History of Vascular Plugs
The advent of vascular plugs for peripheral embolization dates to the early part of this century. To address the deficiencies of coils, the Food and Drug Administration (FDA) approved the first vascular plug, the Amplatzer vascular plug or AVP (Abbott Laboratories, Illinois), in 2004 for peripheral vascular embolization. 1 The original AVP, a derivative of the Amplatzer septal occluder and Amplatzer duct occluder, was first successfully utilized in the cardiac setting for percutaneous closure of aortopulmonary collaterals in a 4.5-month-old infant. 2 Two years later, interventionalists extended the use of the AVP to treat peripheral vascular malformations in patients with congenital heart disease. 3 The following year, the AVPII was released, which was capable of occluding vessels up to 17 mm in diameter, allowing for expanded clinical applications. As popularity and demand increased for the AVP models, advancements in vascular plug technology followed suit over the past 15 years. Today, vascular plugs have many unique attributes, including various shapes and constructs, microcatheter applications, membranes, and deployment methods ( Tables 1 and 2 ).
Table 1. Advantages, disadvantages, and cost of main plug components.
| Advantages | Disadvantages | Cost | Market examples | |
|---|---|---|---|---|
| Material | ||||
| Nitinol | Exhibits shape memory and superelasticity. Most common and readily available plug material on market |
Shown to have variable rates of recanalization. Not shown to have significant intradevice collagenous healing postembolization |
$$ | AVP, MVP, Caterpillar, AZUR |
| Polyurethane | Responds to more physical and chemical triggers than nitinol as a shape memory polymer. Shown to promote stable clot formation long term with reduce rates of recanalization compared with coils and nitinol plugs. Less expensive than nitinol plugs |
Relatively new in the market with limited microcatheter applications. Current models are on the higher side of the spectrum in regard to device length |
$ | IMPEDE |
| Membrane | ||||
| PTFE | Provides immediate mechanical blockage that is not dependent on thrombogenesis | Slightly adds to the overall cost of the device | $ | MVP, Caterpillar, IMPEDE, AZUR |
Table 2. Advantages, disadvantages, and cost of plug deployment methods.
| Advantages | Disadvantages | Cost | Market examples | |
|---|---|---|---|---|
| Plug deployment method | ||||
| Mechanical: Screw release |
Most common and readily available modality. Most cost efficacious |
Can cause unnecessary strain and torque on nearby vessels upon deployment | $ | AVP, MVP, Caterpillar |
| Mechanical: Button release | Easier and smoother deployment technique than screw release mechanism | Limited current options and research in regard to treatable vessel ranges. Slightly increases the overall price of a plug |
$$ | LOBO, AZUR |
| Electrolytic | Most precise delivery method | Most expensive deployment technique. Takes time for setup Also, not readily available in many current models |
$$$ | MVP, AZUR |
General Vascular Plug Technology
Vascular plugs offer effective embolization upon deployment mainly by two complementary mechanisms: (1) providing a mechanical occlusion and (2) serving as a porous embolic scaffold. Plugs are high-volume embolic materials that have been shown to slow down blood flow by effectively filling a void without exerting excessive radial force on nearby vasculature. 4 Through this resistance in flow, vascular plugs then serve as a nidus for thrombogenesis by inducing stasis which initiates the body's natural coagulation cascade at the deployment site. Newer vascular plugs have been shown to induce an acute inflammatory response in conjunction with the above mechanisms, further enhancing collagenous connective tissue deposition and mitigating intradevice recanalization 5 ( Fig. 1 ). Thrombosis typically occurs within 10 to 15 minutes for most vascular plugs, but occlusion times are highly dependent on vascular plug design, vessel size, concomitant use of anticoagulants such as heparin, and speed of incoming blood flow pertaining to the embolization. 6
Fig. 1.
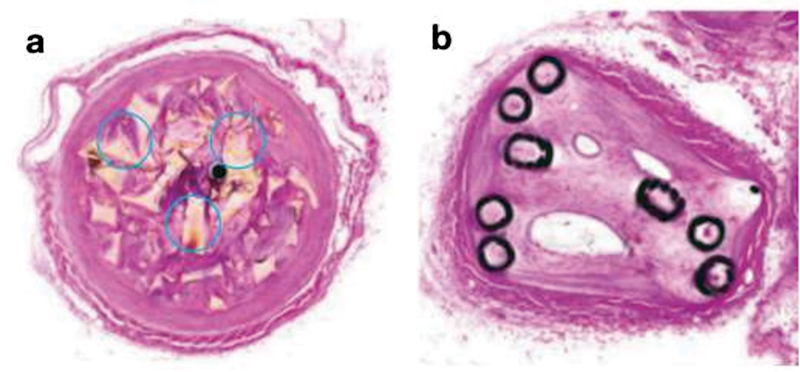
Plug histology. Porcine blood vessels treated with the IMPEDE Embolization plug (Shape Memory Medical, California) ( a ) vs. coils ( b ) at 60 days. Collagen granule (circles) formation at the site of plug deployment helps create a long-term stable clot, offsetting the higher degree of vessel recanalization seen in coils long term. (Images used with permissions from Shape Memory Medical.)
Patient Selection
Vascular plug embolization acts analogous to surgical ligation by causing complete occlusion of vessels. A vascular plug's subsequent thrombogenic properties depend on the patient's ability to initiate the coagulation cascade. As a result, vascular plugs, like coils, are most effective in a patient with an intact innate ability to coagulate. In coagulopathic patients, these devices may not induce complete occlusion, for example, in those with platelet dysfunction, clotting cascade impairments, or severe thrombocytopenia. 7
Materials
Most FDA-approved and clinically utilized vascular plugs are analogous in design to the AVP. The general blueprint of this device consists of a self-expanding, cylindrical nitinol cage with two platinum radiopaque marker bands on both ends that allow for visualization; the vascular plug is also attached to a stainless-steel micro screw and a nitinol delivery wire. Nitinol, an alloy of nickel and titanium, makes for an ideal embolization agent because it readily conforms in shape to its target vessel given its two unique properties of shape memory and superelasticity. 8 Furthermore, nitinol makes for a “smart material” given its ability to easily actuate in stress, strain, and heat. 9
Newer plugs have utilized polyurethane instead of nitinol in their design in a shape memory polymer (SMP) scaffold ( Fig. 2 ). These SMPs collectively respond to a wider range of chemical (i.e., pH or biological stimuli) and physical (i.e., heat, light, or magnetic fields) triggers than their shape memory alloy counterparts, allowing versatility in different embolization scenarios. 10 Additionally, these SMPs are cheaper, more strain-resistant, and less dense than alloys such as nitinol. 10 Recent studies have also shown these plugs to exhibit lower rates of recanalization and increased intra-device collagenous healing compared to traditional nitinol plugs. 5 However, the development of these SMPs is in its early stages, as there are currently no microcatheter applications, limiting their use in the embolization of smaller and more tortuous vascular anatomy.
Fig. 2.

Types of plug materials and membranes. ( a ) Nitinol cage framework of the AVP. ( b ) Polyurethane shape memory polymer of the IMPEDE plug. ( c ) PTFE membrane (red arrow) of the MVP (Medtronic, Dublin, Ireland). (Images used with permissions from Abbott, Shape Memory Medical, and Medtronic.)
Besides chemical composition, the other major component of modern plugs is the presence or absence of a membrane in their design. This membrane is usually made from polytetrafluoroethylene (PTFE) but can also be derived from polyethylene terephthalate (PET). The main advantage of these membranes is that they provide immediate mechanical blockage of flow that does not require thrombogenesis; this may be especially helpful in scenarios with high-stake bleeding (i.e., splenic artery rupture) where embolization is time-sensitive. Membranes marginally increase the overall cost of the plug device.
Shapes and Sizes
Vascular plugs are available in a host of different diameters, lengths, shapes, and braiding. Akin to other embolization devices, it is imperative that interventionalists are familiar with conventional notation on the plug device label regarding sizing. Briefly, the three-lettered model abbreviation comes first followed by a particular model number. The next number can either correspond to the diameter (usually in mm) of the entire plug complex or the diameter of the intended vessel the plug can treat. Following this, the total unconstrained device length (also in mm) is written. In the current market, vascular plug diameters range from 3 to 22 mm, with treatable vessel diameters ranging from 1.5 to 17 mm. When fully deployed to optimal diameter, vascular plug lengths range from 6 to 18 mm. Examples of different plug shapes include cylindrical, lobular, multilobular, and oblong designs. To add another layer of differentiation, vascular plugs can also come in various braiding designs (i.e., single- or multilayered) that can augment their overall occlusive and thrombogenic properties.
Deployment Method
Vascular plugs can be precisely and securely deployed after being advanced by a delivery wire. Most vascular plugs on the market employ mechanical detachment via a screw or button release mechanism ( Fig. 3 ). With the screw release mechanism, after final confirmation in location, the plug can be released by 360-degree counterclockwise rotation of the delivery wire. Unfortunately, this mechanism can theoretically place undue stress and torque on the surrounding vessel, further damaging the vasculature and leading to unintended consequences (i.e., vessel rupture or spasm). Additionally, the plug can sometimes be difficult to release in tortuous anatomy due to catheter redundancy. This makes it especially important to confirm beforehand that the vascular plug chosen for embolization is properly measured in all parameters for the intended vessel, as discussed later in the “General Technical Considerations” section. The button release mechanism unlatches the delivery wire from the plug upon the slide or press of a button. This technique drastically reduces the friction experienced in the screw release method, but only the newer vascular plug models, with limited sizing applications, are equipped with this option. Further research and development are necessary to broaden the scope of plug deployment mechanisms.
Fig. 3.
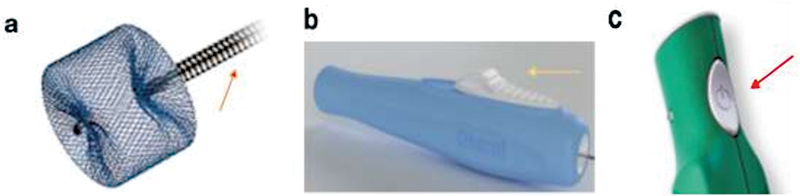
Types of plug deployment methods. ( a ) Screw (orange arrow) release of the AVP. ( b ) Button slide (yellow arrow) release of the LOBO occluder (Okami Medical, California). ( c ) Button press (red arrow) release of the AZUR plug (Terumo, New Jersey). (Images used with permissions from Abbott, Okami Medical, and Terumo Interventional Systems.)
Recently, some vascular plugs have begun employing electrolytic detachment, which utilizes a small electrical current to separate the delivery wire from the plug at their weld point, like some detachable coils. The major advantage of this technique is that it requires no motion (unlike mechanical detachment), allowing operators to deliver plugs with controlled precision; this can be particularly useful in tortuous anatomy where pinpoint deployment is difficult yet vital. Like their coil counterparts, these electrolytic detachable plugs have several drawbacks. These include being more expensive than mechanically detachable plugs, requiring increased setup time before delivery, and hypothetically adding more complication risks (e.g., electric current melting the tip of a catheter). 11 12 Nevertheless, as the technology expands, these electrolytic vascular plug models will have an integral role in the interventionalists' arsenal where precise delivery is of paramount importance.
General Technical Considerations
Preprocedural Planning, Catheter Selection, and Vascular Plug Release Strategies
As with any interventional procedure, deliberate and careful preprocedural planning is vital to successful vascular plug embolization. An interventionalist should be cognizant of not only the mechanics of a chosen vascular plug but also the appropriate tools necessary for successful utilization of that plug. Typically, each plug comes with a personalized table, provided by the manufacturer, which lists pertinent plug dimensions, and the minimum sheath, guide catheter, or microcatheter size requirement for that plug. On average, a 4-Fr sheath or 5-Fr guiding catheter is required to treat vessels greater than 5 mm in diameter. Furthermore, a 0.021-inch microcatheter is necessary to treat vessels 1.5 to 3 mm in diameter, while a 0.027-inch microcatheter is generally utilized for vessel diameters ranging from 3 to 5 mm. Additionally, some of the newer plugs require braided microcatheters which are more rigid and robust.
It is important to keep in mind that both the plug and its delivery wire will also have to be passed through the sheath or guide catheter. The delivery wires for many plugs are quite stiff (as are the devices themselves), which can lead to navigation problems in tortuous vasculature. To combat this, two strategies can be employed. 6 One tactic is to use a guide catheter within a sheath to improve stability for plug advancement. 6 13 The other method relies on utilizing a larger introducing sheath, which offers flexibility and support, to gain better access to the landing zone. 6 13
The geometry of intended deployment site is an important consideration on plug choice. Some devices, such as those with a membrane, function best in straight vessels which allow the membrane to open fully while others can be deployed in complex anatomy if the shape of the deployed device can conform to bifurcations or sharp angulation. Extremely tortuous anatomy is problematic for all plugs. In general, a coil can be successfully deployed in any vessel which can be catheterized; the larger or braided catheters required for many plugs may not be ideal for distal catheterization and in some instances, devices simply cannot be advanced through very tortuous anatomy to a desired target.
To initiate plug deployment, many operators utilize the “pull-back” technique (akin to inferior vena cava deployment) once a plug is appropriately positioned in its desired location with the distal radiopaque marker lined up with the catheter tip. 14 This strategy involves “pulling” versus pushing the plug out from the delivery catheter/sheath to avoid kinking or damaging the plug. 14 If positioning is unsatisfactory, plugs, like detachable coils, can be relocated by stabilizing the delivery wire and readvancing the guide catheter; contrast medium is also typically injected before final detachment to ensure proper plug positioning.
Oversizing
One important concept for vascular plug embolization is oversizing. This is essential for ensuring device stability and maximizing blood flow impedance and the available surface area for thrombogenesis. It is currently recommended that vascular plugs be oversized by 30 to 50% relative to the diameter of the intended target vessel. Oversizing is especially important for durable results, as studies have reported some plugs to reduce in shape and length by 50% after 5 months given the elasticity of nitinol. 15 Therefore, consideration of proper plug size and length is crucial for ensuring long-term plug integrity and avoiding recanalization. Undersized vascular plugs risk migration and nontarget embolization, although sometimes they can be retrieved with a snare. 16 In short landing zones, oversized vascular plugs can protrude and distort nearby structures. For these cases, it is possible to employ the “stuffing technique,” which suggests for the initial guide sheath to be advanced over an already deployed plug to allow for the proximal end (still in the sheath) to invaginate into the device's distal end before final detachment. 17
Anchoring Scaffold/“The Immobilizer” and Combination Occlusion
In large vessels, vascular plugs can serve to anchor or “immobilize” coils behind them to prevent mass coil migration, and consequently improve overall embolic packing density within a vessel 12 ( Fig. 4 ). This functionality has been reported in the embolization of dialysis fistulas and the treatment of intracranial vascular lesions. 18 19 Many operators also frequently utilize plugs in tandem with other embolizing agents (i.e., coils, glue, or gelatin particles) given the variable and delayed occlusion times for many vascular plugs. 20 Although there are many reports of improved embolization times using a combination of agents, operators must be wary of not overly bulking up a plug's profile, which can impede device deployment. 18 20 21 Furthermore, this can result in increased fluoroscopy time, procedure time, and cost when combining agents of embolization. 20 Although vascular plugs usually trap other agents and often prevent distal embolization, it is imperative for operators to be careful when utilizing small particles in critical areas (e.g., carotid artery or pulmonary circulation), as any subsequent migration can lead to devastating consequences. 20
Fig. 4.
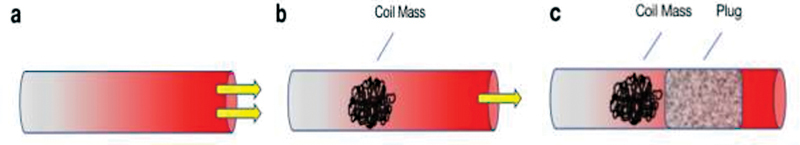
“The immobilizer” function of plugs. ( a ) High flow shown in a turbulent vessel. ( b ) Coil complex helps reduce blood flow, but instability and residual flow can cause coil migration. ( c ) A plug helps anchor the coils to prevent distal, nontarget embolization, further stabilizing and strengthening the intended embolization site.
Complications of Vascular Plug Occlusion
Generally, most vascular plugs are highly effective and safe embolic devices that can be utilized in a plethora of clinical scenarios with no absolute contraindications ( Figs. 5 6 7 8 ). Complications are quite rare; however, there have been reported cases of persistent patency, migration, and recanalization. 22 23 24 In cases of persistent patency and migration, complications are often secondary to incomplete angiographic evaluation before and after vascular plug placement resulting in inappropriate plug selection and sizing. 22 23 Technique and patient anatomy are often the main determinants in occlusion failure. 24 These issues further emphasize the importance of preprocedural planning, and consideration of the specific technical considerations of vascular plugs discussed earlier. Other potential adverse events associated with plug embolization include nontarget embolization, hematoma at the site of entry, vessel perforation or dissection, skin erosion of superficial vessels, spinal cord ischemia, pulmonary embolism, stroke, myocardial infarction, and, in rare cases, mortality.
Fig. 5.
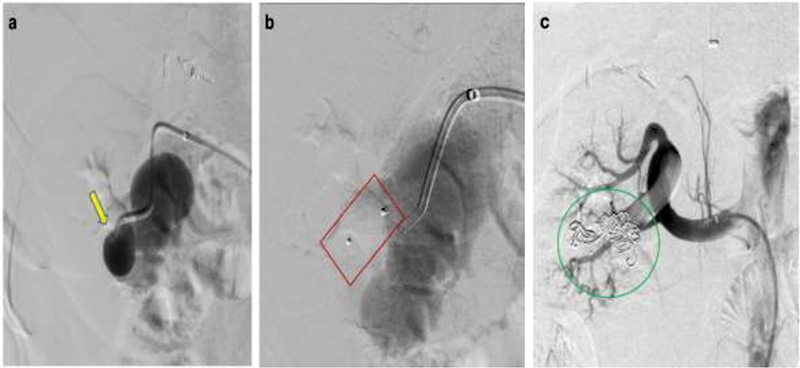
Utilizing combination occlusion to successfully embolize a right renal arteriovenous malformation (AVM). ( a ) Preprocedural angiogram demonstrates a high-flow, large right lower pole renal AVM (yellow arrow). Embolization with an AVP plug ( b , red box) and coils ( c , green circle) helps achieve stasis of inflow into the AVM with minimal residual outflow vessels.
Fig. 6.
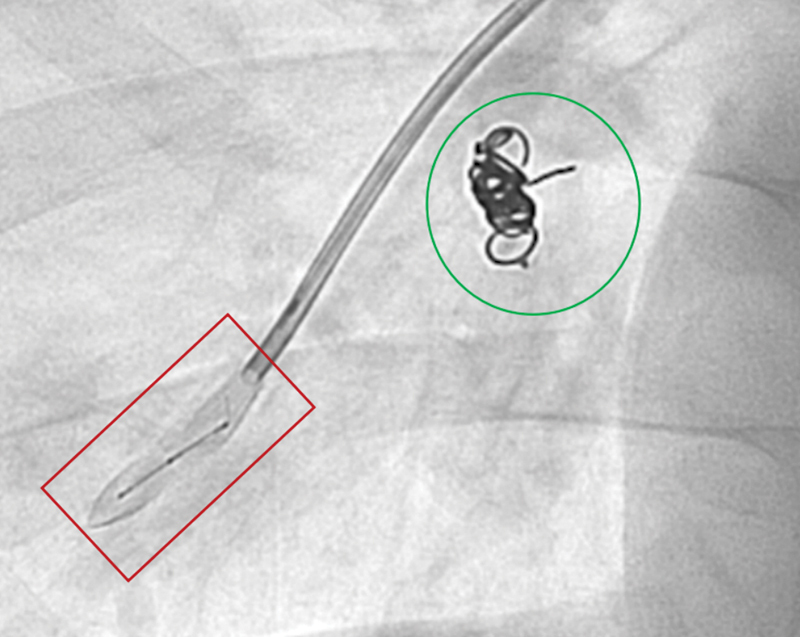
Embolization of a pulmonary AVM. Deployment of a Caterpillar (BD, New Jersey) embolization plug (red box) into a pulmonary AVM. Note previously deployed AVP plug with coils (green circle).
Fig. 7.
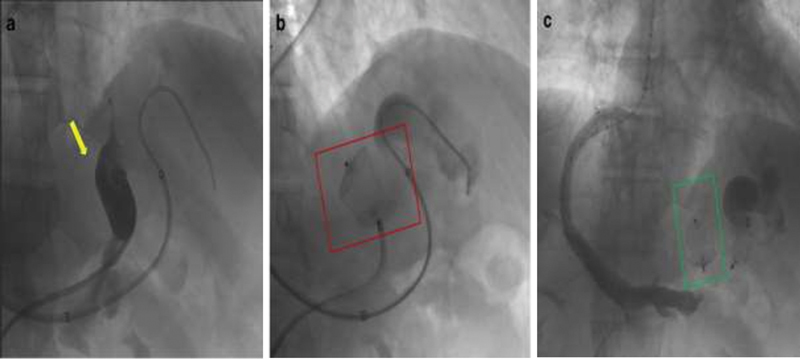
Double-plug embolization of a gastric varix and associated gastrorenal shunt. ( a ) Preprocedural venogram demonstrates flow into a gastric varix (yellow arrow). Placement of one AVP ( b , red box) at the base of the gastrorenal shunt and another AVP ( c , green box) within the afferent posterior gastric vein helps cease flow into the varix.
Fig. 8.
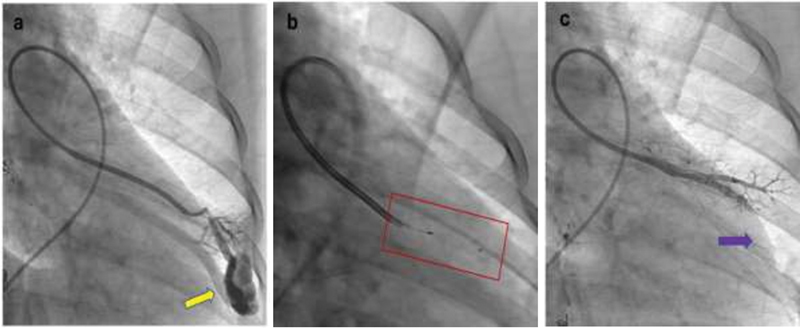
Occlusion of the feeding artery of a pulmonary AVM. ( a ) Selective pulmonary angiogram shows a simple pulmonary AVM (yellow arrow). Fluoroscopic image shows the deployment of the LOBO ( b , red box) which helps occlude the AVM ( c , purple arrow) on postembolization angiogram.
The “Perfect” Plug
Despite the advances and progress over the past two decades, the “perfect” vascular plug still does not exist. The ideal vascular plug will center on being affordable, easy to use, 100% reliable in occlusion, available in all vessel sizing applications, and ultimately require no additional embolization agent. This will be quite difficult; however, an interventionalist familiar with the unique advantages and disadvantages of each plug, along with other embolic agents, will certainly be able to find the “ideal” plug for any clinical scenario.
Future Plug Applications
Several new plugs are in development. One such device is the EMBA Medical's Hourglass (Dublin, Ireland) device. This device is a nitinol-based, membranous plug with a side port that allows for the injection of contrast or embolic material distally. Additionally, this plug is delivered over a 0.018-inch guidewire in a similar fashion to an endovascular stent. This hypothetical ease in deliverability combined with the potential to introduce other agents (i.e., gelatin particles) distally without significant operator maneuvering makes this plug a highlight for the near future. Further studies will certainly be necessary to judge the effectiveness of this vascular plug versus current devices in tortuous anatomy, and other difficult yet clinically relevant scenarios.
Conclusion
Vascular plugs are an important vehicle for embolization and have undergone significant advances since their advent in the early 2000s. Knowledge of their different specifications, technical considerations, and everexpanding applications will be crucial for maximizing their future role in interventional procedures.
Acknowledgements
None. Society of Interventional Radiology Foundation Summer Medical Internship Program (2022).
Footnotes
Conflicts of Interest B.F.: Consultant, Okami Medical.
R.J.L.: Consultant, BD, Boston Scientific, Varian.
References
- 1.Xiao N, Lewandowski R J. Embolic agents: coils. Semin Intervent Radiol. 2022;39(01):113–118. doi: 10.1055/s-0041-1740939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hijazi Z M. New device for percutaneous closure of aortopulmonary collaterals. Catheter Cardiovasc Interv. 2004;63(04):482–485. doi: 10.1002/ccd.20228. [DOI] [PubMed] [Google Scholar]
- 3.Hill S L, Hijazi Z M, Hellenbrand W E, Cheatham J P. Evaluation of the AMPLATZER vascular plug for embolization of peripheral vascular malformations associated with congenital heart disease. Catheter Cardiovasc Interv. 2006;67(01):113–119. doi: 10.1002/ccd.20555. [DOI] [PubMed] [Google Scholar]
- 4.Horn J, Hwang W, Jessen S L. Comparison of shape memory polymer foam versus bare metal coil treatments in an in vivo porcine sidewall aneurysm model. J Biomed Mater Res B Appl Biomater. 2017;105(07):1892–1905. doi: 10.1002/jbm.b.33725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jessen S L, Friedemann M C, Ginn-Hedman A M. Microscopic assessment of healing and effectiveness of a foam-based peripheral occlusion device. ACS Biomater Sci Eng. 2020;6(05):2588–2599. doi: 10.1021/acsbiomaterials.9b00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu X, Tam M D, Pierce G. Utility of the Amplatzer Vascular Plug in splenic artery embolization: a comparison study with conventional coil technique. Cardiovasc Intervent Radiol. 2011;34(03):522–531. doi: 10.1007/s00270-010-9957-0. [DOI] [PubMed] [Google Scholar]
- 7.Kauvar D S, Schechtman D W, Thomas S B. Endovascular embolization techniques in a novel swine model of fatal uncontrolled solid organ hemorrhage and coagulopathy. Ann Vasc Surg. 2021;70:143–151. doi: 10.1016/j.avsg.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert H B, Webster R J., III Rapid, reliable shape setting of superelastic nitinol for prototyping robots. IEEE Robot Autom Lett. 2016;1(01):98–105. doi: 10.1109/LRA.2015.2507706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holman H, Kavarana M N, Rajab T K. Smart materials in cardiovascular implants: shape memory alloys and shape memory polymers. Artif Organs. 2021;45(05):454–463. doi: 10.1111/aor.13851. [DOI] [PubMed] [Google Scholar]
- 10.Wen C, Yu X, Zeng W. Mechanical behaviors and biomedical applications of shape memory materials: a review. AIMS Mater Sci. 2018;5:559–590. [Google Scholar]
- 11.Lee C Y, Yim M B, Benndorf G.Mechanical detachment of Guglielmi detachable coils after failed electrolytic detachment: rescue from a technical complicationNeurosurgery 2008;63(4, Suppl 2):293–294, discussion 294 [DOI] [PubMed]
- 12.Pickett G E, Cora A. Electrothermal coil detachment failure in flow diverter-assisted coiling of a small blister aneurysm: technical considerations and possible solutions. Neurointervention. 2021;16(02):171–174. doi: 10.5469/neuroint.2020.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdel Aal A K, Hamed M F, Biosca R F, Saddekni S, Raghuram K. Occlusion time for Amplatzer vascular plug in the management of pulmonary arteriovenous malformations. AJR Am J Roentgenol. 2009;192(03):793–799. doi: 10.2214/AJR.08.1534. [DOI] [PubMed] [Google Scholar]
- 14.Giurazza F, Ierardi A M, Contegiacomo A, Corvino F, Carrafiello G, Niola R. Embolization with MVP (Micro Vascular Plug ® ): experience on 104 patients in emergent and elective scenarios . CVIR Endovasc. 2021;4(01):59. doi: 10.1186/s42155-021-00246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheridan B, Ward C, Justo R. Reconfiguration of the Amplatzer Vascular Plug II 5 months after occlusion of venovenous collateral in a bidirectional cavopulmonary circulation. Catheter Cardiovasc Interv. 2010;75(06):857–860. doi: 10.1002/ccd.22459. [DOI] [PubMed] [Google Scholar]
- 16.Singh H, Luthra M, Bharadwaj P, Kumar R. Interventional rerouting of scimitar vein to left atrium using an Amplatzer vascular plug. Congenit Heart Dis. 2007;2(04):265–269. doi: 10.1111/j.1747-0803.2007.00113.x. [DOI] [PubMed] [Google Scholar]
- 17.Peynircioglu B, Cil B. Amplatzer stuffing technique in the treatment of an iatrogenic mesenteric arteriovenous fistula. Cardiovasc Intervent Radiol. 2009;32(06):1247–1251. doi: 10.1007/s00270-009-9614-7. [DOI] [PubMed] [Google Scholar]
- 18.Owens C A, Bui J T, West D L, Sepahdari A. Use of the Amplatzer Vascular Plug as a coil constrainer during endovascular occlusion of a dialysis shunt. Cardiovasc Intervent Radiol. 2007;30(04):754–756. doi: 10.1007/s00270-007-9065-y. [DOI] [PubMed] [Google Scholar]
- 19.Hoit D A, Schirmer C M, Malek A M.Use of the Amplatzer vascular plug as an anchoring scaffold for coil-mediated parent vessel occlusion: technical case reportNeurosurgery 2006;59(1, Suppl 1):ONSE171-2, discussion ONSE171-2 [DOI] [PubMed]
- 20.Wang W, Tam M D, Spain J, Quintini C. Gelfoam-assisted Amplatzer vascular plug technique for rapid occlusion in proximal splenic artery embolization. AJR Am J Roentgenol. 2013;200(03):677–681. doi: 10.2214/AJR.12.8949. [DOI] [PubMed] [Google Scholar]
- 21.Glatz A C, Petit C J, Gillespie M J. Novel use of a modified Amplatzer Vascular Plug to occlude a patent ductus arteriosus in two patients. Catheter Cardiovasc Interv. 2008;72(01):82–86. doi: 10.1002/ccd.21546. [DOI] [PubMed] [Google Scholar]
- 22.White H A, Travis S J. The Amplatzer vascular plug. Cardiovasc Intervent Radiol. 2008;31(02):448–449. doi: 10.1007/s00270-007-9259-3. [DOI] [PubMed] [Google Scholar]
- 23.Maleux G, Rega F, Heye S, Troost E, Budts W. Asymptomatic migration of a first-generation Amplatzer vascular plug into the abdominal aorta: conservative management may be an option. J Vasc Interv Radiol. 2011;22(04):569–570. doi: 10.1016/j.jvir.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 24.Tapping C R, Ettles D F, Robinson G J. Long-term follow-up of treatment of pulmonary arteriovenous malformations with AMPLATZER Vascular Plug and AMPLATZER Vascular Plug II devices. J Vasc Interv Radiol. 2011;22(12):1740–1746. doi: 10.1016/j.jvir.2011.08.029. [DOI] [PubMed] [Google Scholar]


