM, Scheinker D. Improved individual and population-level HbA1c estimation using CGM data and patient characteristics. J Diabetes Complications 2021;35:107950 10.1016/j.jdiacomp.2021.107950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adusumalli S, Westover JE, Jacoby DS, et al. Effect of passive choice and active choice interventions in the electronic health record to cardiologists on statin prescribing: a cluster randomized clinical trial. JAMA Cardiol 2021;6:40–8 10.1001/jamacardio.2020.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maddox TM. Clinical decision support in statin prescription-what we can learn from a negative outcome. JAMA Cardiol 2021;6:48–9 10.1001/jamacardio.2020.4756. [DOI] [PubMed] [Google Scholar]
- 14.Kjaer K, Kowalsky R, Rubin LA, et al. A grassroots approach to protecting physicians against burnout and building an engaging practice environment. NEJM Catal Innov Care Deliv 2021;2(12) 10.1056/CAT.21.0275. [DOI] [Google Scholar]
- 15.Jackson S, Yaqub M, Li CX. The agile deployment of machine learning models in healthcare. Front Big Data 2019;1:7 10.3389/fdata.2018.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]


