To the Editor:
Lee et al1 evaluated multiple cases of severe transient vision loss after receiving an intravitreal injection of aflibercept from a prefilled syringe (PFS). They demonstrated that injection speed and syringe design both influence the injection force required to depress the syringe. Their data show that injection speed is directly proportional to injection force and recommend that physicians perform intravitreal injections at a slower rate.
However, this study does not fully explain why patients getting aflibercept with a PFS are experiencing severe transient vision loss. Vision loss after an intravitreal injection is likely related to an acute increase in intraocular pressure owing to excessive medication dosing, over the target of 0.05 ml.
As cited in Lee’s article, Gallagher et al2 showed that the internal cross sectional area of aflibercept PFS is nearly double that of a 1 ml tuberculin (TB) syringe. This means that any error in alignment of the syringe stopper with the mark on the syringe barrel results in a 2-fold greater error in volume delivered with the aflibercept syringe when compared with a 1 ml TB syringe. The authors expand on the etiology of injection variability by demonstrating that, even with near perfect alignment of the syringe (using a telecentric lens system), the force required to depress the syringe varies significantly with different speeds of injection. However, they do not show directly why this variable force provides variability in the injection volume.
I hypothesize that the variability of the intravitreal injection volume depends on the fact that the aflibercept PFS, and to a lesser extent the ranibizumab PFS, have syringe stoppers that deform significantly into the syringe dead space, allowing excess medication to be expressed.
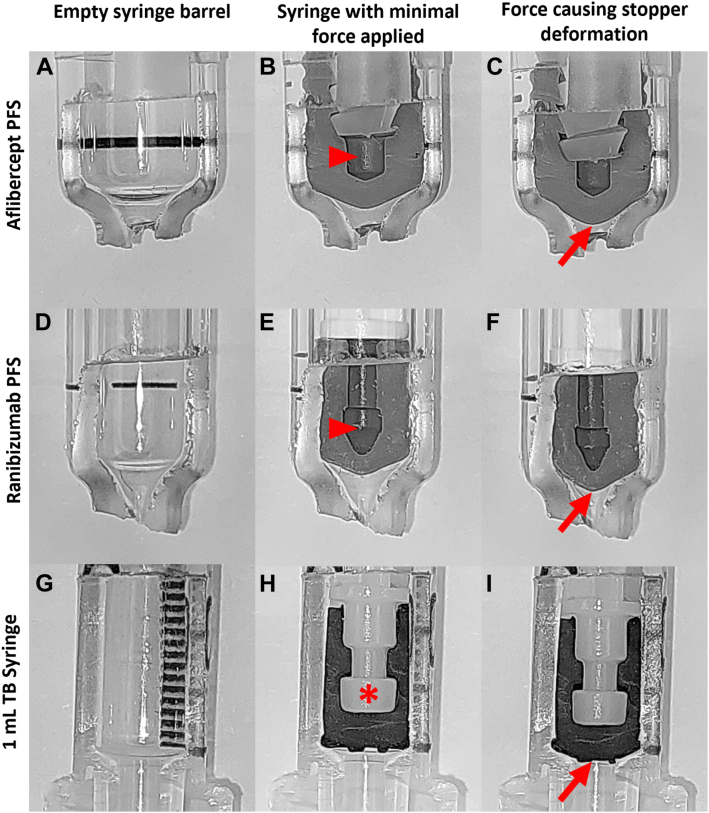
The aflibercept and ranibizumab PFS both have a syringe stopper that is hollow (Fig 1 B and E, red arrowheads), which allows it to deform significantly (Fig 1 C and F). This finding contrasts with the TB syringe, which has a solid stopper, in which the hollow portion is completely filled with the syringe plunger (Fig 1H, asterisk) so that there is no portion that is filled with void. Also, the design of the syringe stopper and distal aspect of the syringe barrel differs (Fig 1 A, D, and G). The aflibercept and ranibizumab PFS have a tapered stopper and a correspondingly tapered barrel. In contrast, the TB syringe has a relatively flat stopper and correspondingly flat distal aspect of the syringe barrel. This difference may allow more fluid to be expressed for the following two reasons.
Figure 1.
(A) Aflibercept prefilled syringe (PFS), (B) ranibizumab PFS, and a (H) 1 ml tuberculin (TB) barrel, and (B, E, H) with syringe stoppers fully depressed with minimal force. Each syringe had force applied by hand and the syringe stoppers can be seen deforming (red arrows C, F, and I) into the syringe barrel, decreasing the dead space in the distal aspect of the syringe. Of note, the stoppers in the aflibercept and ranibizumab PFS have a void (red arrowheads) in comparison to the TB stopper which has no void (asterisk).
First, the wide tapering distal aspect of the PFS allows more of the stopper to deform into the dead space of the barrel. This could lead to more fluid being expressed when the syringe stopper contacts the distal aspect of the syringe barrel and additional force is applied (Fig 1 C, F, and I). Second, the aflibercept and ranibizumab PFS have a soft stop at the completion of the injection, such that the physician may not be exactly sure when the medication has been expressed fully. In contract, TB syringes have a hard stop, making it easier for the physician to know when the injection is complete. If a physician continues to apply force to a syringe, a significant amount of excess medication can be expressed as the syringe plunger deforms (Fig 1 C, F, and I).
The deformation of the syringe stopper also explains the fact that the injection force for the 1 ml tuberculin (TB) syringe requires a relatively constant amount of force applied during the entire extension of the injection. In contrast, the ranibizumab and aflibercept PFS show that an increase in force is required to expel fluid as the syringe stopper approaches the distal aspect of the syringe barrel (Fig 4 A–D the Lee et al article). I hypothesize that this finding of the study is also caused by the syringe stopper deforming as it begins to contact the syringe barrel.
I agree with the conclusions that Lee et al1 came to, but also believe their patients are having severe transient vision loss owing to overdosing of medication with the aflibercept PFS likely due to the syringe stopper deforming into the syringe dead space, allowing for more medication to be delivered, well beyond the target of 0.05 ml.
Footnotes
Disclosure(s): All authors have completed and submitted the ICMJE disclosures form.
The authors have no proprietary or commercial interest in any materials discussed in this article.
References
- 1.Lee D.J., Scruggs B.A., Sánchez E., et al. Transient vision loss associated with prefilled aflibercept syringes. Ophthalmol Sci. 2022;2 doi: 10.1016/j.xops.2022.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallagher K., Raghuram A.R., Williams G.S., et al. Pre-filled aflibercept syringes—variability in expressed fluid volumes and a case series of transient central retinal artery occlusions. Eye. 2021;35:2899–2900. doi: 10.1038/s41433-020-01211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]