Abstract
Cancer immunotherapies have revolutionized the treatment of numerous cancers, with exciting results often superior to conventional treatments, such as surgery and chemotherapy. Despite this success, limitations such as limited treatment persistence and toxic side effects remain to be addressed to further improve treatment efficacy. Biomaterials offer numerous advantages in the concentration, localization and controlled release of drugs, cancer antigens, and immune cells in order to improve the efficacy of these immunotherapies. This review summarizes and highlights the most recent advances in the use of biomaterials for immunotherapies including drug delivery and cancer vaccines, with a particular focus on biomaterials for immune cell delivery.
NOMENCLATURE
- ACT
Adoptive cell therapy
- APC
Antigen-presenting cells
- BSA
Bovine serum albumin
- CAR
Chimeric antigen receptor
- CDN
Cyclic dinucleotides
- CpG ODN
(Cytosine–guanine) oligonucleotides
- CTLA-4
Cytotoxic T lymphocyte antigen-4
- DC
Dendritic cells
- GM–CSF
Granulocyte–macrophage colony-stimulating factor
- ICI
Immune checkpoint inhibitors
- IL-2
Interleukin 2
- MHC
Major histocompatibility complex
- MSR
Mesoporous silica rod
- MSM
Mesoporous silica microspheres
- NK
Natural killer cells
- PBMC
Peripheral blood mononuclear cells
- PCL
Polycaprolactone
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed death-ligand 1
- PEI
Polyethyleneimine
- PIC
Polyisocyanopeptide
- PLGA
Poly (lactide-co-glycolide) acid
- ROS
Reactive oxygen species
- STING
Stimulator of interferon genes
- TIL
Tumor-infiltrating lymphocytes
- TLS
Tertiary lymphoid structure
- T-VEC
Talimogene laherparepvec
I. INTRODUCTION
Despite continuous progress in detection and treatment, cancer remains one of the leading causes of death worldwide.1 Cancer immunotherapy—where cancer treatment is achieved by harnessing and assisting patients' own immune systems—has revolutionized oncology, allowing previously impossible precision in targeting tumor cells compared to conventional treatments and showing impressive results in the previously untreatable disease.2 Immunotherapy exploits the inherent response of the host immune system, which can detect the foreign antigens created by cancerous cells and recognize and eliminate malignant cells.
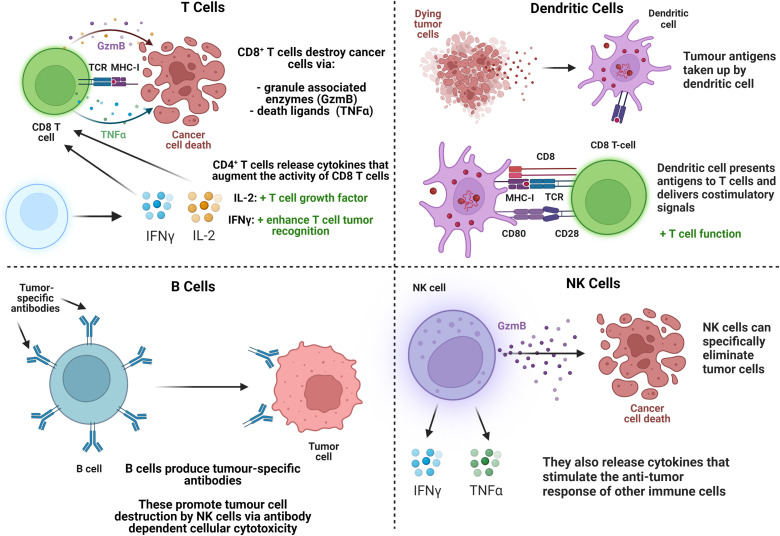
Cell-mediated immunity is the most relevant part of the immune system in the response to cancer, which in innate immunity consists of polymorphonuclear cells (neutrophils, eosinophils, basophils, and mast cells), phagocytic cells [monocytes, macrophages, and dendritic cells (DCs)], and natural killer (NK) cells. B and T lymphocytes (commonly referred to as B and T cells)—of which there are multiple subtypes—are the primary immune cells involved in adaptive immunity. Immunotherapy relies on the interaction of cells, such as T cells, B cells, DC, and NK cells, with cancers3–7 briefly explained in Fig. 1. This process has been described as the cancer immunity cycle, where antigens from necrotic or apoptotic cancer cells are captured by antigen-presenting cells (APCs). The APC subsequently stimulate cytotoxic T lymphocytes to specifically target and destroy the associated tumor cells via apoptosis.8 Unfortunately, alone, its efficacy is often limited by the inhibitory effect of the tumor microenvironment and hence the evasion of the immune system by cancer cells.
FIG. 1.
Summary of the main immune cell types relevant to cancer immunotherapy and their functions. GzmB: granzyme B, TNFα: tumor necrosis factor α, IL-2: interleukin-2, IFNγ: interferon γ, TCR: T cell receptor, MHC: major histocompatibility complex.
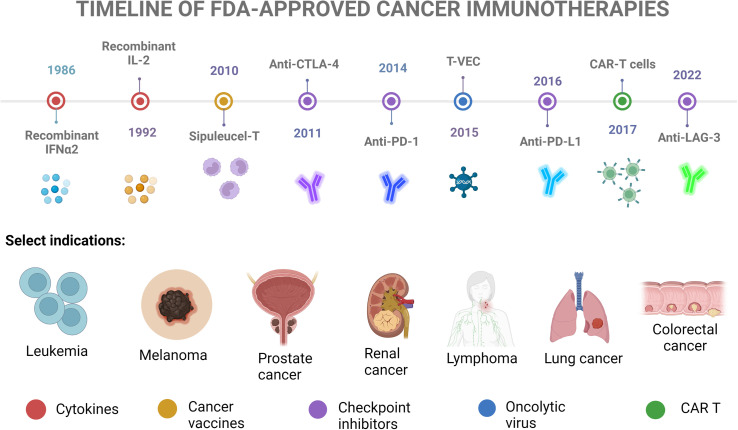
Numerous immunotherapies have been developed based on this principle of stimulating and augmenting the immune system. Some are based on drugs enhancing the T cell response, such as Interleukin 2 (IL-2), or immunomodulatory drugs targeting immune checkpoint inhibitors (ICIs). Some treatments encourage the development of immune cells specialized against the tumor (so-called cancer vaccines9). Oncolytic viruses (OVs) can both directly destroy cancer cells and produce immunostimulatory cytokines.10 Others consist of the injection of cytotoxic T cells, referred to as adoptive cell therapy (ACT).11 There are countless ongoing clinical trials, of which it is not possible (and not the aim of this review) to summarize here. Selected FDA-approved immunotherapies are highlighted in Fig. 2 and are explained in detail where relevant in Sec. II.
FIG. 2.
Timeline of selected FDA-approved cancer immunotherapies. Adapted with permission from Cancer Research Institute, see https://www.cancerresearch.org/fda-approval-timeline-of-active-immunotherapies for “FDA Approval Timeline of Active Immunotherapies-Cancer Research Institute 2022.”16
Despite promising results and numerous clinical studies, the efficacy of these strategies is still limited due to diverse factors, which will be summarized later. A current trend is to combine multiple therapies, particularly a combination of anti-programmed cell death protein 1 (PD-1) and either anti-cytotoxic T lymphocyte antigen-4 (CTLA-4)12 or the newly approved ICI targeting lymphocyte-activation gene 3 (LAG-3),13 or the combination of these checkpoint inhibitors with ACT for melanoma and ovarian cancers.14,15 These combinations are a major focus for current clinical trials with the goal of establishing best practice, and it seems likely that future gold-standard treatments will comprise multiple immunotherapies, perhaps alongside conventional treatments, as combination therapies. Another trend is the increasing use of biomaterials to improve the efficiency of immunotherapies and decrease their toxic effects. This review will focus on the potential of biomaterials (as cell scaffolds or controlled delivery systems for antibodies or tumor antigens) to further enhance the efficacy and decrease the toxicity of immunotherapy.
II. BIOMATERIALS IN IMMUNOTHERAPY
A. Introduction and rationale
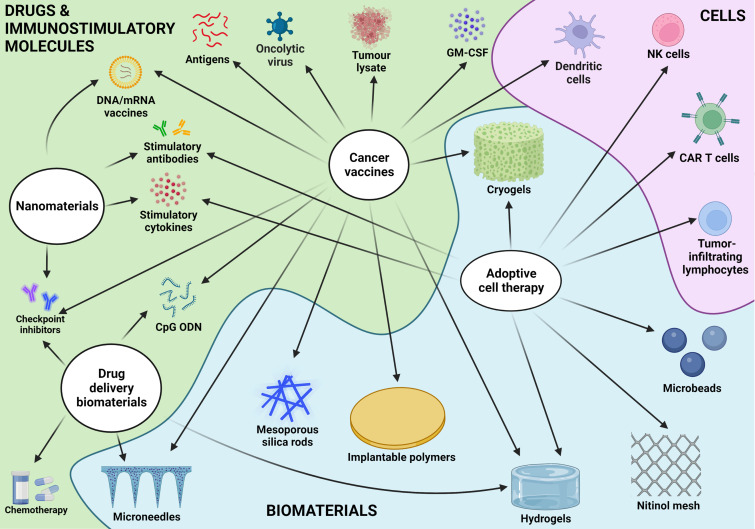
Biomaterials, defined as “materials designed to take a form that can direct, through interactions with living systems, the course of any therapeutic or diagnostic procedure”17 have long been widely studied in numerous biomedical applications.18 We can differentiate biomaterials by their source (natural or synthetic), their class (metals, ceramics, or polymers), and their stability (permanent or biodegradable). Biomaterials have been used in several oncology treatments, for example, for local administration of chemotherapy in urothelial carcinoma19 or the embolization of blood vessels for palliative treatment of hepatic cancers,20 to name just a few. In the present review, we will limit ourselves to immunotherapies. While only comparatively recently have developments in immunotherapy supported their use in oncology, biomaterials offer numerous possibilities to augment the efficacy of immunotherapies or limit their harmful side effects, as summarized in Fig. 3. In brief, they can achieve this through controlled spatial and temporal release of the cells and immunotherapeutic agents, which can result in dose-dependent and off-target toxicities in current immunotherapies.21 They can also create a “niche” for the activation of endogenous or exogenous APC, with potential additional anti-tumor efficacy coming from the biomaterial itself, such as their pro-inflammatory effects or reactive oxygen species (ROS) generation.22
FIG. 3.
Summary of immunotherapies and the potential role of biomaterials.
Due to the interlinked nature of the immune system, there is extensive crossover in the components and mechanisms of action of immunotherapeutic biomaterials. However, two broad categories that can be proposed are cell-delivery biomaterials, directly incorporating cells such as lymphocytes and DC as localized immunotherapies,23 and cell-free biomaterials, which can incorporate a combination of immune adjuvants, antigens and even additional cancer therapeutics such as chemotherapeutic agents alongside the inherent immunomodulatory effects of certain biomaterials.22,24 This review will focus on biomaterials in these categories with a particular focus on lymphocytes as antitumoral agents, either through direct administration and stimulation of lymphocytes or by their indirect stimulation by other cells or immunomodulatory components.
B. Cell-free biomaterials
1. Aim and design criteria
The aim of cell-free scaffolds for immunotherapies is to provide, either through their own chemical composition or through attached or encapsulated biological factors, cellular cues to direct and encourage a favorable immune response toward a given stimulus, or alternatively to locally deliver anti-cancer drugs. In the case of a biomaterial for cancer immunotherapy, this would implicate either the inclusion of immune agonists and/or immunotherapeutic drugs, within a material matrix suitable for the administration and controlled delivery of these factors, such that a strong anti-cancer response is achieved, and/or the utilization of the inherent immune response to a particular matrix to recruit and activate APC.
These systems should be composed of a biocompatible material, which according to the definition of biocompatibility must not elicit adverse biological effects (cytotoxicity, carcinogenicity, hypersensitivity, etc.), but should promote an “appropriate host response in a specific application,”17 namely, here help recruit and activate the APC or be at least immunologically inert to allow the function of its immunomodulatory components. Indeed, in this particular case detailed here, provoking some immune response is deemed beneficial in the anti-cancer response and some inflammation could even be encouraged.
Such a material should also be:
-
•
Injectable through a small needle to enable minimally invasive procedures.
-
•
Persistent over the necessary timescale for maximum efficacy of its drug or molecule of choice and ideally be degraded afterwards.
-
•
For drug delivery, the release rate must be well controlled by the scaffold properties and degradation rate.
-
•
The use of simple materials, potentially already used in FDA-approved medical devices or therapies, is generally desired for easier regulatory approval.
Sections II B 2–II B 5 detail the principles and review the cases of biomaterials used for cancer vaccines and controlled release of OV or immunotherapeutic drugs.
2. Biomaterials for cancer vaccines
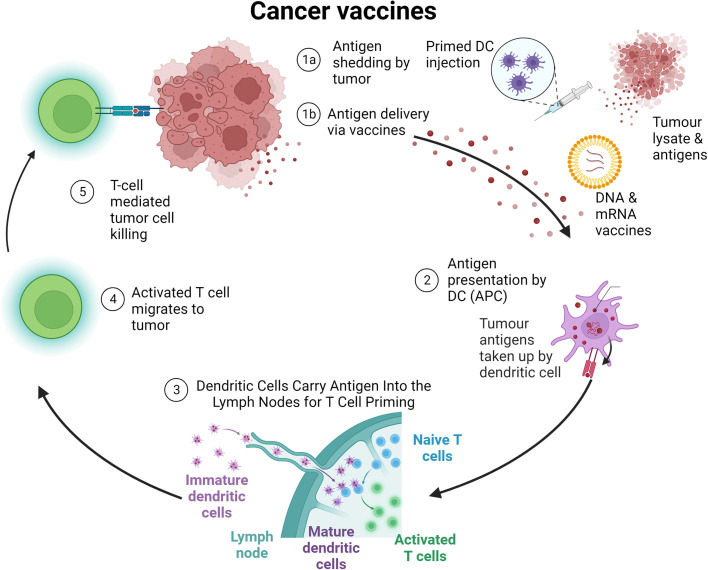
Cancer vaccines can include dead tumor cells or lysate, DC, antigens, or nucleic acids, such as mRNA, with DC the most widely studied form of cancer vaccine to date.25 Whatever their component, the vaccines—generally administered subcutaneously, intramuscularly, or intravenously—act to supplement or improve tumor-specific lymphocyte activity via improved antigen presentation, lymphocyte activation, and localization of the immune response to the tumor.9 A summary of cancer vaccine mechanisms is shown in Fig. 4.
FIG. 4.
Cancer vaccine mechanisms. Tumor antigens from tumor cell death or administered vaccines are taken up by dendritic cells for priming of tumor-specific T cells, which can then specifically eliminate cancer cells, continuing the cycle.
Exogenous cells can be added to the antigens injected, to ensure the formation of activated DC. This is the case of Sipuleucel-T (Provenge®), the first immune cell therapy approved by the FDA as a cancer treatment.26 Sipuleucel T improved antitumor CD8+ cell response and survival in a clinical trial in patients with castration-resistant prostate cancer.27 Despite this initial success, limited progress has since been made in the clinical use of cancer vaccines, with other candidate vaccines failing to demonstrate clinical efficacy and leaving Sipuleucel-T as still the only FDA-approved cancer vaccine.28 Nevertheless, research continues with recent developments, such as neoantigen vaccines, which target patient-specific antigens rather than tumor-associated antigens that are common between patients and associated with higher immune tolerance.
To be effective, cancer vaccines must have two main properties. First, they must stimulate the appropriate specific immune responses against the correct target. Second, the immune responses must be powerful enough to overcome the barriers that cancer cells use to protect themselves. Therefore, sustained delivery (to avoid rapid clearance) and an appropriate structure for the APC to interface with the vaccine component is key.
Biomaterials have thus been used to create a physical structure loaded with vaccine components which stimulate APC cells in situ. These will then disperse to lymph nodes and activate resident T cells which then travel to the tumor site and eliminate malignant cells. We can distinguish between implantable and injectable scaffolds. Figure 5 describes the two main approaches, while Table I summarizes the main biomaterials used for the different vaccines.
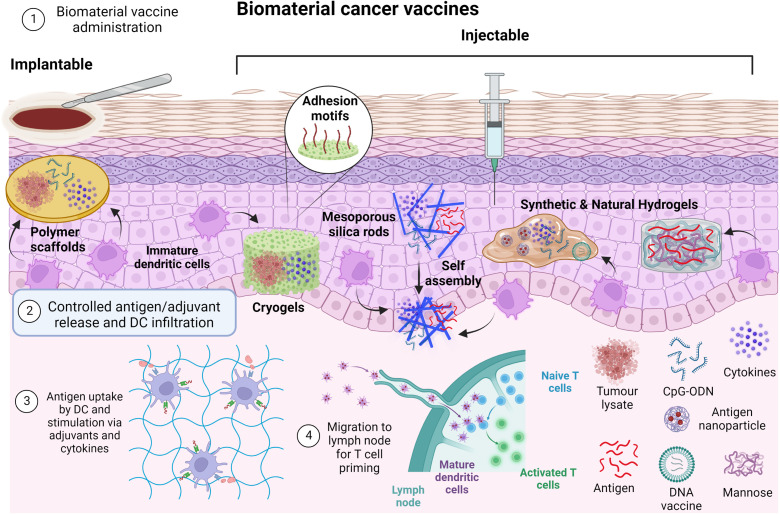
FIG. 5.
Principles of biomaterial vaccines. Biomaterials containing tumor lysates, antigens, cytokines, and adjuvants are implanted or injected. Antigens and adjuvants are released in a controlled manner and DC infiltrate the biomaterial niche where antigen uptake can occur. DC then migrate to lymph nodes where T cell priming occurs.
TABLE I.
Biomaterials used for cancer vaccines.
| Materials | Therapeutic agents | Injectable? | Immunotherapy model and outcomes | References |
|---|---|---|---|---|
| Poly (lactide-co-glycolide) acid (PLGA) | Tumor lysate antigens, GM-CSF, (cytosine-guanine) oligonucleotides (CpG ODN), PD-1 or CTLA-4, monophosphoryl lipid A, polyinosinic:polycytidylic acid | No | Mouse melanoma models | 29–31 |
| Up to 90% survival increase with vaccine + GM-CSF + CpG ODN | ||||
| Enhanced effect in combination with PD-1 or CTLA-4 | ||||
| Methacrylated alginate (MA-alginate) | Tumor lysate antigens, GM–CSF, (cytosine–guanine) oligonucleotides (CpG ODN) | Yes | Mouse melanoma models | 32, 33 |
| Up to 80% survival with biomaterial vaccine + GM–CSF + CpG ODN vs 60% with bolus vaccine | ||||
| MA–alginate + methacrylated polyethylene glycol (MA–PEG) | Tumor lysate antigens, GM–CSF, (cytosine–guanine) oligonucleotides (CpG ODN) | Yes | Mouse leukemia models | 34 |
| 100% survival with chemotherapy + biomaterial vaccine | ||||
| Alginate + tumor lysate | Tumor lysate antigens, GM–CSF, anti-PD-1 | No | Mouse pancreatic cancer model | 35 |
| Five-fold decrease in tumor volume compared to treatment with PBS, additive effect with anti-PD-1 | ||||
| Mesoporous silica rods (MSR) | Tumor antigen, GM–CSF, anti-PD-1, polyethyleneimine (PEI) | Yes | Mouse lymphoma, melanoma, and lung cancer models | 36–38 |
| Increased DC recruitment and survival with MSR vaccines | ||||
| PCL–PEG–PCL | GM–CSF, OVA nanoparticles | Yes | Improved DC recruitment in vivo, increased T cell response to EG7-OVA in vitro | 39 |
| Bovine serum albumin (BSA) + PCL–PEG–PCL | DNA vaccine | Yes | Improved DC recruitment and increased T cell response to amyloid-β Alzheimer antigen in vivo | 40 |
| CpG-modified carboxymethyl chitosan and partially oxidized mannan | OVA antigen | Yes | Mouse melanoma model | 41 |
| Reduced tumor growth and improved survival with gel vaccine | ||||
| Catechol chitosan + calcium phosphate nanosheet | OVA antigen | Yes | Increased DC activation and antigen presentation | 42 |
Biomaterial vaccines were among the first biomaterial-delivered immunotherapies to be researched, with pioneering work from Mooney's group being essential in this field and influential in its development.
The first development was an implantable PLGA scaffold incorporating tumor lysate antigens (from destroyed cancer cells), GM–CSF as a stimulatory cytokine for DC and (cytosine–guanine) oligonucleotides (CpG ODN) a toll-like receptor 9 (TLR-9) agonist that also activates DC.29 The interconnected pores in the scaffold create an environment in which infiltrating DC are activated and process the tumor lysate antigens before migrating to lymph nodes where they can prime antigen-specific T cells. The scaffold also regulates the release of antigens and adjuvants to encourage a persistent immune response. This biomaterial-based vaccine paved the way for future developments in biomaterial immunotherapies and has the distinction of being the first such biomaterial to enter clinical trials, where the above vaccine is being tested in a phase I clinical trial to determine its safety and feasibility and to confirm its biological activity when used to treat metastatic melanoma.43 The study completion date is in 2022. The group has further developed the scaffold, showing that the vaccine is even more effective in combination with checkpoint inhibitors.30 Furthermore, alternative vaccine adjuvants, such as monophosphoryl lipid A and polyinosinic:polycytidylic acid, also preventively and therapeutically reduced tumor growth in mouse melanoma models.31
Later, the team developed an MA–alginate “cryogel” scaffold functionalized with RGD binding sites and using the same immunomodulatory factors, which demonstrated both therapeutic and preventative effects in mice melanoma models, with a further benefit of injectability as opposed to the previous PLGA scaffold.32 However, this requires quite large diameter needles (16G). According to in vitro tests, approximately 80% of the encapsulated vaccine compounds (GM–CSF and CpG ODN) were released within the first 4 days, followed by slow and sustained release over the next month. The interconnected macroporous structure allowed cellular infiltration and immune cell trafficking in situ. Further work interestingly showed that the vaccine maintained its efficacy regardless of whether the injection site was adjacent or distal to the tumor and draining lymph nodes.33 This group also developed a similar cryogel using a combination of MA–alginate and PEG to eradicate established acute myeloid leukemia in mice.34 Lu et al. developed a relatively similar cryogel, which incorporates tumor cell lysates during hydrogel preparation and significantly reduced the growth of a secondary tumor after surgical resection of the primary tumor.35
All these 3D biomaterials are, however, fabricated ex vivo and require either surgical placement in the body or large invasive needles for implantation. Moreover, they are not biodegradable and their preformed structures could limit the capability of host cells to organize themselves.36
To solve this issue, Mooney's group proposed self-assembling mesoporous silica rods (MSR) that are more easily injectable (18G needle), biodegradable, and create more macroporous 3D structures for better interaction with immune cells. The MSRs are injected with a needle and spontaneously assemble in vivo, degrading over time and allowing a slow release of vaccine antigens and adjuvants.36 These self-assembled MSR scaffolds increased the number of recruited cells compared to previously reported preformed macro-porous polymer scaffolds, with 20 × 106 cells in the MSR vaccine after 5 days in vivo vs only 6 × 106 in the MA–alginate cryogel vaccine after 6 days. In later work, the MSR vaccine was coated with polyethyleneimine (PEI) to enhance the effect of neoantigen peptides.37 MSR–PEI vaccines significantly enriched the DC population, roughly doubling the number of recruited DC, and enhanced host DC activation and T cell responses compared to the existing MSR vaccine. The MSR vaccines eradicated established E7-ovalbumin (OVA) tumors in 80% of mice and showed efficacy in reducing tumor growth in melanoma and carcinoma cell lines, with an additive effect when combined with CTLA-4 injection, demonstrating the potential of biomaterials-based vaccines to function alongside other immunotherapies such as checkpoint inhibitors.37 Further work using MSR vaccines has applied the same principle for alternative peptide antigens, such as gonadotropin-releasing hormone (GnRH) and HER2/neu, implicated in multiple cancers including breast cancer.38
Other groups have developed biomaterial vaccines with similar stimulatory molecules, using other commonly used biomaterials such as chitosan, polycaprolactone (PCL), and even bovine serum albumin (BSA). For example, Sun et al. developed an injectable thermosensitive PCL–PEG–PCL hydrogel encapsulated GM–CSF and chitosan-coated ovalbumin (OVA) nanoparticles to create the immune cell niche for DC activation,39 where the chitosan itself may induce DC maturation via the Stimulator of Interferon Genes (STING) signaling pathway.44 STING is a transmembrane protein that interacts with cyclic dinucleotides (CDNs) produced in response to cytosolic double-stranded DNA, resulting in the production of IFN-β and other cytokines that promote the in vivo anti-tumor T cell response.45 In another study by Giang Phan et al., BSA was crosslinked with a similar PCL–PEG–PCL hydrogel to deliver a DNA vaccine against the amyloid-β Alzheimer antigen.40 Both PCL–PEG–PCL hydrogel vaccines induced strong immune responses and improved DC recruitment. Note that hydrogels are particularly interesting as they often present shear-thinning behavior. Moreover, some can undergo reversible volume phase transitions or sol-gel phase transitions in response to external physical or chemical stimuli—such as temperature, pH, ionic strength, light, and electromagnetic radiation—called stimuli-sensitive or intelligent hydrogels and allowing in situ gelation and hence minimally invasive treatment.
Chitosan seems to be gaining popularity for use in cancer vaccines, with an injectable gel formed by a Schiff base reaction between CpG-modified carboxymethyl chitosan and partially oxidized mannan increasing DC infiltration and maturation and significantly reducing tumor growth in a B16-F10 mouse melanoma model.41 Furthermore, though not tested in tumor models, an OVA-antigen-containing “nanosheet” was formed through the simple mixing of catechol-modified chitosan, PBS, and CaCl2.42 The nanosheet significantly increased DC activation and antigen presentation compared to free antigen and so could be another promising future carrier of antigens as a cancer vaccine. Carroll et al. showed that chitosan, a cationic polymer, can engage the cGAS-STING pathway to mediate the selective production of type I IFN and interferon-stimulated genes, which were then responsible for mediating the activation of DC and induction of cellular immunity.44 More generally, these studies show that the choice of the biomaterial composition and porosity of the scaffold is key to induce successful recruitment and activation of DC. Moreover, hydrogels have the advantage of being injectable and can play the dual role of being a vaccine carrier with sustained release and a platform for recruiting DCs.
While clinical results are still absent, all these results suggest that biomaterials will play a key role in the development of cancer vaccines and that, alongside other immunotherapies, vaccines will have a role to play in therapeutic cancer treatment. Furthermore, while still at an early stage, the prophylactic vaccination of cancers with known antigens, such as human epidermal growth factor receptor-2-positive breast cancers, is an intriguing possibility.46 Prophylactic vaccination could be targeted to genetically high-risk populations, or alternatively used to prevent cancer recurrence and metastases.47 Cancer vaccines may also be pioneering precursor technologies, demonstrating the benefit of antigens, adjuvants, and DC, which can then be included as additional components of other immunotherapies.
3. Oncolytic viruses
OV are genetically modified to infect and lyse only cancer cells that sacrifice their normal antiviral defenses in order to grow more rapidly.10 OV can, thus, selectively infect and kill cancer cells, while leaving surrounding non-cancerous cells unharmed. While the precise mechanisms of their anti-tumor effect remain to be defined, they include direct tumor cell lysis, recruitment of APC, and tumor infiltrating lymphocytes as well as the release of pro-inflammatory cytokines from lysed tumor cells (Fig. 6). In addition, some OV have been genetically modified to express these cytokines in order to augment their anti-tumor activity.48 They are a somewhat niche area of cancer immunotherapy, with only one OV to date approved by the FDA: talimogene laherparepvec (T-VEC), a herpes simplex virus that showed a significant clinical response in the treatment of unresectable melanoma, with the virus modified to also express granulocyte–macrophage colony-stimulating factor (GM–CSF).49 Recent work has proposed the use of biomaterials in the form of nanofibers50 or nanoparticles51 for targeted delivery of OV, with bulk biomaterials for OV delivery summarized in Table II and subsequently explained further.
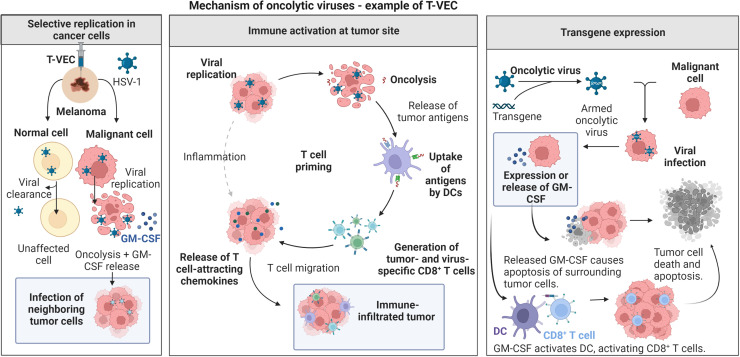
FIG. 6.
Oncolytic virus mechanisms: Oncolytic viruses such as T-VEC are selectively replicated in cancer cells, eliminating tumor cells via oncolysis and stimulating the immune system via the antigen release of destroyed tumor cells and expressed transgene products such as GM–CSF.
TABLE II.
Bulk biomaterials for oncolytic virus delivery.
| Materials | Therapeutic agents | Injectable? | Immunotherapy model and outcomes | References |
|---|---|---|---|---|
| Alginate | Oncolytic adenovirus | Yes | Mouse xenografts of human cervical cancer and glioma | 52 |
| Increased OV accumulation in the tumor, reduced tumor growth, and reduced OV accumulation in off-target tissues | ||||
| Gelatin-hydroxyphenyl propionic acid hydrogel | Oncolytic adenovirus | Yes | Hamster models with pancreatic carcinoma | 53 |
| Reduced tumor growth, reduced OV accumulation in off-target tissues, reduced anti-OV immune response | ||||
| Silk elastin-like protein polymer hydrogel | Oncolytic adenovirus | Yes | Mouse xenograft of human head and neck cancer | 54 |
| Reduced tumor growth and greater OV persistence at the tumor site | ||||
| Polyurethane-sulfamethazine hydrogel | Oncolytic adenovirus | Yes | Mouse xenograft of human lung cancer | 55 |
| Reduced tumor growth and greater OV persistence at the tumor site |
Choi et al. used an alginate gel to encapsulate OV which was shown to double their accumulation in the tumor and reduced tumor growth by half compared to non-encapsulated OV in mouse xenograft models of human C33A (cervical) and U343 (glioma) cancer cell lines. Gel encapsulation also reduced OV accumulation in off target tissues.52 Work from Yun’s group showed similar improvements in OV efficacy, with a gelatin-hydroxyphenyl propionic acid (GHPA) hydrogel also showing a twofold increase in anti-tumor efficacy, reduced accumulation in off target tissues, and a reduced anti-OV immune response in hamster models with the HaP-T1 hamster pancreatic carcinoma cell line.53 In murine xenograft models of localized OV delivery, both a recombinant silk elastin-like hydrogel in a head and neck cancer model and a polyurethane-sulfamethazine injectable hydrogel in a lung cancer model also reduced tumor growth by around half compared to non-encapsulated OV. Histological analysis also demonstrated greater persistence of the OV at the tumor site in these cases.54,55
Clearly, biomaterials have the potential to improve OV retention in tumors, protect them from elimination by the immune system, and hence improve their efficacy. Clinical trials of locally delivered OV will no doubt be awaited with interest as this immunotherapy experiences a resurgence.
4. Drug delivery biomaterials
Biomaterials for simple local delivery of anti-cancer drugs have also been investigated, as with numerous other medical domains.56 Drug delivery scaffolds and the principles underlying the design of hydrogel drug delivery systems, focusing on the physical and chemical properties of the hydrogel network and the hydrogel–drug interactions, have been reviewed previously and will not be detailed here.57–61 Biomaterial scaffolds allow local and controlled release of drugs and molecules, therefore allowing lower doses for equivalent efficacy and limiting dose-related toxicities. Spatial control also has the double benefit of increasing the payload of the drug or molecule at the relevant site, while ensuring a minimum is lost to non-tumor sites where it would be ineffective or even potentially cause off-target toxicity.
Whatever drugs and molecules are being delivered, the drug dosage, mechanism of release, and ease of administration of the biomaterial are of greater importance. Here, the ability to chemically conjugate molecules of interest to the material, and easier administration, such as with an injectable material, would be highly desirable.
In terms of immunotherapeutic drugs, immune checkpoint inhibitors (ICIs) are among the most popular for local drug delivery, often combined with other drugs. ICI are monoclonal antibodies targeted toward so-called “immune checkpoints”—inhibitory immune pathways that downregulate T cell activation when their receptors on immune cells are activated that aim to maintain organism self-tolerance and avoid autoimmunity. Cancer cells themselves can develop the ability to activate these receptors and hence escape the downregulated immune system, for example, programmed death-ligand 1 (PD-L1) expressed on tumor cells (see Fig. 7). The most commonly targeted ICI are CTLA-4, which competes with CD28 to bind CD80/CD86 and hence downregulates T cells,62 PD-1, which has an inhibitory effect on T cell activation while stimulating Treg cells, which suppress the anti-tumoral immune response, and the ligand of PD-1, PD-L1, which can be found on the surface of tumor cells as well as endogenous epithelial and immune cells and contributes to the downregulating of T cells in the tumor microenvironment. LAG-3, a new addition to the ICI portfolio, is a cell surface protein expressed on immune cells that downregulates T cell proliferation and function.63
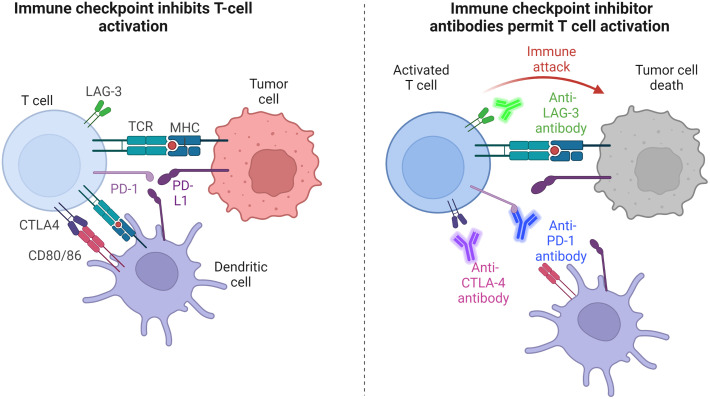
FIG. 7.
Mechanism of checkpoint blockade: antibodies inhibit binding of immune checkpoint pathway receptors, such as PD-1, CTLA-4, and LAG-3, preventing T cell inhibition and hence allowing tumor attack by activated T cells.
ICI have become one of the most successful immunotherapies and dominate among FDA-approved immunotherapies and immunotherapy clinical trials, with Ipilimumab the first commercially approved ICI, targeting CTLA-4. Nivolumab, targeting PD-1, followed. Both treatments demonstrated improved survival over chemotherapeutic agents as treatments for metastatic melanoma patients, especially PD-1 that has the advantage of reduced toxicity compared to CTLA-4.64 ICI have had a revolutionary impact in oncology, although durable responses to ICI remain limited for certain cancers, and their associated toxicities remain a limitation to be addressed.65
Dosage and localization clearly contribute to the limitations associated with ICI, with ICI autoimmunity resulting from the systemic delivery of the treatment. For this reason, localized delivery of the drugs could result in more widely usable and safer treatments. However, the rapid and variable release of drugs in conventional delivery means that carriers, such as biomaterial scaffolds, are required for the controlled release and sustained delivery of therapeutics.58 Selected biomaterials for immunotherapeutic drug delivery are summarized in Table III and explained further below.
TABLE III.
Biomaterials for immunotherapeutic drug delivery.
| Materials | Therapeutic agents | Injectable? | Immunotherapy model and outcomes | References |
|---|---|---|---|---|
| Alginate | Celecoxib, anti-PD-1 | Yes | Mouse melanoma model | 66 |
| Reduced tumor growth and increased mouse survival, enhanced effect with dual-drug administration with hydrogel | ||||
| ROS-degradable poly(vinyl alcohol) hydrogel | Gemcitabine, anti-PD-L1 | Yes | Mouse melanoma model | 67 |
| Improved mouse survival, ROS-scavenging effects | ||||
| Multidomain peptide hydrogel | Anti-PD-1, CDN | Yes | Mouse oral carcinoma model | 69, 70 |
| Fewer tumor lesions in hydrogel-treated mice, improved mouse survival | ||||
| Fibrin gel | Doxorubicin (DOX)-loaded platelet-derived extracellular vesicles, anti-PD-L1 | No (sprayable) | Mouse melanoma model | 71 |
| Increased mouse survival, decreased tumor growth in local and distal tumors | ||||
| Implantable polycarbonate optical fiber | Anti-CTLA-4, anti-PD-1 | No | Mouse melanoma and breast cancer models | 72 |
| Reduced tumor growth and increased mouse survival, treatment guided by tumor impedance | ||||
| Polyvinyl alcohol + chitosan microneedles | 1-methyl-D,L-tryptophan, anti-PD-L1, indocyanine green | No | Mouse melanoma model | 73, 74 |
| Greater anti-PD-L1 retention at the administration site, decreased tumor growth, and greater survival. Improved effect with indocyanine green photosensitizer |
For example, an alginate hydrogel incorporating anti-PD-1 and the anti-inflammatory drug celecoxib reduced tumor growth by 90% compared to a blank hydrogel in a mouse B16-F10 melanoma model.66 The sustained co-delivery of celecoxib and PD-1 enhanced their effects in a synergistic manner, where PD-1 augmented the inhibition of tumor angiogenesis provoked by celecoxib, whereas the anti-inflammatory effect of celecoxib downregulates inflammatory genes which may otherwise interfere with the therapeutic effect of PD-1. Another interesting example consisted of a poly(vinyl alcohol) hydrogel, crosslinked with a compound that can be oxidized and hydrolyzed in the presence of ROS such as H2O2 in the tumor microenvironment.67 This led to gel degradation at the tumor site where ROS are highly prevalent, and the subsequent controlled release of the chemotherapy drug gemcitabine and anti-PD-L1 antibodies. The local release of this drug combination resulted in over 60-day mouse survival in a B16-F10 melanoma model, where no mice in any untreated or single-treatment control groups survived after two months. In addition to controlling the drug release, the hydrogel acts as a ROS scavenger that may limit the ROS-induced differentiation of macrophages to the tumorigenic “M” phenotype.
An example of biomaterials gaining popularity for immunotherapeutic drug delivery is multidomain peptide (MDP) hydrogels. One example developed by Hartgerink's group consists of an amphiphilic core of amino acids, which self-assemble into nanofibers, and gels upon interaction with a negatively charged multivalent.68 This hydrogel was used to locally deliver anti-PD-1 in a sustained and controlled manner, which was significantly more effective and durable than systemic anti-PD-1 in preventing oral carcinomas in mice, with 20% of hydrogel-treated mice showing high-grade lesions 5 weeks after treatment compared to 60% of control mice.69
The gel was further developed with the addition of CDN as a STING agonist as well as synthesizing another MDP that included an inhibitor of the pro-tumorigenic enzyme inducible nitric oxide synthase.70 The cationic gel further slowed and controlled CDN release for longer drug persistence compared to the previous MDP and resulted in the greatest tumor size reduction and survival among treatment groups.
Alternative biomaterial formats for immunotherapeutic drug delivery have also been developed. For example, Zhao et al. designed a sprayable fibrin gel containing doxorubicin (DOX)-loaded platelet-derived extracellular vesicles (which preferentially target circulating tumor cells) and anti-PD-L1,71 which permitted sustained and controlled release of the two therapies at the tumor site in a murine B16-F10 model. Their synergistic effect greatly decreased tumor growth and increased survival compared to single treatments. Furthermore, tumor growth was also limited in a distal tumor, showing the abscopal effect of the treatment.
In another study, Chin et al. developed an implantable optical fiber device, which could simultaneously deliver ICI locally and measure tumor growth in real time by measuring tumor impedance.72 The device included photodynamic therapy as a combination therapy, where an administered photosensitizer will create ROS that can both damage tumor cells and vasculature in response to light and improve intratumoral drug retention. Its combination with repeated ICI delivery directed by impedance measurements resulted in a durable anti-tumor response in a range of mouse melanoma and breast cancer models, and though the device is relatively invasive its real-time tumor growth monitoring to direct treatment is advantageous and it could be seen as analogous to routine medical devices such as insulin-delivery pumps.75
Another relatively novel biomaterial drug delivery method is the use of microneedles, micrometer sized needles that are associated with faster action, increased efficacy, and better patient compliance compared to transdermal injection.76 Microneedle patches, composed of a polyvinyl alcohol core containing the melanoma drug 1-methyl-D,L-tryptophan, surrounded by a chitosan needle shell containing anti-PD-L1 showed improved anti-PD-L1 retention at the administration site as well as decreased tumor growth and greater survival compared to untreated mice and mice with intra-tumor drug delivery using conventional needles.73 Later, indocyanine green, a photosensitizer used in photothermal therapy was added to further stimulate the anti-tumor immune response.74 These microneedles had an even greater anti-tumor effect and a further survival increase compared to the previous microneedles.
These results demonstrate the still unexplored potential of novel drug delivery systems in immunotherapy, which will only become more necessary as these treatments improve and are approved for other malignancies.
5. Nano-scale biomaterials
While their mechanisms differ from localized bulk biomaterials, nanoparticles have seen interest in both cancer vaccine and tumor drug delivery, due to their ability to act systemically but in a highly targeted manner.25 With targets and functions similar to certain bulk biomaterials used for cancer immunotherapy, nanoparticles represent an alternative material form and perhaps an intermediate treatment classified somewhere between systemic therapies and localized biomaterials for cancer immunotherapy. Below are a few examples of the use of nanoparticles in immunotherapy, either as drug or OV delivery systems or means to activate the immune response. Selected nanoparticles used for immunotherapy are summarized in Table IV and explained further below.
TABLE IV.
Nanoparticles for immunotherapy.
| Nanoparticle | Immunotherapeutic agent(s) | Immunotherapy model and outcomes | Reference |
|---|---|---|---|
| Lipid nanoparticle | IL-15 superagonist, IL-21, Pmel-1 T cells | Mouse melanoma model | 77 |
| Complete tumor elimination with cytotoxic T cells + nanoparticles | |||
| PEG–PLGA | PD-1, antitumor necrosis factor receptor superfamily member 4 | Mouse melanoma model | 78 |
| Threefold increased survival with dual-drug nanoparticle delivery | |||
| Chitosan | Anti-PD-L1 | Mouse melanoma model | 79 |
| Increased anti-PD-L1 retention, mouse survival and fewer metastases | |||
| Iron-dextran + quantum dots | CD28, major histocompatibility complex-peptide complexes, cognate T cells | Mouse melanoma model | 80 |
| Significant tumor growth reduction | |||
| PEGylated oligopeptide-modified poly(β-aminoester) | Oncolytic adenovirus | Murine xenograft of human pancreatic cancer | 81 |
| Three-fold increased circulation time, reduced immune neutralization of OV and significantly reduced tumor growth | |||
| Methoxy PEG-b-poly{N-[N-(2-aminoethyl)-2-aminoethyl]-l-glutamate} + PEG | Oncolytic adenovirus | Murine xenograft of human fibrosarcoma and lung cancer | 83 |
| Increased accumulation in the tumor, reduced OV accumulation in off-target tissues, tumor growth reduced two- to three-fold | |||
| Polymeric micelle + PEG | Oncolytic adenovirus, paclitaxel | Murine xenograft of human breast cancer | 84 |
| Higher viral replication in tumor, 12-fold increased blood retention and 2.5-fold anti-tumor efficacy. Synergistic effect of paclitaxel + OV | |||
| Chitosan–PEG folate | Oncolytic adenovirus | Murine xenograft of human epithelial cancer | 85 |
| Increased blood circulation time, two-fold reduced tumor growth | |||
| Lipid nanoparticle | mRNA cancer vaccine | Mouse melanoma and colon cancer models | 86 |
| Completely inhibited tumor growth | |||
| Clinical trial for malignant melanoma: De novo T cell responses against the cancer |
Liposomic nanoparticles conjugated to stimulatory factors such as IL15/IL21 allowed complete tumor elimination, compared to limited survival increase with ACT and systemic stimulatory factors without nanoparticles.77 PEG–PLGA nanoparticle-conjugated PD-1, combined with antitumor necrosis factor receptor superfamily member 4, ensured simultaneous binding to the two molecules and showed a tumor-free survival rate of 30% after biomaterial treatment, compared to 10% after treatment with a mix of NP-conjugated and free drugs in a murine B16F10 melanoma tumor model.78 Nanoparticles also offer interesting possibilities for the treatment of lung cancer: inhaled cationic chitosan nanoparticles conjugated to anti-PD-L1 were able to adhere to the lung mucus layer to prolong anti-PD-L1 retention as well as act as an immune adjuvant due to the inherent immunostimulatory qualities of chitosan.79 Increased mouse survival and fewer metastases were seen in a mouse model of B16-F10 lung metastases as compared to free drugs or nanoparticles alone.
Nanoparticles have been utilized as synthetic APCs, such as a study where iron-dextran and quantum dot nanoparticles were conjugated to CD28 and major histocompatibility complex (MHC)–peptide complexes to form synthetic APC, leading to a significant reduction in tumor growth in mice.80
Some groups have conjugated nanoparticles with OV, to prolong their circulation time and improve their antitumor efficacy. Brugada-Vilà et al. developed an OV conjugated with PEGylated oligopeptide-modified poly(β-aminoester)s that showed a three-fold increased circulation time, reduced immune neutralization of OV, and significantly reduced tumor growth in murine xenograft models compared to unconjugated OV using the human pancreatic cell lines PANC-1 or MIA PaCa-2.81 Methoxy poly(ethylene glycol)-b-poly{N-[N-(2-aminoethyl)-2-aminoethyl]-l-glutamate} and PEG grafted to the OV increased accumulation in the tumor and reduced OV accumulation in off-target tissues.82 Tumor growth was also reduced two- to three-fold compared to unconjugated OV in murine xenograft models using HT1080 human fibrosarcoma and A549 lung cancer cell lines.83 OV + paclitaxel conjugated with PEG and a polymeric micelle demonstrated higher viral replication in tumor, 12-fold increased blood retention and 2.5-fold increased anti-tumor efficacy compared to unconjugated OV in murine xenograft models using the human breast cancer cell line MCF-7 compared to free OV + PTX.84 Chitosan PEG folate nanoparticles greatly increased blood circulation time and showed a two-fold reduced tumor growth compared to unconjugated OV in murine xenograft models using the KB human epithelial carcinoma cell line.85
Nanoparticles have also been used in cancer vaccines, interestingly including mRNA delivery as the vaccine technology. Lipid nanoparticles for mRNA vaccine delivery showed potent anti-tumor effects in mice and some success in clinical trials to treat melanoma.86 In this case, mRNA coding for cancer antigens was encapsulated in negatively charged lipid nanoparticles, which protect the mRNA from elimination by the immune system and efficiently transport the mRNA to APC in the spleen. The mRNA expression appears to mimic infection with RNA viruses where free DNA and RNA are sensed by APC via receptors such as TLR7, which in turn stimulates the production of IFN-α that activates DC, NK, B, and T cells. Furthermore, the RNA is internalized and translated by DC resident in the spleen, producing antigens that are then presented by the DC to activate and stimulate the proliferation of antigen-specific T cells. This technology was also one of the precursors to the revolutionary and now ubiquitous mRNA vaccines developed by Pfizer and Moderna against COVID-19.87
C. Scaffolds for immune cell delivery
1. Principles of ACT
Another therapeutic strategy for which biomaterials could bring strong benefit is ACT. Conventional ACT consists of the intravenous administration of immune cells, mostly of T lymphocytes, which are expanded ex vivo and implanted into the patient to harness their specific anti-tumor effects. Presently, there are two main types of ACT treatment, depending on the sources of immune cells: tumor infiltrating lymphocytes (TILs) and genetically modified T cells with chimeric antigen receptors (CARs) or with tumor-specific TCRs.
TIL used are obtained from the patient tumor, due to their well-established, inherent tumor-specific response, considerably more abundant than peripheral lymphocytes. Since its first adoption in 1988 by the team of Rosenberg et al., TIL-ACT has shown great promise in the treatment of metastatic melanoma. In this treatment, TIL must be isolated from the tumor and expanded in vitro to reach 1–20 × 109 cells, before being intravenously reinjected in the patient. IL-2 is an essential growth factor for T cell proliferation, though other growth factors, such as 4–1BB and TGF-β1, have been proposed as additional factors to improve cell growth and the anti-tumor response.88,89 Current ACT with TIL also uses a rapid expansion protocol, where minimally cultured and non-selected “young” TIL have been shown to be more effective than TIL cultured over a longer time period and selected in vitro based on antigenic stimulation,11 though a limited number of treatment centers are able to both rapidly culture young TIL and select them for tumor specificity through the identification of unique tumor mutations via whole exome sequencing.90 Lymphodepletion via a combination of chemotherapy and total body radiation, prior to T cell injection, is also considered to have a beneficial effect on the anti-tumor activity. High dose IL-2 is also administered in order to maintain T cell activation in vivo, which is a source of the toxicity and negative side effects of current ACT.91 The main steps of ACT are summarized in Fig. 8.
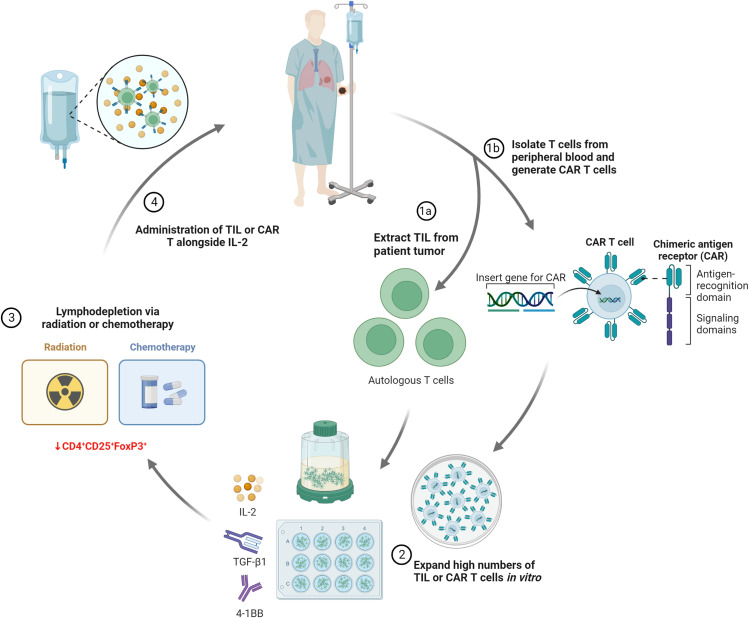
FIG. 8.
Typical phases of in clinical ACT: TIL or CAR are obtained and expanded in vitro, before lymphodepletion and re-administration alongside growth factors, such as high- or low-dose IL-2. CD4+ CD25+ FoxPE+ cells are immunosuppressive regulatory T cells that downregulated the activity of cytotoxic T lymphocytes in ACT.
Another large area of interest is in so-called CAR-T cells, where T cells are genetically modified to express CARs in addition to the native T cell receptors. Their advantage lies in the fact that their recognition is not MHC-dependent, and they can recognize a wider variety of antigens than T cell receptors (which are limited to short peptides). Several pitfalls, however, keep these promising treatments from becoming mainstream.
One significant limitation of ACT is the large numbers of T cells that must be obtained, as many are lost to non-cancerous sites of inflammation during systemic administration and only a small fraction is reaching the tumor. This expansion can be lengthy, expensive, and difficult to achieve for some patients, causing many to lose treatment eligibility because of their deteriorating condition. Cells can also struggle to persist and survive in the immunosuppressive tumor microenvironment.92–94 Even CAR-T cells, despite their success, show similar problems to ACT with TIL, including toxicities, such as cytokine release syndrome and neurotoxicity, as well as limited efficacy in solid tumors.95
These limitations call for reduced dosage and local cell delivery, in order to reduce the number of cells needed and avoid IL-2 associated toxicity, resulting in safer treatments. Simple cell injection around tumors is not sufficient, due to rapid cell loss due to dispersion, inflammation, and anoikis.96 Biomaterial scaffolds could increase the number of cells at the target tumor site, while decreasing the overall doses and numbers required.58 Furthermore, the inclusion of drugs or molecules to improve T cell persistence and anti-tumor activity, as well as the improved physical retention of T cells at the tumor site when delivered via a biomaterial, could improve cell persistence and functionality as well.
2. Design criteria for immune cell scaffolds
Cell-delivery scaffolds refer to biomaterials used as structures with defined architecture and composition, used for the delivery, retention, and support of cells, possibly combined with therapeutic molecules. Only recently have cell-delivery scaffolds been proposed to enhance the efficacy and overcome the limitations of ACT treatments. In terms of the requirements for an immune cell delivery scaffold, the scaffold should satisfy several criteria:18,97
-
1.
Ease of administration—the scaffold must ideally be capable of minimally invasive implantation, via either a catheter or injection of the scaffold through a needle, to avoid the complications of surgical insertion and reach any location within the body.
-
2.
Easy and simple homogenous mixing with cells, with a matrix capable of cell protection from applied shear stress during injection through standard needles or catheters.
-
3.
Rapid stability in vivo—after minimally invasive implantation the scaffold must form a mechanically stable structure, stationary in the location it has been delivered in the body to ensure cell retention close to the target site.
-
4.
Cell compatibility—in addition to the general biocompatibility of the scaffold, it must support cell encapsulation. Once in situ the scaffold must support the survival, growth, anticancer function, and escape of immune cells over a timeframe sufficient for cancer treatment.
-
5.
Porosity—the scaffold must have a porosity that allows access to nutrients and waste removal for encapsulated cells and allows immune cells to escape and perform their anti-cancer functionality, eliminating cancerous cells while other immune cells, such as DC and B cells, could colonize scaffolds to form protective tertiary lymphoid tissue.
-
6.
Biodegradability—the scaffold should biodegrade into nontoxic, metabolizable products. In the case of cancer immunotherapy, degradability such that when the tumor is eliminated the biomaterial will also degrade and disappear would be desirable.
-
7.
Formulation—the scaffold should ideally be chemically simple to formulate and avoid toxic chemical processes and to ease fabrication and approval by regulatory bodies, such as the FDA in the USA or the European Medicines Agency in Europe.
-
8.
Sterilizability—the biomaterial should be capable of sterilization using standard methods, such as autoclaving, ethylene oxide, or gamma radiation, prior to cell mixing and injection.
Table V gives a summary of recent or current studies of cell delivery scaffolds for cancer immunotherapy, which run from simple in vitro studies to preclinical murine models and use lymphocytes, DC, and even in one case NK cells. These are detailed further in Sections C3 and 4 a selection are shown in Fig. 9.
TABLE V.
Cell-delivery biomaterial scaffolds for immunotherapies.
| Materials | Cells | Therapeutic agents | Injectable? | Immunotherapy model and outcomes | Reference |
|---|---|---|---|---|---|
| Chitosan–PEG | Human PBMC | None | Yes | In vitro glioblastoma cells | 98 |
| Chitosan–PEG | Human CAR-T cells | Self-expression of IL-15 | Yes | Human retinoblastoma | 99 |
| Elimination of tumors and 100% survival with gel-delivered IL-15 CAR | |||||
| PCL–PEG–PPG copolymer | Human CD4+ T cells | None | Yes | Human CD4+ T cell survival over 5 days | 100 |
| PEG–heparin | Human CD4+ T cells | CCL21 | Yes | Increased human CD4+ T cell proliferation | 101 |
| PEG | Human CD3+ T cells | None | Yes | Proliferation upon restimulation of escaped T cells | 102 |
| Chitosan | Human PBMC and TIL | None | Yes | In vitro renal cancer, breast cancer and melanoma | 103 |
| Polyisocyanopeptide (PIC) + GRGDS peptide | Human T cells, DC and NK, mouse T cells | None | Yes | Migration in mice in vivo without tumors | 104 |
| Hyaluronic acid | Human CAR-T cells | None | Yes | In vitro glioma cell line | 105 |
| Alginate + GFOGER collagen-like peptide | Human CAR-T cells | IL-15 agonist, CD137, CD28, CD3 | No | Mouse breast cancer resection model | 106 |
| Regression in 60% of treated mice vs 0% survival in untreated mice | |||||
| Alginate + GFOGER collagen-like peptide | Human CAR-T cells | IL-15 agonist, CD137, CD28, CD3, STING agonist | No | Mouse pancreatic cancer and melanoma models | 107 |
| Complete pancreatic tumor elimination in 40% of treated mice, with persistent immunity on tumor rechallenge. | |||||
| Fibrin-coated Nitinol | Human CAR-T cells | IL-15 agonist, CD137, CD28, CD3 | No | Mouse ovarian cancer model | 108 |
| 2.7-fold survival increase with CAR-T-loaded Nitinol film vs untreated | |||||
| Fibrin | Murine DC | None | Yes | Mouse lung cancer model | 109 |
| Encapsulated DC treatment more effective than non-encapsulated | |||||
| RADA16 peptide | Murine DC | Tumor antigens, anti-PD-1 | No | Mouse lymphoma model | 110 |
| Survival improvement with gel-encapsulated DC vs untreated mice | |||||
| α-CD/PEG | Murine DC | DOX, CpG, B16 tumor cells | Yes | Mouse melanoma model | 111,112 |
| Significant survival improvement with gel-encapsulated DC with CpG + B16 vs single treatments | |||||
| Gelatin-hydroxyphenyl propionic acid | Murine DC | OV | Yes | Mouse lung cancer model | 113 |
| Increased survival compared to single treatments of DC/OV | |||||
| Hyaluronic acid | Human CAR NK cells | None | No | Mouse leukemia and breast cancer models | 114 |
| Significant survival improvement with gel-encapsulated CAR NK vs non-encapsulated NK | |||||
| Alginate microspheres | Human CAR-T cells | IL-15, hemoglobin | Yes | Mouse renal and ovarian cancer models | 115 |
| Greatly improved mouse survival with alginate-encapsulated CAR-T cells compared to untreated mice | |||||
| Fibrin | Human CAR-T cells | None | No | Mouse lymphoma and glioma models | 116 |
| Significantly improved mouse survival with fibrin-encapsulated CAR-T cells compared to mice treated with non-encapsulated CAR-T cells | |||||
| Methacrylated hyaluronic acid | Human CAR-T cells | IL-15 nanoparticles, anti-PD-L1 platelets | No | Mouse model of human melanoma | 117 |
| Extensive tumor elimination with CAR + IL-15 + anti-PD-L1 | |||||
| Alginate | Monocytes | Tumor antigens, anti-PD-1 | Yes | Mouse breast cancer modelSignificant preventative and therapeutic anti-tumor effects | 118 |
FIG. 9.
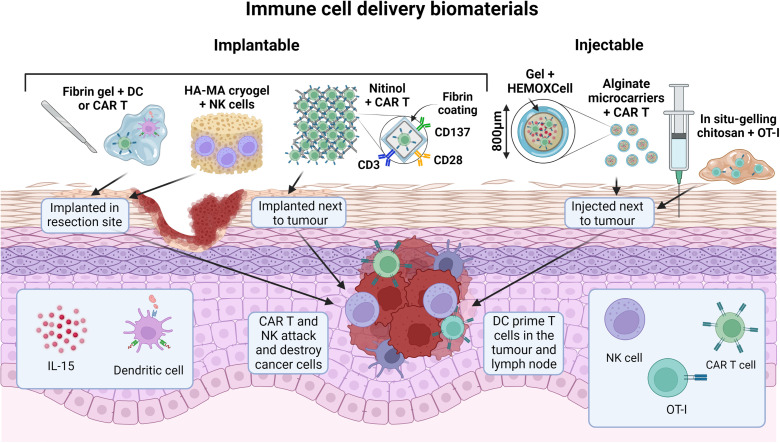
Selected biomaterials for immune cell delivery. Implantable or injectable gels, meshes, and microcarriers are loaded with dendritic cells, NK cells, or CAR T cells to improve cell delivery and persistence in and around the tumor. Cells progressively escape from the scaffolds toward the tumor and immune system, while the biomaterials and additions, such as stimulatory antibodies or cytokines, augment treatment efficacy.
3. T lymphocyte-loaded scaffolds for ACT
Cell-delivery scaffolds offer improvements to systemic cell delivery using inherent biomaterial advantages, such as localization and the incorporation of immunostimulatory factors. T cell-delivery scaffolds have been the most studied approach, with a range of both implantable and injectable materials been investigated with varying progress from in vitro work to in vivo models.
a. Implantable scaffolds
Stephan’s group, which is prominent in this area, developed oxidized (and therefore biodegradable) alginate gels grafted with a collagen-mimetic peptide to locally deliver CAR-T cells.106 In vitro studies demonstrated the benefit of this adhesive peptide, which increased lymphocyte migration within the gel and lymphocyte escape into a surrounding collagen gel, as well as increasing cell viability compared to unmodified alginate. Lipid-coated mesoporous silica microspheres (MSM) incorporating an IL-15 agonist as well as the immunostimulatory antibodies CD3, CD28, and CD137, similar to those present in the Dynabeads Human T-Activator commonly used for in vitro T cell expansion, were also included, and the scaffold was lyophilized to create an implantable porous matrix that was seeded with T cells immediately before implantation. The scaffold led to very promising in vitro and in vivo results in mouse models. In breast cancer resection model, it reduced tumor relapse compared to conventional intravenous or peritoneal treatments and supported tumor-targeting T cells throughout resection beds and associated lymph nodes, while it triggered much stronger regression in a multifocal ovarian cancer model resulting in greater mouse survival than locally delivered cells without the scaffold.
The same group further developed this model in 2017, adding cyclic di-GMP as a STING agonist, again loaded into the MSM, to activate DC and further enhance the immune response.107 The combination showed increased efficacy in treating solid pancreatic cancer and melanoma tumors in mice, with the STING agonist addition resulting in complete tumor remission in some cases. The authors conclude that the codelivery of STING agonists can stimulate the immune responses to eliminate tumor cells that are not recognized by the adoptively transferred lymphocytes and thus improve the CAR-T cell therapy and help protect against the emergence of escape variants. However, the scaffold is not injectable and its numerous complicated fabrication steps were a strong limitation toward clinical transfer. More importantly it lacked a well controlled porosity to favor T cell survival and proliferation.
More recently, Stephan's group adapted similar modifications to a fibrin-coated nickel–titanium alloy (nitinol) porous mesh with well controlled porosity, that can be placed on tumor lesions and release and functionally support tumor-targeted T cells.108 These micropatterned thin films (approximately 10 μm), formed via magnetron sputtering, were designed to improve oxygen and nutrient transfer to the T cells which was a limiting factor in their previous alginate gels. Nitinol is inherently bioinert due to the thin layer of titanium oxide formed on its surface, though in this work the nitinol was functionalized through fibrin coating which allowed lymphocyte adhesion and migration as well as coupling to the CD3, CD28, and CD137 antibodies. The system elicited robust proliferation of the seeded T cells in vitro and in vivo, leading to a huge increase in T cell on the site compared to intravenous and locally injected cells. The authors also showed impressively enhanced tumor elimination compared to locally or intravenously injected CAR-T cells.
The necessity to implant this scaffold is still a potential limitation compared to injectable cell delivery scaffolds for immunotherapy. However, the mesh can be incorporated into a variety of implant configurations, such as an endovascular stent for minimally invasive administration. Applicability to human patients may depend on tumor location and feasibility of surgical implantation. Moreover, the practical constraints for producing and manipulating sterile nitinol films, and their subsequent seeding with T cells followed by implantation or catheter administration may be limiting for practical clinical use.
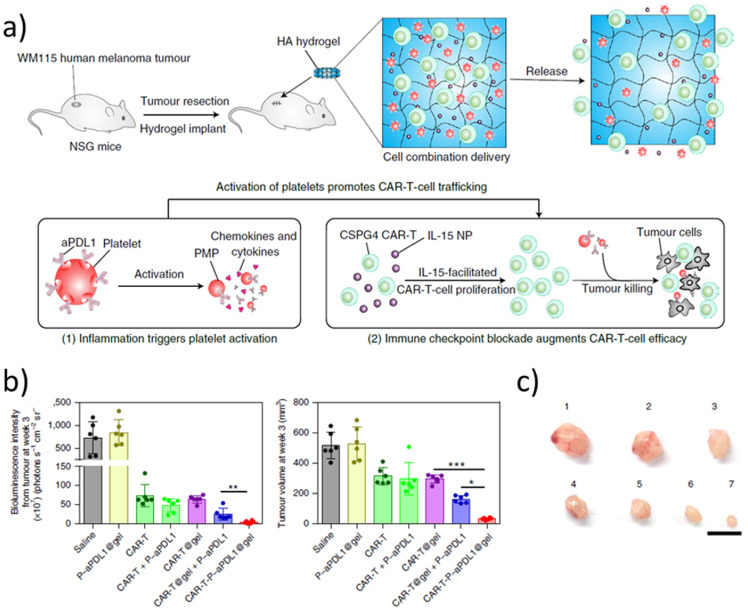
Another interesting study is a methacrylated hyaluronic acid hydrogel developed by Gu’s group for CAR-T cell encapsulation, using freeze-drying to create implantable porous hydrogels with a similar method to the previously described cryogel vaccines.117 The gel also incorporated IL-15 loaded PLGA nanoparticles, and platelets conjugated to anti-PD-L1. While the effect of the CAR-T-loaded hydrogel was quite limited, almost complete tumor elimination was achieved with the group with co-encapsulated CAR, IL-15, and PD-L1 in a mouse model using WM115 human melanoma cells [Fig. 10(b)]. They also demonstrated by bioluminescence the persistence and growth of T cells in vivo, and the scaffold triggered an abscopal effect inhibiting distal tumor growth.
FIG. 10.
Cryogel for CAR-T cell delivery. (a) Schema of the tumor model and cryogel. (b) Tumor bioluminescence (left) and volume (right) 3 weeks after treatment (mean ± SD, n = 6 mice per group). Statistical analysis was performed using one-way ANOVA followed by Tukey's HSD post-hoc test. (c) Representative photos of tumors at three weeks. Groups: (1) saline; 2, P-aPDL1@gel; (3) CAR-T; (4) CAR-T + P-aPDL1; (5) CAR-T@gel; (6) CAR-T@gel + P-aPDL1; and (7) CAR-T-P-aPDL1. Scale bar, 1 cm. PMP: platelet-derived microparticles, P-aPDL1: PDL1 antibody covalently conjugated to the cell surface of human platelets. Reproduced with permission from Hu et al., Nat. Biomed. Eng. 5, 1038–1047 (2021). Copyright 2021 Springer Nature.
b. Injectable scaffolds
Several efforts have also been made to create injectable T lymphocyte delivery scaffolds. A thermosensitive chitosan–PEG hydrogel, prepared by alkylation of chitosan followed by Schiff base formation, showing loading, survival, escape and anti-cancer activity of T lymphocytes in vitro against a glioblastoma cell line.98 However, the scaffold had a gelation time of 8–12 min at 37 °C, which is quite long to avoid cell dispersion at the time of in vivo injection. The same gel was later applied to CAR-T cells targeted against GD2, an antigen overexpressed in retinoblastoma.99 The gel prolonged CAR persistence within the tumor and gel-encapsulated CAR significantly reduced tumor growth. Impressively, further modification of the CAR to induce self-expression of IL-15 resulted in tumor elimination and a 100% survival rate. Our group also developed a thermosensitive porous chitosan gel for T cell delivery, demonstrating significantly faster gelation at 37 °C than the chitosan–PEG gel and human PBMC viability and growth over 2 weeks, as well as specific anti-cancer functionality in transwell models.103 This physical gel requires no chemical modification or cross-linking of chitosan, a natural biomaterial already used in FDA-approved medical devices. It is completely biodegradable and can be prepared by simple mixing of two sterilized solutions following by the addition of the cells prior to injection through small diameter syringes (up to 23G). These are important advantages for the potential clinical transfer of such a cell delivery scaffold for immunotherapy. In vivo tests in MC38-OVA murine subcutaneous tumor models have shown some efficacy to reduce tumor growth in vivo, even when administering a reduced cell number compared to systemic treatment.119 However, the effect is limited in time and the absence of cell proliferation inside the scaffold in vivo calls for further gel optimization.
Perez del Rio et al. developed a PEG-heparin hydrogel containing lymphocyts,101 where heparin allowed the conjugation of the cytokine CCL21 which is present in the lymph nodes and is known to enhance T cell proliferation and migration.120 The hydrogels increased CD4+ T cell proliferation, though were demonstrated primarily as T cell culture scaffolds rather than a delivery platform. A PCL–PEG–poly(propylene glycol) copolymer has also been demonstrated for T cell encapsulation, though only showed cell survival over 5 days and no proliferation was demonstrated in this case.100 Yan et al. demonstrated a PEG hydrogel formed via Diels–Alder cycloaddition of fulvene and maleimide functionalized PEG precursors, which has tunable stiffness and degradation.102 However, the gel showed relatively slow gelation (>15 min) and limited cell viability after several days even with RGD functionalization, though cells escaped from the gel showed proliferation after recovery and re-stimulation.
In the Netherlands, Figdor's group investigated RGD grafted polyisocyanopeptide (PIC) gels to culture human T cells and observe the in vivo escape of murine T cells.104 PIC, a relatively new class of hydrogel, are synthetic hydrogels formed through nickel(II)-catalyzed polymerization of the two monomers—triethylene glycol functionalized isocyano-(D)-alanyl-(L)-alanine (monomer 1) and monomer 2, an azide-appended version of monomer 1. This water-soluble polymer exhibits thermosensitive gelation, remaining liquid below 16 °C and forming soft hydrogels in a matter of minutes above this temperature.121 Interestingly, its mechanical properties can be tuned to reach similar mechanical properties and stress-stiffening behavior to biological polymers such as collagen.122 The authors showed that GRGDS peptide grafting allowed greater T cell migration within the gel compared to unmodified gel and in vitro viability and proliferation up to 72 h.104 Scaffold–encapsulated T cells migrated out of the gels over 3–4 weeks in mice, though with many T cells escaping the gel in the first week after injection. Furthermore, the study was performed simply to demonstrate T cell escape from the gel in mice without tumors and so has not demonstrated efficacy in a mouse tumor model. Atik et al. developed a proprietary, low-viscosity hyaluronic acid and gelatin-based gel as a substrate to deliver CAR-T for glioblastoma using convection enhanced delivery, in which an intracranial catheter is placed into the tumor that infuses the agent with positive-pressure over time.105 The encapsulated CAR-T cells migrate from the gel and carry out their cytotoxic function in vitro. The gel itself showed no toxicity when injected in mice, though again this was performed in mice without tumors and so has not demonstrated anti-tumor efficacy.
Other groups are also beginning to investigate alternative materials and biomaterial formats for ACT delivery. Fibrin gel-mediated CAR-T cell delivery was, thus, tested for glioblastoma by inoculating fibrin solution with CAR-T cells in the cavity followed by immediate addition of thrombin solution. However such a method does not ensure complete encapsulation due to cell dispersion prior to gel formation and fibrin gel is known to degrade very rapidly.116
The main challenge with injectable scaffolds is to create macroporous structures which allow good access to oxygen and nutrients and the possibility for the cells to escape the scaffold. Several studies including Stephan's work with nitinol films have shown that the small physical size of microscaffolds is beneficial for nutrient supply and cell migration.108,123 Another alternative is to encapsulate T cells in small microspheres that allow better diffusion of oxygen and nutrient, and potential vascularization in between the microspheres.124 Thus, Luo et al. created an injectable hydrogel-encapsulated porous immune-microchip system (i-G/MC) with the capabilities of enhancing CAR-T cell survival and proliferation.115 Interestingly, they incorporated the HEMOXCell molecule in their alginate microspheres, a marine hemoglobin with a high oxygen storage capacity, to counteract the hypoxic effect of the tumor microenvironment. However, the long and complex preparation steps required (including multiple lyophilisations and several days immersion in PBS) may prevent the use of such systems in clinical trials.
Altogether, despite impressive developments and a variety of approaches, and very promising results in mice models, there is presently no ideal scaffold for T cell delivery which would combine well controlled porosity and injectability and clinically feasible manufacturing processes. Moreover, efficacy against distal or metastatic tumors and long-term tumor elimination will ideally need to be demonstrated for eventual progression to clinical trials.
In several cases, the authors demonstrate further increase in mice survival when adding debris from eradicated tumor cells to convert the implant to not only act as a cell delivery but also a “self” vaccine site. Thus, to mount a robust anticancer response, an ideal scaffold should probably not only allow sustained growth and delivery of a sufficient number of stimulated T cells that destroy tumor cells but also contain significant amounts of tumor antigens which become available to endogenous APCs and eventually high concentrations of stimulants that activate these APCs.
4. Scaffolds for other immune cells
Biomaterials have also shown promise to assist DC-based immunotherapies, with DC probably the most commonly delivered immune cell via biomaterial scaffolds after T cells. Such DC loaded scaffolds can overcome the lack of recruitment of host DCs to create more efficient vaccine-like immune cell niches. DC can be stimulated prior to their addition in the gel or injected in combination with the tumor antigen, and eventually other drugs, as detailed below.
Tumor antigen-stimulated DCs in a fibrin gel showed significantly reduced tumor growth in mice with the cell scaffold construct compared to injected DC alone.109 DC were also delivered using an injectable self-assembled peptide hydrogel,110 based on RADA16, a synthetic peptide consisting of 16 alternating hydrophobic and hydrophilic amino acids which self-assembles into a nanofibrous, nanoporous hydrogel in the presence of neutral pH solution.125 DC and antigens were mixed with the hydrogel on ice before injection. The gel was biocompatible with DC, did not activate DC by itself (being non-immunogenic) and when containing DC and antigens it improved both therapeutic and prophylactic efficacy in reducing tumor growth in a mouse lymphoma model, compared to intravenous and subcutaneous injection, with even greater efficacy observed in conjunction with an anti-PD-1 checkpoint inhibitor. In addition to anti-PD-1, Yang et al. showed that the chemotherapeutic DOX in the form of nanoparticles, also potentially conjugated to the immune adjuvant CpG, could be incorporated in an injectable α-cyclodextrin/PEG hydrogel along with DC. The gel significantly reduced tumor growth in a B16 melanoma mouse model compared to single treatment or control groups when treated with the full complement of DC, DOX and CpG-loaded gel.111 Subsequent work from this group also showed the further beneficial effect of including dying B16 tumor cells into the DC scaffold vaccine.112 A similar trend was also observed with monocytes encapsulated in alginate droplets using a microfluidic system.118
The combination of IL-12 and GM–CSF-expressing OV with DC was tested using a gelatin-hydroxyphenyl propionic acid hydrogel, enzymatically cross-linked via horseradish peroxidase (HRP) and hydrogen peroxide (H2O2). Gel-encapsulated OV + DC significantly increased survival in a murine Lewis lung carcinoma model compared to single treatments of a DC/OV combination without gel, with impressive 100% survival in mice treated with Gel OV + DC. All this work indicates the range of materials and drugs beneficial for monocyte and DC delivery, as with T cell ACT.
Another interesting recent development is a hyaluronic acid-based scaffold that was used for the delivery of NK cells as a cancer immunotherapy. The scaffold was formed from a blend of methacrylate-modified HA and methacrylate-modified oxidized HA, where the methacrylate-modified oxidized HA acted as a highly degradable sacrificial component to create greater porosity and allow NK cell clustering which improves cell activation and viability.126 The scaffold upregulated NK cell proliferation and tumor killing activity in vitro and resulted in fewer metastases and increased mouse survival in vivo.114 This work may well be the first of many investigations into NK cell-carrier biomaterials as cancer immunotherapies, though the scaffold is non-injectable and must be implanted so it would be interesting to examine some of the above injectable scaffolds as NK cell carriers.
III. CONCLUSIONS AND PERSPECTIVES
In this review, we summarize how biomaterials can enhance cancer immunotherapies by addressing some of the challenges of these approaches, which have been at the forefront of pharmaceutical breakthroughs in recent years and will continue to be so. Through their localized action and controlled release of cells, cancer antigens, drugs, immunomodulatory molecules, or combinations thereof, biomaterials can overcome the problem of rapid cell and antigen dispersion, as well as toxicity related to systemic delivery, and allow the combination of several products, with or without exogenous cells to eliminate cancer more efficiently.
For cancer vaccines, biomaterials can address numerous limitations that have so far hindered their progress toward the clinic. Multiple studies indicate increased potency using biomaterial vaccines compared to free vaccine components intravenously or subcutaneously injected, one factor of which is likely the “immune niche” created in porous biomaterials. This environment encourages antigen uptake by DC, often in a space that is protected from the immunosuppressive tumor microenvironment to concurrently improve DC persistence. Furthermore, biomaterials allow the inclusion of immunostimulatory adjuvants or drugs, in addition to the antigens or tumor cell lysates, and the controlled release of these factors further increases their potency. Exogenous DC cells can also be added to ensure a greater number of activated DC cells, although this implies more complicated preparation steps and regulations. Such products will most probably be used in the future, at least to prevent cancer recurrence or fight metastasis. The main limitations at this stage are on one hand, the absence of ideal injectable porous scaffolds, and on the other, limited persistence and long-term efficacy, with a lack of clinical data.
Biomaterial scaffolds for drug delivery offer similar benefits in terms of controlled and localized release of therapeutics, which increases the potency of ICI and allows combination therapies, for instance with chemotherapies. Localized biomaterial delivery also allows reduced dosages and hence a reduction in the toxicity associated with both chemotherapies and immunotherapies. Furthermore, innovative carriers, such as the sprayable gel, theranostic optic fibers, and inhaled nanoparticles, expand the range of possible methods to administer treatments.
While scaffolds for T lymphocyte growth and delivery remain a significant and underexplored research area, they are potentially advantageous compared to systemic lymphocyte delivery to reduce the numbers of required cells and decrease cell loss. Furthermore, their flexibility in incorporating a range of stimulatory factors or drugs, such as ICI, to better prime the cells and help them avoid cancer immune escape shows great promise for current and future treatments. Certain commonalities between the highest performing scaffolds, such as the inclusion of IL-15 superagonist, indicate priority molecules to include for successful treatment. One can also imagine the development of artificial tertiary lymphoid structures (TLSs) combining several immune cell types, as commonly seen around tumors and associated with a favorable prognosis.127 Other scientific developments, such as 3D printing and bioprinting, also offer hope for further improvements in therapy efficacy and control within immunotherapy, by creating complex implantable 3D structures with spatially defined cell organization. For example, Jin et al. showed improved T cell proliferation and reduced cell exhaustion induced by coaxial 3D printed alginate fibers.128 This could be beneficial in the creation of spatially defined artificial TLS with distinct stromal, T and B cell zones, previously attempted and reviewed elsewhere.129,130
There are, however, still several challenges. One of them is that delivered cells must access the lymphatic or vascular system for efficacy against metastatic tumors. Some groups have shown the presence of delivered cells in the blood and draining lymph nodes, though the mechanism of how exogenous cells reached the vascular system or lymph nodes is rarely investigated and could be examined in future work. The immunosuppressive microenvironment that limits intravenous ACT treatments seems to have been counteracted in many of these examples as well as in some of the discussed cancer vaccines. However, a challenge of immunotherapy linked to tumor immunosuppression is its limited applicability outside certain cancers. It would be interesting to see these localized treatments applied to xenografts of immune-resistant cancers as opposed to the cell lines generally used so far to see if their potency is retained.
Another challenge is the difficulty to satisfy all the requirements of cell growth, delivery, and persistence as well as optimal cell–cell contact and simple, easy to transfer technologies which are injectable, sterilizable and perhaps even with off-the-shelf capabilities. Quick, simple manipulation by clinicians is also of high importance.131 The highest performing T cell scaffolds discussed here show impressive cell encapsulation or seeding, cell growth, and potent anti-tumor effects, but their design may be too complex for clinical translation. A compromise may be necessary between efficacy and feasibility. The requirements for regulatory approval must also be considered if these biomaterial-aided treatments are ever to progress to regular clinical use.132 The vast majority of the immunotherapeutic treatments described above would follow the FDA approval pathway of a biologic drug as the primary mode of action of any immunotherapy is inherently biologic in nature. Therapies using cells or tissues would also have the designation of human cell, tissue, and cellular and tissue-based products with combinations of drugs and materials further classified as combination products. Biologic drugs must demonstrate safety, purity, and potency, while the biomaterial component of combination products must satisfy further criteria associated with the medical device approval pathway.133 In particular, the biomaterial scaffold must pass ISO 10993 biocompatibility requirement, where material cytotoxicity, sensitization, carcinogenicity, and degradability, among other parameters, are to be assessed.134 The device's potential for sterilization, mass production, and batch-to-batch variability should all be considered. Further considerations include the need for aseptic processing, transmissible disease testing, and traceability if cellular products are used as well as Good Laboratory Practice, Good Manufacturing Practice, and Good Clinical Practice throughout research and development, manufacturing, and clinical testing.135 All this requires a large extent of in vitro and in vivo pre-clinical models and clinical trials all demonstrating safety and efficacy for the approval of these products, although candidate treatments can benefit from a range of expedited programs, such as Fast Track, Breakthrough Therapy, and Accelerated Approval, where clinical or non-clinical data indicate a safety or efficacy improvement compared to existing treatments for serious conditions.135
As demonstrated along this review, biomaterials will likely be part of the future approaches to support and continue the revolutionary effect that immunotherapy has had on cancer treatment. As cancer immunotherapy is still at a relatively young stage in its development and scientific understanding, further progress in understanding cancer immunity and each sector of the immune system will guide future developments, such as the inclusion of ICI, adjuvants, and immunostimulatory factors with or without immune cells in biomaterial-based delivery systems. Cell delivery scaffolds represent a growing but still under-investigated area and may hold the key to more effective and durable future cancer treatments.
ACKNOWLEDGMENTS
The authors acknowledge funding from The Natural Sciences and Engineering Research Council of Canada (NSERC, Grant No. RGPIN-2020-06684), the Institut du Cancer de Montréal (ICM), and Quebec Cell, Tissue and Gene Therapy Network—ThéCell (a thematic network supported by the Fonds de recherche du Québec–Santé). Original figures were created with BioRender.com.
AUTHOR DECLARATIONS
Conflict of Interest
The authors have no conflicts to disclose.
Ethics Approval
Ethics approval is not required.
Author Contributions
Nicholas Cunningham: Conceptualization (equal); Writing – original draft (equal). Réjean Lapointe: Conceptualization (supporting); Supervision (supporting); Writing – review & editing (supporting). Sophie Lerouge: Conceptualization (equal); Supervision (equal); Writing – original draft (equal); Writing – review & editing (equal).
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A. et al. , “ Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries,” Ca-Cancer J. Clin. 71(3), 209–249 (2021). 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Farkona S., Diamandis E. P., and Blasutig I. M., “ Cancer immunotherapy: The beginning of the end of cancer?,” BMC Med. 14, 73 (2016). 10.1186/s12916-016-0623-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martínez-Lostao L., Anel A., and Pardo J., “ How do cytotoxic lymphocytes kill cancer cells?,” Clin. Cancer Res. 21(22), 5047–5056 (2015). 10.1158/1078-0432.CCR-15-0685 [DOI] [PubMed] [Google Scholar]
- 4. Kennedy R. and Celis E., “ Multiple roles for CD4+ T cells in anti-tumor immune responses,” Immunol. Rev. 222(1), 129–144 (2008). 10.1111/j.1600-065X.2008.00616.x [DOI] [PubMed] [Google Scholar]
- 5. Anguille S., Smits E. L., Bryant C., Van Acker H. H., Goossens H., Lion E. et al. , “ Dendritic cells as pharmacological tools for cancer immunotherapy,” Pharmacol. Rev. 67(4), 731–753 (2015). 10.1124/pr.114.009456 [DOI] [PubMed] [Google Scholar]
- 6. Sharonov G. V., Serebrovskaya E. O., Yuzhakova D. V., Britanova O. V., and Chudakov D. M., “ B cells, plasma cells and antibody repertoires in the tumour microenvironment,” Nat. Rev. Immunol. 20(5), 294–307 (2020). 10.1038/s41577-019-0257-x [DOI] [PubMed] [Google Scholar]
- 7. Shimasaki N., Jain A., and Campana D., “ NK cells for cancer immunotherapy,” Nat. Rev. Drug Discovery 19(3), 200–218 (2020). 10.1038/s41573-019-0052-1 [DOI] [PubMed] [Google Scholar]
- 8. Chen Daniel S. and Mellman I., “ Oncology meets immunology: The cancer-immunity cycle,” Immunity 39(1), 1–10 (2013). 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 9. Shemesh C. S., Hsu J. C., Hosseini I., Shen B.-Q., Rotte A., Twomey P. et al. , “ Personalized cancer vaccines: Clinical landscape, challenges, and opportunities,” Mol. Ther. 29(2), 555–570 (2021). 10.1016/j.ymthe.2020.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi A. H., O'Leary M. P., Fong Y., and Chen N. G., “ From benchtop to bedside: A review of oncolytic virotherapy,” Biomedicines 4(3), 18 (2016). 10.3390/biomedicines4030018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Itzhaki O., Hovav E., Ziporen Y., Levy D., Kubi A., Zikich D. et al. , “ Establishment and large-scale expansion of minimally cultured ‘young’ tumor infiltrating lymphocytes for adoptive transfer therapy,” J. Immunother. 34(2), 212–220 (2011). 10.1097/CJI.0b013e318209c94c [DOI] [PubMed] [Google Scholar]
- 12. Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J. J., Cowey C. L., Lao C. D. et al. , “ Combined nivolumab and ipilimumab or monotherapy in untreated melanoma,” N. Engl. J. Med. 373(1), 23–34 (2015). 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tawbi H. A., Schadendorf D., Lipson E. J., Ascierto P. A., Matamala L., Castillo Gutiérrez E. et al. , “ Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma,” N. Engl. J. Med. 386(1), 24–34 (2022). 10.1056/NEJMoa2109970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mullinax J. E., Hall M., Prabhakaran S., Weber J., Khushalani N., Eroglu Z. et al. , “ Combination of ipilimumab and adoptive cell therapy with tumor-infiltrating lymphocytes for patients with metastatic melanoma,” Front. Oncol. 8, 44 (2018). 10.3389/fonc.2018.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kverneland A. H., Pedersen M., Westergaard M. C. W., Nielsen M., Borch T. H., Olsen L. R. et al. , “ Adoptive cell therapy in combination with checkpoint inhibitors in ovarian cancer,” Oncotarget 11(22), 2092–2105 (2020). 10.18632/oncotarget.27604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.See Cancer Research Institute, https://www.cancerresearch.org/fda-approval-timeline-of-active-immunotherapies “ FDA approval timeline of active immunotherapies” (2022).
- 17. Ghasemi-Mobarakeh L., Kolahreez D., Ramakrishna S., and Williams D., “ Key terminology in biomaterials and biocompatibility,” Curr. Opin. Biomed. Eng. 10, 45–50 (2019). 10.1016/j.cobme.2019.02.004 [DOI] [Google Scholar]
- 18. O'Brien F. J., “ Biomaterials & scaffolds for tissue engineering,” Mater. Today 14(3), 88–95 (2011). 10.1016/S1369-7021(11)70058-X [DOI] [Google Scholar]
- 19. Matin S. F., Pierorazio P. M., Kleinmann N., Gore J. L., Shabsigh A., Hu B. et al. , “ Durability of response to primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel: OLYMPUS trial final report,” J. Urol. 207(4), 779–788 (2022). 10.1097/JU.0000000000002350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raoul J.-L., Forner A., Bolondi L., Cheung T. T., Kloeckner R., and de Baere T., “ Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence,” Cancer Treat. Rev. 72, 28–36 (2019). 10.1016/j.ctrv.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 21. Koshy S. T. and Mooney D. J., “ Biomaterials for enhancing anti-cancer immunity,” Curr. Opin. Biotechnol. 40, 1–8 (2016). 10.1016/j.copbio.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cai S. S., Li T., Akinade T., Zhu Y., and Leong K. W., “ Drug delivery carriers with therapeutic functions,” Adv. Drug Delivery Rev. 176, 113884 (2021). 10.1016/j.addr.2021.113884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leach D. G., Young S., and Hartgerink J. D., “ Advances in immunotherapy delivery from implantable and injectable biomaterials,” Acta Biomater. 88, 15–31 (2019). 10.1016/j.actbio.2019.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang H. and Mooney D. J., “ Biomaterial-assisted targeted modulation of immune cells in cancer treatment,” Nat. Mater. 17(9), 761–772 (2018). 10.1038/s41563-018-0147-9 [DOI] [PubMed] [Google Scholar]
- 25. Riley R. S., June C. H., Langer R., and Mitchell M. J., “ Delivery technologies for cancer immunotherapy,” Nat. Rev. Drug Discovery 18(3), 175–196 (2019). 10.1038/s41573-018-0006-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Madan R. A., Antonarakis E. S., Drake C. G., Fong L., Yu E. Y., McNeel D. G. et al. , “ Putting the pieces together: Completing the mechanism of action jigsaw for sipuleucel-T,” JNCI, J. Natl. Cancer Inst. 112(6), 562–573 (2020). 10.1093/jnci/djaa021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Antonarakis E. S., Small E. J., Petrylak D. P., Quinn D. I., Kibel A. S., Chang N. N. et al. , “ Antigen-specific CD8 lytic phenotype induced by sipuleucel-T in hormone-sensitive or castration-resistant prostate cancer and association with overall survival,” Clin. Cancer Res. 24(19), 4662–4671 (2018). 10.1158/1078-0432.CCR-18-0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Antonarelli G., Corti C., Tarantino P., Ascione L., Cortes J., Romero P. et al. , “ Therapeutic cancer vaccines revamping: Technology advancements and pitfalls,” Ann. Oncol. 32(12), 1537–1551 (2021). 10.1016/j.annonc.2021.08.2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ali O. A., Huebsch N., Cao L., Dranoff G., and Mooney D. J., “ Infection-mimicking materials to program dendritic cells in situ,” Nat. Mater. 8(2), 151–158 (2009). 10.1038/nmat2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ali O. A., Lewin S. A., Dranoff G., and Mooney D. J., “ Vaccines combined with immune checkpoint antibodies promote cytotoxic T-cell activity and tumor eradication,” Cancer Immunol. Res. 4(2), 95 (2016). 10.1158/2326-6066.CIR-14-0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ali O. A., Verbeke C., Johnson C., Sands R. W., Lewin S. A., White D. et al. , “ Identification of immune factors regulating antitumor immunity using polymeric vaccines with multiple adjuvants,” Cancer Res. 74(6), 1670 (2014). 10.1158/0008-5472.CAN-13-0777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bencherif S. A., Sands R. W., Ali O. A., Li W. A., Lewin S. A., Braschler T. M. et al. , “ Injectable cryogel-based whole-cell cancer vaccines,” Nat. Commun. 6, 7556 (2015). 10.1038/ncomms8556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Najibi A. J., Shih T.-Y., and Mooney D. J., “ Cryogel vaccines effectively induce immune responses independent of proximity to the draining lymph nodes,” Biomaterials 281, 121329 (2022). 10.1016/j.biomaterials.2021.121329 [DOI] [PubMed] [Google Scholar]
- 34. Shah N. J., Najibi A. J., Shih T.-Y., Mao A. S., Sharda A., Scadden D. T. et al. , “ A biomaterial-based vaccine eliciting durable tumour-specific responses against acute myeloid leukaemia,” Nat. Biomed. Eng. 4(1), 40–51 (2020). 10.1038/s41551-019-0503-3 [DOI] [PubMed] [Google Scholar]
- 35. Lu Y., Wu C., Yang Y., Chen X., Ge F., Wang J. et al. , “ Inhibition of tumor recurrence and metastasis via a surgical tumor-derived personalized hydrogel vaccine,” Biomater. Sci. 10(5), 1352–1363 (2022). 10.1039/D1BM01596F [DOI] [PubMed] [Google Scholar]
- 36. Kim J., Li W. A., Choi Y., Lewin S. A., Verbeke C. S., Dranoff G. et al. , “ Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy,” Nat. Biotechnol. 33(1), 64–72 (2015). 10.1038/nbt.3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li A. W., Sobral M. C., Badrinath S., Choi Y., Graveline A., Stafford A. G. et al. , “ A facile approach to enhance antigen response for personalized cancer vaccination,” Nat. Mater. 17(6), 528–534 (2018). 10.1038/s41563-018-0028-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dellacherie M. O., Li A., Lu B. Y., Verbeke C. S., Gu L., Stafford A. G. et al. , “ Single-shot mesoporous silica rods scaffold for induction of humoral responses against small antigens,” Adv. Funct. Mater. 30(38), 2002448 (2020). 10.1002/adfm.202002448 [DOI] [Google Scholar]
- 39. Sun Z., Liang J., Dong X., Wang C., Kong D., and Lv F., “ Injectable hydrogels coencapsulating granulocyte-macrophage colony-stimulating factor and ovalbumin nanoparticles to enhance antigen uptake efficiency,” ACS Appl. Mater. Interfaces 10(24), 20315–20325 (2018). 10.1021/acsami.8b04312 [DOI] [PubMed] [Google Scholar]
- 40. Giang Phan V. H., Duong H. T. T., Thambi T., Nguyen T. L., Turabee M. H., Yin Y. et al. , “ Modularly engineered injectable hybrid hydrogels based on protein-polymer network as potent immunologic adjuvant in vivo,” Biomaterials 195, 100–110 (2019). 10.1016/j.biomaterials.2018.12.034 [DOI] [PubMed] [Google Scholar]
- 41. Liang X., Li L., Li X., He T., Gong S., Zhu S. et al. , “ A spontaneous multifunctional hydrogel vaccine amplifies the innate immune response to launch a powerful antitumor adaptive immune response,” Theranostics 11(14), 6936–6949 (2021). 10.7150/thno.58173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pei M., Liang J., Zhang C., Wang X., Zhang C., Ma G. et al. , “ Chitosan/calcium phosphates nanosheet as a vaccine carrier for effective cross-presentation of exogenous antigens,” Carbohydr. Polym. 224, 115172 (2019). 10.1016/j.carbpol.2019.115172 [DOI] [PubMed] [Google Scholar]
- 43.See https://ClinicalTrials.gov/show/NCT01753089 for “ Dendritic cell activating scaffold in melanoma.”
- 44. Carroll E. C., Jin L., Mori A., Muñoz-Wolf N., Oleszycka E., Moran H. B. T. et al. , “ The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons,” Immunity 44(3), 597–608 (2016). 10.1016/j.immuni.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Corrales L., Glickman L. H., McWhirter S. M., Kanne D. B., Sivick K. E., Katibah G. E. et al. , “ Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity,” Cell Rep. 11(7), 1018–1030 (2015). 10.1016/j.celrep.2015.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Palladini A., Thrane S., Janitzek C. M., Pihl J., Clemmensen S. B., de Jongh W. A. et al. , “ Virus-like particle display of HER2 induces potent anti-cancer responses,” Oncoimmunology 7(3), e1408749 (2018). 10.1080/2162402X.2017.1408749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Crews D. W., Dombroski J. A., and King M. R., “ Prophylactic cancer vaccines engineered to elicit specific adaptive immune response,” Front. Oncol. 11, 626463 (2021). 10.3389/fonc.2021.626463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zeng J., Li X., Sander M., Zhang H., Yan G., and Lin Y., “ Oncolytic viro-immunotherapy: An emerging option in the treatment of gliomas,” Front. Immunol. 12, 721830 (2021). 10.3389/fimmu.2021.721830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Raman S. S., Hecht J. R., and Chan E., “ Talimogene laherparepvec: Review of its mechanism of action and clinical efficacy and safety,” Immunotherapy 11(8), 705–723 (2019). 10.2217/imt-2019-0033 [DOI] [PubMed] [Google Scholar]
- 50. Badrinath N., Jeong Y. I., Woo H. Y., Bang S. Y., Kim C., Heo J. et al. , “ Local delivery of a cancer-favoring oncolytic vaccinia virus via poly (lactic-co-glycolic acid) nanofiber for theranostic purposes,” Int. J. Pharm. 552(1), 437–442 (2018). 10.1016/j.ijpharm.2018.10.020 [DOI] [PubMed] [Google Scholar]
- 51. Lee J-j, Kang J. A., Ryu Y., Han S.-S., Nam Y. R., Rho J. K. et al. , “ Genetically engineered and self-assembled oncolytic protein nanoparticles for targeted cancer therapy,” Biomaterials 120, 22–31 (2017). 10.1016/j.biomaterials.2016.12.014 [DOI] [PubMed] [Google Scholar]
- 52. Choi J. W., Kang E., Kwon O. J., Yun T. J., Park H. K., Kim P. H. et al. , “ Local sustained delivery of oncolytic adenovirus with injectable alginate gel for cancer virotherapy,” Gene Ther. 20(9), 880–892 (2013). 10.1038/gt.2013.10 [DOI] [PubMed] [Google Scholar]
- 53. Jung B.-K., Oh E., Hong J., Lee Y., Park K. D., and Yun C.-O., “ A hydrogel matrix prolongs persistence and promotes specific localization of an oncolytic adenovirus in a tumor by restricting nonspecific shedding and an antiviral immune response,” Biomaterials 147, 26–38 (2017). 10.1016/j.biomaterials.2017.09.009 [DOI] [PubMed] [Google Scholar]
- 54. Jung S.-H., Choi J.-W., Yun C.-O., Yhee J. Y., Price R., Kim S. H. et al. , “ Sustained local delivery of oncolytic short hairpin RNA adenoviruses for treatment of head and neck cancer,” J. Gene Med. 16(5–6), 143–152 (2014). 10.1002/jgm.2770 [DOI] [PubMed] [Google Scholar]
- 55. Le T. M. D., Jung B.-K., Li Y., Duong H. T. T., Nguyen T. L., Hong J. W. et al. , “ Physically crosslinked injectable hydrogels for long-term delivery of oncolytic adenoviruses for cancer treatment,” Biomater. Sci. 7(10), 4195–4207 (2019). 10.1039/C9BM00992B [DOI] [PubMed] [Google Scholar]
- 56. Li J. and Mooney D. J., “ Designing hydrogels for controlled drug delivery,” Nat. Rev. Mater. 1(12), 16071 (2016). 10.1038/natrevmats.2016.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Correa S., Grosskopf A. K., Lopez Hernandez H., Chan D., Yu A. C., Stapleton L. M. et al. , “ Translational applications of hydrogels,” Chem. Rev. 121(18), 11385–11457 (2021). 10.1021/acs.chemrev.0c01177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fenton O. S., Olafson K. N., Pillai P. S., Mitchell M. J., and Langer R., “ Advances in biomaterials for drug delivery,” Adv. Mater. 30(29), 1705328 (2018). 10.1002/adma.201705328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sponchioni M., Capasso Palmiero U., and Moscatelli D., “ Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering,” Mater. Sci. Eng., C 102, 589–605 (2019). 10.1016/j.msec.2019.04.069 [DOI] [PubMed] [Google Scholar]
- 60. Ahsan S. M., Thomas M., Reddy K. K., Sooraparaju S. G., Asthana A., and Bhatnagar I., “ Chitosan as biomaterial in drug delivery and tissue engineering,” Int. J. Biol. Macromol. 110, 97–109 (2018). 10.1016/j.ijbiomac.2017.08.140 [DOI] [PubMed] [Google Scholar]
- 61. Eskandari S., Guerin T., Toth I., and Stephenson R. J., “ Recent advances in self-assembled peptides: Implications for targeted drug delivery and vaccine engineering,” Adv. Drug Delivery Rev. 110–111, 169–187 (2017). 10.1016/j.addr.2016.06.013 [DOI] [PubMed] [Google Scholar]
- 62. Hodi F. S., O'Day S. J., McDermott D. F., Weber R. W., Sosman J. A., Haanen J. B. et al. , “ Improved survival with ipilimumab in patients with metastatic melanoma,” N. Engl. J. Med. 363(8), 711–723 (2010). 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Workman C. J. and Vignali D. A. A., “ Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223),” J. Immunol. 174(2), 688 (2005). 10.4049/jimmunol.174.2.688 [DOI] [PubMed] [Google Scholar]
- 64. Haanen J. B. A. G., Thienen H., and Blank C. U., “ Toxicity patterns with immunomodulating antibodies and their combinations,” Semin. Oncol. 42(3), 423–428 (2015). 10.1053/j.seminoncol.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 65. Wilky B. A., “ Immune checkpoint inhibitors: The linchpins of modern immunotherapy,” Immunol. Rev. 290(1), 6–23 (2019). 10.1111/imr.12766 [DOI] [PubMed] [Google Scholar]
- 66. Li Y., Fang M., Zhang J., Wang J., Song Y., Shi J. et al. , “ Hydrogel dual delivered celecoxib and anti-PD-1 synergistically improve antitumor immunity,” Oncoimmunology 5(2), e1074374 (2015). 10.1080/2162402X.2015.1074374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang C., Wang J., Zhang X., Yu S., Wen D., Hu Q. et al. , “ In situ formed reactive oxygen species–responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy,” Sci. Transl. Med. 10(429), eaan3682 (2018). 10.1126/scitranslmed.aan3682 [DOI] [PubMed] [Google Scholar]
- 68. Aulisa L., Dong H., and Hartgerink J. D., “ Self-assembly of multidomain peptides: Sequence variation allows control over cross-linking and viscoelasticity,” Biomacromolecules 10(9), 2694–2698 (2009). 10.1021/bm900634x [DOI] [PubMed] [Google Scholar]
- 69. Shi Y., Xie T-x, Leach D. G., Wang B., Young S., Osman A. A. et al. , “ Local anti–PD-1 delivery prevents progression of premalignant lesions in a 4NQO-oral carcinogenesis mouse model,” Cancer Prev. Res. 14(8), 767–778 (2021). 10.1158/1940-6207.CAPR-20-0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Leach D. G., Dharmaraj N., Lopez-Silva T. L., Venzor J. R., Pogostin B. H., Sikora A. G. et al. , “ Biomaterial-facilitated immunotherapy for established oral cancers,” ACS Biomater. Sci. Eng. 7(2), 415–421 (2021). 10.1021/acsbiomaterials.0c01575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhao J., Ye H., Lu Q., Wang K., Chen X., Song J. et al. , “ Inhibition of post-surgery tumour recurrence via a sprayable chemo-immunotherapy gel releasing PD-L1 antibody and platelet-derived small EVs,” J. Nanobiotechnol. 20(1), 62 (2022). 10.1186/s12951-022-01270-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chin A. L., Jiang S., Jang E., Niu L., Li L., Jia X. et al. , “ Implantable optical fibers for immunotherapeutics delivery and tumor impedance measurement,” Nat. Commun. 12(1), 5138 (2021). 10.1038/s41467-021-25391-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang P., Lu C., Qin W., Chen M., Quan G., Liu H. et al. , “ Construction of a core-shell microneedle system to achieve targeted co-delivery of checkpoint inhibitors for melanoma immunotherapy,” Acta Biomater. 104, 147–157 (2020). 10.1016/j.actbio.2019.12.037 [DOI] [PubMed] [Google Scholar]
- 74. Yang P., Chen M., Qin W., Shi C., Bai X., Quan G. et al. , “ Effective photothermal therapy mediated by indocyanine green nanoparticle tip-loaded microneedles to enhance checkpoint inhibitor immunotherapy for melanoma treatment,” ACS Appl. Nano Mater. 4(6), 5921–5931 (2021). 10.1021/acsanm.1c00832 [DOI] [Google Scholar]
- 75. Kesavadev J., Saboo B., Krishna M. B., and Krishnan G., “ Evolution of insulin delivery devices: From syringes, pens, and pumps to DIY artificial pancreas,” Diabetes Ther. 11(6), 1251–1269 (2020). 10.1007/s13300-020-00831-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Waghule T., Singhvi G., Dubey S. K., Pandey M. M., Gupta G., Singh M. et al. , “ Microneedles: A smart approach and increasing potential for transdermal drug delivery system,” Biomed. Pharmacother. 109, 1249–1258 (2019). 10.1016/j.biopha.2018.10.078 [DOI] [PubMed] [Google Scholar]
- 77. Stephan M. T., Moon J. J., Um S. H., Bershteyn A., and Irvine D. J., “ Therapeutic cell engineering with surface-conjugated synthetic nanoparticles,” Nat. Med. 16(9), 1035–1041 (2010). 10.1038/nm.2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mi Y., Smith C. C., Yang F., Qi Y., Roche K. C., Serody J. S. et al. , “ A dual immunotherapy nanoparticle improves T-cell activation and cancer immunotherapy,” Adv. Mater. 30(25), 1706098 (2018). 10.1002/adma.201706098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jin Q., Zhu W., Zhu J., Zhu J., Shen J., Liu Z. et al. , “ Nanoparticle-mediated delivery of inhaled immunotherapeutics for treating lung metastasis,” Adv. Mater. 33(7), 2007557 (2021). 10.1002/adma.202007557 [DOI] [PubMed] [Google Scholar]
- 80. Perica K., De León Medero A., Durai M., Chiu Y. L., Bieler J. G., Sibener L. et al. , “ Nanoscale artificial antigen presenting cells for T cell immunotherapy,” Nanomedicine 10(1), 119–129 (2014). 10.1016/j.nano.2013.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Brugada-Vilà P., Cascante A., Lázaro M. Á., Castells-Sala C., Fornaguera C., Rovira-Rigau M. et al. , “ Oligopeptide-modified poly(beta-amino ester)s-coated AdNuPARmE1A: Boosting the efficacy of intravenously administered therapeutic adenoviruses,” Theranostics 10(6), 2744–2758 (2020). 10.7150/thno.40902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lee J. Y., Hong J. W., Thambi T., Yoon A.-R., Choi J.-W., Li Y., Bui Q. N., Lee D. S., and Yun C.-O., “ Optimizing active tumor targeting biocompatible polymers for efficient systemic delivery of adenovirus,” Cells 10(8), 1896 (2021). 10.3390/cells10081896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kim J., Li Y., Kim S. W., Lee D. S., and Yun C.-O., “ Therapeutic efficacy of a systemically delivered oncolytic adenovirus – Biodegradable polymer complex,” Biomaterials 34(19), 4622–4631 (2013). 10.1016/j.biomaterials.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 84. Kasala D., Lee S.-H., Hong J. W., Choi J.-W., Nam K., Chung Y. H. et al. , “ Synergistic antitumor effect mediated by a paclitaxel-conjugated polymeric micelle-coated oncolytic adenovirus,” Biomaterials 145, 207–222 (2017). 10.1016/j.biomaterials.2017.08.035 [DOI] [PubMed] [Google Scholar]
- 85. Kwon O.-J., Kang E., Choi J.-W., Kim S. W., and Yun C.-O., “ Therapeutic targeting of chitosan–PEG–folate-complexed oncolytic adenovirus for active and systemic cancer gene therapy,” J. Controlled Release 169(3), 257–265 (2013). 10.1016/j.jconrel.2013.03.030 [DOI] [PubMed] [Google Scholar]
- 86. Kranz L. M., Diken M., Haas H., Kreiter S., Loquai C., Reuter K. C. et al. , “ Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy,” Nature 534, 396–401 (2016). 10.1038/nature18300 [DOI] [PubMed] [Google Scholar]
- 87. Patel R., Kaki M., Potluri V. S., Kahar P., and Khanna D., “ A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson,” Hum. Vaccines Immunother. 18(1), 2002083 (2022). 10.1080/21645515.2021.2002083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chacon J. A., Pilon-Thomas S., Sarnaik A. A., and Radvanyi L. G., “ Continuous 4–1BB co-stimulatory signals for the optimal expansion of tumor-infiltrating lymphocytes for adoptive T-cell therapy,” Oncoimmunology 2(9), e25581 (2013). 10.4161/onci.25581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Liu S., Etto T., Rodríguez-Cruz T., Li Y., Wu C., Fulbright O. J. et al. , “ TGF-β1 induces preferential rapid expansion and persistence of tumor antigen-specific CD8+ T cells for adoptive immunotherapy,” J. Immunother. 33(4), 371–381 (2010). 10.1097/CJI.0b013e3181cd1180 [DOI] [PubMed] [Google Scholar]
- 90. Lu Y.-C., Zheng Z., Robbins P. F., Tran E., Prickett T. D., Gartner J. J. et al. , “ An efficient single-cell RNA-seq approach to identify neoantigen-specific T cell receptors,” Mol. Ther. 26(2), 379–389 (2018). 10.1016/j.ymthe.2017.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Atkins M. B., Lotze M. T., Dutcher J. P., Fisher R. I., Weiss G., Margolin K. et al. , “ High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993,” J. Clin. Oncol. 17(7), 2105 (1999). 10.1200/JCO.1999.17.7.2105 [DOI] [PubMed] [Google Scholar]
- 92. Wu R., Forget M. A., Chacon J., Bernatchez C., Haymaker C., Chen J. Q. et al. , “ Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: Current status and future outlook,” Cancer J. 18(2), 160–175 (2012). 10.1097/PPO.0b013e31824d4465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Goff S. L., Smith F. O., Klapper J. A., Sherry R., Wunderlich J. R., Steinberg S. M. et al. , “ Tumor infiltrating lymphocyte therapy for metastatic melanoma: Analysis of tumors resected for TIL,” J. Immunother. 33(8), 840–847 (2010). 10.1097/CJI.0b013e3181f05b91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Shapira-Frommer R. and Schachter J., “ Adoptive immunotherapy of advanced melanoma,” Curr. Treat. Options Oncol. 13(3), 340–353 (2012). 10.1007/s11864-012-0203-7 [DOI] [PubMed] [Google Scholar]
- 95. Neelapu S. S., Tummala S., Kebriaei P., Wierda W., Gutierrez C., Locke F. L. et al. , “ Chimeric antigen receptor T-cell therapy – Assessment and management of toxicities,” Nat. Rev. Clin. Oncol. 15(1), 47–62 (2018). 10.1038/nrclinonc.2017.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Roche E. T., Hastings C. L., Lewin S. A., Shvartsman D. E., Brudno Y., Vasilyev N. V. et al. , “ Comparison of biomaterial delivery vehicles for improving acute retention of stem cells in the infarcted heart,” Biomaterials 35(25), 6850–6858 (2014). 10.1016/j.biomaterials.2014.04.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Guyot C. and Lerouge S., “ Can we achieve the perfect injectable scaffold for cell therapy?,” Future Sci. OA 4(4), FSO284 (2018). 10.4155/fsoa-2017-0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tsao C.-T., Kievit F. M., Ravanpay A., Erickson A. E., Jensen M. C., Ellenbogen R. G. et al. , “ Thermoreversible poly(ethylene glycol)-g-chitosan hydrogel as a therapeutic T lymphocyte depot for localized glioblastoma immunotherapy,” Biomacromolecules 15(7), 2656–2662 (2014). 10.1021/bm500502n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wang K., Chen Y., Ahn S., Zheng M., Landoni E., Dotti G. et al. , “ GD2-specific CAR T cells encapsulated in an injectable hydrogel control retinoblastoma and preserve vision,” Nat. Cancer 1(10), 990–997 (2020). 10.1038/s43018-020-00119-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Brewer K., Gundsambuu B., Facal Marina P., Barry S. C., and Blencowe A., “ Thermoresponsive poly(ε-caprolactone)-poly(ethylene/propylene glycol) copolymers as injectable hydrogels for cell therapies,” Polymers 12(2), 367 (2020). 10.3390/polym12020367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pérez del Río E., Santos F., Rodriguez Rodriguez X., Martínez-Miguel M., Roca-Pinilla R., Arís A. et al. , “ CCL21-loaded 3D hydrogels for T cell expansion and differentiation,” Biomaterials 259, 120313 (2020). 10.1016/j.biomaterials.2020.120313 [DOI] [PubMed] [Google Scholar]
- 102. Yan J., Gundsambuu B., Krasowska M., Platts K., Facal Marina P., Gerber C. et al. , “ Injectable Diels–Alder cycloaddition hydrogels with tuneable gelation, stiffness and degradation for the sustained release of T-lymphocytes,” J. Mater. Chem. B. 10, 3329 (2022). 10.1039/D2TB00274D [DOI] [PubMed] [Google Scholar]
- 103. Monette A., Ceccaldi C., Assaad E., Lerouge S., and Lapointe R., “ Chitosan thermogels for local expansion and delivery of tumor-specific T lymphocytes towards enhanced cancer immunotherapies,” Biomaterials 75, 237–249 (2016). 10.1016/j.biomaterials.2015.10.021 [DOI] [PubMed] [Google Scholar]
- 104. Weiden J., Voerman D., Dölen Y., Das R. K., van Duffelen A., Hammink R. et al. , “ Injectable biomimetic hydrogels as tools for efficient T cell expansion and delivery,” Front. Immunol. 9, 2798 (2018). 10.3389/fimmu.2018.02798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Atik A. F., Suryadevara C. M., Schweller R. M., West J. L., Healy P., Herndon Ii J. E. et al. , “ Hyaluronic acid based low viscosity hydrogel as a novel carrier for convection enhanced delivery of CAR T cells,” J. Clin. Neurosci. 56, 163–168 (2018). 10.1016/j.jocn.2018.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Stephan S. B., Taber A. M., Jileaeva I., Pegues E. P., Sentman C. L., and Stephan M. T., “ Biopolymer implants enhance the efficacy of adoptive T cell therapy,” Nat. Biotechnol. 33(1), 97–101 (2015). 10.1038/nbt.3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Smith T. T., Moffett H. F., Stephan S. B., Opel C. F., Dumigan A. G., Jiang X. et al. , “ Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors,” J. Clin. Invest. 127(6), 2176–2191 (2017). 10.1172/JCI87624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Coon M. E., Stephan S. B., Gupta V., Kealey C. P., and Stephan M. T., “ Nitinol thin films functionalized with CAR-T cells for the treatment of solid tumours,” Nat. Biomed. Eng. 4, 195–206 (2019). 10.1038/s41551-019-0486-0 [DOI] [PubMed] [Google Scholar]
- 109. Verma V., Kim Y., Lee M.-C., Lee J.-T., Cho S., Park I.-K. et al. , “ Activated dendritic cells delivered in tissue compatible biomatrices induce in-situ anti-tumor CTL responses leading to tumor regression,” Oncotarget 7(26), 39894–39906 (2016). 10.18632/oncotarget.9529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yang P., Song H., Qin Y., Huang P., Zhang C., Kong D. et al. , “ Engineering dendritic-cell-based vaccines and PD-1 blockade in self-assembled peptide nanofibrous hydrogel to amplify antitumor T-cell immunity,” Nano Lett. 18(7), 4377–4385 (2018). 10.1021/acs.nanolett.8b01406 [DOI] [PubMed] [Google Scholar]
- 111. Yang A., Dong X., Bai Y., Sheng S., Zhang Y., Liu T. et al. , “ Doxorubicin/CpG self-assembled nanoparticles prodrug and dendritic cells co-laden hydrogel for cancer chemo-assisted immunotherapy,” Chem. Eng. J. 416, 129192 (2021). 10.1016/j.cej.2021.129192 [DOI] [Google Scholar]
- 112. Yang A., Bai Y., Dong X., Ma T., Zhu D., Mei L. et al. , “ Hydrogel/nanoadjuvant-mediated combined cell vaccines for cancer immunotherapy,” Acta Biomater. 133, 257–267 (2021). 10.1016/j.actbio.2021.08.014 [DOI] [PubMed] [Google Scholar]
- 113. Oh E., Oh J.-E., Hong J., Chung Y., Lee Y., Park K. D. et al. , “ Optimized biodegradable polymeric reservoir-mediated local and sustained co-delivery of dendritic cells and oncolytic adenovirus co-expressing IL-12 and GM-CSF for cancer immunotherapy,” J. Controlled Release 259, 115–127 (2017). 10.1016/j.jconrel.2017.03.028 [DOI] [PubMed] [Google Scholar]
- 114. Ahn Y. H., Ren L., Kim S. M., Seo S.-H., Jung C.-R., Kim D. S. et al. , “ A three-dimensional hyaluronic acid-based niche enhances the therapeutic efficacy of human natural killer cell-based cancer immunotherapy,” Biomaterials 247, 119960 (2020). 10.1016/j.biomaterials.2020.119960 [DOI] [PubMed] [Google Scholar]
- 115. Luo Z., Liu Z., Liang Z., Pan J., Xu J., Dong J. et al. , “ Injectable porous microchips with oxygen reservoirs and an immune-niche enhance the efficacy of CAR T cell therapy in solid tumors,” ACS Appl. Mater. Interfaces 12(51), 56712–56722 (2020). 10.1021/acsami.0c15239 [DOI] [PubMed] [Google Scholar]
- 116. Ogunnaike E. A., Valdivia A., Yazdimamaghani M., Leon E., Nandi S., Hudson H. et al. , “ Fibrin gel enhances the antitumor effects of chimeric antigen receptor T cells in glioblastoma,” Sci. Adv. 7(41), eabg5841 (2021). 10.1126/sciadv.abg5841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hu Q., Li H., Archibong E., Chen Q., Ruan H., Ahn S. et al. , “ Inhibition of post-surgery tumour recurrence via a hydrogel releasing CAR-T cells and anti-PDL1-conjugated platelets,” Nat. Biomed. Eng. 5(9), 1038–1047 (2021). 10.1038/s41551-021-00712-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tian Y., Xu C., Feng J., Huangfu Y., Wang K., and Zhang Z.-L., “ Personalized gel-droplet monocyte vaccines for cancer immunotherapy,” Lab Chip 21(22), 4414–4426 (2021). 10.1039/D1LC00646K [DOI] [PubMed] [Google Scholar]
- 119. Cunningham N., Malaret T., Stagg J., Thébault P., Trudel D., Lapointe R., et al. , “ Injectable T cell-loaded hydrogel as a localised immunotherapy reduces tumour growth in mice,” in Society For Biomaterials Annual Meeting and Exposition, 2021. [Google Scholar]
- 120. Flanagan K., Moroziewicz D., Kwak H., Hörig H., and Kaufman H. L., “ The lymphoid chemokine CCL21 costimulates naïve T cell expansion and Th1 polarization of non-regulatory CD4+ T cells,” Cellular Immunology 231(1), 75–84 (2004). 10.1016/j.cellimm.2004.12.006 [DOI] [PubMed] [Google Scholar]
- 121. Das R. K., Gocheva V., Hammink R., Zouani O. F., and Rowan A. E., “ Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels,” Nat. Mater. 15(3), 318–325 (2016). 10.1038/nmat4483 [DOI] [PubMed] [Google Scholar]
- 122. Zimoch J., Padial J. S., Klar A. S., Vallmajo-Martin Q., Meuli M., Biedermann T. et al. , “ Polyisocyanopeptide hydrogels: A novel thermo-responsive hydrogel supporting pre-vascularization and the development of organotypic structures,” Acta Biomater. 70, 129–139 (2018). 10.1016/j.actbio.2018.01.042 [DOI] [PubMed] [Google Scholar]
- 123. Canaple L., Rehor A., and Hunkeler D., “ Improving cell encapsulation through size control,” J. Biomater. Sci. Polym. Ed. 13(7), 783–796 (2022). 10.1163/156856202760197410 [DOI] [PubMed] [Google Scholar]
- 124. Alinejad Y., Bitar C. M. E., Martinez Villegas K., Perignon S., Hoesli C. A., and Lerouge S., “ Chitosan microbeads produced by one-step scalable stirred emulsification: A promising process for cell therapy applications,” ACS Biomater. Sci. Eng. 6(1), 288–297 (2020). 10.1021/acsbiomaterials.9b01638 [DOI] [PubMed] [Google Scholar]
- 125. Cormier A. R., Ruiz-Orta C., Alamo R. G., and Paravastu A. K., “ Solid state self-assembly mechanism of RADA16-I designer peptide,” Biomacromolecules 13(6), 1794–1804 (2012). 10.1021/bm300313h [DOI] [PubMed] [Google Scholar]
- 126. Kim M., Kim T.-J., Kim H. M., Doh J., and Lee K.-M., “ Multi-cellular natural killer (NK) cell clusters enhance NK cell activation through localizing IL-2 within the cluster,” Sci. Rep. 7(1), 40623 (2017). 10.1038/srep40623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cabrita R., Lauss M., Sanna A., Donia M., Skaarup Larsen M., Mitra S. et al. , “ Tertiary lymphoid structures improve immunotherapy and survival in melanoma,” Nature 577(7791), 561–565 (2020). 10.1038/s41586-019-1914-8 [DOI] [PubMed] [Google Scholar]
- 128. Jin Z., Li X., Zhang X., DeSousa P., Xu T., and Wu A., “ Engineering the fate and function of human T-cells via 3D bioprinting,” Biofabrication 13(3), 035016 (2021). 10.1088/1758-5090/abd56b [DOI] [PubMed] [Google Scholar]
- 129. Aoyama S., Nakagawa R., Mulé J. J., and Mailloux A. W., “ Inducible tertiary lymphoid structures: Promise and challenges for translating a new class of immunotherapy,” Front. Immunol. 12, 675538 (2021). 10.3389/fimmu.2021.675538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kobayashi Y., Kato K., and Watanabe T., “ Synthesis of functional artificial lymphoid tissues,” Discov. Med. 12(65), 351–362 (2011). [PubMed] [Google Scholar]
- 131. Wang L., Guo X., Chen J., Zhen Z., Cao B., Wan W. et al. , “ Key considerations on the development of biodegradable biomaterials for clinical translation of medical devices: With cartilage repair products as an example,” Bioact. Mater. 9, 332–342 (2022). 10.1016/j.bioactmat.2021.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.FDA, see https://www.fda.gov/regulatory-information/search-fda-guidance-documents/principles-premarket-pathways-combination-products for “ Principles of premarket pathways for combination products” (2022). [DOI] [PubMed]
- 133. Oberweis C. V., Marchal J. A., López-Ruiz E., and Gálvez-Martín P., “ A worldwide overview of regulatory frameworks for tissue-based products,” Tissue Eng., Part B 26(2), 181–196 (2020). 10.1089/ten.teb.2019.0315 [DOI] [PubMed] [Google Scholar]
- 134.FDA, see https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-international-standard-iso-10993-1-biological-evaluation-medical-devices-part-1-evaluation-and for “ Use of International Standard ISO 10993-1, ‘Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process’” 2020.
- 135. Mendicino M., Fan Y., Griffin D., Gunter K. C., and Nichols K., “ Current state of U.S. Food and Drug Administration regulation for cellular and gene therapy products: Potential cures on the horizon,” Cytotherapy 21(7), 699–724 (2019). 10.1016/j.jcyt.2019.04.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.