Abstract
Background
Colorectal cancer screening programmes worldwide have been disrupted during the COVID-19 pandemic. We aimed to estimate the impact of hypothetical disruptions to organised faecal immunochemical test-based colorectal cancer screening programmes on short-term and long-term colorectal cancer incidence and mortality in three countries using microsimulation modelling.
Methods
In this modelling study, we used four country-specific colorectal cancer microsimulation models–Policy1-Bowel (Australia), OncoSim (Canada), and ASCCA and MISCAN-Colon (the Netherlands)—to estimate the potential impact of COVID-19-related disruptions to screening on colorectal cancer incidence and mortality in Australia, Canada, and the Netherlands annually for the period 2020–24 and cumulatively for the period 2020–50. Modelled scenarios varied by duration of disruption (3, 6, and 12 months), decreases in screening participation after the period of disruption (0%, 25%, or 50% reduction), and catch-up screening strategies (within 6 months after the disruption period or all screening delayed by 6 months).
Findings
Without catch-up screening, our analysis predicted that colorectal cancer deaths among individuals aged 50 years and older, a 3-month disruption would result in 414–902 additional new colorectal cancer diagnoses (relative increase 0·1–0·2%) and 324–440 additional deaths (relative increase 0·2–0·3%) in the Netherlands, 1672 additional diagnoses (relative increase 0·3%) and 979 additional deaths (relative increase 0·5%) in Australia, and 1671 additional diagnoses (relative increase 0·2%) and 799 additional deaths (relative increase 0·3%) in Canada between 2020 and 2050, compared with undisrupted screening. A 6-month disruption would result in 803–1803 additional diagnoses (relative increase 0·2–0·4%) and 678–881 additional deaths (relative increase 0·4–0·6%) in the Netherlands, 3552 additional diagnoses (relative increase 0·6%) and 1961 additional deaths (relative increase 1·0%) in Australia, and 2844 additional diagnoses (relative increase 0·3%) and 1319 additional deaths (relative increase 0·4%) in Canada between 2020 and 2050, compared with undisrupted screening. A 12-month disruption would result in 1619–3615 additional diagnoses (relative increase 0·4–0·9%) and 1360–1762 additional deaths (relative increase 0·8–1·2%) in the Netherlands, 7140 additional diagnoses (relative increase 1·2%) and 3968 additional deaths (relative increase 2·0%) in Australia, and 5212 additional diagnoses (relative increase 0·6%) and 2366 additional deaths (relative increase 0·8%) in Canada between 2020 and 2050, compared with undisrupted screening. Providing immediate catch-up screening could minimise the impact of the disruption, restricting the relative increase in colorectal cancer incidence and deaths between 2020 and 2050 to less than 0·1% in all countries. A post-disruption decrease in participation could increase colorectal cancer incidence by 0·2–0·9% and deaths by 0·6–1·6% between 2020 and 2050, compared with undisrupted screening.
Interpretation
Although the projected effect of short-term disruption to colorectal cancer screening is modest, such disruption will have a marked impact on colorectal cancer incidence and deaths between 2020 and 2050 attributable to missed screening. Thus, it is crucial that, if disrupted, screening programmes ensure participation rates return to previously observed rates and provide catch-up screening wherever possible, since this could mitigate the impact on colorectal cancer deaths.
Funding
Cancer Council New South Wales, Health Canada, and Dutch National Institute for Public Health and Environment.
Research in context.
Evidence before this study
Colorectal cancer screening programmes worldwide have been disrupted due to the COVID-19 pandemic. Screening for colorectal cancer is known to reduce long-term colorectal cancer incidence and mortality. Any disruption to screening would reduce these health benefits. Longer waiting times between a primary screening and a diagnostic follow-up colonoscopy decrease the effectiveness of screening. A delay of up to 12 months in screening or diagnostic follow-up can result in a loss in life-years gained from screening of up to 10%. It has been estimated that a 6-month delay to predicted usual colorectal cancer diagnoses and treatment would result in 11 excess deaths in the next 5 years in Australia attributable to shifts from stage 1 to stage 2 alone. In England, it is estimated that 2-week delays in diagnosis for all patients for 12 months could lead to up to 1563 additional deaths from colorectal cancer, which is corroborated by existing microsimulation modelling on the long-term impact of delays to diagnosis and warnings from the US National Cancer Institute.
We searched PubMed and MEDLINE to identify modelling studies about disruption in colorectal cancer screening. Our search yielded five studies, but none included a comparative modelling study between different countries or modelled catch-up scenarios.
Added value of this study
This comparative modelling study using four well calibrated and validated models (MIcrosimulation SCreening ANalysis for colorectal cancer, Adenoma and Serrated pathway to Colorectal CAncer, Policy1-Bowel, and OncoSim) provided a unique opportunity to predict short-term and long-term health and resource impacts of disruptions to colorectal cancer screening caused by the COVID-19 pandemic. By considering a range of hypothetical possibilities for the effect of COVID-19 on screening, we generated representative results that illustrate the scale and scope of both disruption to screening, and the effect of measures to mitigate the health impact. Our findings indicated excess colorectal cancer cases (specified by stage), colorectal cancer deaths, and resource use (ie, number of individuals invited, participating, and participating in diagnostic follow-up) occurring annually between 2020 and 2024 and cumulatively between 2020 and 2050 due to disruptions to screening caused by the COVID-19 pandemic.
Implications of all the available evidence
The results indicate that disruption to screening programmes will have a substantial effect on the absolute number of colorectal cancer deaths between 2020 and 2050. Without catch-up screening, a 6-month disruption would result in 678–881 additional deaths in the Netherlands, 1961 in Australia, and 1319 in Canada. However, with immediate catch-up screening, the impact of such disruption would be minimised to a relative increase in excess deaths of 0·1% in all countries. Where screening programmes have been disrupted, it is crucial that participation rates return to observed rates as soon as possible and, if possible, strategies to catch-up screening, to mitigate the impact of any disruption.
Introduction
The COVID-19 pandemic represents a considerable global health crisis. As of Jan 29, 2021, COVID-19 has caused more than 2 166 000 deaths, and the impact of the pandemic will continue to evolve in the coming months and years.1 During the crisis, many elective and preventive health services have been suspended, including some organised colorectal cancer screening programmes. Population-level colorectal cancer screening programmes typically consist of inviting individuals for primary screening using a faecal test sent to their home. Individuals with positive faecal results are then invited for diagnostic colonoscopy done by a trained professional.2 In some countries, such primary screening has been paused, and the number of diagnostic colonoscopy procedures have been minimised or suspended. In the Netherlands and Canada, primary screening was disrupted between March and May, 2020.3, 4 In the Netherlands, colonoscopy capacity decreased by around 65% and recovered in September3 and colorectal cancer diagnoses among people aged 55–75 years (ie, those eligible for screening) were lower than expected between March and June, 2020.5 In Australia, no disruption to primary screening has occurred during the pandemic, but many diagnostic colonoscopy follow-up services were affected by constraints on the health system, whereby the number of diagnostic colonoscopies decreased by 55% between March and April, 2020, which was followed by signs of recovery, although these procedures were not all associated with the organised screening programme.6 No change in screening participation could be directly attributed to the COVID-19 pandemic according to the Australian Institute of Health and Welfare.7 Outside of any formal disruption, there might have also been changes to participation rates for both primary screening and diagnostic follow-up, due to behavioural changes (such as perceived risks of seeking diagnostic care) and reduced health system capacity. Detailed data regarding the extent of these informal disruptions is not yet available and might not be for some time.
The effectiveness of population-level colorectal cancer screening is well established, reducing long-term colorectal cancer incidence by up to 24% and colorectal cancer mortality by up to 31%.8 Modelling studies have indicated that decreases in screening participation or longer waiting times between a positive screen and a diagnostic follow-up colonoscopy reduce the effectiveness of screening.9, 10 Delays in diagnostic colonoscopy of up to 12 months can result in a loss in screening benefits (ie, life-years gained) of up to 10%,11 and modelling of cancer stage shifting at treatment initiation has already been applied to delays in diagnosis attributable to COVID-19.12 However, these studies did not assess the effect of disruptions to primary screening, and although data regarding the short-term impact of colorectal cancer screening disruptions have started to become available,13 the long-term impact of these disruptions will not be apparent for years or decades since precancerous lesions that would otherwise be removed at routine screening might instead develop into colorectal cancer in later life. Additionally, any impact will be difficult to measure because of confounding factors, existing trends in colorectal cancer incidence and survival, and the absence of any meaningful comparator for worldwide disruption to such services.
Recognising the need for long-term evidence to inform public health decision making, and the potential for models to provide this evidence, the COVID-19 and Cancer Global Modeling Consortium (CCGMC) was established to support decision making in cancer control both during and after the COVID-19 crisis. Modelling provides a unique opportunity to predict short-term and long-term health and resource impacts of disruptions to screening. Additionally, modelling enables comparison between hypothetical scenarios to assist health-care providers and policy makers in timely recovery planning when complete real-world data is not yet available.
The aim of this study was to model and assess the short-term and long-term impact of the disruption to colorectal cancer screening due to the COVID-19 pandemic in the Netherlands, Australia, and Canada, to illustrate the scale and scope of disruptions to screening, and the effect of measures to mitigate the health impact and guide health-care providers and policy decision makers.
Methods
Modelling approach
We used four microsimulation models from different institutions participating in the CCGMC to simulate colorectal cancer development and the national colorectal cancer screening programmes in the Netherlands, Australia, and Canada. These countries have similar burdens of disease and use faecal immunochemical test (FIT)-based national colorectal cancer screening programmes. A number of hypothetical disruption scenarios were simulated to reflect the impact of a range of potential disruptions to screening due to the COVID-19 pandemic. Table 1 provides an overview of each model and simulated national screening programme, and further details on the national screening programmes, model assumptions, and characteristics are provided in the appendix (pp 2–4).
Table 1.
COVID-19 and colorectal cancer burden, screening programmes, and modelling characteristics for the Netherlands, Australia, and Canada
| Netherlands | Australia* | Canada† | |
|---|---|---|---|
| Population size | 17·3 million14 | 25·4 million14 | 37·6 million14 |
| COVID-19 incidence (per 100 000 people)‡ | 1058·0 | 107·1 | 484·4 |
| COVID-19 mortality (per 100 000 people)‡ | 38·4 | 3·5 | 25·5 |
| Age-standardised colorectal cancer incidence (per 100 000 people)§ | 41·0 | 33·1 | 31·2 |
| Age-standardised colorectal cancer mortality (per 100 000 people)§ | 13·5 | 8·9 | 9·9 |
| 2020 screening cohort size | 2 260 000 | 1 320 000 | 2 020 000 |
| Screening test (cutoff value¶) | Biennial FIT (47 μg/g) | Biennial two-sample FIT (20 μg/g)‖ | Biennial FIT (20 μg/g) |
| Screening age, years | 55–75 | 50–74 | 50–74 |
| Screening participation rate | 73%15 | 41%16 | 41%17 |
| Models | MISCAN-Colon, ASCCA | Policy1-Bowel | OncoSim |
FIT=faecal immunochemical test. MISCAN-Colon=MIcrosimulation SCreening ANalysis for colorectal cancer. ASCCA=Adenoma and Serrated pathway to Colorectal CAncer.
Included data were obtained from the National Bowel Cancer Screening Program of Australia;16 a high proportion of screening is known to occur outside of national programmes in Australia, but this screening cannot be quantified.
OncoSim models the most common screening practice of Canada; across Canada, screening varies by the number of samples used for FIT, FIT cutoff values, and screening frequency used.
According to the WHO coronavirus disease (COVID-19) dashboard as of Oct 14, 2020.1
According to the 2020 WHO world standardised population.18
Tests with a lower threshold are more sensitive for the detection of advanced adenomas and cancer, but also have higher false-positive rates.
In Australia, individuals are invited to complete a two-sample FIT, with a positive result on either test sufficient for diagnostic follow-up, whereas in the Netherlands and Canada, a one-sample FIT is offered.
This comparative modelling analysis was done using four country-specific colorectal cancer microsimulation models: MIcrosimulation SCreening ANalysis for colorectal cancer (MISCAN-Colon; the Netherlands), Adenoma and Serrated pathway to Colorectal CAncer (ASCCA; the Netherlands), Policy1-Bowel (Australia), and OncoSim (Canada). The models' structure, underlying assumptions, calibration, and validation have been described elsewhere.10, 19, 20, 21, 22 Colorectal cancer incidence and mortality data used for the models were obtained from the Netherlands Cancer Registry, the Australian Institute of Health and Welfare Cancer Registry, and the Canadian Cancer Registry. Prevalence data were based on autopsy studies, which have been described previously.10, 19, 20, 21, 22
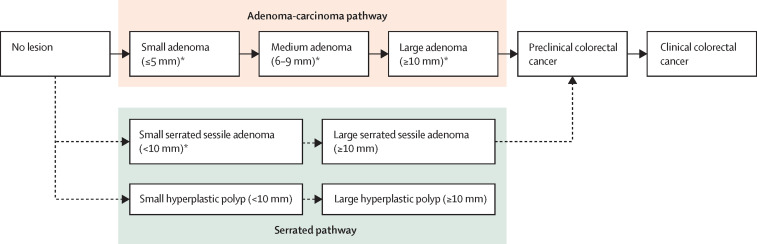
All models simulate the natural history of colorectal cancer. MISCAN-Colon and OncoSim assume all colorectal cancers develop from conventional adenomas via the adenoma-carcinoma pathway (figure 1 ), whereas ASCCA and Policy1-Bowel assume that 85% of colorectal cancers develop from conventional adenomas and 15% develop from serrated lesions. Simulated individuals start their modelled lifetime free of disease, and over time might develop one or more adenomas or serrated lesions. These lesions might grow and transition into preclinical colorectal cancer. A preclinical colorectal cancer might become symptomatic, leading to clinical detection. An individual with colorectal cancer has a probability of dying from the colorectal cancer. This probability is calibrated to local colorectal cancer survival data and dependent on the stage of the colorectal cancer and whether colorectal cancer was detected through screening or clinically (outside of screening). Each model includes a screening component to simulate the local screening programme, including primary FIT-based screening and diagnostic colonoscopy follow-up. This component will lead to some cancers being prevented completely by the detection and removal of adenomas, and other cancers detected at earlier stages with more favourable survival. The models also tracked the following outcomes: false-positive FIT test results, complications associated with colonoscopy, increased detection of precursor lesions that would not have progressed to cancer, and false-negative test results. Further assumptions on test characteristics, risk of colonoscopy complications, and screening participation, including screening uptake and adherence, are listed in the appendix (pp 2–4).
Figure 1.
Natural history of colorectal cancer simulated by MISCAN-Colon, ASCCA, Policy1-Bowel, and OncoSim models
MISCAN-Colon and OncoSim only simulate the adenoma-carcinoma pathway, whereas ASCCA and Policy1-Bowel models include both the adenoma-carcinoma pathway and the serrated pathway. *ASCCA and Policy1-Bowel additionally assume that small and medium adenomas with high-grade dysplasia or villous structure and serrated sessile adenomas can progress to preclinical colorectal cancer.
Modelled scenarios
Each scenario consists of two periods: a disruption period and a recovery period. During the disruption period, we assumed that all primary screening and follow-up were paused for 3, 6, or 12 months. The recovery period was defined as a 6-month or 12-month period after the disruption period. To estimate the impact of screening disruptions, we simulated a comparator scenario with typical country-specific screening and follow-up participation rates for 2020 (ie, no disruption or recovery period) based on data obtained from the national screening programmes of the Netherlands, Australia, and Canada.15, 16, 17
To estimate the effects of different durations of disruption, and recovery periods (including post-disruption participation and catch-up screening), we simulated a base case scenario in which a 6-month disruption period from April to September, 2020, was assumed, with no catch-up screening or changes to participation in the recovery period. We designed three groups of scenarios by varying the following parameters: length of screening disruption (3, 6, or 12 months); decreases in participation rates for primary screening and diagnostic follow-up during the recovery period following a 6-month disruption (0%, 25%, or 50%); and simulation of catch-up scenarios, which required an increase in capacity and increased workload, whereby both individuals who missed screening during the disruption period and individuals scheduled for usual screening were invited for screening to reduce the backlog caused by the disruption in the 6-month post-disruption period (yes or no; table 2 ).
Table 2.
Disruption scenarios
| Disruption period | Catch-up screening | Participation with primary screening during recovery period | Participation with diagnostic follow-up during recovery period | ||
|---|---|---|---|---|---|
| Comparator | NA | NA | No changes | No changes | |
| Base case | 6-month disruption to screening (April–September, 2020, inclusive) | None | No changes | No changes | |
| Duration of disruption | |||||
| 3 months | 3-month disruption to screening (April–June, 2020, inclusive) | None | No changes | No changes | |
| 12 months | 12-month disruption to screening (April, 2020–March, 2021 inclusive) | None | No changes | No changes | |
| Reduced participation during recovery period | |||||
| Participation reduced by 50% in first 3 months, and by 25% in second 3 months | 6-month disruption to screening (April–September, 2020, inclusive) | No catch-up screening | Participation reduced by 50% in the first 3 months and by 25% in the second 3 months | Participation reduced by 50% in the first 3 months and by 25% in the second 3 months | |
| Participation reduced by 50% for 6 months | 6-month disruption to screening (April–September, 2020, inclusive) | No catch-up screening | Participation reduced by 50% for 6 months | Participation reduced by 50% for 6 months | |
| Inclusion of catch-up screening | |||||
| Immediate catch-up | 6-month disruption to screening (April–September, 2020, inclusive) | Catch-up screening at usual participation rates for 6 months after the disruption | No changes | No changes | |
| Delayed catch-up | 6-month disruption to screening (April–September, 2020, inclusive) | All screening is delayed by 6 months, including for people not affected by the disruption; new cohorts invited for screening at usual time | No changes | No changes | |
NA=not applicable.
We also simulated six additional scenarios to combine different aspects such as the length of screening disruption, decreases in participation, and catch-up scenarios from the primary scenarios (appendix p 4).
Outcomes
Outcomes for the comparator scenario are reported as absolute numbers for individuals aged 50 years and older, and the outcomes for each disruption scenario are represented as both absolute and relative changes compared with the comparator scenario. Relative changes were presented as percentage change. The number of individuals eligible for, and participating in, primary screening and the number of colonoscopies done are presented for 2020 and 2021. Annual colorectal cancer incidence by stage were calculated for the period 2020 to 2024. Cumulative totals for colorectal cancer incidence by stage, colorectal cancer mortality, and life-years lost compared with undisrupted screening were calculated for the period 2020–50. Additionally, age-group specific cumulative totals were calculated for colorectal cancer incidence, colorectal cancer mortality, and life-years lost for three scenarios with 6-months disruption: the base case scenario, one scenario with 50% reduced participation during the 6-month recovery period, and an immediate catch-up scenario.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to the data in the study and the final responsibility to submit for publication.
Results
For the comparator scenario (no disruption to services), MISCAN-Colon predicted 12 512 colorectal cancer diagnoses and 4112 colorectal cancer deaths in 2020 in the Netherlands, and 169 016 colorectal cancer deaths between 2020 and 2050 (table 3 ; figure 2 ). ASCCA predicted slightly more colorectal cancer diagnoses and deaths in the Netherlands, with 13 562 diagnoses and 5208 deaths in 2020, and 142 621 total colorectal cancer deaths between 2020 and 2050 (table 3; figure 2). Policy1-Bowel predicted 17 391 colorectal cancer diagnoses and 6198 colorectal cancer deaths in Australia in 2020, and 196 336 colorectal cancer deaths between 2020 and 2050 (table 3; figure 2). OncoSim predicted 21 721 colorectal cancer diagnoses and 8134 colorectal cancer deaths in Canada in 2020, and 311 133 colorectal cancer deaths between 2020 and 2050 (table 3; figure 2).
Table 3.
Changes in colorectal cancer diagnoses and deaths between 2020 and 2050 relative to undisrupted screening for MISCAN-Colon, ASCCA, Policy1-Bowel, and OncoSim
| Comparator scenario | 6-month disruption, no recovery period, and no catch-up screening | 3-month disruption, no recovery period, and no catch-up screening | 12-month disruption, no recovery period, and no catch-up screening | 6-month disruption, 6-month recovery period (participation reduced by 50% in first 3 months and 25% in second 3 months), and no catch-up screening | 6-month disruption, 6-month recovery period (participation reduced by 50%), and no catch-up screening | 6-month disruption, no recovery period, and immediate catch-up screening | 6-month disruption, no recovery period, and delayed catch-up screening | ||
|---|---|---|---|---|---|---|---|---|---|
| MISCAN-Colon (Netherlands) | |||||||||
| Change in number of colorectal cancer diagnoses | |||||||||
| 2020 | 12 512 | −1558 (−12·5%) | −759 (−6·1%) | −2412 (−19·3%) | −2198 (−17·6%) | −2198 (−17·6%) | −626 (−5·0%) | −1408 (−11·3%) | |
| 2021 | 12 970 | 363 (2·8%) | 183 (1·4%) | −166 (−1·3%) | 194 (1·5%) | −31 (−0·2%) | 895 (6·9%) | 555 (4·3%) | |
| 2022 | 12 977 | 737 (5·7%) | 363 (2·8%) | 1312 (10·1%) | 1178 (9·1%) | 850 (9·4%) | −125 (−1·0%) | 1620 (12·5%) | |
| 2023 | 13 400 | 102 (0·8%) | 43 (0·3%) | 495 (3·7%) | 310 (2·3%) | 433 (3·2%) | −50 (−0·4%) | −287 (−2·1%) | |
| 2024 | 13 526 | 323 (2·4%) | 153 (1·1%) | 547 (4·0%) | 462 (3·4%) | 481 (3·6%) | −25 (−0·2%) | −104 (−0·4%) | |
| 2020–50 | 451 697 | 803 (0·2%) | 414 (0·1%) | 1619 (0·4%) | 1147 (0·2%) | 1255 (0·3%) | −36 (0·0%) | −67 (0·0%) | |
| Change in number of colorectal cancer deaths | |||||||||
| 2020–50 | 169 016 | 678 (0·4%) | 324 (0·2%) | 1360 (0·8%) | 975 (0·6%) | 609 (0·6%) | −7 (0·0%) | −52 (0·0%) | |
| ASCCA (Netherlands) | |||||||||
| Change in number of colorectal cancer diagnoses | |||||||||
| 2020 | 13 562 | −1866 (−13·8%) | −933 (−6·9%) | −2799 (−20·6%) | −2559 (−18·9%) | −2566 (−18·9%) | −932 (−6·9%) | −1760 (−13·0%) | |
| 2021 | 13 473 | 309 (2·3%) | 154 (1·1%) | −457 (−3·4%) | 54 (0·4%) | −266 (−2·0%) | 1261 (9·4%) | 757 (5·6%) | |
| 2022 | 13 146 | 1104 (8·4%) | 552 (4·2%) | 1807 (13·7%) | 1610 (12·2%) | 1657 (12·6%) | −131 (−1·0%) | 1982 (15·1%) | |
| 2023 | 13 213 | 125 (0·9%) | 63 (0·5%) | 739 (5·6%) | 416 (3·1%) | 606 (4·6%) | −19 (−0·1%) | −282 (−2·1%) | |
| 2024 | 13 043 | 451 (3·5%) | 226 (1·7%) | 737 (5·7%) | 627 (4·8%) | 640 (4·9%) | −37 (−0·3%) | −107 (−0·8%) | |
| 2020–50 | 405 025 | 1803 (0·4%) | 902 (0·2%) | 3615 (0·9%) | 2883 (0·7%) | 3091 (0·8%) | 74 (0·0%) | 267 (0·1%) | |
| Colorectal cancer deaths | |||||||||
| 2020–50 | 142 621 | 881 (0·6%) | 440 (0·3%) | 1762 (1·2%) | 1395 (1·0%) | 1500 (1·1%) | 38 (0·0%) | 131 (0·1%) | |
| Policy1-Bowel (Australia) | |||||||||
| Change in number of colorectal cancer diagnoses | |||||||||
| 2020 | 17 391 | −1537 (−8·8%) | −759 (−4·4%) | −2264 (−13·0%) | −2092 (−12·0%) | −2075 (−11·9%) | −773 (−4·4%) | −1516 (−8·7%) | |
| 2021 | 17 397 | 313 (1·8%) | 176 (1·0%) | −286 (−1·6%) | 111 (0·6%) | −129 (−0·7%) | 892 (5·1%) | 551 (3·2%) | |
| 2022 | 18 458 | 492 (2·7%) | 243 (1·3%) | 548 (3·0%) | 767 (4·2%) | 816 (4·4%) | 20 (0·1%) | 534 (2·9%) | |
| 2023 | 18 548 | 202 (1·1%) | 78 (0·4%) | 456 (2·5%) | 409 (2·2%) | 456 (2·5%) | 8 (0·0%) | 307 (1·7%) | |
| 2024 | 18 618 | 308 (1·7%) | 138 (0·7%) | 454 (2·4%) | 452 (2·4%) | 492 (2·6%) | 7 (0·0%) | 305 (1·6%) | |
| 2020–50 | 618 564 | 3552 (0·6%) | 1672 (0·3%) | 7140 (1·2%) | 5365 (0·9%) | 5831 (0·9%) | 205 (0·0%) | 1177 (0·2%) | |
| Change in number of colorectal cancer deaths | |||||||||
| 2020–50 | 196 336 | 1961 (1·0%) | 979 (0·5%) | 3968 (2·0%) | 2897 (1·5%) | 3233 (1·6%) | 126 (0·1%) | 423 (0·2%) | |
| OncoSim (Canada) | |||||||||
| Change in number of colorectal cancer diagnoses | |||||||||
| 2020 | 21 721 | −1417 (−6·5%) | −832 (−3·8%) | −1737 (−8·0%) | −1551 (−7·1%) | −1628 (−7·5%) | −827 (−3·8%) | −1084 (−5·0%) | |
| 2021 | 21 970 | 406 (1·8%) | 302 (1·4%) | −238 (−1·1%) | 170 (0·8%) | −41 (−0·2%) | 1169 (5·3%) | 622 (2·8%) | |
| 2022 | 22 295 | 785 (3·5%) | 1124 (5·0%) | 1080 (4·8%) | 923 (4·1%) | 1050 (4·7%) | −53 (−0·2%) | 470 (2·1%) | |
| 2023 | 22 295 | 762 (3·4%) | 1101 (4·9%) | 1080 (4·8%) | 923 (4·1%) | 1050 (4·7%) | −53 (−0·2%) | 470 (2·1%) | |
| 2024 | 22 295 | 785 (3·5%) | 1124 (5·0%) | 1080 (4·8%) | 923 (4·1%) | 1050 (4·7%) | −53 (−0·2%) | 493 (2·2%) | |
| 2020–50 | 841 511 | 2844 (0·3%) | 1671 (0·2%) | 5212 (0·6%) | 3733 (0·4%) | 4393 (0·5%) | 142 (0·0%) | 229 (0·0%) | |
| Change in number of colorectal cancer deaths | |||||||||
| 2020–50 | 311 133 | 1319 (0·4%) | 799 (0·3%) | 2366 (0·8%) | 1707 (0·5%) | 2002 (0·6%) | 65 (0·0%) | −66 (0·0%) | |
Data are presented as absolute change in individuals aged 50 years and older compared with the comparator scenario of undisrupted screening; numbers in parentheses show percentage change compared with the comparator scenario. MISCAN-Colon=MIcrosimulation SCreening ANalysis for colorectal cancer. ASCCA=Adenoma and Serrated pathway to Colorectal CAncer.
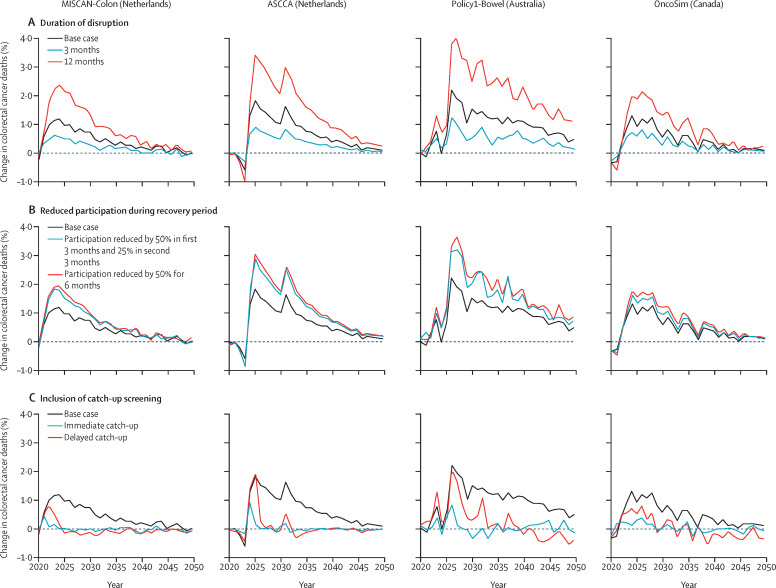
Figure 2.
Projected changes in colorectal cancer mortality among individuals aged 50 years and older relative to the comparator scenario according to MISCAN-Colon, ASCCA, Policy1-Bowel and OncoSim models
For the base case scenario, a 6-month disruption period from April to September, 2020, was assumed, with no catch-up or changes to participation in the recovery period. The predicted number of colorectal cancer deaths in 2020 in the comparator scenario was 4112 according to MISCAN-Colon, 5208 according to ASCCA, 6198 according to Policy1-Bowel, and 8134 according to OncoSim. MISCAN-Colon=MIcrosimulation SCreening ANalysis for colorectal cancer. ASCCA=Adenoma and Serrated pathway to Colorectal Cancer.
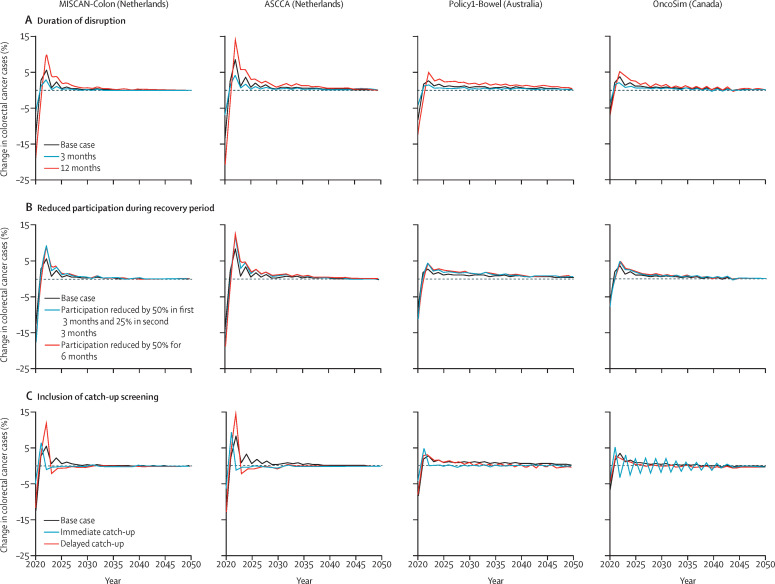
With 6-month disruption and no catch-up screening, for the Netherlands, MISCAN-Colon and ASCCA predicted a decrease in colorectal cancer diagnoses of 12·5% and 13·8%, respectively, in 2020 (table 3; figure 3 ). Cumulatively for the period 2020–50, MISCAN-Colon estimated 803 additional colorectal cancer diagnoses (relative increase 0·2% among individuals aged 50 years and older between 2020 and 2050) and 678 additional deaths (relative increase 0·4%), and ASCCA estimated 1803 (relative increase 0·4%) additional colorectal cancer diagnoses and 881 additional deaths (relative increase 0·6%) in the Netherlands. For Australia, colorectal cancer diagnoses were estimated to decrease in 2020 by 8·8%, with 3552 additional colorectal cancer diagnoses (relative increase 0·6%) and 1961 additional deaths (relative increase 1·0%) between 2020 and 2050. In Canada, compared with the comparator scenario, a relative decrease in colorectal cancer diagnoses of 6·5% was predicted to occur in 2020, and 2844 (relative increase 0·3%) additional colorectal cancer diagnoses and 1319 (relative increase 0·4%) additional deaths were estimated for the period 2020–50. The decrease in colorectal cancer diagnoses in 2020 was followed by a corresponding peak in colorectal cancer incidence in all four models in 2022, followed by a sustained increase in the number of diagnoses. Age-group specific results for these models estimated that the impact of disruptions increased with increasing age. In the 50–59 year age group, the impact of disruptions to screening services on colorectal cancer incidence and mortality was estimated to be small (table 4 ).
Figure 3.
Projected changes in colorectal cancer incidence among individuals aged 50 years and older relative to the comparator scenario according to MISCAN-Colon, ASCCA, Policy1-Bowel and OncoSim models
For the base case scenario, a 6-month disruption period from April to September, 2020 was assumed, with no catch-up screening or changes to participation in the recovery period. The predicted number of colorectal cancer cases in 2020 for the comparator scenario was 12 512 according to MISCAN-Colon, 13 562 according to ASCCA, 17 391 according to Policy1-Bowel, and 21 721 according to OncoSim. MISCAN-Colon=MIcrosimulation SCreening ANalysis for colorectal cancer. ASCCA=Adenoma and Serrated pathway to Colorectal Cancer.
Table 4.
Outcomes for 6-month disruption scenarios relative to the comparator scenario (undisrupted screening) by age group for MISCAN-Colon, ASCCA, and Policy1-Bowel models (2020–50)
|
MISCAN-Colon (Netherlands) |
ASCCA (Netherlands) |
Policy1-Bowel (Australia) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparator scenario | No recovery period and no catch-up screening | 6-month recovery period (participation decreased by 50%) and no catch-up screening | No recovery period and immediate catch-up screening | Comparator scenario | No recovery period and no catch-up screening | 6-month recovery period (participation decreased by 50%) and no catch-up screening | No recovery period, and immediate catch-up screening | Comparator scenario | No recovery period and no catch-up screening | 6-month recovery period (participation decreased by 50%) and no catch-up screening | No recovery period and immediate catch-up screening | |
| Colorectal cancer diagnoses | ||||||||||||
| 50–59 years | 56 828 | 56 719 | 56 669 | 56 862 | 69 525 | 69 373 | 69 225 | 69 462 | 83 961 | 83 866 | 83 783 | 83 986 |
| 60–69 years | 100 862 | 101 085 | 101 227 | 100 900 | 101 916 | 102 276 | 102 629 | 101 959 | 162 450 | 162 868 | 163 238 | 162 486 |
| ≥70 years | 294 006 | 294 696 | 295 057 | 293 899 | 233 584 | 235 179 | 236 262 | 233 677 | 409 569 | 412 798 | 414 787 | 409 714 |
| Colorectal cancer deaths | ||||||||||||
| 50–59 years | 13 124 | 13 138 | 13 119 | 13 121 | 19 149 | 19 149 | 19 150 | 19 151 | 22 854 | 22 903 | 22 919 | 22 880 |
| 60–69 years | 25 746 | 25 896 | 25 916 | 25 754 | 32 178 | 32 340 | 32 484 | 32 199 | 42 846 | 43 185 | 43 382 | 42 847 |
| ≥70 years | 130 146 | 130 660 | 130 589 | 130 090 | 91 294 | 92 013 | 92 488 | 91 309 | 139 964 | 141 536 | 142 598 | 140 062 |
| Life-years lost compared with undisrupted screening | ||||||||||||
| 50–59 years | .. | −103 | −65 | −48 | .. | −8 | −29 | 0 | .. | 142 | 151 | 87 |
| 60–69 years | .. | 707 | 1120 | 92 | .. | 629 | 1133 | 102 | .. | 2032 | 2912 | 296 |
| ≥70 years | .. | 7290 | 10 476 | 333 | .. | 4358 | 7725 | 302 | .. | 17 501 | 28 030 | 1134 |
Age-group specific results could not be provided for the OncoSim model due to underlying code. MISCAN-Colon=MIcrosimulation SCreening ANalysis for colorectal cancer. ASCCA=Adenoma and Serrated pathway to Colorectal CAncer.
Without catch-up screening or any long-term decrease in participation, the effect of a disruption on colorectal cancer incidence and mortality was proportional to the length of the disruption, with a 3-month disruption having roughly half the impact of a 6-month disruption, and a 12-month disruption having roughly twice the impact of a 6-month disruption (table 3; Figure 2, Figure 3).
A reduction in participation during the recovery period increased the impact of the disruption. For scenarios with a 6-month disruption followed by a 50% reduction in participation during the 6-month recovery period, colorectal cancer incidence was predicted to increase by 0·3–0·9% and mortality by 0·6–1·6% in the modelled cohorts between 2020 and 2050 (Figure 2, Figure 3).
Offering catch-up screening to individuals who missed screening during the disruption period mitigated the impact of the disruption: a catch-up screen within 6 months after the disruption increased the number of colorectal cancer diagnoses, matching that for the comparator scenario by 2022 for all four models, and increased colorectal cancer deaths by less than 0·1% between 2020 and 2050 in all countries (Figure 2, Figure 3). In the catch-up scenario in which screening was delayed by 6 months, the models predicted that annual colorectal cancer incidence would return to that of the comparator scenario after 6 years in the Netherlands, and after approximately 10 years in Australia and Canada. In this scenario, the increase in colorectal cancer deaths is slightly higher than scenarios in which immediate catch-up was offered, but the total increase in deaths remained less than 0·2% between 2020 and 2050 for all four models. Full results, including colorectal cancer incidence by stage, are shown in the appendix (pp 5–8).
Additional outcomes such as the number of individuals eligible, number of individuals participating, diagnostic follow-up colonoscopies, colorectal cancer incidence by stage, colorectal cancer deaths, and life-years lost are included in the appendix (pp 9–12). For scenarios with catch-up screening, additional resource demand was predicted for primary FIT screening and diagnostic colonoscopies, with colonoscopy demand nearly doubling during the 6-month recovery period with immediate catch-up screening. The additional outcomes also highlight the stage shift, with much of the increase in colorectal cancer incidence occurring for stage 4 colorectal cancers (up to 0·8% increase in the Netherlands, 2·8% increase in Australia, and 0·5% increase in Canada between 2020 and 2050 with a 6-month disruption; appendix pp 5–8). This shift in incidence to stages with worse survival rates explains the corresponding increase in colorectal cancer mortality observed. No changes to survival were assumed for delays in diagnosis that did not result in a stage shift.
In the additional scenarios modelled, the results for different combinations of disruption length, participation decrease, and catch-up screening were consistent with the results observed in the main scenarios (appendix pp 9–12).
Discussion
This study predicts that should the COVID-19 pandemic disrupt colorectal cancer screening services, the long-term impact could lead to thousands of additional colorectal cancer deaths. Disruptions of up to 12 months to colorectal cancer screening services could result in up to 3968 excess deaths in Australia, 2366 in Canada, and 1360–1762 in the Netherlands. Furthermore, reduced participation in screening during the post-disruption recovery period was estimated to result in hundreds of additional deaths. To minimise the long-term impact of disruptions in colorectal cancer screening services, it is important to catch-up disrupted screening invitations either immediately or with a delay, since it is estimated that such an approach would reduce the impact to less than a 0·2% relative increase in excess colorectal cancer deaths compared with undisrupted screening for all three countries.
Our findings are consistent with existing studies, which demonstrated the importance of ensuring cancer diagnoses continue during the COVID-19 pandemic. For instance, Degeling and colleagues estimated that 3-month delays to melanoma, breast, colorectal, and lung cancer diagnoses and treatment would result in 90 excess deaths over the next 5 years in Australia for patients diagnosed in 2020, and a 6-month delay would result in 350 excess deaths.12 A similar study estimated thousands of excess deaths attributable to diagnostic delays in England for breast, colorectal, oesophageal, and lung cancer, which is consistent with microsimulation modelling on the long-term impact of delays to diagnosis and warnings from the US National Cancer Institute.9, 13, 23
When restrictions lift and health systems regain capacity, governments face unique challenges to ensure a safe and effective return to screening. This modelling study has presented a clear argument for the health benefits of catch-up screening, but in practice administering modifications to existing population screening programmes could prove challenging. Individuals might skip screening regardless of whether screening programmes were paused, due to a real or perceived increased risk associated with screening or follow-up colonoscopy, and it might prove difficult to ensure these people return to screening. When it becomes possible, governments must make a concerted effort to ensure individuals have both the opportunity and confidence to catch up or resume their cancer screening as soon as they can.
The differences observed in the projected impact of screening disruptions on health outcomes across the four models can be explained by three key differences: colorectal cancer incidence, screening programmes (eg, eligibility criteria, participation rates, screening test performance), and model assumptions. Canada and Australia use the same age thresholds for screening eligibility, have similar screening participation rates, and use similar FIT cutoff values to recall individuals for follow-up colonoscopy; however, Australia uses a two-sample FIT test, which is much more sensitive than the one-sample FIT test used in the Netherlands and most parts of Canada. The Dutch programme targets a slightly older population and has a much higher screening participation rate than Australia and Canada (>70%). Any pause to screening would have a greater impact in a population with higher colorectal cancer incidence, screening participation rates, and test sensitivity, which was observed in this study. ASCCA and MISCAN-Colon simulated the Dutch population, and their cumulative results are similar but annual results differed slightly, as a result of differences in the underlying model structure and assumptions–eg, ASCCA simulates both the adenoma-carcinoma and serrated polyp pathways whereas MISCAN-Colon assumes non-bleeding adenomas that are systematically missed by FIT.24 Such differences in modelling assumptions are likely to be present in all models used, but are most clearly illustrated by the differences in findings identified by the ASCCA and MISCAN-Colon models, since they both projected estimates for the Netherlands. By using models that were developed wholly or in part by independent groups, we reduced the risk of systematic bias.
Colonoscopy capacity is a key concern as demand often exceeds supply, leading to long waiting times (although this demand is not always generated by the screening programme).25 Catch-up screening might temporarily increase colonoscopy demand to nearly twice that of normal levels, which might exceed the capacity of health systems. Further studies are required and have been planned to assess the viability of targeted catch-up screening strategies, such as using higher FIT cutoff values at screening during the recovery period to prioritise individuals who are at increased risk.
Ongoing monitoring of participation rates during and after the pandemic will demonstrate the effect of changing patient behaviours on screening levels. Our findings suggest that COVID-19 associated decreases in screening participation can lead to worse health outcomes. This finding is underlined by previous studies that have estimated that higher screening participation rates markedly increase the health benefits of screening, while remaining cost-effective.10 The task of ensuring individuals understand the importance of screening and have the confidence to return to screening safely is key, especially in countries with relatively low participation rates such as Canada and Australia. Disseminating findings that highlight the benefits of screening such as those included in this study is crucial, to enable individuals to make informed decisions about balancing cancer screening and COVID-19 risk. Previous studies have shown that mass media campaigns can improve colorectal cancer screening participation, have positive effects on long-term health impacts, and are highly cost-effective.23, 26 Similar media campaigns might be necessary to return participation rates to pre-2020 levels. Programmes that require general practitioner (GP) attendance for primary screening could consider transitioning to a postal-based system similar to that currently used in Australia and the Netherlands, to reduce the requirement for in-person interaction, alleviate strain on GP services, and relieve patient fear regarding the possibility of infection.
A key strength of this study is the use of well-established microsimulation models. These models have all been calibrated and validated using the best available data in their settings and have been used to inform local screening guidelines.10, 19, 21, 22, 27 By modelling the same scenario across multiple countries, this study highlights the scale of the possible impact of disruptions to screening on long-term health outcomes. Although the models varied in the absolute number of projected excess deaths due to the disruption, all models had similar conclusions. We infer from this agreement in modelling results that our observations are applicable to a range of settings and disruption periods and disruptions are likely to impact the efficacy of colorectal cancer screening programmes in other high-income countries in a similar way. Future work could use these methods and results and estimate outcomes for other countries.
This study has several limitations. The reported outcomes were model projections and not observed outcomes, since real-world data on programme disruptions are not available at present. Modelling can be completed quickly, whereas data for population-level screening programmes often requires months or years to collect and disseminate. We were also not able to incorporate any effects attributable to delays to colorectal cancer treatment, which is beyond the scope of the models used and appropriate data that could be incorporated into our modelling is not yet available to inform any such delay. Additionally, the modelled scenarios in this study, including effects on participation rates, and the duration of these effects, are based on conjecture, and were chosen to present a range of possibilities. In particular, any reduction in participation is currently modelled as being uniform across participants, but in reality changes to participation could differ by population factors including socioeconomic status, rural or urban location, and sex. It is unlikely that any particular scenario is representative of a true disruption in any of the settings, but the range of outcomes will provide a guide to the expected effect. As real-world data on screening disruption, changes to diagnostic follow-up, and variations in participation rates become available, future modelling studies will be required to model such data. In this study, the range of the scenarios modelled (including the additional disruption scenarios included in the appendix) act as a sensitivity analysis for the unknown impact of the true disruption. Sensitivity analyses for other model parameters, such as colorectal cancer survival, were not included in this study, but have been reported for each model previously.10, 19, 20, 21, 22 The comparative analysis with four structurally different models across different global settings can be considered as a sensitivity analysis on biological parameters. All three included countries are high-income countries; thus, the projected impact might not be generalisable to low-income and middle-income countries due to differences in cancer incidence and screening accessibility. It was not possible to model any less favourable outcomes for patients with colorectal cancer attributable to the additional risk of nosocomial COVID-19 infections. Such results would allow health service providers to balance COVID-19-related health risks against the benefits of screening, but this is beyond the scope of currently available data and the existing models.
As the COVID-19 pandemic continues, reprioritisation of health services globally has led to extraordinary and complex challenges. The results of this study clearly demonstrate the impact of screening interruptions on colorectal cancer burden, and highlight the importance of continuing colorectal cancer screening, either uninterrupted or via catch-up screening. Ensuring crucial cancer prevention continues where possible without exposing patients and medical professionals to unjustifiable risks is a difficult task, and one that will continue to evolve alongside the pandemic. Where disruptions are unavoidable, it is essential that screening is restarted as soon as possible afterwards, and catch-up screening is held for those that missed screening, to prevent avoidable colorectal cancer burden.
Data sharing
No primary data were collected for this study. Colorectal cancer incidence and mortality data used for the models were obtained from national cancer registries. Prevalence data were based on previously published autopsy studies. Other parameters in the models were based on previously published literature.
Contributors
LdJ, JW, FvW, JL, MJEG, EF, KC, VMHC, and IL-V conceptualised the study. LdJ, JW, FvW, NI, and JHEY did the analysis. LdJ and JW wrote the draft version of the manuscript, with supervision from IL-V. All authors contributed to the methodology, interpretation of results, and reviewing drafts of the manuscript, and all authors approved the final manuscript draft. LdJ, JW, FvW, and IL-V accessed and verified the data.
Declaration of interests
KC is coprincipal investigator of an unrelated investigator-initiated trial of cervical screening in Australia (Compass; ACTRN12613001207707 and NCT02328872), which is done and funded by the VCS Foundation, a government-funded health promotion charity. The VCS Foundation received equipment and a funding contribution from Roche Molecular Systems USA. However, neither KC nor her institution on her behalf (Cancer Council NSW) receives direct funding from industry for this trial or any other project. All other authors declare no competing interests.
Supplementary Material
References
- 1.WHO WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int/
- 2.International Agency for Research on Cancer . International Agency for Research on Cancer; Lyon: 2017. IARC Handbooks of Cancer Prevention: colorectal cancer screening. [Google Scholar]
- 3.Van den Brink C, Van den Ende C, Eeuwijk J, et al. National Institute for Public Health and Environment; Bilthoven: 2020. Kort-cyclische rapportage indirecte effecten COVID-19 op zorg en gezondheid. [Google Scholar]
- 4.Lee J. CBC Canada; Toronto, ON: May 27, 2020. Thousands of cancer screening tests halted during pandemic restart in Alberta.https://www.cbc.ca/news/canada/calgary/alberta-cancer-screening-resumes-after-pandemic-suspension-1.5586513 [Google Scholar]
- 5.Dinmohamed AG, Visser O, Verhoeven RHA, et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21:750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Australian Government. Cancer Australia Review of the impact of COVID-19 on medical services and procedures in Australia utilising MBS data: skin, breast and colorectal cancers, and telehealth services. September, 2020. https://www.canceraustralia.gov.au/sites/default/files/publications/review-impact-covid-19-medical-services-and-procedures-australia-utilising-mbs-data-skin-breast-and/pdf/review-of-the-impact-of-covid-19-on-medical-services-and-procedures-in-australia-utilising-mbs-data.pdf
- 7.Australian Institute of Health and Welfare . Australian Institute of Health and Welfare; Canberra: 2020. Cancer screening and COVID-19 in Australia. [Google Scholar]
- 8.Jodal HC, Helsingen LM, Anderson JC, Lytvyn L, Vandvik PO, Emilsson L. Colorectal cancer screening with faecal testing, sigmoidoscopy or colonoscopy: a systematic review and network meta-analysis. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-032773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutter CM, Kim JJ, Meester RGS, et al. Effect of time to diagnostic testing for breast, cervical, and colorectal cancer screening abnormalities on screening efficacy: a modeling study. Cancer Epidemiol Biomarkers Prev. 2018;27:158–164. doi: 10.1158/1055-9965.EPI-17-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lew J-B, St John DJB, Xu X-M, et al. Long-term evaluation of benefits, harms, and cost-effectiveness of the National Bowel Cancer Screening Program in Australia: a modelling study. Lancet Public Health. 2017;2:e331–e340. doi: 10.1016/S2468-2667(17)30105-6. [DOI] [PubMed] [Google Scholar]
- 11.Meester RG, Zauber AG, Doubeni CA, et al. Consequences of increasing time to colonoscopy examination after positive result from fecal colorectal cancer screening test. Clin Gastroenterol Hepatol. 2016;14:1445–1451. doi: 10.1016/j.cgh.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Degeling K, Baxter NN, Emery J, et al. An inverse stage-shift model to estimate the excess mortality and health economic impact of delayed access to cancer services due to the COVID-19 pandemic. medRxiv. 2020 doi: 10.1101/2020.05.30.20117630. published online May 30. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The World Bank Data for Australia, Netherlands, Canada. https://data.worldbank.org/?locations=AU-NL-CA
- 15.Landelijk Evaluatie team voor Colorectaal kanker bevolkingsonderzoek Landelijke monitoring en evaluatie van het bevolkingsonderzoek naar darmkanker in Nederland. 2014–2017. https://www.rivm.nl/sites/default/files/2019-03/monitor-evaluatie-darm-2014-2017.pdf
- 16.Australian Institute of Health and Welfare . Australian Institute of Health and Welfare; Canberra: 2019. National Bowel Cancer Screening Program: monitoring report 2019. [Google Scholar]
- 17.Statistics Canada Cancer Screening. 2017. https://www150.statcan.gc.ca/n1/pub/82-625-x/2018001/article/54977-eng.htm
- 18.International Agency for Research on Cancer. WHO Cancer today: population fact sheets. 2020. https://gco.iarc.fr/today/fact-sheets-populations
- 19.Loeve F, Boer R, van Oortmarssen GJ, van Ballegooijen M, Habbema JD. The MISCAN-COLON simulation model for the evaluation of colorectal cancer screening. Comput Biomed Res. 1999;32:13–33. doi: 10.1006/cbmr.1998.1498. [DOI] [PubMed] [Google Scholar]
- 20.van Hees F, Zauber AG, van Veldhuizen H, et al. The value of models in informing resource allocation in colorectal cancer screening: the case of The Netherlands. Gut. 2015;64:1985–1997. doi: 10.1136/gutjnl-2015-309316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greuter MJ, Xu XM, Lew JB, et al. Modeling the Adenoma and Serrated pathway to Colorectal CAncer (ASCCA) Risk Anal. 2014;34:889–910. doi: 10.1111/risa.12137. [DOI] [PubMed] [Google Scholar]
- 22.Coldman A, Pader J, Gauvreau C, et al. Simulating results from trials of sigmoidoscopy screening using the OncoSim microsimulation model. J Cancer Policy. 2018;15:52–58. [Google Scholar]
- 23.Worthington J, Lew J-B, Feletto E, et al. Improving Australian National Bowel Cancer Screening Program outcomes through increased participation and cost-effective investment. PLoS One. 2020;15 doi: 10.1371/journal.pone.0227899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Meulen MP, Lansdorp-Vogelaar I, van Heijningen EM, et al. Nonbleeding adenomas: evidence of systematic false-negative fecal immunochemical test results and their implications for screening effectiveness-a modeling study. Cancer. 2016;122:1680–1688. doi: 10.1002/cncr.29952. [DOI] [PubMed] [Google Scholar]
- 25.van Turenhout ST, Terhaar sive Droste JS, Meijer GA, Masclée AA, Mulder CJ. Anticipating implementation of colorectal cancer screening in The Netherlands: a nation wide survey on endoscopic supply and demand. BMC Cancer. 2012;12:46. doi: 10.1186/1471-2407-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durkin S, Broun K, Guerin N, Morley B, Wakefield M. Impact of a mass media campaign on participation in the Australian bowel cancer screening program. J Med Screen. 2020;27:18–24. doi: 10.1177/0969141319874372. [DOI] [PubMed] [Google Scholar]
- 27.Lew J-B, Greuter MJE, Caruana M, et al. Validation of microsimulation models against alternative model predictions and long-term colorectal cancer incidence and mortality outcomes of randomized controlled trials. Med Decis Making. 2020;40:815–829. doi: 10.1177/0272989X20944869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No primary data were collected for this study. Colorectal cancer incidence and mortality data used for the models were obtained from national cancer registries. Prevalence data were based on previously published autopsy studies. Other parameters in the models were based on previously published literature.