Abstract
Purpose of Review
To summarize the targeted therapies and immunotherapy of Kirsten rat sarcoma viral oncogene homolog (KRAS)-mutant non-small cell lung cancer (NSCLC), and discuss the ongoing clinical trials.
Recent Findings
KRAS mutations occur in about 30% of patients with NSCLC and are the second most frequent genetic variation in lung cancer. It has been considered “undruggable” for 40 years until the discovery of a direct inhibitor of KRAS G12C. The promising direct KRAS G12C inhibitors such as sotorasib and MRTX849 have made a breakthrough with promising anti-tumor effects in patients with KRAS G12C-mutant advanced/metastatic NSCLC post one prior line of therapy. Following the success of immune checkpoint inhibitors (ICIs) in NSCLC, many patients harboring KRAS mutations can benefit from ICIs. However, due to disease heterogeneity, the prognosis of patients remains unsatisfactory, leaving room for personalized treatment options, such as new targeted therapies and other therapies.
Summary
In this review, we aim to dissect the strategies of clinical trials in these tumors, shifting from a few chemotherapy options to targeted and immunotherapy, in the context of molecular selection of KRAS-mutant NSCLC subtypes.
Keywords: non-small cell lung cancer, KRAS G12C mutations, targeted therapy, immunotherapy
Molecular Features and Genetic Heterogeneity of KRAS-Mutant NSCLC
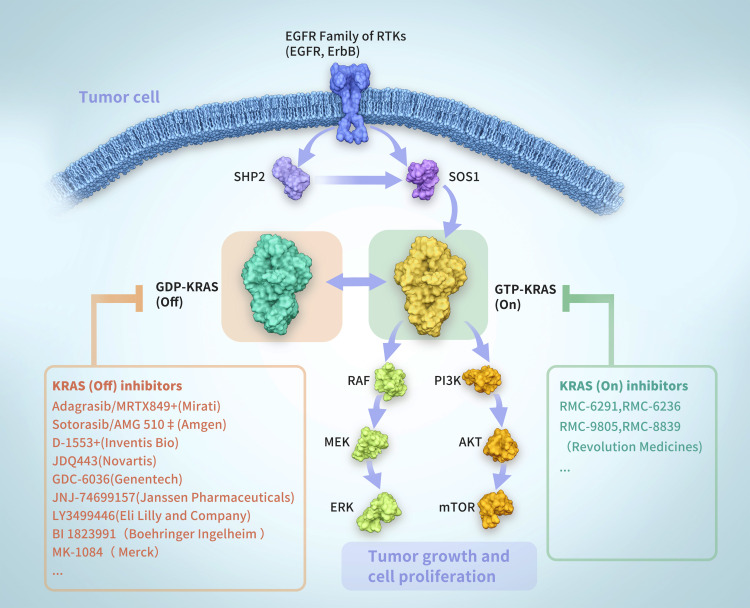
KRAS encodes a membrane-bound guanosine triphosphatase (GTPase), which plays a molecular switching role by converting guanosine triphosphate (GTP) molecules into guanosine diphosphate (GDP) molecules. Under normal conditions, KRAS is inactive when bounds to GDP and active when bounds to GTP (Figure 1). The activation/deactivation process of KRAS involves two regulatory proteins: (1) guanine nucleotide exchange factor (GEF), which promotes the binding of KRAS and GTP to activate Son of Sevenless (SOS) protein etc.; and (2) GTPase activating protein (GAP), which promotes the binding between GDP and KRAS and GTP hydrolysis.2 When KRAS mutations occur (codons 12, 13, and 61), the mutated KRAS proteins primarily maintain the KRAS-GTP active state, which disrupts the GTP hydrolysis and guanine exchange rates of RAS proteins. Therefore, it would lead to unregulated oncogenic signaling and tumorigenesis.2
Figure 1.
The activation and deactivation process of KRAS.
Abbreviations: KRAS, Kirsten rat sarcoma viral oncogene; GTP, guanosine triphosphate; GDP, guanosine diphosphate; GEF, guanine nucleotide exchange factor; GAP, GTPase activating protein.
KRAS-mutant NSCLC is heterogeneous for the different subtypes and frequently co-mutations with other master genes. KRAS-mutant NSCLC is composed of distinct subtypes, with the majority (97%) found in the 12th or 13th amino acid residues. The frequency common subtypes include G12D, G12V, G12C, G12A, and G13D.3 Of the above subtypes, KRAS G12C is one of the most common genetic mutations in NSCLC, and the incidence varies in different races, ranging from ~13% in Western countries4 to 3–5% in Asia countries.5
KRAS often have co-mutations with other master genes rather than only a single KRAS mutation. Genetic co-mutations varied with different KRAS clusters, including: (1) KP subgroup (+TP53 mutation); (2) KL subgroup (+STK11/LKB1 mutation); and (3) KC subgroup (+CDKN2A/B inactivation plus low TTF1). Different subgroups may show distinct biology, patterns of immune-system engagement, and therapeutic vulnerabilities.6
Pre-Clinical Development: Sotorasib (AMG-510) and Adagrasib (MRTX849)
A preclinical study demonstrates that sotorasib is highly selective for impairing cell viability in pancreatic and lung adenocarcinoma cell lines compared to non-KRAS G12C cell lines.7 In multiple in vitro and in vivo models of KRAS G12C-mutant (such as cell line-derived xenografts, syngeneic mouse models, and patient-derived xenografts), sotorasib is found to inhibit ERK phosphorylation. What’s more, sotorasib is also found to restore an efficient immune tumor response.8,9
Similar to sotorasib, adagrasib selectively impairs cell viability and inhibits ERK phosphorylation without affecting AKT activation.10 A preclinical study reveals that adagrasib decreases intratumoral myeloid-derived suppressor cells (MDSCs) and increases M1-macrophages, dendritic cells, and CD4+/CD8+ T cells.11
The Breakthrough in Targeted Therapy for KRAS G12C-Mutant NSCLC
Better insights into KRAS structural biochemistry allow researchers to discover a covalent inhibitor drug handle in the KRAS G12C protein. The investigation of the crystal structure of the mutant protein binding to GDP revealed a new pocket beneath the small molecule-type drugs that bind the switch II region. This led to the development of first-in-class KRAS G12C off-state inhibitors: sotorasib/Lumakras™ (Amgen®) and adagrasib (Mirati Therapeutics). In May 2021, the Food and Drug Administration (FDA) announced the accelerated approval of sotorasib for the treatment of patients with KRAS G12C-mutant locally advanced or metastatic NSCLC who had received at least one prior systemic therapy.12
Sotorasib
Sotorasib is a small molecule that irreversibly and selectively binds to the mutant C12 in a small pocket (P2) on the KRAS G12C protein 2, locking the KRAS G12C-mutant protein in an inactive state, thus preventing oncogenic signaling without affecting wild-type KRAS.13
The Phase I/II study (CodeBreak 100: NCT03600883) showed a favorable safety profile of sotorasib monotherapy. Pharmacokinetics (PK) analysis demonstrated that the half-life of sotorasib was approximately 5.5 hours, and brief exposure to sotorasib (960 mg) was expected to completely inhibit KRAS G12C mutations throughout the dosing interval.14 Meanwhile, sotorasib showed encouraging anticancer activity in previously treated metastatic NSCLC, colorectal cancer (CRC), and other tumor types, with a median number of previous anticancer treatment lines of 3 (range: 0–11).
Recently, a Phase II study was published by American Association for Cancer Research (AACR) in 2022. A total of 174 patients with KRAS G12C-mutant locally advanced or metastatic NSCLC were enrolled, of whom 82.8% had previously received platinum-based chemotherapy and programmed cell death-1 (PD-1)/PD-1 ligand 1 (PD-L1) inhibitors. Sotorasib was orally administered at 960 mg once daily until disease progression. The efficacy and safety of sotorasib for metastatic NSCLC were promising, with an objective response rate (ORR) of 40.7% and a disease control rate (DCR) of 83.7% (Table 1). In these patients with NSCLC, the median duration of response, median progression-free survival (PFS), median overall survival (OS), and 1-year OS rate were 12.3 months, 6.3 months, 12.5 months, and 50.8%, respectively.
Table 1.
Preliminary Data from Targeted Therapy
| Drugs | Study | Phase | Setting | No. of Patients | Outcome |
|---|---|---|---|---|---|
| Sotorasib | CodeBreak 10015 | I/II | ≥2 line | 174 | ORR, 40.7%; DCR, 83.7%; mPFS, 6.3 months; mOS, 12.5 months; 1-year OS rate, 50.8% |
| CodeBreak 20016 | III | ≥1 line | 171 | ORR, 28.1%; DCR, 82.5%; mPFS, 6.5 months; 1-year PFS rate, 24.8% | |
| 174 | ORR, 13.2%; DCR, 83.7%; mPFS, 4.5 months; 1-year PFS rate, 10.1% | ||||
| Adagrasib | KRYSTAL-118 | I/II | ≥2 line | 116 | ORR, 43%; DCR, 80%; mPFS, 6.5 months; mOS, 12.6 months; 1-year OS rate, 51% |
| D-155319 | I | ≥2 line | 59 | ORR, 40.4%; DCR, 90.4% |
Abbreviations: ORR, objective response rate; DCR, disease control rate; mPFS, median progression-free survival; mOS, median overall survival; OS, overall survival; No, number.
For sotorasib, treatment-related adverse events (TRAEs) were generally mild and manageable. Grade 3 and grade 4 TRAEs were reported in 20% and 1% of patients, respectively, and no fatal TRAEs were reported.15
Furthermore, the global Phase III study (CodeBreak 200) of sotorasib versus docetaxel for previously treated NSCLC with KRAS G12C mutation was published by the European Society for Medical Oncology (ESMO) in 2022.16 The results showed that sotorasib significantly improved the primary endpoint of PFS compared to docetaxel (5.6 vs 4.5 months, HR=0.66, p=0.002). Besides, sotorasib was well-tolerated with fewer grade ≥3 TRAEs than docetaxel (Table 1).
Besides, the phase II trial (CodeBreak 201) of sotorasib in treatment-naïve stage IV NSCLC patients with KRAS G12C mutations, PD-L1 tumor proportion score (TPS) <1%, and/or harboring a serine/threonine kinase 11 (STK11) co-mutation is ongoing.14
MRTX849
Another KRAS G12C inhibitor is under development. According to the data disclosed in phase I/II study (KRYSTAL-1: NCT03785249), adagrasib (MRTX849) showed a favorable safety profile and significant clinical activity in heavily pretreated patients. The PK analysis demonstrated that the half-life of adagrasib was approximately 24.7 hours, and the phase II dose (RP2D) was 600 mg twice daily (BID).17
Furthermore, a total of 116 patients with KRAS G12C-mutant locally advanced or metastatic NSCLC were enrolled in a phase II study (KRYSTAL-1-CohortA) of adagrasib, of whom 98% had received previous treatment. Adagrasib was orally administered at 600 mg BID until disease progression. The efficacy and safety of adagrasib for metastatic NSCLC (n=116) were encouraging, with an ORR of 43% and a DCR of 80% (Table 1). The median duration of response, median PFS, median OS, and 1-year OS rate were 8.5 months, 6.5 months, 12.6 months, and 51%, respectively. Regarding safety, grade 3–4 TRAEs were reported in 43% of patients, and 2 patients reported grade 5 TRAE (heart failure, n=1; pulmonary hemorrhage, n=1).18
Furthermore, a phase II study (KRYSTAL-7) of adagrasib in combination with pembrolizumab for newly treated NSCLC patients harboring KRAS G12C mutations who cannot be treated locally or with metastases is ongoing.17
D-1553
D-1553 is a novel and selective KRAS G12C inhibitor. A Phase I study assessed the safety and efficacy of D-1553 in treating patients with NSCLC harboring KRAS G12C mutations. A total of 59 KRAS G12C-mutant NSCLC patients who had received at least one systemic therapy were enrolled. The ORR was 40.4% and the DCR was 90.4% (Table 1).19
So far, from the initial data of sotorasib and adagrasib, we have not seen deeper and more durable responses observed from other targeted agents such as epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase gene (ALK) inhibitors. The ORR of the KRAS G12C inhibitor was about 40%, with a median improvement in PFS of around 6 months and OS of about 1 year. TRAEs were generally tolerable and manageable.
The less-than-ideal response may come from the following reasons: (1) patients included in the trial had advanced disease and had received several lines of prior treatment; (2) disease heterogeneity (many tumors harbor co-occurring mutations and cross-talk with other pathways); and (3) the development of acquired resistance. The unsatisfactory responses keep drug research in KRAS-mutant populations uninterrupted.
KRAS-mutant tumors are characterized by the evasion of immune surveillance. In addition, the oncogenic KRAS signaling induces the expression of several immunomodulatory factors (nuclear factor kappa-Β [NF-kB], signal transducer and activator of transcription 3 [STAT3], and certain suppressive inflammatory cytokines such as interleukin 6 [IL-6], interleukin-1B [IL-1b], and granulocyte-macrophage colony-stimulating factor [GM-CSF]), resulting in an immune-suppressive tumor microenvironment.20 Other mechanisms in this tumor type consist of interference (IFNG), PD-L1, and PD-1, and CD8 expressions were higher in KRAS-mutant lung cancer.21
The Progress in Immunotherapy for KRAS-Mutant NSCLC
Anti-PD-(L) 1 Single Agent
Several published research has found that patients with KRAS mutations can benefit from immune monotherapy (Table 2). A meta-analysis including 9 studies with 1716 cases of NSCLC, 694 cases of KRAS mutations, and 1022 cases of KRAS wild-type showed that patients with KRAS gene mutations can benefit more (ORR [mutant vs wild type]: HR=1.51 [95%: 1.17–1.96]) from immune monotherapy.1 In the Checkmate 057 study, 62 patients with KRAS mutations benefited more from nivolumab than chemotherapy (mOS: HR=0.52 [95% CI: 0.29–0.95]). In the OAK study, 59 patients with KRAS mutations tended to benefit from atezolizumab treatment (mOS: HR=0.71 [95% CI: 0.38–1.35]).22 In the KEYNOTE-042 study, 301 patients were analyzed, of whom 69 possessed KRAS mutations while 29 possessed KRAS G12C mutations. Compared with KRAS wild-type, pembrolizumab significantly prolonged OS in patients with KRAS mutations (HR=0.86 [95% CI: 0.63–1.18] vs HR=0.42 [95% CI: 0.22–0.81]). Meanwhile, patients with KRAS G12C mutations seem to benefit more in OS (HR=0.28 [95% CI: 0.09–0.86]); it should be noted that all patients enrolled in this study had a PD-L1 expression of greater than 1%.23 The IMMUNTRGET study included 246 NSCLC patients with KRAS mutations who received immune monotherapy. The ORR was 26%, the median PFS was 3.2 months (95% CI: 2.7–4.5), and the median OS was 13.5 months (95% CI: 9.4–15.6), which suggested that patients with KRAS mutations could benefit from immunotherapy.24 For patients with KRAS mutations, more large-scale studies are needed to prove the survival benefits of immunotherapy.
Table 2.
Preliminary Data from Immunotherapy
| Study | Phase | Setting | Arms | No. of Patients with KRAS Mutant | Outcome | |
|---|---|---|---|---|---|---|
| ICIs alone | Keynote04223 | III | 1 line | Pembromab | 69 | KRAS mutant vs wild type: ORR, 56.7 vs 29.1%; mOS, HR=0.42 (95% CI: 0.22–0.81) |
| IMMUNTRGET24 | – | ≥2 line | ICIs | 246 | ORR, 26%; mPFS, 3.2 months; mOS, 13.5 months | |
| Checkmate 05731 | III | ≥2 line | Nivolumab vs docetaxel | 62 | mOS, 12.2 vs 9.4 months, HR=0.52 (95% CI: 0.29–0.95) | |
| OAK study22 | III | ≥2 line | Atezolizumab vs docetaxel | 59 | mOS, 13.8 vs 9.6 months, HR=0.71 (95% CI: 0.38–1.34) | |
| Meta analysis1 | – | 1 line/2 line | ICIs alone | 694 | KRAS mutant vs wild type: HR=1.51 (95% CI: 1.17–1.96), p=0.002 | |
| ICIs combined therapy | Keynote-18925 | III | 1 line | Pembromab plus chemotherapy | 89 | KRAS mutant vs wild type: ORR, 40.7 vs 47.6%; mOS, HR=0.79 (95% CI: 0.45–1.38) |
| IMPOWER 15026 | III | 1 line | Atezolizumab plus chemotherapy | 226 | KRAS mutant (ABCP vs BCP): mPFS, HR=0.42 (95% CI: 0.29–0.61); OS, HR=0.50 (95% CI: 0.34–0.72) | |
| Meta analysis1 | – | 1 line/2 line | ICIs plus others | 386 | ICI plus CT vs CT: OS, 0.59 months (95% CI: 0.49–0.72), p <0.00001; PFS, 0.58 months (95% CI: 0.43–0.78), p=0.0003 |
Abbreviations: ICIs, immune checkpoint inhibitors; KRAS, Kirsten rat sarcoma viral oncogene; HR, hazard ratio; BCP, bevacizumab plus carboplatin plus paclitaxel; ABCP, atezolizumab plus BCP; 95% CI, 95% confidence interval; ORR, objective response rate; mPFS, median progression-free survival; mOS, median overall survival; OS, overall survival; No, number.
Anti-PD-(L) 1 Combined Therapy
A meta-analysis of 6 studies showed that immunotherapy combined with chemotherapy significantly prolonged the OS (HR=0.59 [95% CI: 0.49–0.72]; p<0.00001) and PFS (HR=0.58 [95% CI: 0.43–0.78]; p=0.0003) in patients with KRAS-mutant NSCLC compared with chemotherapy alone, and the OS of patients with KRAS mutations was significantly longer than that in the KRAS wild-type group (p=0.001) (Table 2).1 In the Keynote-189 study, 89 patients with KRAS mutations (37 with KRAS G12C mutations) were analyzed, and there was no significant difference in OS (HR=0.79 [95% CI: 0.45–1.38]) between the pembrolizumab combined chemotherapy and chemotherapy. Besides, for patients with KRAS G12C mutations, the OS between the two groups was similar (HR=1.14 [95% CI: 0.45–2.92]).25 In the IMPOWER 150 study, ABCP (atezolizumab, bevacizumab, carboplatin, and paclitaxel) showed more benefit in OS and PFS than ACP (atezolizumab, carboplatin, and paclitaxel) or BCP (bevacizumab, carboplatin, and paclitaxel) in patients with KRAS mutations; however, ABCP or ACP showed limited improvement in OS compared with BCP in the KRAS-WT population.26
The published data suggested that ICIs could confer a survival benefit on the KRAS-mutant population. For patients with KRAS-mutant NSCLC, the ICIs monotherapy as first-line treatment contributed to a median improvement in PFS of approximately 12 months and OS of about 2 years. The OS of ICIs monotherapy as second-line treatment was about 1 year. Moreover, the anti-PD-(L) 1 combined chemotherapy contributed to a median improvement in PFS of approximately 9 months and OS of about 21 months.
Outlook
KRAS mutations consist of different subtypes, with the KRAS G12C mutations being a small subset of the population. Patients beyond KRAS G12C mutations still have unmet needs, promoting a new research era, focusing on finding more effective ways to target the KRAS pathway. The development of drug strategies around KRAS mutations can be divided into 3 categories, including KRAS G12C (OFF) inhibitors, KRAS (ON) inhibitors, and rat sarcoma viral oncogene (RAS) companion inhibitors.
The KRAS G12C mutations drive these cancers by shifting the cellular equilibrium of KRAS toward the GTP-bound (active state, KRAS G12C [ON]), which in turn increases signaling output to initiate and support an oncogenic state. KRAS G12C (OFF) inhibitors (such as sotorasib) work via sequestration of the GDP-bound (inactive state, KRAS G12C [OFF]). The research progress of KRAS G12C (OFF) inhibitors is at the forefront, and the less ideal response drives the KRAS (ON) inhibitors to form a tri-complex (KRAS variant, KRAS [ON] inhibitors, and chaperone [such as cyclophilin A]) in the presence of the compound. The chaperone protein sterically occludes the target protein (such as KRAS [ON]) and prevents interaction with affiliated proteins (such as the RAS effector kinase-rapidly accelerated fibrosarcoma [RAF]), which was required for propagating oncogenic signals. Besides, KRAS companion inhibitors suppress cooperating targets and pathways that sustain RAS-addicted cancers, such as SOS Ras/Rac Guanine Nucleotide Exchange Factor 1(SOS1), Src homology-2 domain containing protein tyrosine phosphatase-2 (SHP2), mechanistic target of rapamycin complex 1 (mTORC1), and immune checkpoints.27–29
This article reviews the latest progress in the treatment of KRAS-mutant NSCLC, aiming at understanding the biological diversity and potential clinical significance of KRAS-mutant NSCLC, to provide the basis for individualized treatment of KRAS-mutant NSCLC.
The efficacy achieved in the current KRAS-mutant populations focuses on targeted (targeting KRAS G12C mutations) therapy and immunotherapy. Meanwhile, the tumor heterogeneity and immunomodulatory effects of KRAS-mutant lung cancers exhibit different sensitivity to different therapies. The ongoing clinical design study provides insights into the path to personalized treatment of KRAS mutation by inhibiting different isoforms of KRAS via KRAS (OFF) G12C inhibitors and KRAS (ON) inhibitors (Table 3). Moreover, based on KRAS inhibitors, the combination of multiple treatment methods (such as in combination with EGFR inhibitors or MEK inhibitors, or anti-PD-1)28,30 is an inevitable trend in the future.
Table 3.
Ongoing Drug Development Strategies for KRAS Mutant Populations
| Classification | Programs (Company) | Combination Therapies Under Investigation | Phase |
|---|---|---|---|
| KRAS G12C (OFF) inhibitors32 | Adagrasib/MRTX849+ (Mirati) | PD-1, PD-L1, SHP2, MEK | III |
| Sotorasib/AMG 510‡ (Amgen) | Pan-ErbB, EGFR, mTOR | III | |
| D-1553+ (Inventis Bio) | CDK4/6, chemotherapy | I/II | |
| JDQ443 (Novartis) | PD-L1, EGFR, pan-ErbB, SHP2 | I/II | |
| GDC-6036 (Genentech) | Chemotherapy | I | |
| JNJ-74699157 (JanssenPharmaceuticals) | PD-1, SHP2 | I | |
| LY3499446 (Eli Lilly and Company) | PD-1, VEGF, EGFR | The study terminated due to toxicity | |
| BI 1823991 (Boehringer Ingelheim) | SOS1 | Ia/Ib | |
| MK-1084 (Merck) | Pembrolizumab | I | |
| KRAS (ON) inhibitors27,28 | RMC-9805 KRASG12D (Revolution Medicines) | - | Preclinical |
| RMC-8839 KRASG13C (Revolution Medicines) | EGFR, CDK4/6 | Preclinical | |
| RMC-6291 KRASG12C (Revolution Medicines) | - | Preclinical | |
| RMC-6236 KRASMULTI (Revolution Medicines) | - | Preclinical | |
| RAS companion inhibitors27,28 | RMC-4630 SHP2 (Revolution Medicines) | SHP2 or SOS1 inhibitors, ICIs | II |
| mTORC1 | - | I | |
| Immune checkpoints | MEK, ERK, KRAS G12C, RTK, ICIs | - | |
| mRNA vaccine and RNAi | - | I/II |
Abbreviations: KRAS, Kirsten rat sarcoma viral oncogene; RAS, rat sarcoma viral oncogene; ICIs, immune checkpoint inhibitors; PD-1, programmed cell death-1; PD-L1, programmed death ligand 1; SHP2, Src homology-2 domain containing protein tyrosine phosphatase-2; MEK, MAPK/ERK kinase; EGFR, epidermal growth factor receptor; mTOR, mammalian target of rapamycin; CDK, cyclin-dependent kinase; VEGF, vascular endothelial growth factor; SOS1, SOS Ras/Rac guanine nucleotide exchange factor 1; ERK, extracellular signals regulated kinases; RTK, receptor tyrosine kinase; mTORC1, mechanistic target of rapamycin complex 1.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Landre T, Justeau G, Assié JB, et al. Anti-PD-(L)1 for KRAS-mutant advanced non-small-cell lung cancers: a meta-analysis of randomized-controlled trials. Cancer Immunol Immunother. 2022;71(3):719–726. doi: 10.1007/s00262-021-03031-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warren GW, Cummings KM. Tobacco and lung cancer: risks, trends, and outcomes in patients with cancer. American Society of Clinical Oncology educational book American Society of Clinical Oncology Annual Meeting; 2013:359–364. [DOI] [PubMed] [Google Scholar]
- 3.Moore AR, Rosenberg SC, McCormick F, Malek S. Author Correction: RAS-targeted therapies: is the undruggable drugged? Nat Rev Drug Discov. 2020;19(12):902. doi: 10.1038/s41573-020-0089-1 [DOI] [PubMed] [Google Scholar]
- 4.Nassar AH, Adib E, Kwiatkowski DJ. Distribution of KRAS G12C somatic mutations across race, sex, and cancer type. N Engl J Med. 2021;384(2):185–187. doi: 10.1056/NEJMc2030638 [DOI] [PubMed] [Google Scholar]
- 5.Loong HH, Du N, Cheng C, et al. KRAS G12C mutations in Asia: a landscape analysis of 11,951 Chinese tumor samples. Translat Lung Cancer Res. 2020;9(5):1759–1769. doi: 10.21037/tlcr-20-455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbour KC, Jordan E, Kim HR, et al. Effects of co-occurring genomic alterations on outcomes in patients with KRAS-mutant non-small cell lung cancer. Clin Cancer Res. 2018;24(2):334–340. doi: 10.1158/1078-0432.CCR-17-1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fell JB, Fischer JP, Baer BR, et al. Identification of the clinical development candidate MRTX849, a covalent KRASG12C inhibitor for the treatment of cancer. J Med Chem. 2020;63(13):6679–6693. doi: 10.1021/acs.jmedchem.9b02052 [DOI] [PubMed] [Google Scholar]
- 8.Skoulidis F, Li BT, Dy GK, et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med. 2021;384(25):2371–2381. doi: 10.1056/NEJMoa2103695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong D, Bang Y, Barlesi F, et al. 1257O Durability of clinical benefit and biomarkers in patients (pts) with advanced non-small cell lung cancer (NSCLC) treated with AMG 510 (sotorasib). Ann Oncol. 2020;31:S812. doi: 10.1016/j.annonc.2020.08.1571 [DOI] [Google Scholar]
- 10.Hallin J, Engstrom LD, Hargis L, et al. The KRAS(G12C) inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov. 2020;10(1):54–71. doi: 10.1158/2159-8290.CD-19-1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briere DM, Li S, Calinisan A, et al. The KRAS(G12C) inhibitor MRTX849 reconditions the tumor immune microenvironment and sensitizes tumors to checkpoint inhibitor therapy. Mol Cancer Ther. 2021;20(6):975–985. doi: 10.1158/1535-7163.MCT-20-0462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Zhang J, Liu Q, et al. Resistance looms for KRAS G12C inhibitors and rational tackling strategies. Pharmacol Ther. 2022;229:108050. doi: 10.1016/j.pharmthera.2021.108050 [DOI] [PubMed] [Google Scholar]
- 13.Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503(7477):548–551. doi: 10.1038/nature12796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong DS, Fakih MG, Strickler JH, et al. KRAS(G12C) inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383(13):1207–1217. doi: 10.1056/NEJMoa1917239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dy GK, Govindan R, Velcheti V, et al. Abstract CT008: long-term outcomes with sotorasib in pretreated KRASp.G12C-mutated NSCLC: 2-year analysis of CodeBreaK100. Cancer Res. 2022;82(12_Supplement):CT008–CT008. doi: 10.1158/1538-7445.AM2022-CT008 [DOI] [Google Scholar]
- 16.J ML. Presented at European Society for Medical Oncology (ESMO) 2022 Annual Meeting; September 9–13; 2022; Paris, France. [Google Scholar]
- 17.Riely GJ, Ou SI, Rybkin I, et al. 99O_PR KRYSTAL-1: activity and preliminary pharmacodynamic (PD) analysis of adagrasib (MRTX849) in patients (Pts) with advanced non–small cell lung cancer (NSCLC) harboring KRASG12C mutation. J Thoracic Oncol. 2021;16(4):S751–S752. doi: 10.1016/S1556-0864(21)01941-9 [DOI] [Google Scholar]
- 18.Jänne PA, Riely GJ, Gadgeel SM, et al. Adagrasib in Non–Small-Cell lung cancer harboring a KRAS G12C mutation. N Engl J Med. 2022;387(2):120–131. doi: 10.1056/NEJMoa2204619 [DOI] [PubMed] [Google Scholar]
- 19.Luo J, Ostrem J, Pellini B, et al. Overcoming KRAS-mutant lung cancer. Am Soc Clinl Oncol Educ Book. 2022;41:1–11. [DOI] [PubMed] [Google Scholar]
- 20.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8(1):33–40. doi: 10.1038/nrd2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang XC, Wang J, Shao GG, et al. Comprehensive genomic and immunological characterization of Chinese non-small cell lung cancer patients. Nat Commun. 2019;10(1):1772. doi: 10.1038/s41467-019-09762-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a Phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbst R, Lopes G, Kowalski D, et al. LBA4 Association of KRAS mutational status with response to pembrolizumab monotherapy given as first-line therapy for PD-L1-positive advanced non-squamous NSCLC in Keynote-042. Ann Oncol. 2019;30:xi63–xi64. doi: 10.1093/annonc/mdz453.001 [DOI] [Google Scholar]
- 24.Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30(8):1321–1328. doi: 10.1093/annonc/mdz167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gadgeel S, Rodriguez-Abreu D, Felip E, et al. KRAS mutational status and efficacy in KEYNOTE-189: pembrolizumab (pembro) plus chemotherapy (chemo) vs placebo plus chemo as first-line therapy for metastatic non-squamous NSCLC. Ann Oncol. 2019;30:xi64–xi65. doi: 10.1093/annonc/mdz453.002 [DOI] [Google Scholar]
- 26.West H, Cappuzzo F, Reck M, et al. 1265P IMpower150: a post hoc analysis of efficacy outcomes in patients with KRAS, STK11 and KEAP1 mutations. Ann Oncol. 2020;31:S817–S818. doi: 10.1016/j.annonc.2020.08.1579 [DOI] [Google Scholar]
- 27.Nagasaka M, Azmi AS. Clinical progress of KRAS-targeted therapies: what next? Future Med Chem. 2022;14(15):1107–1110. doi: 10.4155/fmc-2022-0128 [DOI] [PubMed] [Google Scholar]
- 28.Désage AL, Léonce C, Swalduz A, Ortiz-Cuaran S. Targeting KRAS mutant in non-small cell lung cancer: novel insights into therapeutic strategies. Front Oncol. 2022;12:796832. doi: 10.3389/fonc.2022.796832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LoRusso PM. SY20-KRAS anniversary session: novel mechanisms for targeting KRAS-The clinical successes and challenges of drugging KRAS. American Association for Cancer Research Annual Meeting 2022 Itinerary Planner Home; 2022. [Google Scholar]
- 30.Canon J, Rex K, Saiki AY, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575(7781):217–223. doi: 10.1038/s41586-019-1694-1 [DOI] [PubMed] [Google Scholar]
- 31.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostrem JM, Shokat KM. Targeting KRAS G12C with Covalent inhibitors. Annual Rev Cancer Biol. 2022;6:49–64. doi: 10.1146/annurev-cancerbio-041621-012549 [DOI] [Google Scholar]