Abstract
Upper extremity deep vein thrombosis (UEDVT) is responsible for 4 to 10% of all deep vein thrombosis (DVT). Untreated UEDVT can lead to significant disability secondary to the postthrombotic syndrome. To date, there are no randomized trials specifically comparing different therapeutic strategies. Ultimately, optimal management of UEDVT depends on the underlying etiology, patient symptoms, and degree of thrombosis, with supporting evidence primarily extrapolated from lower extremity DVT data. This article will review the classification, presentation, and diagnosis of both primary and secondary UEDVT. In addition, it will discuss updates in clinical guidelines, anticoagulation, endovascular and surgical treatment strategies.
Keywords: upper extremity deep vein thrombosis, postthrombotic syndrome, endovenous catheter-directed therapy, venous thoracic outlet syndrome
Upper extremity deep vein thrombosis (UEDVT) accounts for 4 to 10% of all DVT, with an annual estimated incidence of 16 per 100,000. 1 2 The prevalence of UEDVT appears to be increasing due to more venous instrumentation or the placement of indwelling venous devices. 1 UEDVT is defined as either primary or secondary.
Primary UEDVT, also known as spontaneous or idiopathic UEDVT, is less common than secondary UEDVT and results from genetic hypercoagulable states or anatomic abnormalities predisposing patients to thrombosis. Typically, primary UEDVT is effort-induced and is distinguished from secondary UEDVT by lack of prior venous instrumentation. Up to 80% of patients presenting with primary UEDVT report a history of strenuous upper extremity activity or repetitive motions. Risk factors include young age, athletes, prolonged strenuous upper extremity activity with repetitive hyperabduction, congenital or acquired thoracic outlet narrowing, and primary thrombophilia.
Primary UEDVT from anatomic compression of a narrowed thoracic outlet is also known as venous thoracic outlet syndrome (VTOS), Paget-Schroetter syndrome, or effort thrombosis. The venous thoracic outlet is a narrow space between the clavicle superiorly, the first rib inferiorly, the costoclavicular ligament anteromedially, and the anterior scalene muscle posterolaterally. The subclavian vein, which travels in this space, can be stretched and compressed by these adjacent structures during arm abduction and neck extension, causing repetitive mechanical stress on the vein. The stress is especially pronounced in individuals with variant ligament or rib anatomy (such as cervical ribs) or a hypertrophied scalene muscle (due to sports or activities). Such trauma leads to a cycle of intimal damage and healing, resulting in UEDVT and fibrosis. In adults, the incidence of VTOS in men is twice that of women. However, in the pediatric population VTOS is equally seen across genders. 3
Genetic hypercoagulability is another important etiology of primary UEDVT, particularly in the pediatric population. Small case series of children with primary UEDVT found incidences of 8 to 61% of primary thrombophilia after workup. 4 The most common acquired thrombophilias include antiphospholipid (31% lupus anticoagulant, 13% anticardiolipin antibodies), factor V Leiden (13%), and prothrombin G20210A mutation (20%). Less frequent contributors include high plasma homocysteine levels and protein S deficiency. 4
Secondary UEDVT is far more common than primary UEDVT, responsible for approximately 80% of all UEDVT. 5 Secondary UEDVT is related to indwelling or implanted venous devices such as catheters or leads, surgery, trauma, or acquired hypercoagulable states related to malignancy, oral contraceptive use or pregnancy. The reported incidence of clinically overt UEDVT related to indwelling venous catheters has a wide variation of incidence (5–28%), 2 depending on the catheter location and type. Peripherally inserted central catheters (PICCs) are associated with a higher risk of UEDVT than central venous catheters (CVCs). 6 Nonetheless, an indwelling CVC is associated with a 14.0-fold increased risk of UEDVT. 6 It is also highest in those with malignancy, as these patients more frequently require central venous access and are often in a prothrombotic state. Malposition of the catheter tip in the brachiocephalic vein can also increase UEDVT risk, although most were asymptomatic. 7
Implantable cardioverter defibrillator (ICD) leads are a less frequent cause of secondary clinically overt UEDVT. In the early postoperative period, UEDVT was reported only in 0.2 to 0.7% of cases after ICD implantation and replacement. Long-term symptomatic UEDVT, occurring more than 2 months after device placements (typically years later), occurred at 0.9 per 100,000 in a recent 2021 meta-analysis. The overall pooled incidence of asymptomatic UEDVT was 8% in the same patient population. 8 Overall, catheter-related and acquired hypercoagulability states are more frequent causes of secondary UEDVT.
Clinical Presentations and Complications
Clinical presentation varies based on degree, chronicity, and cause of thrombosis. Patients with primary VTOS-related UEDVT commonly report strenuous arm use within 24 hours. Between 70 and 80% of patients will report discomfort in the neck, shoulder, and axilla, arm heaviness, pain, and swelling. 9 The pain and swelling improve with rest and worsens with elevating the arm over the head. Some also report sentinel symptoms of “heaviness” or “weakness” after repetitive over-the-head activities that improve with rest before presenting with acute thrombosis. VTOS can also be associated with neurologic or arterial thoracic outlet syndrome, which most commonly manifests as numbness or weakness in the ulnar distribution, blue discoloration of the hand, cold hands, and hand and/or arm pain.
Catheter-related UEDVT can range from entirely asymptomatic to debilitating. The phlebitis-related inflammatory reaction of thrombus is more commonly seen in superficial vein thrombosis related to PICCs. It can cause pain but tends to be self-limited and resolves once the catheter is removed. 10
On a physical exam, patients with acute thrombosis will have classic findings of acute DVT, including cyanosis, edema, and tenderness. 11 Phlegmasia cerulea dolens and rhabdomyolysis occur rarely. 12 Collateral veins over the chest (Urschel's sign) can develop in those with underlying chronic central venous stenosis (as with VTOS or implantable devices). A palpable venous cord can be apparent with superficial thrombophlebitis. Provocative maneuvers for thoracic outlet syndromes, such as the Adson test, often evaluate concomitant neurogenic or arterial thoracic outlet syndrome but are not specific or sensitive for VTOS. 3 11
Complications of UEDVT include pulmonary embolism (PE) and postthrombotic syndrome (PTS). In adults, UEDVT is associated with less clinically overt PTS compared to those with lower-extremity DVT (9.0 vs. 29%). 13 At 3 months, the risks of major bleeding, recurrent DVT, and recurrent PE were not significantly different from that of lower extremity DVT. Those with underlying malignancy tend to have worsened outcomes. 13 Another study found far lower risks of PE related to UEDVT of only 2% 14 with no PE-related mortality. The risk of symptomatic PE after removal of a CVC in those with CVC-associated UEDVT is low and does not increase when removed early (within 48 hours of diagnosis). 15
Postthrombotic syndrome is a long-standing, potentially disabling consequence of DVT, with symptoms of pain, heaviness, swelling, and paresthesias. It occurs in 7 to 46% of cases with conservative management alone and is more frequently seen with axillary and subclavian vein thrombosis and thrombophilia and less with catheter-related UEDVT. 16 A recent meta-analysis found higher rates of PTS after primary UEDVT compared to secondary UEDVT; however, the rate of recurrent DVT was higher in patients with secondary UEDVT. 17
Diagnosis and Imaging Findings
In patients with suspected UEDVT, ultrasound with Doppler imaging is considered the best initial screening tool and has greater than 80% sensitivity and specificity 18 ( Fig. 1 ). While ultrasound cannot directly evaluate central occlusions, signs such as dampening of respiratory variations can reliably suggest central venous occlusion from VTOS or malignancy. 18
Fig. 1.

Color and pulse wave Doppler examination of the left upper extremity subclavian vein demonstrating echogenic, expansile thrombus with no flow. These findings are consistent with acute upper extremity deep vein thrombosis (UEDVT).
Magnetic resonance venogram (MRV) and computed tomography venography (CTV) with contrast are noninvasive alternatives to conventional venography in diagnosing UEDVT and have different advantages and disadvantages. MRV can assess vessel lumen, vessel inflammation, and intraluminal occlusions. It is also excellent in identifying causes of extrinsic compression of central veins, such as lymphadenopathy or tumor. Furthermore, MRV can potentially differentiate from acute versus chronic thrombus. 19 However, MRV can be limited by breathing and flow artifacts that impair diagnosis and is insensitive for evaluating skeletal anomalies. CTV is excellent in identifying all causes of extrinsic compression and diagnosing DVT but uses ionizing radiation ( Fig. 2 ).
Fig. 2.

Computed tomography venography demonstrates compression of the left subclavian (arrows) as it traverses the thoracic outlet posterior to the clavicle and anterior to the left first rib.
Catheter venography remains the gold standard for evaluating VTOS and is usually performed after noninvasive imaging often as part of endovascular treatment. It is more sensitive than either MRV or CTV in identifying collateral veins. In addition, it can assess flow with arm adduction and abduction to aid the diagnosis of nonocclusive VTOS. Most importantly, a venogram allows subsequent interventions, including thrombolysis, thrombectomy, and venoplasty. 18
Therapeutic Options
To date, there are no randomized controlled trials to evaluate the optimal treatment for UEDVT. Consensus guidelines have derived their recommendations from data extrapolated from studies with lower extremity DVT. The 2016 American College of Chest Physicians (ACCP) suggested thrombolysis in those who have (1) severe symptoms, (2) thrombus involving most of the subclavian and axillary veins, (3) symptoms less than 14 days, (4) good functional status, (5) life expectancy of at least 1 year, and (6) low risk of bleeding. In addition, a 3-month course of anticoagulation was recommended regardless of whether a patient underwent thrombolysis. 20 Of note, the second update on antithrombotic therapy for VTE from the ACCP did not address updates specific to UEDVT. 21
Anticoagulation
Treatment of VTOS is often multifaceted, including treating the acute thrombosis and symptoms and the underlying inciting cause. Whether or not the patient receives endovascular catheter-directed therapies (CDTs), the patient should be started on anticoagulation at the time of presentation to maintain patency of collateral veins, reduce thrombus propagation, and prevent the risk of PE. However, patients treated with anticoagulation alone often suffer from ongoing symptoms and complications related to residual thrombus and fibrosis. 22 Studies directly comparing anticoagulation alone to anticoagulation with thrombolysis demonstrated improved outcomes and symptom reduction in the thrombolysis group compared to conservative therapy with anticoagulation only. 23 24 These have led to the recommendation against treating VTOS with axillary-subclavian vein thrombosis with anticoagulation alone if CDT can be safely performed. 20 Generally, anticoagulation is administered for 3 months after initial thrombosis and longer for recurrent thrombosis per extrapolation from ACCP guidelines. However, it can be as short as 4 to 8 weeks of anticoagulation after thrombolysis with subsequent surgical decompression for VTOS. 25 26 Physical therapy and sleeve compression can be adjunctive conservative treatments. 27
On the other hand, observation, anticoagulation, and removal of thrombogenic stimulus can be sufficient for catheter-related and other secondary causes of UEDVT. ACCP recommends anticoagulation if DVT involves axillary or more proximal veins. 20 Catheters may stay in place if there is an ongoing need for use as long as the device is functional and in a good position. This is often the case in the setting of PICC-related thrombus. A duration of 3 months of anticoagulation is also recommended if there is no contraindication to anticoagulation. 21 There is no anticoagulation recommendation for asymptomatic catheter-related thrombosis, although catheter removal is often generally accepted to prevent clot propagation. Early catheter removal does not increase the risk of clinically overt PE. 15
There is no specific anticoagulation regimen recommended for UEDVT. In 2021, ACCP recommended oral Xa inhibitor (apixaban, edoxaban, or rivaroxaban) and dabigatran over vitamin K antagonist for DVT of leg or PE. For cancer-associated thrombosis, oral Xa inhibitors are recommended over low-molecular-weight heparin (LWMH) for initiation and treatment phases, with the remark that apixaban or LMWH is preferred for luminal gastrointestinal (GI) malignancies as edoxaban and rivaroxaban are associated with higher risks of major GI bleeding. 21 These recommendations are based on and designed for lower extremity DVT and PE but are generally extrapolated for use in UEDVT. Prophylactic anticoagulation is not routine for patients with a CVC.
Endovascular Intervention
Venous Thoracic Outlet Syndrome
Catheter-directed thrombolysis (CDT) has become the mainstay therapy for patients with VTOS and has replaced systemic therapy for DVT because it requires lower doses of lytic agents ( Fig. 3 ). The goal of CDT is to limit the development of PTS. Data supporting CDT are again extrapolated from data supporting CDT in iliofemoral DVT. The CaVenT study demonstrated a 14% reduction in postthrombotic syndrome at the 2-year mark. Five-year follow-up demonstrated an absolute risk reduction for PTS of 28%. 28 The ATTRACT trial also showed reduced PTS severity in those with iliofemoral DVT that underwent CDT. Fewer patients experienced moderate-or-severe PTS after CDT, although there was no demonstrable difference in the total incidence of PTS. 29 Unfortunately, a randomized controlled trial evaluating anticoagulation alone versus CDT for VTOS-related UEDVT is unlikely due to the infrequent nature of this disease and because most presenting patients are young and active with a low risk of bleeding. As such, reducing the potential risk of long-term disability from PTS in these patients is paramount.
Fig. 3.

A 26-year-old female softball player presenting with acute left upper extremity pain and swelling. ( a ) Initial venogram demonstrates occlusion of the subclavian and axillary vein with extensive collateralization. The brachiocephalic is patent via collateral blood flow from the left internal jugular vein. ( b ) Initiation of overnight thrombolysis with the catheter in place across the occlusion. ( c ) Pharmacomechanical thrombolysis/thrombectomy was subsequently performed the next day, given persistent occlusion using the AngioJet Zelante (Boston Scientific, Marlborough, MA) catheter. ( d ) Final venogram demonstrates a patent subclavian and axillary vein with a marked decrease in the filling of collaterals.
If surgical decompression is intended, the exact timing of thrombolysis related to surgical decompression is controversial and is detailed in section “Surgical Management.” Doyle et al have shown that thrombolysis is less likely to be successful in patients with symptoms lasting more than 2 weeks. 30 At this point, the chronicity of this late subacute thrombus leads to vessel scarring. 26
Placement of venous stents prior to surgery is contraindicated due to the high risk of stent compression and fracture at the level of the thoracic outlet. 31 This is due to external compression and flexion on the stent. Even after first rib resection, venous stents placement is discouraged due to the persistent high risk of compression from the residual first rib stump. 26
Other endovascular therapies, including pharmacomechanical thrombectomy (PMT) or mechanical aspiration thrombectomy, have also been described in small case reports and series. Kärkkäinen et al reported successfully performing PMT in 22 out of 27 consecutive patients with the Trellis-8 peripheral infusion system (Covidien, Dublin, Ireland), which is no longer on the market. 32 Singh et al reported successful mechanical thrombectomy with the Indigo Aspiration System (Penumbra, Inc., Alameda, CA) for intraluminal thrombus that did not resolve after 12 hours of thrombolysis. 33 Sýkora et al reported successful mechanical thrombectomy in two patients presenting with rethrombosis after surgical decompression using the Indigo Aspiration System and the Aspirex device (Straub Medical, Wangs, Switzerland). 34 Since mechanical thrombectomy does not require thrombolytic, it should be considered in patients with a contraindication to CDT such as recent hemorrhage. Additionally, mechanical thrombectomy may be appropriate in the immediate postoperative period for cases of rethrombosis and in patients with more late-subacute thrombus that does not respond to CDT.
Secondary UEDVT
CDT may also be appropriate for patients suffering from severe symptoms after acute secondary UEDVT involving most or all axillary and subclavian veins. 21 However, given that the primary purpose of CDT is to reduce long-term symptoms, the risks of CDT should be carefully weighed against the patients' long-term prognosis relative to their underlying medical conditions. In general, CDT can be considered if patients have severe symptoms that do not respond to anticoagulation, have an active lifestyle that will require vigorous upper extremity movement, and are expected to live long enough for PTS to impact their quality of life significantly. Patients with malignancy should have a CT or MRI of the brain to rule out intracranial neoplasms, which are a contraindication to CDT. Patients with malignant superior vena cava (SVC) syndromes are an exception to the above generalization. Malignant SVC syndrome itself can be life-threatening and often requires urgent CDT and/or endovascular stenting ( Fig. 4 ).
Fig. 4.
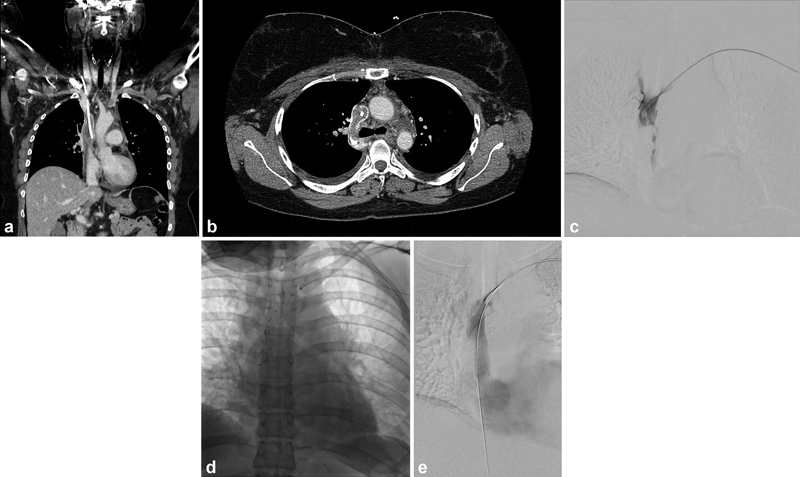
A 27-year-old female with a history of Hodgkin's lymphoma presenting with acute upper extremity and facial swelling. A CT scan was performed demonstrating significant thrombus surrounding the port catheter causing superior vena cava (SVC) occlusion ( a —coronal CT, b —axial CT). ( c ) Initial venogram demonstrates extensive thrombus within the SVC. ( d ) The patient underwent aspiration thrombectomy followed by CDT using EKOS. ( e ) The following day the patient underwent SVC stenting with a 16 mm × 60 mm Zilver Vena stent (Cook Medical, Bloomington, IN).
Superior Vena Cava Filters
There are no guidelines for SVC filter placement for patients with UEDVT and may be considered in patients who cannot be anticoagulated and are at high risk for clinically overt PE ( Fig. 5 ). It is important to note that the risk of PE from UEDVT is five times lower than that of LEDVT. However, due to SVC's location and short length, SVC filters are much more challenging to place correctly, and carries a greater risk of life-threatening complications. Risks include thoracic aortic perforation, SVC perforation, pericardial tamponade, and pneumothorax. Placing the filter in a safe location is especially challenging given the pericardium may extend as high as 5 cm above the superior cavoatrial junction. 35 Usoh et al, one of the largest studies, showed that filter strut perforated the SVC in three patients, causing cardiac tamponade in all of them. 36 As such, there is currently inadequate evidence to suggest that the benefit of placing an SVC filter outweighs the risk of filter placement and the risk of PE secondary to an UEDVT. 37
Fig. 5.

A 50-year-old male with extensive upper and lower extremity deep vein thrombosis, pulmonary embolism, and contraindication to thrombolysis s/p superior vena cava filter placement.
Surgical Management
Surgical management of VTOS is highly controversial in how, when, and whether it should be performed. To date, there are no official surgical guidelines for UEDVT. The surgical approach varies depending on the plan for reconstruction but includes resection of the anterior aspect of the first rib, anterior scalene muscle, subclavian muscle, and costoclavicular ligament. Traditionally this was done via a transaxillary or supraclavicular approach. However, more recently, an infraclavicular and a paraclavicular approach has also been described. 38 39 The key to all surgical approaches is access to the medial portion of the subclavian vein. Sometimes surgery may also include endovenectomy, vein patch angioplasty, and rarely venous reconstruction to address or resect the abnormal fibrotic portion of the vein and prevent VTOS recurrence. 26 40 When patch venoplasty is performed, it should span from a normal innominate vein to a normal axillary vein. 26
The timing of surgery ranges from a staged approach to immediately after thrombolysis. Some studies recommend early surgical decompression to avoid recurrent thrombosis, which may require repeat thrombolysis. Others recommend postponing surgery to after 14 days (typically months later) to allow time for vessel remodeling. 41 However, such a recommendation often requires prolonged anticoagulation. A recent systematic review of 126 patients (six articles) compared outcomes after early (≤2 weeks) versus late (>2 weeks) surgical decompression. Preoperative and postoperative rethrombosis occurred more frequently in the late surgical group (11 vs. 7%, preoperative; 31 vs. 18%, postoperative). Most perioperative bleeding complications come from a single study that performed surgery within 24 hours of CDT. Patients had hemothoraces, one requiring surgical decortication, and wound hematomas requiring drainage. Ultimately, however, the two groups had no difference in long-term PTS. 41 This study suggests that both techniques are safe and effective, except for surgical decompression within 24 hours of CDT.
More recently, some argue that anticoagulation and CDT alone are sufficient for VTOS, especially for asymptomatic patients after CDT. An argument for surgical intervention has been that the underlying anatomical compression remains, and that thrombolysis followed by anticoagulation alone has a reported rate of rethrombosis of 30%. 26 32 However, surgical decompression patients also have a 13 to 21% rethrombosis rate. 41 In addition, surgery carries a high complication rate of 5 to 21%, including brachial nerve injury, chronic pain syndrome, hemo- or chylothorax, and arterial injuries. 42 43
There are studies supporting both strategies. The meta-analysis by Lugo et al found more symptom relief among those with first rib resection than those treated conservatively (95 vs. 54%). 44 A critique of their method is that it did not assess lengths of follow-up and anticoagulation management. 42 Lee et al managed patients conservatively for a month and opted for nonoperative follow-up for those with minimal symptoms. They showed that all patients with minimal symptoms after a month (41% of all-comers) could resume their regular activity after 3 months of anticoagulation with minimal symptoms with a mean follow-up of 25.8 months. In addition, subsequent ultrasound at the last follow-up showed recanalization and patency of treated axillary and subclavian veins. 25 In 2021, a retrospective case series by Silverberg et al showed that 94% of patients did not develop PTS during a mean follow-up of 109 months after CDT and at least 6 months of anticoagulation; 78% of patients were completely asymptomatic. A mild difference in arm circumference of 1 to 2 cm was reported in 61% of patients. None had impaired quality of life secondary to the affected arm. Follow-up ultrasound also showed venous patency in all patients. This study showed long-term safety of nearly a decade of follow-up after nonoperative management. 42
Endovascular Procedural Technique
CDT technique has been previously described in detail by Carlon et al published in 2017 and has not significantly changed. 45 However, since this publication, several novel mechanical thrombectomy devices have become available on the market. The ClotTriever system (Inari Medical, Irvine, CA) has been successfully used for UEDVT. 46 It uses a 13-Fr specialty sheath with a flared funnel. As such, a minimal vein caliber of 6 mm is generally recommended. The Penumbra Indigo Aspiration System (Penumbra, Inc.) also has new sizes in 7 and 12 Fr for mechanical aspiration.
Conclusion
Despite the increasing incidence of UEDVT, there has been no large scientific breakthroughs or prospective data specific to the treatment of UEDVT. Updates in ACCP guidelines for anticoagulation and CDT have been specific to LEDVT but are nevertheless widely applied for UEDVT. Anticoagulation with and without CDT remains the first-line treatment for axillary-subclavian DVT regardless of the underlying etiology and is safe and effective. Similarly, data for surgical treatments of VTOS remain limited. The surgical strategy appears to evolve and trend toward a more delayed and conservative approach. Ultimately, dedicated randomized trials evaluating long-term outcomes with different therapeutic options for different etiologies of UEDVT are needed to improve patient outcomes.
Footnotes
Conflict of Interest None declared.
References
- 1.Isma N, Svensson P J, Gottsäter A, Lindblad B. Upper extremity deep venous thrombosis in the population-based Malmö thrombophilia study (MATS). Epidemiology, risk factors, recurrence risk, and mortality. Thromb Res. 2010;125(06):e335–e338. doi: 10.1016/j.thromres.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Engelberger R P, Kucher N. Management of deep vein thrombosis of the upper extremity. Circulation. 2012;126(06):768–773. doi: 10.1161/CIRCULATIONAHA.111.051276. [DOI] [PubMed] [Google Scholar]
- 3.Modi B P, Chewning R, Kumar R. Venous thoracic outlet syndrome and Paget-Schroetter syndrome. Semin Pediatr Surg. 2021;30(06):151125. doi: 10.1016/j.sempedsurg.2021.151125. [DOI] [PubMed] [Google Scholar]
- 4.Hendler M F, Meschengieser S S, Blanco A N. Primary upper-extremity deep vein thrombosis: high prevalence of thrombophilic defects. Am J Hematol. 2004;76(04):330–337. doi: 10.1002/ajh.20131. [DOI] [PubMed] [Google Scholar]
- 5.Kucher N. Clinical practice. Deep-vein thrombosis of the upper extremities. N Engl J Med. 2011;364(09):861–869. doi: 10.1056/NEJMcp1008740. [DOI] [PubMed] [Google Scholar]
- 6.Winters J P, Callas P W, Cushman M, Repp A B, Zakai N A. Central venous catheters and upper extremity deep vein thrombosis in medical inpatients: the Medical Inpatients and Thrombosis (MITH) Study. J Thromb Haemost. 2015;13(12):2155–2160. doi: 10.1111/jth.13131. [DOI] [PubMed] [Google Scholar]
- 7.Luciani A, Clement O, Halimi P. Catheter-related upper extremity deep venous thrombosis in cancer patients: a prospective study based on Doppler US. Radiology. 2001;220(03):655–660. doi: 10.1148/radiol.2203001181. [DOI] [PubMed] [Google Scholar]
- 8.Duijzer D, de Winter M A, Nijkeuter M, Tuinenburg A E, Westerink J. Upper extremity deep vein thrombosis and asymptomatic vein occlusion in patients with transvenous leads: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:698336. doi: 10.3389/fcvm.2021.698336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deep Vein Thrombosis (DVT) FREE Steering Committee . Joffe H V, Kucher N, Tapson V F, Goldhaber S Z, Deep Vein Thrombosis F SC. Upper-extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110(12):1605–1611. doi: 10.1161/01.CIR.0000142289.94369.D7. [DOI] [PubMed] [Google Scholar]
- 10.Paauw J D, Borders H, Ingalls N. The incidence of PICC line-associated thrombosis with and without the use of prophylactic anticoagulants. JPEN J Parenter Enteral Nutr. 2008;32(04):443–447. doi: 10.1177/0148607108319801. [DOI] [PubMed] [Google Scholar]
- 11.Sanders R J, Hammond S L, Rao N M. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46(03):601–604. doi: 10.1016/j.jvs.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 12.Lee J B, Kurzweil A, Lahham S. A case report on Paget-Schroetter syndrome presenting as acute localized rhabdomyolysis. Clin Pract Cases Emerg Med. 2020;4(03):358–361. doi: 10.5811/cpcem.2020.6.47335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.RIETE Investigators . Muñoz F J, Mismetti P, Poggio R. Clinical outcome of patients with upper-extremity deep vein thrombosis: results from the RIETE Registry. Chest. 2008;133(01):143–148. doi: 10.1378/chest.07-1432. [DOI] [PubMed] [Google Scholar]
- 14.Levy M M, Albuquerque F, Pfeifer J D. Low incidence of pulmonary embolism associated with upper-extremity deep venous thrombosis. Ann Vasc Surg. 2012;26(07):964–972. doi: 10.1016/j.avsg.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Houghton D E, Billett H H, Gaddh M. Risk of pulmonary emboli after removal of an upper extremity central catheter associated with a deep vein thrombosis. Blood Adv. 2021;5(14):2807–2812. doi: 10.1182/bloodadvances.2021004698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elman E E, Kahn S R. The post-thrombotic syndrome after upper extremity deep venous thrombosis in adults: a systematic review. Thromb Res. 2006;117(06):609–614. doi: 10.1016/j.thromres.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 17.Thiyagarajah K, Ellingwood L, Endres K. Post-thrombotic syndrome and recurrent thromboembolism in patients with upper extremity deep vein thrombosis: a systematic review and meta-analysis. Thromb Res. 2019;174:34–39. doi: 10.1016/j.thromres.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Expert Panel on Vascular Imaging Desjardins B, Hanley M, Steigner M L.ACR Appropriateness Criteria® suspected upper extremity deep vein thrombosis J Am Coll Radiol 202017(5S):S315–S322. [DOI] [PubMed] [Google Scholar]
- 19.Theia Study Group . van Dam L F, Dronkers C EA, Gautam G. Magnetic resonance imaging for diagnosis of recurrent ipsilateral deep vein thrombosis. Blood. 2020;135(16):1377–1385. doi: 10.1182/blood.2019004114. [DOI] [PubMed] [Google Scholar]
- 20.Kearon C, Akl E A, Ornelas J. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149(02):315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Stevens S M, Woller S C, Kreuziger L B. Antithrombotic therapy for VTE disease: second update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160(06):e545–e608. doi: 10.1016/j.chest.2021.07.055. [DOI] [PubMed] [Google Scholar]
- 22.Alla V M, Natarajan N, Kaushik M, Warrier R, Nair C K. Paget-Schroetter syndrome: review of pathogenesis and treatment of effort thrombosis. West J Emerg Med. 2010;11(04):358–362. [PMC free article] [PubMed] [Google Scholar]
- 23.AbuRahma A F, Sadler D, Stuart P, Khan M Z, Boland J P. Conventional versus thrombolytic therapy in spontaneous (effort) axillary-subclavian vein thrombosis. Am J Surg. 1991;161(04):459–465. doi: 10.1016/0002-9610(91)91112-v. [DOI] [PubMed] [Google Scholar]
- 24.Aburahma A F, Sadler D L, Robinson P A. Axillary subclavian vein thrombosis. Changing patterns of etiology, diagnostic, and therapeutic modalities. Am Surg. 1991;57(02):101–107. [PubMed] [Google Scholar]
- 25.Lee W A, Hill B B, Harris E J, Jr, Semba C P, Olcott C I V. Surgical intervention is not required for all patients with subclavian vein thrombosis. J Vasc Surg. 2000;32(01):57–67. doi: 10.1067/mva.2000.107313. [DOI] [PubMed] [Google Scholar]
- 26.Molina J E, Hunter D W, Dietz C A. Protocols for Paget-Schroetter syndrome and late treatment of chronic subclavian vein obstruction. Ann Thorac Surg. 2009;87(02):416–422. doi: 10.1016/j.athoracsur.2008.11.056. [DOI] [PubMed] [Google Scholar]
- 27.Edo Fleta G, Torres Blanco Á, Gómez Palonés F, Ortiz Monzón E. Combined non-surgical treatment for Paget-Schröetter syndrome: a case report. J Med Case Reports. 2016;10:171. doi: 10.1186/s13256-016-0940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CaVenT Study Group . Haig Y, Enden T, Grøtta O. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematol. 2016;3(02):e64–e71. doi: 10.1016/S2352-3026(15)00248-3. [DOI] [PubMed] [Google Scholar]
- 29.ATTRACT Trial Investigators . Comerota A J, Kearon C, Gu C S. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis. Circulation. 2019;139(09):1162–1173. doi: 10.1161/CIRCULATIONAHA.118.037425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doyle A, Wolford H Y, Davies M G. Management of effort thrombosis of the subclavian vein: today's treatment. Ann Vasc Surg. 2007;21(06):723–729. doi: 10.1016/j.avsg.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Meier G H, Pollak J S, Rosenblatt M, Dickey K W, Gusberg R J.Initial experience with venous stents in exertional axillary-subclavian vein thrombosis J Vasc Surg 19962406974–981., discussion 981–983 [DOI] [PubMed] [Google Scholar]
- 32.Kärkkäinen J M, Nuutinen H, Riekkinen T. Pharmacomechanical thrombectomy in Paget-Schroetter syndrome. Cardiovasc Intervent Radiol. 2016;39(09):1272–1279. doi: 10.1007/s00270-016-1376-4. [DOI] [PubMed] [Google Scholar]
- 33.Singh A, Kaur M, Singh A, Bhatti A, Patel R. Paget-Schroetter syndrome (PSS) and adjunctive treatment with mechanical aspiration thrombectomy and catheter directed thrombolysis: a case report and review of literature. Cureus. 2022;14(04):e24437. doi: 10.7759/cureus.24437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sýkora J, Zeleňák K, Vorčák M. Percutaneous thrombectomy in the management of early rethrombosis in venous thoracic outlet syndrome: two case reports. CVIR Endovasc. 2021;4(01):61. doi: 10.1186/s42155-021-00250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bayer O, Schummer C, Richter K, Fröber R, Schummer W. Implication of the anatomy of the pericardial reflection on positioning of central venous catheters. J Cardiothorac Vasc Anesth. 2006;20(06):777–780. doi: 10.1053/j.jvca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Usoh F, Hingorani A, Ascher E. Long-term follow-up for superior vena cava filter placement. Ann Vasc Surg. 2009;23(03):350–354. doi: 10.1016/j.avsg.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Owens C A, Bui J T, Knuttinen M G, Gaba R C, Carrillo T C. Pulmonary embolism from upper extremity deep vein thrombosis and the role of superior vena cava filters: a review of the literature. J Vasc Interv Radiol. 2010;21(06):779–787. doi: 10.1016/j.jvir.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 38.Samoila G, Twine C P, Williams I M. The infraclavicular approach for Paget-Schroetter syndrome. Ann R Coll Surg Engl. 2018;100(02):83–91. doi: 10.1308/rcsann.2017.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki T, Kimura H, Matsumura N, Iwamoto T.Surgical approaches for thoracic outlet syndrome: a review of the literatureJournal of Hand Surgery Global Online2022 [DOI] [PMC free article] [PubMed]
- 40.Elixène J B, Sadaghianloo N, Mousnier A, Brizzi S, Declemy S, Hassen-Khodja R. Long-term functional outcomes and subclavian vein patency in patients undergoing thoracic outlet surgery for Paget-Schroetter syndrome. J Cardiovasc Surg (Torino) 2017;58(03):451–457. doi: 10.23736/S0021-9509.16.08177-5. [DOI] [PubMed] [Google Scholar]
- 41.de Kleijn R JCMF, Schropp L, Westerink J, de Borst G J, Petri B J. Timing of thoracic outlet decompression after thrombolysis for primary upper extremity deep venous thrombosis: a systematic review. Ann Vasc Surg. 2020;66:654–661. doi: 10.1016/j.avsg.2020.01.083. [DOI] [PubMed] [Google Scholar]
- 42.Silverberg D, Fish M, Lubetsky A. Long-term outcome after nonsurgical management of Paget-Schroetter syndrome. J Vasc Surg Venous Lymphat Disord. 2021;9(01):170–177. doi: 10.1016/j.jvsv.2020.04.027. [DOI] [PubMed] [Google Scholar]
- 43.Peek J, Vos C G, Ünlü Ç, van de Pavoordt H DWM, van den Akker P J, de Vries J PM. Outcome of surgical treatment for thoracic outlet syndrome: systematic review and meta-analysis. Ann Vasc Surg. 2017;40:303–326. doi: 10.1016/j.avsg.2016.07.065. [DOI] [PubMed] [Google Scholar]
- 44.Lugo J, Tanious A, Armstrong P. Acute Paget-Schroetter syndrome: does the first rib routinely need to be removed after thrombolysis? Ann Vasc Surg. 2015;29(06):1073–1077. doi: 10.1016/j.avsg.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Carlon T A, Sudheendra D. Interventional therapy for upper extremity deep vein thrombosis. Semin Intervent Radiol. 2017;34(01):54–60. doi: 10.1055/s-0036-1597764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harmon D, Dabaja W, Qaqi O. A novel interventional approach to upper extremity swelling. J Vasc Surg Cases Innov Tech. 2020;6(02):209–211. doi: 10.1016/j.jvscit.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


