Abstract
Introduction
Periodontitis is a chronic inflammatory disease, resulting due to host immune response against subgingival biofilm. Most conventional treatment protocols aim to control the subgingival biofilm by mechanical means, such as dental scaling and root planning, and frequently accompanied by antibacterial co‐adjuvant therapies, including antibiotics, antiseptics, or probiotics. Local drug delivery facilitates administration of a lower dose of the drug to the target site, but at higher concentration, thereby reducing systemic adverse effects and toxicity. The present systematic review was conducted with the aim of identifying and reporting nanoparticle based periodontal drug delivery systems, with a specific focus on current trends and future perspectives in this field.
Materials & methods
Comprehensive literature search, restricted to published reports in English language between January 2000 and February 2022, was done electronically and manually. Search queries were addressed to the following electronic databases including, PubMed (MEDLINE), Science Direct (Elsevier), Cochrane Library, Web of Science (Clarivate Analytics) and Scholar (Google). Database search returned 780 results which were screened based on title, author names and publication dates, to identify 13 studies fulfilling the review criteria.
Results
Data from the 13 included studies were reviewed and tabulated, elaborating the type of nanoparticle used, drug delivered and tissues/cells/subcellular components targeted by periodontal drug delivery. While majority of the studies were conducted in vitro, there were 3 in vivo studies and 3 clinical studies. Using nanotechnology for drug delivery resulted in better inhibition of bacterial growth, inflammatory modulation favoring resolution of periodontitis and capability for early tissue regeneration.
Conclusion
Recent developments in nanotechnology have enabled targeted local delivery of drugs and anti-inflammatory biomolecules, in synergy with nanoparticles, towards periodontal pathogens, inflammatory cells and periodontal tissues. Further research evaluating clinical periodontal disease management through nanoparticle based local drug delivery drugs is highly recommended.
Keywords: Nanoparticles, Nanotechnology, Local drug delivery, Periodontal diseases
1. Introduction
The chronicity of periodontitis is often attributed to the development of host inflammatory response to the sub gingival biofilm (Van Dyke, 2007). Migration of the gingival epithelium due to pocket formation, leads to alveolar bone destruction and attachment apparatus degeneration (Hajishengallis and Lamont, 2012). Eradication of the periodontopathic biofilm and prevention of recurrence with supportive therapy remains the current focus of periodontal disease management (Kinane and Bartold, 2007). Based on an understanding of the pathophysiology behind periodontitis and the contributing role of the host immune response, the conventional treatment modalities of scaling and root planning focus on controlling the sub-gingival biofilm. Evidence from literature suggest proven success in decreasing the subgingival biofilm with the usage of routine mechanical cleaning, irrigation devices and syringes with dentifrices or mouth rinses. Nonetheless, long term patient compliance and permanent periodical controls are having a definitive influence on the recovery and health of periodontal tissues (John et al., 2017).
In the last two decades, the conventional periodontal managements, namely mechanical debridement and routine oral hygiene procedures, have often been supported by antibiotics, antiseptics or probiotics to enhance the treatment outcomes (John et al., 2017). However, the mode of delivering these therapeutic agents to the target site remains a challenge (Aminu et al., 2013). Usage of systemic antibiotics for management of periodontal diseases is often discouraged due to the impending side effects and lack of adequate target site concentration. In addition, sub-therapeutic levels of antibiotics due to rapid decrease in plasma concentration, gastrointestinal intolerance, microbial resistance and hypersensitivity have also been reported (Osorio et al., 2016). Efforts to mitigate these side effects have led to the emergence of new effective local drug delivery systems, which can achieve higher target site concentration of drug molecules with more effectiveness and minimal systemic adverse effects or toxicity (Benatti et al., 2012). These drug delivery systems aim to achieve a safe therapeutic level of the prescribed pharmacological formulations in the periodontium through innovative approaches and technologies (Muhamad et al., 2015).
Ideally, local drug delivery systems safely transport the active compound(s) to the target site, tissue or cells with enhanced residence time and also enable optimal mucosal contact with the periodontium (Yao et al., 2015). Furthermore, in order to facilitate epithelial transport of inefficiently absorbed drugs, local delivery systems should also establish stable contact with the junctional epithelium. This approach helps ascertain regeneration of the deteriorated periodontal tissues (Hau et al., 2014). In the recent times, conventional therapies have further been augmented with direct immunomodulatory strategies, including administration of antimicrobial vaccines or anti-inflammatory molecules, through local delivery (Benatti et al., 2012). The primary objective here is to inhibit the pro-inflammatory axis of immune response or increase the regulatory immune response (Duarte et al., 2012). Similar to locally delivered drugs, the sustained presence of an effective concentration of these anti-inflammatory agents at the target site, is imperative for long term success. Current advances in nanotechnology and nanoparticle based drug delivery approaches have enabled researchers and clinicians achieve the aforementioned goal. Wherein, drug molecules in the form of nanoparticles and scaffolds are encapsulated or loaded onto carriers, which help releasing them in the targeted location in a sustained and controlled manner (Dubar et al., 2021).
In addition to providing a mechanism of sustained and targeted delivery of drug molecules or bioactive therapeutic compounds, local drug delivery systems also aim at reducing dosing frequency as a means of enhancing patient compliance and quality of life (Toker et al., 2019). This is very well achieved with the advanced nanotechnology based drug carriage systems, which provide the benefit of autonomous regulation of drug release from the site of placement (Rathor et al., 2017). Additionally, advanced production technologies have led to the synthesis of nanoparticle scaffolds and nanostructured carriers, which are completely biodegradable, and require no removal after their intended therapeutic drug release action is complete (Cafferata et al., 2020). All of the above have resulted in the increased usage of nanocarriers for drugs, antigens or macromolecules, which can be entrapped in a polymeric matrix, encapsulated in a liquid core, surrounded by a polymeric membrane, or bound to the nanoparticle surface by adsorption (Jung et al., 2000). Furthermore, the presence of a larger specific surface area which can be exposed to the biological environment and the inherent diverse biological distribution potential at cellular and tissue levels have improved the bioactive properties of nanocarriers (Naskar et al., 2021). Nevertheless, economic liability and time consumption associated with the production of specific nanotechnology based controlled release drug formulations, effective in achieving the desired pharmacodynamics, have restricted their clinical use (Kamaly et al., 2016). Therefore, the present systematic review was conducted with the aim of identifying and reporting nanoparticle based periodontal drug delivery systems, with a specific focus on current trends and future perspectives which could be clinically translated. This review also envisages to analyze systems delivering immunomodulatory therapies, in the form of nanocarriers composed of different polymers such as polylactic-co-glycolic acid (PLGA), chitosan, gelatin, silica, gold, silver and similar materials.
2. Materials & methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews (PRISMA-2020) guidelines. A literature searches with publications restricted to English language was done both electronically and manually. From the collected data, we have conceived a summary of the research findings about the design and architecture of nanomaterials, as well as their applications and advantages in periodontal local drug delivery systems.
The following focused review question were addressed during the review process.
-
1.
What are the nanoparticle based drug delivery systems for periodontal diseases, reported in the literature?
-
2.
What is the efficacy of nanoparticle based drug delivery systems in the management of periodontal disease conditions?
The PICOS strategy for the review was as follows:
Population: Patients with acute or chronic periodontal conditions, and animal models, cells and/or bacterial cultures mimicking the periodontal disease milieu and process.
Intervention: Nanoparticle and nanotechnology based local drug delivery systems.
Comparison: Conventional systemic and localized drug delivery systems.
Outcome: Efficacy of the drug in alleviating the periodontal condition in terms of (but not restricted to) periodontal tissue and bone healing and regeneration, antimicrobial efficacy against periodontal pathogens, anti-inflammatory action, and stimulation of growth factor expression, osteoblasts and fibroblasts.
Studies: In vitro and in vivo studies, and clinical randomized and non-randomized trials.
2.1. Search strategy
Search queries were addressed to the following electronic databases including, PubMed (MEDLINE), ScienceDirect (Elsevier), Cochrane Library, Web of Science (Clarivate Analytics) and Scholar (Google), using a combinations of keywords such as new, newer, novel, nano, nanoparticle, nanotechnology, “drug delivery”, “drug delivery system”, perio, “periodontal tissue”, “periodontal disease”, “periodontal regeneration”, along with Boolean operators AND/OR. For instance, the following search strategy was used in PubMed without any limit or filter, and then adapted for the other databases: ((new OR newer OR novel) AND (nano OR nanoparticle OR nanotechnology) AND (“drug delivery” OR “drug delivery system”) AND (perio OR “periodontal disease” OR “periodontal regeneration’ OR “periodontal tissue”)).
Articles and monographs falling within the search criteria and published in English between January 2000 and February 2022 were taken into consideration. The selected articles were downloaded onto a citation management software ENDNOTE X7, and the titles were screened for relevance and removal of duplicates. Abstracts of the screened article titles were thoroughly reviewed based on the selection criteria, to identify the full text manuscripts for final systematic review. The preliminary electronic search was complemented by a manual search of the list of references of the items included, and searching in Cochrane database of systematic reviews, Cited articles (snow balling), Gray Literature (https://www.opengray.eu) and clinical trials registry (https://www.clinicaltrails.gov). In order to standardize the review process, 2 independent calibrated reviewers with an inter-rater reliability value (K = 0.71), evaluated the selected full text manuscripts. An article was adjudicated for systematic review only when both reviewers were in agreement based on the selection criteria, and any disagreement was resolved through discussion and rereading.
2.2. Selection criteria
-
(a)
Inclusion criteria.
-
•
Original studies evaluating the efficacy of nanotechnology and nanoparticle based periodontal drug delivery system
-
•
Published in indexed literature from January 2001 until February 2022
-
•
Study comparison done in a homogeneous setting (Intervention and controls reported in the same study)
-
(b)
Exclusion Criteria.
-
•
Case reports, letters to the editor and technical notes
-
•
Non-English reports and publications in grey literature
-
•
Studies with heterogeneity in data collection
2.3. Data extraction
Data extraction was performed using a modified Cochrane data collection form considering the following parameters: author; year of publication; including eligibility, study methods, participants, interventions, outcomes and results. Data extraction was analyzed separately for in vitro, in vivo, and clinical studies.
2.4. Strategy for data synthesis
A narrative analysis of the included studies was conducted, dividing the studies by their design into in vitro, in vivo, and clinical studies. No quantitative analysis was performed. Study outcomes, such as the decrease in antimicrobial activity, periodontal pocket reduction, bone regeneration, minimum inhibitory concentration, and type of nanoparticles were considered, and tabulated. (Table 1).
Table 1.
Detailed characteristics of the studies included in the review.
| Author (year) | Study design | Nature of nanoparticle used | Drug delivered | Target cell/tissue/organism | Outcomes | Conclusions |
|---|---|---|---|---|---|---|
| Wayakanon et al. (2013) | In Vitro | Polymersomes | Metronidazole or doxycycline | Intracellular P. gingivalis within monolayers of keratinocytes and organotypic oral mucosal models | Normal oral keratinocytes (NOKs) and oral fibroblasts (NOFs) were isolated from gingival biopsies, the oral squamous carcinoma cell lines, H357 and TR146 were cultured as test samples. In H357 cells, polymersome-encapsulated metronidazole reduced the number of intracellular bacteria by more than half [2110 CFU/ml to 810 CFU/ml]. Polymersome-encapsulated doxycycline also significantly (p < 0.05) reduced the number of intracellular P.gingivalis in TR146-infected cells compared to controls and is comparatively more effective than encapsulated metronidazole in reducing the intracellular bacteria in TR146. Encapsulated polymersomes reduce intracellular P. gingivalis load in tissue-engineered models of the oral mucosa. |
Polymersomes are effective delivery vehicles for antibiotics that do not normally gain entry to host cells. Effective in recurrent periodontitis or other diseases caused by intracellular dwelling organisms. |
| Madhumathi and Sampath Kumar (2014) | In Vitro | Calcium deficient hydroxyapatite nanocarriers prepared from Ca(OH2) and (NH4)2HPO4 solutions, of different Ca/P ratios CDHA1.55/1.61/1.64 | Tetracycline | S. aureus and E. coli bacteria in human periodontal ligament fibroblast cells (hPDLF). | MIC of pure TC was 20 μg/μl for S. aureus and for E. coli it was 10 μg/μl. TC-CDHA1.61 had an MIC which was 5 times higher (equivalent to 100 μg/μl) than that of pure TC against S. aureus and 9 times higher (equivalent to 90 μg/μl) than that of pure TC against E. coli. The hPDLF cells may also be mildly susceptible to the nano-plate like morphology of the CDHA nanoparticles |
CDHA nanocarriers are ideal for drug delivery and have bone regenerative potential in local periodontal applications. |
| Lee et al. (2016) | In Vitro + In vivo | Poly(d,l-lactide-co-glycolide acid) (PLGA) and chitosan NPs | Tetracycline + Lovastatin | Osteoblast cell cultures (ALP activity and cytotoxicity assay),A.actinomycetemcomitans and P. nigrescens (MIC assay), and beagle dogs (periodontal defect regeneration) | Both A. actinomycetemcomitans and P. nigrescens, had a clear inhibition zone on days 1, 3, 5, and 7 in the PLGA-lovastatin-chitosan-tetracycline 0.3% group and had an inhibitory effect at the first day of incubation, which continued to day 7. Although PLGA-chitosan and PLGA-lovastatin-chitosan-tetracycline NPs showed similar cell viability, the latter had a significantly higher ALP expression. Active plasmacytoid osteoblastic rimming along the trabecular surface of the bone adjacent to the defect was prominent in the experimental group. A statistically significant difference (P = 0.04) in the percentage of new bone formation between the experimental (41.32%) and control (34.01%) groups by micro-CT analysis. |
PLGA-lovastatin-chitosan-tetracycline nanoparticles showed good antibacterial activity, biocompatibility and increased alkaline phosphatase activity. The volumetric analysis from micro-CT revealed significantly increased new bone formation in defects filled with nanoparticles in dogs. |
| Mahmoud and Samy (2016) | In Vitro + Clinical | Nano DOX/chitosan particulate system incorporated in PVA-based films | Doxycycline | Periodontal tissue cells | In-vitro DOX release was sustained for about a week with the nano-structured films showing 23% of the drug released compared to 44% released from DOX films. Placement of nano-DOX and DOX films after SRP in periodontal pockets, lead to significant clinical improvement. |
A significant effect of both nano-structured and DOX films in improving the periodontal parameters compared with the control and placebo groups. |
| Ranjbar-Mohammadi et al. (2016) | In Vitro | Composite PG nanofibers of PLGA and GT were blended by electrospinning and coaxial electrospinning. | Tetracycline hydrochloride | S. aureus and Pseudomonas aeruginosa as model bacteria. | Clear bacterial inhibition rings against Gram-positive S. aureus which is known causes a wide range of suppurative infections and is sensitive to tetracycline. The bacterial inhibition ring was smaller against Gram-negative P. aeruginosa. Compared to TCH, the electrospun scaffolds showed insignificant changes in cell viability after 5 days. PG 75:25 composite nanofibers showed a sustained increase in TCH release up to a period of 25 days, after which it plateaued. For the core shell nanofibers, where TCH was incorporated in the core along with GT, the burst release was significantly lower (19%), compared to both pure PLGA and PG blend nanofibers. PLGA nanofibers exhibited an initial burst release of 23.20% within the first 2 h and reached a plateau within 7 days, by releasing only 35% of TCH content. |
Optimum burst release followed by prolonged drug release by the drug loaded core shell nanofibers make them a promising drug delivery system for periodontal diseases. |
| Emmanuel et al. (2017) | In Vitro |
Justicia glauca (aqueous leaf extract) mediated AuNPs |
Azithromycin and Clarithromycin |
Micrococcus luteus, Bacillus subtilis, Staphylococcus aureus, Streptococcus mutans, Lactobacillus acidophilus, Escherichia coli, Pseudomonas aeruginosa, Saccharomyces cerevisiae and Candida albicans |
The AuNPs and drug conjugated AuNPs showed potential antibacterial and antifungal activity against the oral pathogens. MIC values of biogenic AuNPs conjugated with antibiotics (azithromycin/clarithromycin) were observed in the range of 6.25–25 μg/mL against selected oral pathogens. |
Biogenic drug delivery system for Azithromycin and clarithromycin can be exploited as potential antimicrobial therapy. |
| Lin et al. (2018) | In Vivo | pH-responsive PLGA/chitosan nanospheres | Metronidazole and N-PTB, a host modulator. | Periodontal tissue cells in rat maxilla | The encapsulated drug was released rapidly from the nanospheres without significant initial burst release at pH 5.5. Following induced periodontitis, groups treated with drug encapsulated nanospheres, showed significant (p < 0.05) reduction in PBL after 4 days. After 21 days, group treated with N-PTB encapsulated nanospheres showed significant reduction in PBL. Moreover, application of drug encapsulated nanospheres, resulted in significantly (p < 0.05) reduced inflammation and increased collagen deposition when compared to the negative (periodontitis only) and positive (periodontitis + nanospheres) control groups. |
PLGA/chitosan nanospheres encapsulating metronidazole or N-PTB showed potential for modulating periodontitis progression. |
| Madi et al. (2018) | Clinical | nDOX gel in chitosan polymer matrix polymer, and dispersed in PVA | Doxycycline | Periodontal tissue cells | After a period of 1 and 3 months, the combination of SRP + nDOX showed significant (p < 0.05) reduction in probing depth and attachment gain compared with SRP + DOX and SRP + placebo chitosan. Levels of inflammatory mediators reduced significantly reduced in all treatment groups, after 1 month. | Treatment with nDOX gel as an adjunct to SRP improves clinical parameters and inflammatory markers within a 3 month period. |
| Lecio et al. (2020) | Parallel, double-blind, randomized, placebo-controlled clinical trial | PLGA nanospheres | Doxycycline | Periodontal tissue cells | After 3 months of treatment with 20% DOX containing PLGA nanospheres, the percentage of sites presenting PD reduction and CAL gain ≥ 2 mm was significantly higher (p < 0.05) than that of the control group. Application of PLGA/DOX nanospheres resulted in significant (p < 0.05) increase in anti-inflammatory IL-10 and reduction in IL-8, IFN-y, IL-6, and IL-17 levels. Also, significant (p < 0.05) reduction in periodontal pathogens and mean HbA1C percentage were observed. |
Locally applied PLGA/DOX nanospheres represent an adjunctive therapeutic approach in the treatment of periodontal disease in type-2 diabetes patients. Additionally in deep pockets, they may help in local modulation of anti- and pro-inflammatory cytokines, microbial reduction, improving clinical parameters. |
| Bai et al. (2021) | In Vitro + In Vivo | ROS-responsive drug delivery system based on PDA functionalized mesoporous silica nanoparticles | Minocycline hydrochloride | Macrophages | Synergistic action of PDA and MH in the nanoparticles, polarized macrophages from pro-inflammatory M1 to anti-inflammatory M2 phenotype. In addition PDA exhibited effective scavenging of ROS. Application of PDA/MH NPs in rat periodontitis models resulted in prevention of alveolar bone loss, without any adverse effects. |
Potential for reprogramming the inflammatory microenvironment in periodontitis by polarizing macrophages into the anti-inflammatory M2 phenotype and scavenging ROS. |
| de Carvalho Bernardo et al. (2021) | In Vitro | AgNPs combined with lyophilized HESc plant extract (seed/flower) | Syzygium cumini seeds and flowers | A. naeslundii, C. albicans, F. nucleatum, S. aureus, S. epidermidis, S. mutans, S. oralis and V. dispar | HESc (seed) and HESc (flower) extracts when used alone or in combined with AgNPS were effective against all the studied organisms, albeit in differing MIC levels (HESc extract alone − 648.4–5,187.5 μg/mL; AgNPs + HESc extract − 31.2–2,000 μg/mL). | Hydroalcoholic extracts of S. cumini seeds and flower have antimicrobial action against medical/dental pathogens with species-dependent MIC. Addition of AgNPs to HESc significantly lowered the MIC. |
| Toledano-Osorio et al. (2021) | In Vitro | Composite membrane made from a polymeric blend and 20 nm SiO2 and functionalized with drug. | Zinc or doxycycline | Osteoblast-like cells | The blending of SiO2-NPs in the tested non-resorbable polymeric scaffold improves expression of osteogenic genes over the control membranes. Doxycycline doping of experimental scaffolds attained the best results, encountering up-regulation of BMP-2, ALP, OPG, TGFβ-1 and TGFβ-R1. Membranes with zinc induced a significant increase in the expression of Col-I, ALP and TGF β1. Both, zinc and doxycycline functionalized membranes enormously down-regulated the expression of RANKL. |
Doxycycline doped membranes may be a potential candidate for use in GBR procedures in several challenging pathologies, including periodontal diseases. |
| Dhingra et al. (2022) | In Vitro | Mucoadhesive drug delivery chip fabricated using gelatin, glycerol, sodium alginate and AgNPs | Glutaraldehyde | Pseudomonas aeruginosa (for MIC) and Murine macrophages (for cell viability) | While the AgNPs in the drug delivery chip had a MIC ≥ 7.5 μg/ml against P. aeruginosa, murine macrophage cells exhibited 93% viability after 24 h incubation with mucoadhesive AgNP chips. | The novel AgNP chip showed dimensional stability, minimal effect on murine macrophage cell viability, and significant antimicrobial activity against P. aeruginosa. |
CFU – Colony forming unit(s); MIC – Minimum inhibitory concentration; TC – Tetracycline; NPs – Nanoparticles; CT – Computed tomography; DOX – Doxycycline; PVA – Poly (vinyl alcohol); SRP – Scaling and root planning; PG – Composite of PLGA (Poly lactide glycolic acid) and GT (Gum tragacanth); TCH – Tetracycline hydrochloride; AuNP – Gold nanoparticles; N-PTB – N-phenacylthiazolium bromide; PBL – Periodontal bone loss; nDOX – Nanostructured doxycycline; SRP – Scaling and root planning; PD – Probing depth; CAL – Clinical attachment level; IL – Interleukin; IFN – Interferon, HbA1C – Glycated hemoglobin; ROS – Reactive oxygen species; PDA – Polydopamine; MH – Minocycline hydrochloride; AgNPs – Silver nanoparticles; HESc – Hydroalcoholic extract of Syzygium cumini; SiO2 – Silicon dioxide; BMP – Bone morphogenic protein; ALP – Alkaline phosphatase; OPG- Osteoprotegerin, TGF – Transforming growth factor; Col – Collagen; RANKL - Receptor activator of NF-κB ligand; GBR – Guided bone regeneration.
3. Results
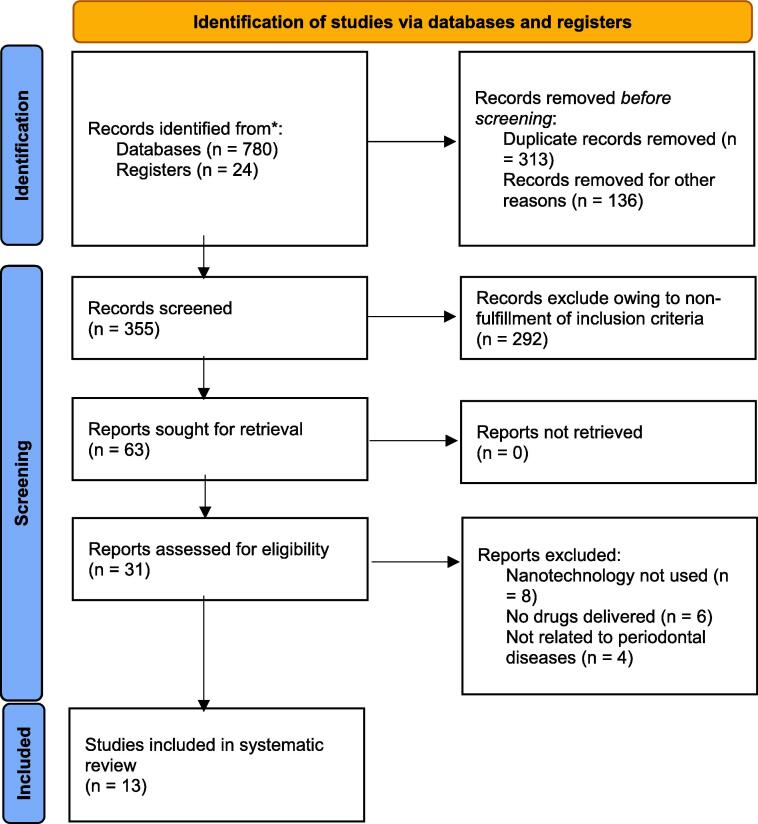
See Fig. 1.
Fig. 1.
PRISMA chart showing the process of study selection for the review. The articles finally included for review were 13 studies reporting about nanoparticle based drug delivery for periodontal conditions including in vitro, in vivo and clinical data (Wayakanon et al., 2013, Madhumathi and Sampath Kumar, 2014, Lee et al., 2016, Mahmoud and Samy, 2016, Ranjbar-Mohammadi et al., 2016, Emmanuel et al., 2017, Lin et al., 2018, Madi et al., 2018, Lecio et al., 2020, Bai et al., 2021, de Carvalho Bernardo et al., 2021, Toledano-Osorio et al., 2021, Dhingra et al., 2022).
3.1. Characteristics of reviewed studies
A total of 780 titles were obtained through database search and an additional 24 titles were included through registry searches. Excluding duplicates and abstracts not fulfilling the inclusion criteria, 63 articles were included for full text review, out which 13 studies were included for the final systematic review. Specific characteristics of the reviewed studies including study design, nature of nanoparticle used, drug(s) delivered, target cell/tissue/organism, outcomes and conclusions are comprehensively elaborated in Table 1.
While majority of the 13 reviewed studies were based on in vitro experiments, 3 studies reported clinical data (Mahmoud and Samy, 2016, Madi et al., 2018, Lecio et al., 2020), one study evaluated in vivo data (Lin et al., 2018), and 2 studies combined in vitro and in vivo analysis (Lee et al., 2016, Bai et al., 2021). All the studies except one (de Carvalho Bernardo et al., 2021), used an antibiotic, antibacterial or antimicrobial drug delivered using nanotechnology to target periodontal pathogens, inflammatory cells and/or periodontal cells. de Carvalho et al. (2021) reported the in vitro use of hydrophilic extracts of herbal (Syzygium cumini) seeds and flowers in combination with silver nanoparticles (AgNPs) against periodontal pathogens. While all the clinical studies evaluated the role nano-doxycycline (nano-DOX) on resolution of periodontitis after scaling and root planning, the in vivo studies employed periodontal tissue sites in rat and beagle dog models. On the contrary, in vitro studies utilized a range of bacterial cultures comprising frequently reported periodontal pathogens, osteoblast cell cultures, periodontal tissue cells and macrophages.
The reviewed studies reported usage of a wide range of nanoparticles (NPs), nanocarriers and nanometric scaffolds for drug delivery. These included nanoscale polymersomes, apatitic nanocarriers composed of calcium deficient hydroxyapatite (CDHA), poly(d,l-lactide-co-glycolide acid) (PLGA) and chitosan NPs, nano-DOX, composite nanofibers of PLGA and gum tragacanth (GT), silver (Ag) and gold (Au) NPs, mesoporous silica (SiO2) NPs, and PLGA nanospheres. Among the drugs delivered in the reviewed studies, tetracycline and doxycycline were the predominantly used antibiotics. Additionally, minocycline, metronidazole, azithromycin, clarithromycin and glutaraldehyde were also delivered using nanoparticles. Although the measured in vitro outcomes varied across the reviewed studies, a general consensus could be inferred regarding the inhibitory role of drug loaded NPs on periodontal pathogens, in addition to enhancing alkaline phosphatase (ALP) activity in osteoblast cells and a potential to favorably modulate the immune microenvironment. While in vivo studies, in general, reported delaying the progression of periodontitis and accelerated new bone formation, clinical studies observed decrease in inflammatory markers of periodontitis, when nanoparticle based drug delivery was used.
4. Discussion
Increased prevalence of periodontitis is attributed to the lack of patient compliance during the maintenance phase and the progressive destruction of the tooth-supporting tissue (Hajishengallis and Lamont 2012). The pathophysiology of the periodontal diseases is associated to the disproportionate host immune response towards the colonization of the bacterial pathogens (Bao et al., 2018). The chronicity of the periodontal diseases is evident upon the release of the pro-inflammatory cytokines and reactive oxygen species (ROS) due to the activation of the recruited immune cells (Alvarez Echazú et al., 2018, Bao et al., 2018). The conventional management protocol for periodontitis involves mechanical debridement of the root surfaces with or without periodontal flap surgeries. Adjuvant therapy with local and systemic medications like antimicrobial agents have also proven efficacious. However, long-term administration of the systemic antibiotics leads to development of resistance and have deteriorated treatment outcomes (McGowan et al. 2018). Thus underlining the need for local drug delivery systems which primarily aim at reduction of the sub-gingival microbiome, and secondarily help modulate the immune response and promote new bone and soft tissue regeneration. Recently, the usage of NPs in drug delivery systems have found to be efficacious in minimizing the pathogenic periodontal microbiome. Therefore the present systematic review was based on the above premise, with specific impetus towards shedding light on the available nanoparticle based local drug delivery systems.
According to Lee et al. (2016), PLGA-derived nanocarriers proved effective when loaded with tetracycline and lovastatin in managing canine models with periodontitis. In human therapy, they were not only biocompatible but also inhibited the bacterial growth significantly (Lee et al., 2016). Interestingly, in spite of being reported as cytotoxic to gingival fibroblasts, 5 mg/ml of Chitosan nanoparticles alone showed significant inhibition of the periodontal pathogens, in an in-vitro study (Arancibia et al., 2013). The reported mechanism of action of chitosan nanoparticles is in reducing the viability of periodontal pathogens like P. gingivalis and modulation of prostaglandin production by the gingival fibroblasts (Arancibia et al., 2013). Similar studies, illustrate that improved periodontal clinical parameters like probing pocket depth, gingival bleeding and clinical attachment were evident with usage of chitosan loaded metronidazole, ornidazole or chlorhexidine. In addition, prolonged bioavailability of the loaded medication in the periodontal tissues were also reported (Joshi et al., 2016). In the present review, PLGA/chitosan NPs were used in two, to deliver combinations of tetracycline and lovastatin, and metronidazole and N-phenacylthiazolium bromide (N-PTB) (Lee et al., 2016, Lin et al., 2018). PLGA/chitosan NPs with tetracycline and lovastatin, not only demonstrated greater in vitro antibacterial activity than PLGA/chitosan alone, but also resulted in enhanced ALP activity, which translated to increased new bone formation, in vivo (Lee et al., 2016). Similarly, PLGA/chitosan NPs with metronidazole and N-PTB significantly modulated progression of periodontal inflammation, as evidenced by reduced PBL after 21 days, in a rat model of induced periodontitis (Lin et al., 2018). Owing to its biocompatibility, chitosan polymer matrix was also reportedly used as a carrier for nano-DOX in all the reviewed clinical studies (Mahmoud and Samy, 2016, Madi et al., 2018, Lecio et al., 2020). Additionally, PLGA was used as a composite nanofiber along with gum tragacanth, a naturally occurring, biodegradable anionic polymer. This blended electrospun nanopolymer with tetracycline was used in vitro to inhibit bacterial growth and demonstrated potential for immediate burst release initially and sustained drug release thereafter (Ranjbar-Mohammadi et al., 2016).
Apatitic NPs such as nanocrystalline hydroxyapatite have proven cytocompatitbility and have been used clinically for periodontal bone regeneration and bone healing (Patel et al., 2020). However, their antibacterial efficacy or immune modulatory role in the treatment of periodontitis has not been reported in the literature. Madhumathi and Sampath Kumar (2014) reported the in vitro use of CDHA nanocarriers for targeted delivery of tetracycline against periodontal pathogens. According to them, delivering tetracycline through CDHA nanocarriers resulted in a fivefold increase in bacterial minimum inhibitory concentration (MIC) and resulted in greater affinity of periodontal fibroblast cells, indicating a potential role for CDHA NPs in tissue regeneration. Metallic NPs of gold, silver and platinum have been postulated to inhibit bacterial growth in cultures, thereby underscoring their antimicrobial potential (Itohiya et al., 2019). Moreover, in vitro studies have reported the non-cytotoxic, biocompatible nature of metallic NPs comprising bismuth-salicylate, calcium and zinc, when cultured alongside human gingival and oral mucosal fibroblasts (Osorio et al., 2016, Vega-Jiménez et al., 2017). While calcium NPs were reported to precipitate calcium phosphate deposits, which might have a role in enhancing bone healing (Osorio et al., 2016), gold NPs are capable of acting as an adjuvant for bone induction (Jadhav et al., 2018). Nevertheless, the use metallic NPs as a means of local drug delivery is sparsely reported in the literature.
An interesting aspect of the present review, was the reporting of drug delivery in synergy with metallic NPs. Emmanuel et al. (2017) reported the synergistic antimicrobial efficacy of azithromycin and clarithromycin incorporated AuNPs against the periodontal microbiome. Similarly, herbal extracts of Syzygium cumini, which are proven to have an antimicrobial effect, had significantly lowered MIC against common periodontal pathogens when combined with AgNPs (de Carvalho Bernardo et al., 2021). Even pathogens implicated in the etiopathology of peri-implantitis, such as A. naeslundii, C. albicans, F. nucleatum, S. aureus, S. epidermidis, S. mutans, S. oralis and V. dispar were effectively inhibited by the combination of AgNPs and Syzygium cumini extracts (de Carvalho Bernardo et al., 2021). Moving a step further, Dhingra et al. (2022) demonstrated significant antimicrobial efficacy of a silver nanoparticle based mucoadhesive glutaraldehyde drug delivery chip. Despite the aforementioned promising results, further studies are needed to investigate these novel formulations containing metallic NPs, especially through in vivo studies and controlled clinical trials.
In Tissue engineering, the production of nanoscale biomimetic scaffolds have been recently revolutionized by Electro-spinning. This has also enabled the incorporation of therapeutic agents into the non-woven nanofiber meshes for controlled drug delivery systems. In association with biomaterials these nanoparticles facilitate sustained release and prolonged residence time of the drugs in the oral cavity. When the AgNPs were loaded into the electrospun nanofibers, they demonstrated enhanced bio-functionality. Hence, this biomaterial is beneficial in reduction of periodontitis and gingivitis when used as an oral wound dressing with local drug delivery systems (Lee et al., 2016). Additionally, these nanofibers are capable of modulating periodontal inflammatory mechanism to reduce bone resorption and accentuate regeneration (Rathor et al., 2017).
The current review also analyzed the efficacy of specific forms of vesicular nano carriers such as liposomes, lipid and polymeric nanoparticles, nanocrystals, dendrimers and nanofibers, which have possible implications in the management of periodontal diseases (Table 2). Based on a review, Erdoğar et al. (2018) reported that vesicular NPs such as polymersomes, nanospheres, liposomes and nanocapsules are excellent drug carriers owing to their hydrophilic nature capable of stabilizing lipid drug molecules. Moreover, vesicular NPs are capable of facilitating oral administration of controlled release drug formulations to achieved desirable pharmacokinetic profiles (Erdoğar et al., 2018). An extended period of antibacterial activity with sustained release of antimicrobial agents has been observed with micro and nanosphere encapsulated peptide loaded PLGA. The rapid release of peptides from PLGA NPs is even capable of disrupting bacterial co-existence, such as in the case of inhibiting P. gingivalis and Streptococcus gordonni induced dual-species biofilm (Mahmoud et al., 2020). The above mechanism was further improvised clinically by addition of doxycycline to PLGA nanospheres. Lecio et al. (2020), demonstrated significant reduction in clinical periodontitis along with decreased pro-inflammatory cytokines (IL-8, IFN-y, IL-6, and IL-17) and increased anti-inflammatory markers (IL-10), when PLGA/DOX nanospheres were locally administered in Type-2 diabetes patient with periodontal disease.
Table 2.
Different forms of Periodontal Nanoparticle Based Local drug delivery systems.
| Delivery form of the Nano particles | Drug | Polymer matrix | Biodegradability | Brand | Mechaniscm of Action |
|---|---|---|---|---|---|
| Fiber | Tetracycline hydrochloride | Ethylene Vinyl Acetate | Non-biodegradable | Actisite | Inhibits Protein synthesis |
| Gel | Doxycycline | Poly(di-lactide) and N methyl pyridine | Bioabsorbable in 21 days | Atridox | Inhibits Protein synthesis |
| Microspheres | Minocycline hydrochloride | Poly(glycolide-co-dl-lactide) | Biodegradable (14–21 days) | Arestin | Inhibits Protein synthesis |
| Chip | Chlorhexidine gluconate | Gelatin crosslinked with glutaraldehyde | Biodegradable (7 to 10 days) |
Periochip | Bactericidal via cytoplasmic contents precipitation and cell wall destruction |
| Gel | Metronidazole benzoate | Glycerylmono-oleate and sesame oil | Biodegradable | Elyzol | Interferes with nucleic acid metabolism |
| Gel | Chlorhexidine | Xanthan gel | Biodegradable | Chlo-Site | Bactericidal via cytoplasmic contents precipitation and cell wall destruction |
| Oinment | Minocycline HCl | Hydroxy-cellulose, aminoalkly-methacrylate, triacetine and Glycerine | Biodegradable | Dentomycin | Inhibits Protein synthesis |
| Type-1 Collagen membrane | Chlorhexidine | Xanthan gel | Biodegradable | Pericol-CG | Bactericidal via cytoplasmic contents precipitation and cell wall destruction |
| Biodegradable mix insyringe | Minocycline | Poly(glycolide-co-dl-lactide) | Biodegradable (14–21 days) | Dentomycine | |
| Film | Chlorhexidine | Xanthan gel | Biodegradable | Periochip | Bactericidal via cytoplasmic contents precipitation and cell wall destruction |
5. Conclusion
The emergence of nanomedicines and their current implications in the successful treatment of periodontal diseases with an altered approach is highlighted in the present review. This is largely made possible through local drug delivery systems reaching the focus tissues, cells, or subcellular compartments in the periodontal pockets. Nanoparticle based local drug delivery systems establish direct contact on the biofilms or host cells and hence warrant more efficacy of the incorporated antibiotic or anti-inflammatory drugs. Promising results in this field of nanotechnology and their increasing use as an adjuvant has revolutionized prognosis and outcomes of conventional periodontal treatment modalities. Although complete regeneration of the periodontal apparatus comprising cementum, periodontal ligament and bone is hard to achieve, introduction of nanoparticle based drug delivery systems could help realize the goal. In spite of popular utilization of the nanocomposites and nano porous materials in the field of dentistry, further research and development of nanomaterials specific for clinical periodontal disease management through local delivery drugs and bioactive molecules is highly recommended.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Acknowledgement
The author acknowledges Department of Periodontology, College of Dentistry, King Saud University, and its Teaching Staff, Colleagues, Patients, Participants, Technicians and any member help to pursue and finish this study. The author expresses sincere gratitude to Dr. Marwa Y. Shaheen for supporting in the review process.
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
References
- Alvarez Echazú M.I., Olivetti C.E., et al. Development of pH-responsive biopolymer-silica composites loaded with Larrea divaricata Cav. extract with antioxidant activity. Colloids Surf. B Biointerfaces. 2018;169:82–91. doi: 10.1016/j.colsurfb.2018.05.015. [DOI] [PubMed] [Google Scholar]
- Aminu N., Baboota S., et al. Development and evaluation of triclosan loaded poly-ε-caprolactone nanoparticulate system for the treatment of periodontal infections. J. Nanopart. Res. 2013;15(11):2075. [Google Scholar]
- Arancibia R., Maturana C., et al. Effects of chitosan particles in periodontal pathogens and gingival fibroblasts. J. Dent. Res. 2013;92(8):740–745. doi: 10.1177/0022034513494816. [DOI] [PubMed] [Google Scholar]
- Bai B., Gu C., et al. Polydopamine functionalized mesoporous silica as ROS-sensitive drug delivery vehicles for periodontitis treatment by modulating macrophage polarization. Nano Res. 2021;14(12):4577–4583. [Google Scholar]
- Bao X., Zhao J., et al. Polydopamine Nanoparticles as Efficient Scavengers for Reactive Oxygen Species in Periodontal Disease. ACS Nano. 2018;12(9):8882–8892. doi: 10.1021/acsnano.8b04022. [DOI] [PubMed] [Google Scholar]
- Benatti B.B., Campos-Júnior J.C., et al. Effects of a Mikania laevigata extract on bone resorption and RANKL expression during experimental periodontitis in rats. J. Appl. Oral Sci. 2012;20(3):340–346. doi: 10.1590/S1678-77572012000300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferata E.A., Terraza-Aguirre C., et al. Interleukin-35 inhibits alveolar bone resorption by modulating the Th17/Treg imbalance during periodontitis. J. Clin. Periodontol. 2020;47(6):676–688. doi: 10.1111/jcpe.13282. [DOI] [PubMed] [Google Scholar]
- de Carvalho Bernardo W.L., Boriollo M.F.G., et al. Antimicrobial effects of silver nanoparticles and extracts of Syzygium cumini flowers and seeds: Periodontal, cariogenic and opportunistic pathogens. Arch. Oral Biol. 2021;125 doi: 10.1016/j.archoralbio.2021.105101. [DOI] [PubMed] [Google Scholar]
- Dhingra K., Dinda A.K., et al. Mucoadhesive silver nanoparticle-based local drug delivery system for peri-implantitis management in COVID-19 era. Part 1: antimicrobial and safety in-vitro analysis. J. Oral Biol. Craniofac. Res. 2022;12(1):177–181. doi: 10.1016/j.jobcr.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte P.M., Napimoga M.H., et al. The expression of antioxidant enzymes in the gingivae of type 2 diabetics with chronic periodontitis. Arch. Oral Biol. 2012;57(2):161–168. doi: 10.1016/j.archoralbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Dubar M., Lizambard M., et al. In-situforming drug-delivery systems for periodontal treatment: current knowledge and perspectives. Biomed. Mater. 2021;16(6) doi: 10.1088/1748-605X/ac254c. [DOI] [PubMed] [Google Scholar]
- Emmanuel R., Saravanan M., et al. Antimicrobial efficacy of drug blended biosynthesized colloidal gold nanoparticles from Justicia glauca against oral pathogens: A nanoantibiotic approach. Microb. Pathog. 2017;113:295–302. doi: 10.1016/j.micpath.2017.10.055. [DOI] [PubMed] [Google Scholar]
- Erdoğar N., Akkın S., et al. Nanocapsules for Drug Delivery: An Updated Review of the Last Decade. Recent Pat. Drug Deliv. Formul. 2018;12(4):252–266. doi: 10.2174/1872211313666190123153711. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G., Lamont R.J. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012;27(6):409–419. doi: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau H., Rohanizadeh R., et al. A mini-review on novel intraperiodontal pocket drug delivery materials for the treatment of periodontal diseases. Drug Deliv. Transl. Res. 2014;4(3):295–301. doi: 10.1007/s13346-013-0171-x. [DOI] [PubMed] [Google Scholar]
- Itohiya H., Matsushima Y., et al. Organic resolution function and effects of platinum nanoparticles on bacteria and organic matter. PLoS ONE. 2019;14(9):e0222634. doi: 10.1371/journal.pone.0222634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav K., Hr R., et al. Phytosynthesis of gold nanoparticles: Characterization, biocompatibility, and evaluation of its osteoinductive potential for application in implant dentistry. Mater. Sci. Eng. C Mater Biol. Appl. 2018;93:664–670. doi: 10.1016/j.msec.2018.08.028. [DOI] [PubMed] [Google Scholar]
- John M.T., Michalowicz B.S., et al. Network meta-analysis of studies included in the Clinical Practice Guideline on the nonsurgical treatment of chronic periodontitis. J. Clin. Periodontol. 2017;44(6):603–611. doi: 10.1111/jcpe.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi D., Garg T., et al. Advanced drug delivery approaches against periodontitis. Drug Deliv. 2016;23(2):363–377. doi: 10.3109/10717544.2014.935531. [DOI] [PubMed] [Google Scholar]
- Jung T., Kamm W., et al. Biodegradable nanoparticles for oral delivery of peptides: is there a role for polymers to affect mucosal uptake? Eur. J. Pharm. Biopharm. 2000;50(1):147–160. doi: 10.1016/s0939-6411(00)00084-9. [DOI] [PubMed] [Google Scholar]
- Kamaly N., Yameen B., et al. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016;116(4):2602–2663. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinane D.F., Bartold P.M. Clinical relevance of the host responses of periodontitis. Periodontology. 2007;2000(43):278–293. doi: 10.1111/j.1600-0757.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- Lecio G., Ribeiro F.V., et al. Novel 20% doxycycline-loaded PLGA nanospheres as adjunctive therapy in chronic periodontitis in type-2 diabetics: randomized clinical, immune and microbiological trial. Clin. Oral Investig. 2020;24(3):1269–1279. doi: 10.1007/s00784-019-03005-9. [DOI] [PubMed] [Google Scholar]
- Lee B.S., Lee C.C., et al. Controlled-release of tetracycline and lovastatin by poly(D, L-lactide-co-glycolide acid)-chitosan nanoparticles enhances periodontal regeneration in dogs. Int. J. Nanomed. 2016;11:285–297. doi: 10.2147/IJN.S94270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.H., Feng F., et al. Modulation of periodontitis progression using pH-responsive nanosphere encapsulating metronidazole or N-phenacylthialzolium bromide. J. Periodontal Res. 2018;53(1):22–28. doi: 10.1111/jre.12481. [DOI] [PubMed] [Google Scholar]
- Madhumathi K., Sampath Kumar T.S. Regenerative potential and anti-bacterial activity of tetracycline loaded apatitic nanocarriers for the treatment of periodontitis. Biomed. Mater. 2014;9(3) doi: 10.1088/1748-6041/9/3/035002. [DOI] [PubMed] [Google Scholar]
- Madi M., Pavlic V., et al. The anti-inflammatory effect of locally delivered nano-doxycycline gel in therapy of chronic periodontitis. Acta Odontol. Scand. 2018;76(1):71–76. doi: 10.1080/00016357.2017.1385096. [DOI] [PubMed] [Google Scholar]
- Mahmoud M.M., Samy W.M. Enhanced Periodontal Regeneration by Novel Single Application Sustained Release Nano-Structured Doxycycline Films. Curr. Drug Deliv. 2016;13(6):899–908. doi: 10.2174/1567201813666151113122752. [DOI] [PubMed] [Google Scholar]
- Mahmoud M.Y., Sapare S., et al. Rapid Release Polymeric Fibers for Inhibition of Porphyromonas gingivalis Adherence to Streptococcus gordonii. Front. Chem. 2020;7 doi: 10.3389/fchem.2019.00926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan K., McGowan T., et al. Optimal dose and duration of amoxicillin-plus-metronidazole as an adjunct to non-surgical periodontal therapy: A systematic review and meta-analysis of randomized, placebo-controlled trials. J. Clin. Periodontol. 2018;45(1):56–67. doi: 10.1111/jcpe.12830. [DOI] [PubMed] [Google Scholar]
- Muhamad, I.I., Selvakumaran, S., et al., 2015. Designing Polymeric Nanoparticles for Targeted Drug Delivery System.
- Naskar S., Das S.K., et al. A Review on Designing Poly (Lactic-co-glycolic Acid) Nanoparticles as Drug Delivery Systems. Pharm. Nanotechnol. 2021;9(1):36–50. doi: 10.2174/2211738508666201214103010. [DOI] [PubMed] [Google Scholar]
- Osorio R., Alfonso-Rodríguez C.A., et al. Bioactive Polymeric Nanoparticles for Periodontal Therapy. PLoS ONE. 2016;11(11):e0166217. doi: 10.1371/journal.pone.0166217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D.K., Jin B., et al. Osteogenic potential of human mesenchymal stem cells on eggshells-derived hydroxyapatite nanoparticles for tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2020;108(5):1953–1960. doi: 10.1002/jbm.b.34536. [DOI] [PubMed] [Google Scholar]
- Ranjbar-Mohammadi M., Zamani M., et al. Electrospinning of PLGA/gum tragacanth nanofibers containing tetracycline hydrochloride for periodontal regeneration. Mater. Sci. Eng., C. 2016;58:521–531. doi: 10.1016/j.msec.2015.08.066. [DOI] [PubMed] [Google Scholar]
- Rathor S., Bhatt D.C., et al. A Comprehensive Review on Role of Nanoparticles in Therapeutic Delivery of Medicine. Pharm. Nanotechnol. 2017;5(4):263–275. doi: 10.2174/2211738505666171113130639. [DOI] [PubMed] [Google Scholar]
- Toker H., Yuce H., et al. The effect of colchicine on alveolar bone loss in ligature-induced periodontitis. Brazil. Oral Res. 2019;33 doi: 10.1590/1807-3107bor-2019.vol33.0001. [DOI] [PubMed] [Google Scholar]
- Toledano-Osorio M., Manzano-Moreno F.J., et al. Doxycycline-doped membranes induced osteogenic gene expression on osteoblastic cells. J. Dent. 2021;109 doi: 10.1016/j.jdent.2021.103676. [DOI] [PubMed] [Google Scholar]
- Van Dyke T.E. Control of inflammation and periodontitis. Periodontol. 2007;2000(45):158–166. doi: 10.1111/j.1600-0757.2007.00229.x. [DOI] [PubMed] [Google Scholar]
- Vega-Jiménez A.L., Almaguer-Flores A., et al. Bismuth subsalicylate nanoparticles with anaerobic antibacterial activity for dental applications. Nanotechnology. 2017;28(43) doi: 10.1088/1361-6528/aa8838. [DOI] [PubMed] [Google Scholar]
- Wayakanon K., Thornhill M.H., et al. Polymersome-mediated intracellular delivery of antibiotics to treat Porphyromonas gingivalis-infected oral epithelial cells. FASEB J. 2013;27(11):4455–4465. doi: 10.1096/fj.12-225219. [DOI] [PubMed] [Google Scholar]
- Yao W., Xu P., et al. RGD functionalized polymeric nanoparticles targeting periodontitis epithelial cells for the enhanced treatment of periodontitis in dogs. J. Colloid Interface Sci. 2015;458:14–21. doi: 10.1016/j.jcis.2015.07.032. [DOI] [PubMed] [Google Scholar]